Recent data from mouse models suggest that some phenotypes of X-linked lymphoproliferative disease (XLP) result from impaired T:B-cell interactions. Hislop and colleagues now provide evidence that this may contribute to abnormal responses to Epstein-Barr virus (EBV) in XLP.1
XLP is a complex immunodeficiency frequently characterized by fatal responses to EBV infection and hemophagocytosis lymphohistiocytosis (HLH).2,3 Patients with XLP also exhibit a wide range of other immune abnormalities that include lymphoproliferative disorders/lymphomas and dys/hypogammaglobulinemias associated with defective germinal center (GC) formation and a lack of memory IgG+ cells. Other phenotypes now recognized include a nearly complete absence of natural killer T (NKT) cells, a rare innate T-cell population. Approximately 12 years ago, it was shown that the majority of cases of XLP are caused by mutations affecting the signaling lymphocytic activation molecule (SLAM)–associated protein (SAP), a small adaptor molecule that binds to and is involved in signaling downstream of the SLAM family of immunomodulatory receptors, including SLAM, 2B4, Ly108, CD84, Ly9, and CRACC.2-4 However, how SAP deficiency causes this diverse range of phenotypes was not readily apparent.
Recent data from studies of antibody-mediated immune defects in a mouse model of SAP deficiency have provided insight into a potential common theme to these pathophysiologies. SAP-deficient mice exhibit defects in long-term humoral (antibody) immunity associated with defective formation of GCs,5 the site where B cells proliferate and undergo antibody gene class switching and hypermutation to generate high-affinity isotypes in response to antigen and signals from T cells, such as CD40L. Similar findings have now been reported in patients with XLP.3 Cell-transfer experiments in mice argued that the GC defect is T-cell intrinsic.4,5 Recently, the use of intravital imaging of GC formation has provided new insight into this phenotype. These studies demonstrated that SAP-deficient T cells exhibit a specific defect in interactions with B cells, while retaining relatively normal interactions with dendritic cells,6 the antigen-presenting cells that first activate T cells. Thus, in response to immunization, SAP-deficient T cells appear to be activated normally, but are unable to deliver signals to B cells due to a selective T:B-cell adhesion defect.6,7 These findings raised the question of whether the phenotypes of XLP are the result of altered T:B-cell interactions.4
In this issue of Blood, Hislop and colleagues1 provide evidence that XLP cytotoxic lymphocytes may indeed have specific defects in cytolysis of B cells compared with other cells. Previous data from several groups had shown that NK and CD8+ T cells from XLP1 patients were unable to kill EBV-infected targets, while maintaining normal or even elevated cytolysis in redirected killing assays or alloantigen assays that used noncognate target cells.3,8,9 Those studies emphasized specific responses to EBV and the role of SLAM family receptor 2B4, whose ligand is highly induced by EBV. By evaluating EBV-specific cytotoxic T lymphocytes (CTL) isolated from XLP patients and normal controls, Hislop and colleagues were able to extend these findings by showing that the XLP1 CTL showed a relative decrease in interferon-γ production and killing of cognate EBV-transformed B cells compared with fibroblast targets pulsed with the same EBV antigen.1 Thus, the specificity of the defective responses to EBV may reflect the B-cell tropism of the virus.
Hislop et al1 further showed that treatment of cells with antibodies against the SLAM family members 2B4 and NTB/A (SlamF6) rescued cytolysis of B-cell targets. However, because these antibodies could potentially be either stimulating or blocking, the issue may be better addressed via expression of SLAM family member ligands on fibroblast targets or the use of RNA interference to reduce 2B4 and NTB/A expression. Similarly, it will be important to examine CD8+ T-cell cytolysis directed against other antigens when presented on B cells versus non-B-cell targets. Such experiments will help determine whether B-cell tropism is truly key to the markedly impaired responses to EBV.
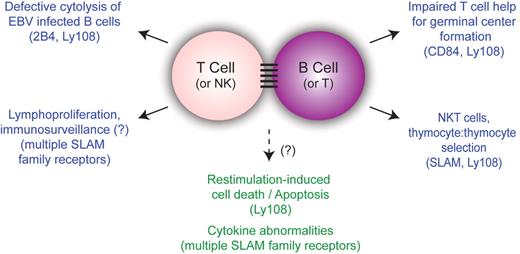
This article provides further evidence supporting a unifying hypothesis for many of the phenotypes of XLP where T cells (and NK cells) are activated, yet fail to deliver effector functions effectively to B-cell targets.4 The specific defect in clearance of EBV may therefore reflect the B-cell affinity of the virus, rather than differences in cellular responses to EBV. Similarly, the defects in humoral immunity can be attributed to impaired T:B-cell interactions preventing GC formation. Such defects may also contribute to lymphomas, if immunosurveillance against transformed B cells is impaired. Finally, it is of interest that NKT cells are selected in the thymus by interactions with other lymphocytes, unlike conventional T cells that are selected on the thymic stroma, suggesting that altered lymphocyte:lymphocyte interactions may be a common theme to these diverse observations.4 Thus, SAP and SLAM family members may be particularly important for mediating interactions between lymphocyte populations (see figure).
Unifying model for phenotypes of X-linked lymphoproliferative syndrome. Although the phenotypes of XLP are diverse, many of them are understood in the context of impaired T:B-cell interactions. SLAM family members implicated in specific phenotypes are shown in parentheses. Phenotypes that have not been directly linked to lymphocyte:lymphocyte interactions are listed in the bottom center in green.
Unifying model for phenotypes of X-linked lymphoproliferative syndrome. Although the phenotypes of XLP are diverse, many of them are understood in the context of impaired T:B-cell interactions. SLAM family members implicated in specific phenotypes are shown in parentheses. Phenotypes that have not been directly linked to lymphocyte:lymphocyte interactions are listed in the bottom center in green.
This unifying theme may not account for all phenotypes in XLP. Recent data from several groups in both humans and mice demonstrate that SAP-deficient T cells have defects in restimulation-induced cell death (RICD) that could contribute to the lymphoproliferation and HLH phenotypes in XLP1 patients.10 Similarly, cytokine abnormalities have also been observed in XLP and SAP-deficient T cells.2 Whether altered lymphocyte: lymphocyte interactions also contribute to these phenotypes is unknown, but this does raise the question of whether lymphocyte:lymphocyte interactions may influence efficiency of RICD.
Nonetheless, by placing the defect in EBV clearance in the context of T:B-cell interactions, the work by Hislop and colleagues1 continues to extend our understanding of the diverse phenotypes of XLP. Intriguingly, recent mutations affecting 2 other proteins, the X-linked inhibitor of apoptosis (XIAP)11 and the IL-2–inducible T-cell kinase (ITK),12 have been linked to fatal infectious mononucleosis and XLP-like phenotypes. Whether altered lymphocyte:lymphocyte interactions also contribute to the pathophysiology associated with these mutations remains to be seen.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal