Abstract
Infection with the human T-cell leukemia virus-1 (HTLV-1) results in a variety of diseases including adult T-cell leukemia/lymphoma (ATL). Although the pathogenesis of these disorders is poorly understood, it involves complex interactions with the host immune system. Activation of infected T cells may play an important role in disease pathogenesis through induction of the oncogenic HTLV-1 Tax transactivator protein. To test this hypothesis, we employed transgenic mice in which Tax is regulated by the HTLV-1 LTR. T-cell receptor stimulation of LTR-Tax CD4+ T cells induced Tax expression, hyper-proliferation, and immortalization in culture. The transition to cellular immortalization was accompanied by markedly increased expression of the antiapoptotic gene, mcl-1, previously implicated as important in T-cell survival. Immortalized cells exhibited a CD4+CD25+CD3− phenotype commonly observed in ATL. Engraftment of activated LTR-Tax CD4+ T cells into NOD/Shi-scid/IL-2Rγ null mice resulted in a leukemia-like phenotype with expansion and tissue infiltration of Tax+, CD4+ lymphocytes. We suggest that immune activation of infected CD4+ T cells plays an important role in the induction of Tax expression, T-cell proliferation, and pathogenesis of ATL in HTLV-1–infected individuals.
Introduction
Human T-cell Leukemia Virus-1 (HTLV-1) is a CD4+ T cell–tropic human deltaretrovirus infecting 10-20 million people worldwide.1,2 HTLV-1 was the first human retrovirus isolated and is the causative agent of diverse diseases such as Adult T-cell Leukemia/Lymphoma (ATL), a fatal CD4+ T-cell Leukemia/Lymphoma and HTLV-1 Acquired Myelopathy/Tropical Spastic Paraparesis (HAM-TSP), a demyelinating disease of the spinal cord. Approximately 3%–5% of those infected with HTLV-1 will develop disease, while the majority of carriers remain asymptomatic. Development of HAM/TSP or ATL occurs after long clinically latent periods and specific disease phenotypes tend to be associated with particular routes and timing of infection.3 The mechanisms responsible for HTLV-1 pathogenesis remain poorly understood.
Development of HTLV-1–associated diseases is dependent upon expression of HTLV-1 gene products, most notably the HTLV-1 viral transcriptional transactivator, Tax4 and other regulatory proteins including the HBZ protein.5,6 Tax expression is required for viral replication, and can induce CD4+ T-cell immortalization. Tax enhances viral gene expression through interactions with cellular transcription factors at the HTLV-1 Long Terminal Repeat (LTR) promoter. Tax also interacts with many host proteins involved in cell survival, proliferation, genomic stability, and cell cycle regulation to promote altered cellular gene expression.4 Thus, Tax plays a critical role in regulating viral gene expression, as well as the host cellular response to infection. Recently, HBZ has also been shown to play an important role in HTLV-1 pathogenesis both through increased viral load, and enhancing T-cell proliferation in culture and transgenic mice.7,8 p12I, an activator of STAT5 antiapoptotic signaling is also likely to play a role in HTLV-1 transformation.9
Determining the cellular and viral events that regulate HTLV-1 gene expression is central to understanding pathogenesis of HTLV-1 infection. Our laboratory has previously shown that Tax expression in chronically infected human CD4+ T cells can be markedly enhanced by immune activation stimuli, such as PMA, PHA, or anti-CD3 antibody.10,11 These data suggest that immune stimulation of infected cells, through T-cell antigen receptor signaling, could regulate a balance of virus-host interactions leading to increased viral gene expression.
To elucidate whether immune activation events contribute to HTLV-1–associated-diseases, study of animal models of pathogenesis will be valuable. Although mouse models of Tax-associated oncogenesis have been described,12-14 a transgenic model of Tax-derived CD4+ T-cell oncogenesis, reflecting regulation of Tax by the authentic HTLV-1 LTR has yet to be established. Transgenic mice expressing Tax under the control of the LTR, are phenotypically normal until approximately 3 months of age, when they develop neurofibromas, and die at approximately 6 months due to wasting and tumor growth.15 These mice do not develop CD4+ T-cell disease and no Tax expression was detected in the thymus. We hypothesized that, as observed in HTLV-1–infected human T cells,10,11 immune activation stimuli might induce Tax expression in the transgenic T cells. This would allow us to examine whether the induction of Tax in these cells would alter CD4+ T-cell gene regulation and model progression of HTLV-1–associated diseases in humans.
There has been little characterization of the T cells of LTR-Tax transgenic mice.16 We sought to establish CD4+ T-cell immune phenotypes, examine whether immune activation could induce Tax expression, and determine whether induction of Tax could affect the properties of these CD4+ T cells. We have shown that activation of T cells from LTR-Tax transgenic mice results in induction of Tax expression, robust proliferation, and expression of multiple activation-associated cytokines. Furthermore, these cells exhibit sustained viability and proliferation in culture and are capable of inducing a leukemia-like phenotype when transferred into immunodeficient mice. Gene expression studies showed increased expression of the antiapoptotic protein, Mcl-1, whose role in HTLV-1 infection has not been established.17 These studies suggest that immune activation can induce Tax expression in T cells containing integrated HTLV-1 sequences, which in turn can lead to T-cell immortalization contributing to HTLV-1 pathogenesis.
Methods
Mice
Transgenic mice containing the HTLV-1 LTR directing Tax expression15 (gift of J. Green, National Cancer Institute, National Institutes of Health, Bethesda, MD) were crossed to the ICR strain. ICR LTR-Tax and littermate control mice were maintained in pathogen free conditions. Transgenic mice were identified by polymerase chain reaction (PCR) on mouse tail genomic DNA followed by gel electrophoresis, using Tax-specific primers. All experiments were approved by the RWJMS Institutional Animal Care and Use Committee. Mice were euthanized by CO2 inhalation, and spleen and lymph nodes were harvested for cell isolation. For histological analysis, organs were fixed in 10% buffered formalin phosphate. Paraffin embedded sections (5- to 6-μm) were stained with hematoxylin and eosin (Cancer Institute of New Jersey Immunohistochemistry Shared Resource) and examined microscopically. For adoptive transfer, LTR-Tax and littermate control CD4+ T cells (20 × 106) were injected intraperitoneally into 6-week-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice18 (The Jackson Laboratory). Upon evidence of disease (moribund status), autopsy was performed and spleen, blood, and lymph nodes were harvested for preparation of cells for flow cytometric analysis, CD4+ T-cell isolation, RNA and DNA isolation to confirm Tax positivity by PCR, and histologic examination.
Flow cytometric analysis and antibodies
Single-cell suspensions of splenocytes, lymph node cells, and peripheral blood were treated with erythrocyte lysis buffer (150mM NH4Cl,10mM KHCO3, 0.1mM Na2EDTA) and analyzed for cell-surface markers using immunofluorescence staining and flow cytometry (FACScan; BD Immunocytometry) by standard methods. Fluorescein isothiocyanate– or phycoerythrin-conjugated antibodies against CD4, CD3, CD25, CD69, CD62L, CD16/32, CD8, CD19, CD11b, and NK1.1 (eBioscience) were used for 2-color analysis. Dead cells were excluded by propidium iodide staining.
Isolation, activation, and long-term culture of CD4+ T cells
CD4+ T cells were purified from splenocytes by immunomagnetic sorting using negative selection (CD4+ T-cell Isolation Kit; Miltenyi Biotech) according to manufacturer's protocol. CD4+ T-cell purity was determined by flow cytometry using phycoerythrin-conjugated anti-CD4. Enriched CD4+ T cells (1 × 106/mL) were stimulated with plastic-bound anti-CD3 (prepared from 2C11 hybridoma) and soluble anti-CD28 (Pharmingen) in complete medium (RPMI 1640 medium supplemented with 10% fetal bovine serum, 2mM glutamine, 100 U/mL penicillin, and 100 g/mL streptomycin [Invitrogen]) with addition of recombinant human interleukin-2 (IL-2; 20 U/mL; Proleukin, Chiron) for 3 days in T-25 flasks as previously described.19 For long-term culture, these activated CD4+ T cells were maintained in the same medium without stimulatory antibodies but with increased IL-2 supplementation (80 U/mL). At biweekly intervals, 10% of the medium was changed, and every month cells were washed and cultures were continued in fresh medium. Cell concentration was adjusted weekly to 1 × 106 cells/mL. Viable cell number was determined by trypan blue exclusion using a hemocytometer.
Proliferation
To measure ongoing cell proliferation, CD4+ T cells (2 × 105 /well) were transferred to 96-well plates, cultured for 8 hours with [3H]thymidine (1μCi/well; Perkin Elmer), and [3H]thymidine incorporation measured by liquid scintillation counting.
Cytokine assay
Cytokines in the supernatant of activated CD4+ T-cell cultures (1 × 106/mL) were assayed by Luminex technology and multiple-analyte microbead-based immunoassay, using a multiplex cytokine assay kit (Bio-Plex; Bio-Rad) for the simultaneous quantitation of IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, tumor necrosis factorα (TNFα), interferonγ (IFNγ), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Assays were performed according to the manufacturer's instruction.
Real-time quantitative PCR
Total RNA was extracted using Tripure Isolation Reagent (Roche) according to manufacturer's protocol and further purified using a RNeasy mini purification kit (QIAGEN) including a DNase treatment step. Quantitative PCR (QPCR) was performed in a 2-step procedure first transcribing cDNA using Superscript III First-Strand Synthesis System (Invitrogen) for RT-PCR and then synthesizing cDNA using the Platinum SYBR Green qPCR Super-Mix-UDG (Invitrogen). Amplification of RNA encoding arginine succinate lyase (ASL), a gene whose RNA levels do not significantly vary in response to either T-cell activation or Tax expression (P. Simon and A.B.R., in preparation), was used for sample normalization. Gene expression relative to ASL was quantitated using the ΔΔCt method.20 Primers prepared by Integrated DNA Technologies, RWJMS core sequencing were: Tax 5′-CTGTAGAGCTGAGCCGATAACGC-3′ and 5′-CCAACACCATGGCCCACTTCC-3′; ASL 5′-CCCCTGTTCTCAGGTGATGT-3′ and 5′-CTCCACTTTATTGGGGAGCA-3′. Mcl1 5′-GACTTCCCAGCTCACAAAGG-3′and 5′-CATGCTTCTTCCCTCCAAAG-3′.
All reactions were performed in triplicate on a Mx4000 (Stratagene) and analyzed using Multiplex Quantitative PCR Systems Software V4.2 (Stratagene). Cycle conditions used were: 50°C for 2:00 (minutes:seconds), 95°C for 2:00; followed by 40 cycles of 95°C for 00:15, 60°C for 00:30, and 72°C for 00:30. A dissociation curve was generated using: 95°C for 1:00 and 41 cycles of 55°C for 00:30 increasing by 1°C every cycle.
PCR
To confirm Tax genotype and identify transferred LTR-Tax CD4+ T cells in NSG mice, DNA (1 ug, extracted from tail or organs) was analyzed by RT-PCR using primers: Tax 5′-CACCTGTCCAGAGCATCAGA-3′ and Tax 5′-CTGTTTCACGGAAATGTTTTTC-3′ using standard cycling conditions. Reactions were resolved by agarose gel electrophoresis.
Quantitative PCR-based superarray analysis
For the comparative measurement of 84 genes involved in apoptosis, Q-PCR was performed using the RT2 Profiler PCR Array System, Mouse apoptosis array (Superarray). Briefly, RNA was isolated using the RT2 qPCR-Grade RNA Isolation Kit. RNA (1 μg) was transcribed into cDNA using the RT2 First Strand Kit, which includes a genomic DNA elimination step. Resulting cDNA was used for Q-PCR with SYBR Green master mix. Ct values obtained from a set threshold were analyzed using the ΔΔCt Method in an Excel-based PCR Array Data Analysis Template (provided on the Superarray Web site, http://www.superarray.com/pcrarraydataanalysis.php), and included controls for Genomic DNA, Reverse Transcription, Positive PCR, and normalization to a panel of housekeeping genes; Gusb, Hprt1, Hsp90ab1, GAPDH, and Actb.
Western blot analysis
Protein extracts were prepared from enriched CD4+ T cells using the Tripure (Roche) purification protocol. Proteins were resolved on a precast 8% polyacrylamide gel (Pierce), and transferred onto a nitrocellulose membrane (Hybond-P; Amersham). Membranes were blocked with 5% blocker grade nonfat dry milk (Bio-Rad) in Tris buffered saline (20mM Tris, 150mM NaCl, pH7.4), probed with antibodies against mouse Mcl-1 (Santa Cruz Biotechnology and Rockland) and Actin (Cell Signal), and developed using an ECL Advance Western Blotting Detection Kit (GE Healthcare).
Statistical analysis
Statistical significance was calculated using the unpaired, 2-tailed Student t test provided in Prism software Version 5.0a (GraphPad). P ≤ .05 was considered significant.
Results
HTLV-1 LTR-Tax CD4+ T cells are hyper-responsive to T-cell receptor stimulation
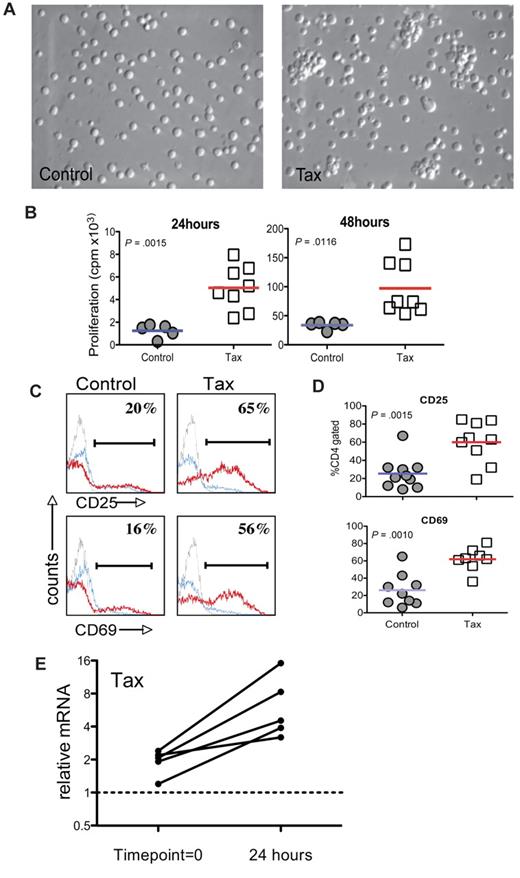
To characterize the HTLV-1 LTR-Tax CD4+ T cells, we examined the response of these cells to immune activation stimuli ex vivo. CD4+T cells enriched from splenocytes of 3- to 4-month-old LTR-Tax mice and age-matched littermate controls were incubated with anti-CD3 and anti-CD28. After 24 hours, the LTR-Tax CD4+ T cells exhibited marked clustering, indicative of T-cell activation, which was not observed at this early time point in control CD4+ T cells (Figure 1A). LTR-Tax CD4+ T cells showed greater proliferation (∼ 4- to 5-fold) compared with control CD4+ T cells after 24 hours and 48 hours (Figure 1B). We analyzed cell-surface expression of CD25 (IL-2 receptorα) and CD69 (T cell–activation marker) by flow cytometry and found that LTR-Tax CD4+ T cells expressed significantly higher CD25 and CD69 after 24 hours of activation (Figure 1C-D). These data show that the LTR-Tax CD4+ T cells are both hyper-proliferative and hyper-responsive to immune stimulation.
LTR-Tax CD4+ T cells are hyper-responsive to immune stimulation. CD4+ T cells enriched from splenocytes of LTR-Tax transgenic or littermate control mice were stimulated with plastic-coated anti-CD3 and soluble anti-CD28 in vitro (1 × 106 cells/mL) and compared after 24 hours. The LTR-Tax CD4+ T cells exhibited enhanced activation compared with control CD4+ T cells as demonstrated by: (A) physical clustering of cells; (B) increased proliferation, as measured by 3H-thymidine incorporation (results for individual animals shown); (C) greater percentages of cells expressing the surface activation markers CD25 or CD69, as detected by immunofluorescence and flow cytometry after activation with anti-CD3 and anti-CD28 (stimulated, red line; unstimulated, blue line; isotype control, gray line) in a representative animal; (D) greater percentages of cells expressing CD25 or CD69 after activation with anti-CD3 and anti-CD28 (all animals shown, lines represent means); and (E) relative Q-PCR analysis of Tax mRNA in LTR-Tax CD4+ T cells, 24 hours after immune stimulation, compared with RNA from time point = 0 (prior to activation). Tax mRNA levels in each sample were normalized to RNA levels of the housekeeping control gene, ASL. Solid lines show normalized Tax mRNA levels for T cells derived from individual mice. Micrographs were obtained by visualization with a 20× objective lens, using a Nikon EclipseTE2000-S microscope, Roper Scientific Photometrics imaging system, and Image Pro plus image acquisition software Version 4.5.0.29.
LTR-Tax CD4+ T cells are hyper-responsive to immune stimulation. CD4+ T cells enriched from splenocytes of LTR-Tax transgenic or littermate control mice were stimulated with plastic-coated anti-CD3 and soluble anti-CD28 in vitro (1 × 106 cells/mL) and compared after 24 hours. The LTR-Tax CD4+ T cells exhibited enhanced activation compared with control CD4+ T cells as demonstrated by: (A) physical clustering of cells; (B) increased proliferation, as measured by 3H-thymidine incorporation (results for individual animals shown); (C) greater percentages of cells expressing the surface activation markers CD25 or CD69, as detected by immunofluorescence and flow cytometry after activation with anti-CD3 and anti-CD28 (stimulated, red line; unstimulated, blue line; isotype control, gray line) in a representative animal; (D) greater percentages of cells expressing CD25 or CD69 after activation with anti-CD3 and anti-CD28 (all animals shown, lines represent means); and (E) relative Q-PCR analysis of Tax mRNA in LTR-Tax CD4+ T cells, 24 hours after immune stimulation, compared with RNA from time point = 0 (prior to activation). Tax mRNA levels in each sample were normalized to RNA levels of the housekeeping control gene, ASL. Solid lines show normalized Tax mRNA levels for T cells derived from individual mice. Micrographs were obtained by visualization with a 20× objective lens, using a Nikon EclipseTE2000-S microscope, Roper Scientific Photometrics imaging system, and Image Pro plus image acquisition software Version 4.5.0.29.
To determine whether immune activation of LTR-Tax CD4+ T cells could lead to increased expression of the Tax transgene, we performed Q-PCR for Tax mRNA expression. After 24 hours of immune stimulation, Tax mRNA was increased 2.5- to 8-fold in comparison with the low basal levels (Figure 1E). These data show that immune activation induces Tax transgene expression in LTR-Tax CD4+ T cells, correlating with early morphologic changes and increased proliferation.
LTR-Tax CD4+ T cells produce multiple T-helper subtype–associated cytokines
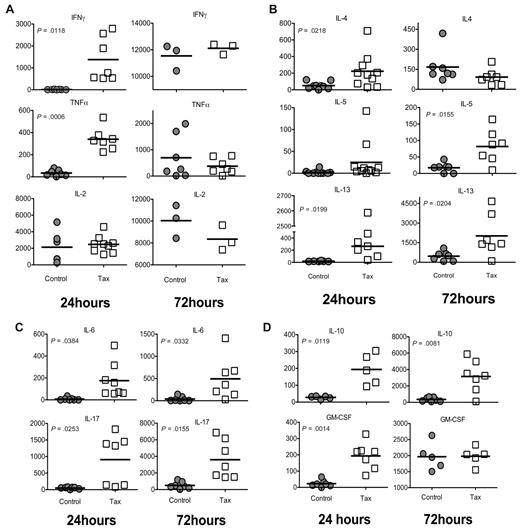
In response to immune stimulation, CD4+ T cells differentiate into distinct subtypes, allowing for effective functional immune responses to diverse potential pathogens.21 These subtypes can be characterized by their cytokine profiles. To determine the predominant subtype response of LTR-Tax CD4+ T cells under unbiased conditions, we evaluated the production of multiple cytokines involved in helper T-cell activation by immunoassay of culture supernatants. LTR-Tax CD4+ T cells secreted significantly higher amounts of the Th1-associated cytokines IFNγ and TNFα at 24 hours, however these levels became comparable by 72 hours (Figure 2A). LTR-Tax CD4+ T cells showed significantly higher levels of the Th2-associated cytokines IL-4, IL-10 (also associated with Tregs), and IL-13 at 24 hours after immune activation (Figure 2B,D). The Tax cells maintained significant elevation of IL-13 and IL-10, and showed increased IL-5, while IL-4 dropped to control levels by 72 hours. The Th17-associated cytokine IL-17, and IL-6, which promotes Th17 differentiation, were significantly increased in Tax cells at both 24 and 72 hours (Figure 2C). GM-CSF, a cytokine involved in leukocyte proliferation and activation, was markedly increased in the Tax CD4+ T-cell cultures at 24 but not 72 hours (Figure 2D).
Activated LTR-Tax CD4+ T cells express cytokines characteristic of multiple CD4+ subtypes. LTR-Tax and control CD4+ T cells were stimulated with anti-CD3 and anti-CD28, as in Figure 1, and supernatants collected at 24 hours (left panel) or 72 hours (right panel) and assayed for the indicated cytokines by multiplexed bead-based immunoassay and Luminex technology (concentration units: pg/mL). (A) Type 1 helper T-cell (Th1)–associated cytokines; (B) Th2-associated cytokines; (C) Th17-associated cytokines; and (D) other cytokines associated with T-cell activation.
Activated LTR-Tax CD4+ T cells express cytokines characteristic of multiple CD4+ subtypes. LTR-Tax and control CD4+ T cells were stimulated with anti-CD3 and anti-CD28, as in Figure 1, and supernatants collected at 24 hours (left panel) or 72 hours (right panel) and assayed for the indicated cytokines by multiplexed bead-based immunoassay and Luminex technology (concentration units: pg/mL). (A) Type 1 helper T-cell (Th1)–associated cytokines; (B) Th2-associated cytokines; (C) Th17-associated cytokines; and (D) other cytokines associated with T-cell activation.
These cytokine profiles suggest immune activated LTR-Tax CD4+ T cells secrete a diverse array of cytokines representative of multiple CD4+ T-cell subtypes, while control CD4+ T cells acquire a predominantly Th1 phenotype as determined by strong production of Th1 cytokines and minimal production of Th2 and Th17 cytokines.
Immortalization of LTR-Tax CD4+ T cells after immune stimulation
To study the effects of the induction of Tax expression on CD4+ T-cell survival in the transgenic cells, we monitored cell morphology, cell number, immune phenotype, and cytokine expression over time after immune activation.
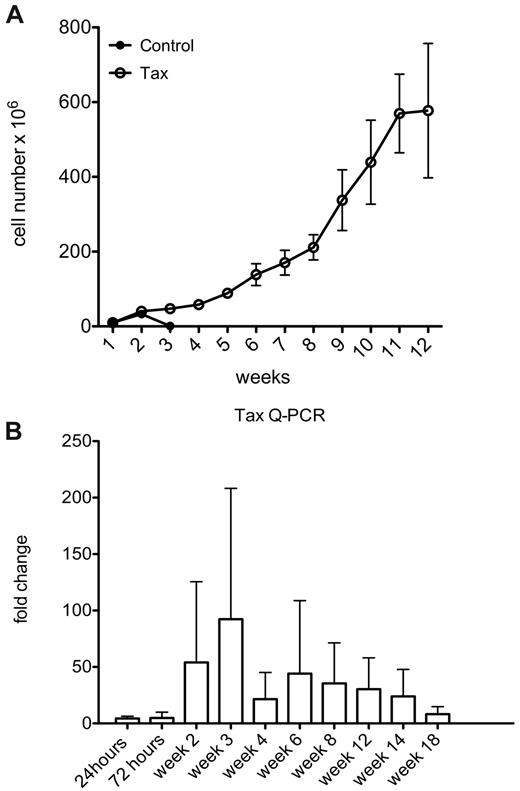
By continued passage of these cultures, we observed that immune activated LTR-Tax CD4+ T cells could be maintained in culture for more than 1 year, while littermate control CD4+ T cells persisted for approximately 3 weeks (Figure 3A). These LTR-Tax CD4+ T cells continued to proliferate in culture supplemented with IL-2 throughout this time course. Unstimulated LTR-Tax CD4+ T cells failed to proliferate (data not shown). To determine the levels of Tax transgene expression longitudinally, we performed Q-PCR for Tax RNA through 18 weeks of culture. Tax RNA levels peaked between weeks 2 and 3, corresponding to the time points when control cells were undergoing population contraction, and remained elevated throughout this period (Figure 3B). Thus, immune activation with its associated induction of Tax expression, is sufficient to induce immortalization of the transgenic LTR-Tax CD4+ T cells in the presence of exogenous IL-2.
Immune activation induces immortalization of LTR-Tax CD4+ T cells. Cultures of stimulated LTR-Tax and control CD4+ T cells (3 independent mice each) were propagated with IL-2 (80 U/mL). (A) Cells numbers were determined weekly for up to 12 weeks (means ± SD). (B) Tax mRNA levels from the LTR-Tax cells were assayed by Q-PCR at the indicated times. Expression increased during weeks 2 and 3 of cell culture, and then decreased with time.
Immune activation induces immortalization of LTR-Tax CD4+ T cells. Cultures of stimulated LTR-Tax and control CD4+ T cells (3 independent mice each) were propagated with IL-2 (80 U/mL). (A) Cells numbers were determined weekly for up to 12 weeks (means ± SD). (B) Tax mRNA levels from the LTR-Tax cells were assayed by Q-PCR at the indicated times. Expression increased during weeks 2 and 3 of cell culture, and then decreased with time.
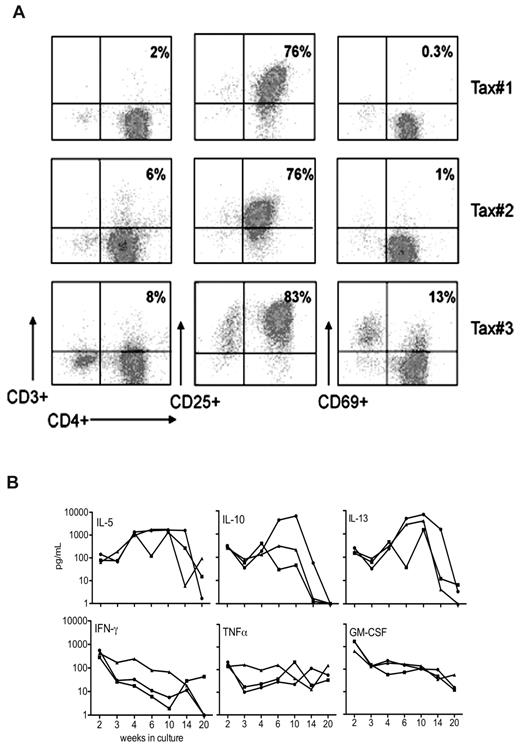
Phenotypic analysis of cell-surface makers expressed by the LTR-Tax CD4+ T-cell cultures showed that by week 4 of culture, these cells acquired a CD4+CD69−CD3− phenotype (Figure 4A). This is similar to the T-cell marker phenotype commonly observed for ATL cells in humans with the disease.22
Phenotypic analysis of long-term surviving LTR-Tax CD4+ T cells. (A) Cell-surface phenotype analysis determined by flow cytometric analysis of CD4+ T-cell cultures propagated for 4 weeks as in Figure 3 from 3 LTR-Tax mice show a similar CD4+CD25+CD3- phenotype. (B) The indicated cytokines were assayed in supernatants from the same cultures of LTR-Tax CD4+ T cells collected at the indicated times between 2 and 20 weeks (concentration units: pg/mL, log-scale).
Phenotypic analysis of long-term surviving LTR-Tax CD4+ T cells. (A) Cell-surface phenotype analysis determined by flow cytometric analysis of CD4+ T-cell cultures propagated for 4 weeks as in Figure 3 from 3 LTR-Tax mice show a similar CD4+CD25+CD3- phenotype. (B) The indicated cytokines were assayed in supernatants from the same cultures of LTR-Tax CD4+ T cells collected at the indicated times between 2 and 20 weeks (concentration units: pg/mL, log-scale).
HTLV-1 transformation of human T cells has been associated with production of multiple cytokines that likely play important roles in pathogenesis.23 We therefore examined the kinetics of cytokine production in the LTR-Tax CD4+ T-cell cultures. Detectable levels of extracellular IL-5, IL-10, IL-13, IFNγ, TNFα, and GM-CSF were found in these cell lines (Figure 4B and supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In general, cytokine levels detected were low compared with those seen at early times after activation (Figure 3A-D), although there was variability between cell lines and over time. In contrast, high levels of IL-5 and IL-13 were detected from weeks 4 to 14. Cytokine secretion appeared decreased by week 20.
Expression of apoptosis-related genes by LTR-Tax CD4+ T cells
These studies demonstrated that immune activation combined with the induction of Tax expression resulted in immortalization of the LTR-Tax CD4+ T cells in culture. This system provided a valuable opportunity to characterize regulatory events leading to prolonged cell survival. We therefore employed multiplex Q-PCR (Superarray) analysis, to compare the expression of 84 different apoptosis-related genes before activation, 24 hours after activation, and 2 weeks after immune activation, when control effector T cells were starting to undergo population contraction, while LTR-Tax cells were continuing to proliferate (supplemental Tables 2-4). We found significant changes in expression of more than half of the pro- and antiapoptotic genes analyzed in immune-stimulated LTR-Tax cells compared with littermate controls (supplemental Tables 2-4). The greatest change was observed for the antiapoptotic Bcl-2 family member, Mcl-1; in the LTR-Tax CD4+ T cells; mcl-1 RNA was up-regulated more than 300-fold in separate experiments.
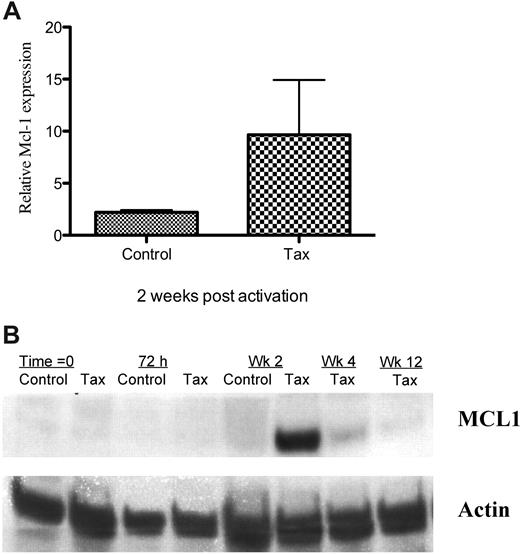
There is little information about the role of Mcl-1 in HTLV-1 infection. Because we found up-regulation of mcl-1 transcription in LTR-Tax CD4+ T cells during the period that control cells were undergoing contraction, we further examined mcl-1 expression by Q-PCR and Western blot analysis. We found increased expression of Mcl-1 protein, as well as mRNA in the LTR-Tax cells at the same 2-week time point (Figure 5A-B and supplemental Figures 1, 2A-B). After 4 weeks in culture, at which time the control CD4+ T cells have markedly reduced viability, Mcl-1 protein and RNA levels in the Tax cells were still readily detectable, although reduced. By 12 weeks, Mcl-1 protein levels returned to the barely detectable levels present prior to activation. Mcl-1 RNA levels were variable (supplemental Figure 1). Mcl-1 induction may be indirect, as levels were decreased 24 hours after LTR-Tax T-cell activation (supplemental Table 3), although Tax expression was already increased.
Increased Mcl-1 expression in immune stimulated LTR-Tax CD4+ T cells. (A) Mcl-1 mRNA was quantitated in Tax and control CD4+ T cells 2 weeks after stimulation. mRNA levels were normalized to basal levels measured at time = 0. Values represent mean ± SEM of 3 separate experiments. (B) Mcl-1 protein expression by Tax and control CD4+ T cells at various times after activation was measured by Western blot analysis. Actin expression was measured as a housekeeping control.
Increased Mcl-1 expression in immune stimulated LTR-Tax CD4+ T cells. (A) Mcl-1 mRNA was quantitated in Tax and control CD4+ T cells 2 weeks after stimulation. mRNA levels were normalized to basal levels measured at time = 0. Values represent mean ± SEM of 3 separate experiments. (B) Mcl-1 protein expression by Tax and control CD4+ T cells at various times after activation was measured by Western blot analysis. Actin expression was measured as a housekeeping control.
Adoptive transfer of LTR-Tax CD4+ T cells into immunodeficient mice
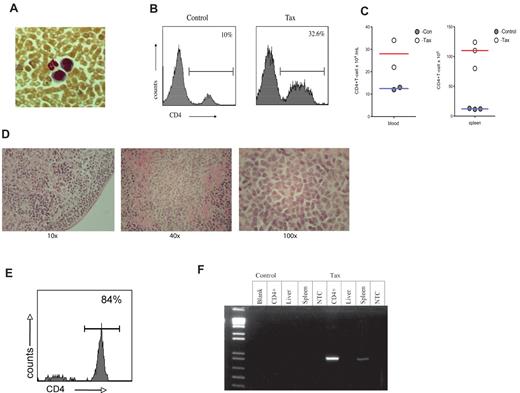
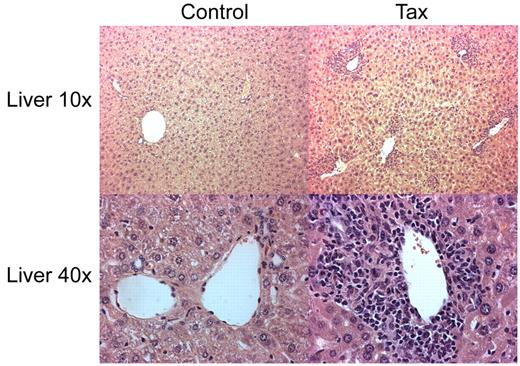
To determine whether the immortalization of immune activated LTR-Tax CD4+ T cells resulted in a potentially oncogenic phenotype, we performed an adoptive transfer of the ex vivo anti-CD3/28 immune-stimulated LTR-Tax and littermate control CD4+ T cells. Stimulated cells, cultured ex vivo for 2 weeks, were injected intra-peritoneally into 6 week old NSG mice. LTR-Tax cell–inoculated NSG mice exhibited wasting and weakness after a period of 2 to 4 weeks; control cell–inoculated mice remained healthy (data not shown). Blood smears from the LTR-Tax CD4+ T-cell mice showed the presence of large cells with large nuclei and scant agranular, basophilic cytoplasm, consistent with a lymphoblastoid phenotype, in addition to mature neutrophils. (Figure 6A). Flow cytometric analysis of peripheral blood cells from LTR-Tax cell–inoculated mice revealed markedly increased numbers of CD4+ T cells compared with control cell–inoculated mice, constituting more than 30% of circulating white blood cells (Figure 6B-C). LTR-Tax cell–recipient NSG mice had enlarged spleens with increased CD4+ T cells (Figure 6C), and small but grossly identifiable lymph nodes (not detected in the control cell–inoculated mice). Histologic analysis of lymph nodes from the LTR-Tax–injected mice demonstrated a diffuse infiltration of lymphoid cells, many with larger and/or irregular nuclei (Figure 6D). Flow cytometry demonstrated that at least 80% of mononuclear cells in lymph nodes were CD4+ (Figure 6E). H&E staining of several organs showed marked infiltration of the parenchyma with lymphocytes exhibiting atypical nuclei, as seen in liver (Figure 7), as well as kidney and lung (data not shown). PCR analysis revealed that CD4+ T cells from the LTR-Tax cell–recipient NSG mice contained the Tax transgene, confirming their origin from the inoculated LTR-Tax cells (Figure 6f). Tax DNA was also detected by PCR in spleen, but not in liver, possibly because of the lower load of infiltrating lymphocytes (Figure 7). Therefore, ex vivo stimulated LTR-Tax CD4+ T cells were capable of inducing a leukemia-phenotype in the NSG mice.
NSG mice adoptively transferred with LTR-Tax CD4+ T cells exhibit CD4+ T-cell proliferation. CD4+ T cells from LTR-Tax or littermate control mice were activated with anti-CD3 and anti-CD28 for 2 weeks in vitro and transferred into NSG mice. Recipient mice were euthanized at 2-4 months. (A) Geimsa staining of blood smear from a representative LTR-Tax CD4+ T cell–inoculated NSG mouse showing the presence of cells exhibiting lymphoblastoid phenotype adjacent to a neutrophil. Micrographs were obtained by visualization with a 40×/0.6 objective lens, using a Nikon EclipseTE2000-S microscope, Roper Scientific Photometrics imaging system, and Image Pro plus image acquisition software Version 4.5.0.29. (B) Flow cytometric analysis of blood leukocytes from NSG mice. 32.6% of cells in LTR-Tax CD4+ cell–inoculated NSG mouse (same as panel A) were CD4+;, compared with 10% in control cell inoculated mice. (C) Total CD4 + T-cell counts in the blood and spleen of control or LTR-Tax cell–inoculated NSG mice, calculated as the percentage of gated CD4+ cells multiplied by the total number of blood leukocytes or splenocytes. (D) Histologic analysis (H&E staining) of a representative lymph node from the LTR-Tax-injected NSG mice, demonstrating diffuse infiltration of lymphoid cells (using 10×/0.2, 40×/0.65, and 100×/1.25 objectives, on a Leica DME microscope, Nikon Coldpix4500 camera and Nikon View 5.0 software). No lymph nodes were detected in littermate control inoculated NSG mice. (E) Percentage of lymph node cells expressing CD4, as determined by flow cytometry (representative specimen). (F) Tax expression in adoptively transferred NSG mice. NSG mice treated with LTR-Tax CD4+ T cells are positive for Tax. PCR for Tax expression (∼ 1kb) was performed from DNA (∼ 1 ug) that was extracted from various tissue taken from NSG mice adoptively transferred with LTR-Tax or littermate control CD4+ T cells that were previously activated ex vivo and cultured for 2 weeks prior to transfer. As shown NSG mice treated with LTR-Tax CD4+ T cells are positive for Tax in spleen and lymph node derived DNA.
NSG mice adoptively transferred with LTR-Tax CD4+ T cells exhibit CD4+ T-cell proliferation. CD4+ T cells from LTR-Tax or littermate control mice were activated with anti-CD3 and anti-CD28 for 2 weeks in vitro and transferred into NSG mice. Recipient mice were euthanized at 2-4 months. (A) Geimsa staining of blood smear from a representative LTR-Tax CD4+ T cell–inoculated NSG mouse showing the presence of cells exhibiting lymphoblastoid phenotype adjacent to a neutrophil. Micrographs were obtained by visualization with a 40×/0.6 objective lens, using a Nikon EclipseTE2000-S microscope, Roper Scientific Photometrics imaging system, and Image Pro plus image acquisition software Version 4.5.0.29. (B) Flow cytometric analysis of blood leukocytes from NSG mice. 32.6% of cells in LTR-Tax CD4+ cell–inoculated NSG mouse (same as panel A) were CD4+;, compared with 10% in control cell inoculated mice. (C) Total CD4 + T-cell counts in the blood and spleen of control or LTR-Tax cell–inoculated NSG mice, calculated as the percentage of gated CD4+ cells multiplied by the total number of blood leukocytes or splenocytes. (D) Histologic analysis (H&E staining) of a representative lymph node from the LTR-Tax-injected NSG mice, demonstrating diffuse infiltration of lymphoid cells (using 10×/0.2, 40×/0.65, and 100×/1.25 objectives, on a Leica DME microscope, Nikon Coldpix4500 camera and Nikon View 5.0 software). No lymph nodes were detected in littermate control inoculated NSG mice. (E) Percentage of lymph node cells expressing CD4, as determined by flow cytometry (representative specimen). (F) Tax expression in adoptively transferred NSG mice. NSG mice treated with LTR-Tax CD4+ T cells are positive for Tax. PCR for Tax expression (∼ 1kb) was performed from DNA (∼ 1 ug) that was extracted from various tissue taken from NSG mice adoptively transferred with LTR-Tax or littermate control CD4+ T cells that were previously activated ex vivo and cultured for 2 weeks prior to transfer. As shown NSG mice treated with LTR-Tax CD4+ T cells are positive for Tax in spleen and lymph node derived DNA.
Parenchymal lymphocytic infiltration in liver of LTR-Tax CD4+ T cell–recipient NSG mice. Histology of liver sections from NSG mice inoculated with LTR-Tax or control CD4+ T cells were analyzed by H&E staining (10-40× magnification). Micrographs were obtained using 10×/0.3 and 40×/0.6 objectives, as indicated, a Nikon EclipseTE2000-S microscope, Roper Scientific Photometrics imaging system, and Image Pro plus image acquisition software Version 4.5.0.29.
Parenchymal lymphocytic infiltration in liver of LTR-Tax CD4+ T cell–recipient NSG mice. Histology of liver sections from NSG mice inoculated with LTR-Tax or control CD4+ T cells were analyzed by H&E staining (10-40× magnification). Micrographs were obtained using 10×/0.3 and 40×/0.6 objectives, as indicated, a Nikon EclipseTE2000-S microscope, Roper Scientific Photometrics imaging system, and Image Pro plus image acquisition software Version 4.5.0.29.
Discussion
Although HTLV-1 and its association with ATL, have been known for close to 30 years, critical details about the pathogenesis of HTLV-1–associated diseases and the interplay between virus, infected cells and the host immune system remains poorly understood. The development of improved animal models could play an important role in elucidating how this virus causes diverse disease phenotypes, and aid in the identification of novel approaches for treatment and prevention.
Previous studies from our laboratory and others have provided evidence that immune activation of HTLV-1–infected CD4+ T cells can induce expression of Tax in infected cells.10,11,24,25 We hypothesized that immune activation of infected T cells may contribute to the pathogenesis of HTLV-1–associated diseases through induction of Tax. To further examine this hypothesis, we revisited a transgenic model of HTLV-1 infection, in which expression of the HTLV-1 Tax gene is directed by the authentic HTLV-1 LTR, mimicking important aspects of viral infection.15 We observed that LTR-Tax CD4+T cells activated through T-cell receptor stimulation, were hyper-proliferative and hyper-responsive to stimulation, correlating with induction of Tax expression. We also identified a unique cytokine profile in stimulated LTR-Tax transgenic CD4+ T cells, characterized by strong expression of Th1-, Th2-, and Th17-associated cytokines. Strikingly, with immune stimulation, LTR-Tax CD4+ T cells became resistant to cell death and continued to proliferate in culture for more than 1 year, exhibiting a CD4+CD25+CD3− phenotype. Over time, there was a shift in cytokine production to predominant expression of Th2-associated cytokines. Finally, adoptive transfer of activated Tax CD4+ T cells into immunodeficient NSG mice, resulted in engraftment and proliferation of these cells, associated with development of wasting and a leukemia-like phenotype.
Previous mouse models of HTLV-1 pathogenesis have successfully shed light on the ability of Tax to promote in vivo malignancy. Transgenic mice with Tax regulated by the granzyme B promoter, expressed Tax in hematopoietic tissues and developed lymphomas of large granular lymphocytes,12 which were dramatically increased with inflammation.26 The Tet-Tax-regulated, lymphocyte-expression mouse model showed that Tax expression promoted a lethal cutaneous disease modeling a pre-leukemic state in HTLV-1–infected patients.27 Tax expression regulated by the proximal Lck promoter resulted in development of thymus-derived, pre–T-cell leukemia,13 and use of the distal Lck promoter resulted in late onset of CD4+ and CD8+ T-cell lymphomas.28 These models demonstrate the ability of Tax to transform lymphoid cells (including CD4+ cells) in vivo, and have shown that inflammation appears to play a key role in disease progression.26,27 These transgenic models are unable to address events related to regulation of Tax expression by the authentic HTLV-1 LTR promoter during HTLV-1 infection, unlike the LTR-Tax mouse. In studies of an inflammatory arthropathy model, LTR-env-pX transgenic rats demonstrated T-cell hyper-responsiveness to TCR activation.29 Most recently, adaptive transfer of human CD34+hematopoietic precursor and stem cells infected by HTLV-1 was able to recapitulate an ATL-like phenotype, providing another model for examining the role of authentic HTLV-1 gene regulation in leukemogenesis in vivo.30
Through studies of CD4+ T cells derived from the LTR-Tax transgenic mice, we have shown a role for immune stimulation in inducing Tax expression, cellular immortalization and a leukemic-like phenotype in engrafted immunodeficient mice, suggesting that immune activation may play an important role in the development of ATL from the pool of HTLV-1–infected cells in vivo. Although ex vivo immune-activated cells from the ICR strain LTR-Tax mice were able to induce a leukemia-like phenotype when engrafted into NSG mice, to date, we have not been able to induce an in vivo T-cell malignancy in LTR-Tax transgenic mice through immune activation, possibly due to the early death of these mice. The LTR-Tax mice die at 5-7 months of age, possibly limiting the accumulation of cooperating genetic events required for lymphoma development. Alternatively, in vivo lymphomagenesis may require additional viral proteins such as HBZ or p12I. Strain differences, known to be important in murine lymphoma models31 may play a role in in vivo lymphoma development. To further explore these issues with a more natural antigen, we have recently bred the LTR-Tax transgene onto the OTII mouse background that expresses a TCR specific for ovalbumin in CD4+ T cells.32 These studies are in progress.
Characterization of immune-activated LTR-Tax CD4+ T cells revealed important similarities to HTLV-1–infected, human cells. The immunophenotype of human ATL cells generally is CD4+, CD8−,CD25+, CD3low, similar to that observed in our long-term surviving LTR-Tax transgenic CD4+ T cells. Upon immune activation, the LTR-Tax CD4+ T cells secreted many cytokines that are markers for several distinct T-cell subtypes, including Th1, Th2, Th17, and Tregs. The expression of a wide array of T-cell associated cytokines, including those characteristic of different T-helper cell subtypes has also been seen in both ATL cells and HTLV-1–transformed human cells, and a number of these cytokines have been shown to be directly regulated by Tax, including IL-5, IL-10, IL-13, and GM-CSF.23,33-35 The relative amounts of Tax mRNA expression do not always directly correlate with the amounts of these cytokines secreted over the time course of cell growth. The complexity of cytokine regulation, involving both transcriptional and posttranscriptional events may obscure such a direct correlation. Interestingly, at 72 hours when the control CD4+ T cells were maximally activated, the levels of IL-2 in LTR-Tax cells were somewhat lower than controls. One possible explanation is that, similar to observations on Treg cells,36 the LTR-Tax cells may be consuming larger amounts of IL-2, associated with higher levels of CD25, thus leading to a relative decrease in IL-2 in the media. Immune activated LTR-Tax CD4+ T cells expressed high levels of IL-4 and IL-10, cytokines associated with virus-specific T-cell anergy, and the establishment of many chronic viral infections.37 The high levels of IL-6 and IL-17 produced by LTR-Tax CD4+ cells suggest that a Th17-type immune response may be induced in HTLV-1 infection, and high IL-17 expression has been previously detected in HTLV-1–infected T cells.38 A predominate Th2-like immune response was seen in the long-term cultures of LTR-Tax CD4+ T cells with particularly high expression of IL-5 and IL-10. Previous studies have suggested a strong correlation between Th-2 responses, co-infection with HTLV-1 and Stercolis strongyloides, and progression of HTLV-1 associated-disease.39,40 In contrast, the relationship between HTLV-1 infection and the Treg subset remains unclear. Some studies have suggested that a subset of ATL cells have the characteristics of Tregs41 ; however, in HAM-TSP, the levels of Tregs have been shown to inversely correlate with disease progression and HTLV-1 infection has been suggested to inhibit development of the Tregs phenotype.42,43 Further studies of the effects of immune activation on the phenotypic differentiation and function of the LTR-Tax CD4+ T cells may help to clarify these issues.
Although we do not yet know all of the mechanisms responsible for the immortalization of the LTR-Tax CD4+ T cells, it was of interest that the antiapoptotic gene, mcl-1, was found increased in the immune stimulated Tax cells, at the same time point that control cells were undergoing population contraction. These data are correlative and do not prove a definitive role for Mcl-1 in HTLV-1 induced T-cell immortalization, however, Mcl-1 has been previously detected in HTLV-1 cell lines and suggested to play a role in their viability.17 Mcl-1 has shown to play a vital role in T-cell development and survival.44,45 Our system may enable a better understanding of the role of Mcl-1 in Tax-mediated, CD4+ T-cell immortalization. The timing of Mcl-1 induction suggests that it may play a role in early stages of HTLV-1 pathogenesis, although it is unlikely to be a direct target of Tax itself. Survivin or Birc5, previously reported to be a Tax target,46 also was increased at 2 weeks and may contribute. In contrast, Bcl-xl, another antiapoptotic protein shown to be a target of Tax,47 showed an early increase in expression 24 hours after stimulation, but not at the 2 week time point, suggesting that it was unlikely to be critical for the survival of the LTR-Tax T cells during population contraction. Despite the induction of important antiapoptotic proteins, the long-term surviving LTR-Tax cells remain dependent on cytokines, including relatively high levels of IL-2 added to the cultures and those present in the conditioned media.
A key observation from these studies was that a single round of immune activation of LTR-Tax CD4+ T cells was sufficient to induce Tax expression, long-term survival past the time when normal T cells undergo immune response shutdown death,48 and establishment of immortalized cells capable of engraftment and leukemogenesis. Interestingly, a recent paper has suggested that Tax alone is insufficient to immortalize human cells in culture.49 In those studies, Tax-transduced cells continued to proliferate for 12-15 months but eventually ceased to grow. In our experiments, we only maintained our cell lines 12-13 months, thus cannot address whether these mouse cells would exhibit even longer-term growth. Furthermore, similar to these recent studies, our long-term proliferating cells remained dependent upon IL-2. A previous study also maintained Tax-transformed human CD4+ cells for up to 8 months in the presence of IL-2, while control cells failed to grow.50
A critical question in HTLV-1 pathogenesis is the identity of factors responsible for the long-term clonal proliferation of infected CD4+ T-cell clones, characteristic of progression to HAM/TSP or ATL. One determinant is clearly the ability of these proliferating cells to escape the potent CTL response directed against Tax, and the strength of the CTL response has been postulated to be a critical determinant of whether individuals progress to disease.51 Our studies suggest that induction of Tax expression through immune stimulation of infected T cells may also contribute to pathogenesis, similar to a previous suggestion that low level antigenic stimulation may induce viral proteins and cell proliferation.52 In our model, activation of infected T cells through antigen or superantigen stimulation of the TCR could induce Tax expression, providing the initial impetus for proliferation of distinct HTLV-1–infected T-cell clones. Not only would Tax induce proliferation and inhibit apoptosis of the infected cells, but Tax induction of immunosuppressive cytokines (as we observed), could potentially protect infected cells from the host cytotoxic T-cell response. Thus, induced Tax expression, T-cell proliferation and immortalization, with elaboration of immunosuppressive cytokines could serve as a critical early step in the pathogenesis of HTLV-1–associated diseases. Progression to ATL or HAM/TSP would be dependent upon secondary events involving both the proliferating clones and the nature and success of the host immune response. Such a model would suggest that immune activation may act as an important early step in HTLV-1 pathogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to J. Green (National Cancer Institute, Bethesda, MD) for the LTR-Tax mice. We thank H.-C. Lin for initial experiments on immune activation and for helpful discussions and advice. We thank J. Friedman and S. Bhat of the Cancer Institute of New Jersey, Tissue Analytical Services Shared Resource, for performing mouse histology. We thank D. Medina, L. Y. Zhang, C. Liu, and the Shi laboratory for helpful discussions and advice, and P. Simon for sharing unpublished observations on the use of ASL as a housekeeping gene and primer design.
This study was supported by US National Institutes of Health grants CA94148 (A.B.R.) and AI057596, DE19413, DE019932 (Y.S.), and National Aeronautics and Space Biomedical Research Institute grant IIH00405 (Y.S.). A.Y.S. was supported by a National Institutes of Health training grant (T32AI007403) and a Predoctoral Fellowship from the New Jersey Commission on Cancer Research.
National Institutes of Health
Authorship
Contribution: A.Y.S. performed the experimental work, assisted in experimental design, and interpreted data; F.K. performed Q-PCR experiments; Y.Z. assisted in examination and interpretation of histology; A.I.R. assisted with immune methodologies and gave advice on the manuscript; S.D. provided advice in experimental design and interpretation; A.B.R. and Y.S. supervised the project including experimental design and interpretation of data; A.S and A.B.R. wrote the manuscript with editing assistance by A.I.R.; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arnold B. Rabson, Child Health Institute of New Jersey, UMDNJ-RWJMS, 89 French St, New Brunswick, NJ 08901; e-mail: rabsonab@umdnj.edu; or Yufang Shi, Department of Molecular Genetics, Microbiology, and Immunology, UMDNJ-RWJMS, 661 Hoes La, Piscataway, NJ 08854; e-mail: shiyu@umdnj.edu.
REFERENCES
Author notes
Y.S. and A.B.R. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal