Abstract
Stem cells exhibit long-term self-renewal by asymmetric division and multipotent differentiation. During embryonic development, cell fate is determined with predictable orientation, differentiation, and partitioning to form the organism. This includes the formation of a hemangioblast from which 2 derivative cell clusters commit to either a hematopoietic or an endothelial lineage. Frequently, it is not clear whether tissue resident stem cells in the adult originate from the bone marrow. Here, we show that blast colony-forming cells exhibiting bilineage (hematopoietic and vascular) potential and long-term self-renewal originate from the uterus in the mouse. This is the first in vitro and in vivo evidence of an adult hemangioblast retained from development in the uterus. Our findings offer new understanding of uterine cell renewal and turnover and may provide insights and opportunities for the study of stem cell maintenance.
Introduction
Stem cells are uncommitted cells capable of self-renewal by asymmetric proliferation, which both retains stemness and allows multipotent differentiation. The hemangioblast is a stem cell that is the common precursor to hematopoietic and endothelial cell types. Hemangioblasts were initially identified through ontogeny studies showing that the cells arose from a common mesoderm/primitive streak to form blood islands containing both hematopoietic and vascular cells.1 Mammalian evidence for the hemangioblast was provided by clonal blast-forming cell assays, which demonstrated the emergence of hematopoietic and vascular cells from murine/human embryonic stem cell-derived embryoid bodies.2-5 The theory that a hemangioblast may be retained (from development) in the adult was inspired by the discovery of endothelial progenitor cells in the circulation.6 This “adult hemangioblast” could be of bone marrow (BM) origin in vivo7,8 and distinguishable in vitro.9,10
In the adult, neovascularization may contribute to pathologies (eg, malignancy, rheumatoid arthritis, atherosclerosis, diabetic retinopathy, and macular degeneration), or to regeneration in response to ischemia, tissue growth, wound healing, and endometrial cycling.11 Such neovascularization may involve mobile derivatives of hemangioblasts in the somatic tissues, but the existence of adult hemangioblasts is still under debate.12
In the adult uterus, physiologic remodeling and neovascularization involve high-volume epithelial and mesenchymal expansion in cyclic endocrine-cued regeneration before menstruation. The uterus is composed of contractile myometrial and highly vascularized endometrial layers housed within the perimetrium and uteroligaments. The highly regenerative nature of the uterus was the basis for speculation about stem cell contributions to the physiology of this organ, as first suggested in a study describing 3 epithelial stem cells sensitive to the follicular hormones estrogen, progesterone, or a combination of both.13 It remains unclear whether those cells were also described by Kearns and Lala as hormone-sensitive decidual cells14 of possible hematopoietic origin.15 Uterine stem cells were proposed to be a component of cyclic endometrial regeneration producing multiple lineages of luminal and glandular epithelia, stroma, and granular and vascular cells.16 This long-held theory of uterine stem cells was partly addressed by Gargett,17 who identified 2 subsets of progenitor cells derived from uterine endometrium with a clonogenic potential and epithelial or mesenchymal differentiation.18
The origin of uterine stem cells has been attributed, at least in part, to cells of BM origin. In BM transplantation studies, differentiated epithelial and stromal cells were reported to originate during uterine turnover at rates from less than 0.01%19 to approximately 0.5%20 to approximately 10%21 of the cells. This cellular integration was temporally restrictive because of irradiation protocols because marrow contribution increased with the number of menses before analysis.22 In murine pregnancy, as much as 80% of the epithelia may be of BM origin; however, a contribution of only 10% to 15% was reported in a human pregnant sex-mismatch marrow recipient.22 Although uterine stem cells have potentially significant implications for health, clear proof of their origin and character remains elusive. In an attempt to address this knowledge gap, the current study reveals that the mouse uterus retains hemangioblasts that are not of BM origin. This is the first definitive proof of a self-renewing hemangioblast that has been reported to arise outside the BM in the adult.
Methods
Animal procedures
All animal procedures were approved by the Animal Care Committee of the Toronto General Research Institute. The following strains of mice were obtained from The Jackson Laboratory: C57BL/6 (wild-type), C57BL/6-Tg (ACTb-EGFP)1Osb/J, and Tg(CAG-DsRed*MST)1Nagy/J. The C57BL/6-Tg (ACTb-EGFP)1Osb/J mouse has a chicken cytoplasmic β-actin promoter with cytomegalovirus enhancer elements driving the expression of an enhanced green fluorescent protein (GFP) cDNA. The Tg(CAG-DsRed*MST)1Nagy/J mouse has a chicken β-actin promoter with cytomegalovirus immediate early enhancer elements driving the expression of an enhanced disheveled red (DsRed) cDNA. In fluorescent mice, all tissues except erythrocytes and hair fluoresce green or red as appropriate.
Tissue preparation and FACS
Female C57BL/6 mice (8-10 weeks old) were anesthetized, intubated, and ventilated with 2% to 3% isoflurane. Blood was collected from the right jugular vein using a 0.5-mL insulin syringe. Mice were continuously perfused with saline through the aorta at physiologic pressures until the liquid flowing from the right atrium was clear. BM was flushed from femurs and tibias. Uteri were collected, minced, and digested with 2 treatments of 0.25% trypsin, 2 mg/mL collagenase, and 0.01% DNAase at 37°C for 1 hour. Hearts were digested with 0.1% collagenase (type II) at 37°C for 30 minutes. Skeletal muscle (gastrocnemius and soleus) was excised, separated from tendons, bone, and fat tissues, and then minced and digested with 0.2% collagenase at 37°C for 45 minutes, followed by 0.1% trypsin for 45 minutes. Red blood cells were removed from blood samples using 1% ammonium chloride lysis buffer. Cells from other tissues were passed through a 70-μm filter. Fluorescence-activated cell sorting (FACS) analysis of the bright cells was performed and used the following antibodies: phycoerythrin-conjugated anti–mouse CD34 (eBioscience), fluorescein isothiocyanate-conjugated anti–mouse c-kit (Santa Cruz Biotechnology), and anti-GFP (Invitrogen). The labeled cells were analyzed with an EPICS XLMCL flow cytometer with Expo32 ADC Xa software (Beckman Coulter).
Cell separation and blast formation
Isolated uterine cells were separated into 2 populations according to the presence or absence of c-kit expression (using magnetic beads conjugated with antibodies against c-kit). The c-kit− population was further separated based on the presence or absence of CD34 expression using a biotin selection kit (StemCell Technologies) according to the manufacturer's instructions.
In vitro blast formation was induced as previously described.2,3 Uterine CD34+/c-kit− or CD34−/c-kit− cells (5 × 104) were cultured in a 35-mm dish containing 1% methylcellulose media with 10% fetal calf serum, 4.5 × 10−4M monothioglycerol, 25 μg/mL ascorbic acid, 2mM glutamine, 200 μg/mL iron saturated transferrin, 50 ng/mL vascular endothelial growth factor (VEGF), 10 ng/mL basic fibroblast growth factor, 100 ng/mL stem cell factor, and 10 ng/mL interleukin-6 (IL-6). The cultures were maintained at 37°C, 5% CO2 in air, and more than or equal to 95% humidity. To determine whether the resulting blast colonies were clonal (originated from a common progenitor cell), a mixture of uterine CD34+/c-kit− cells isolated from GFP+ and red fluorescent protein (RFP)+ mice was cultured in blast formation media (1:1 ratio). The resulting GFP+ and RFP+ blast colonies were independently assessed by fluorescence and phase-contrast microscopy (Nikon Eclipse Ti; NIS-Elements BR software Version 3.0).
Blast cell differentiation
Individual CD34+/c-kit− cell-derived blast colonies were carefully isolated and transferred into differentiation media (Iscove modified Dulbecco medium supplemented with 10% fetal bovine serum and 25% endothelial cell primary culture media containing 10 ng/mL VEGF, 1 ng/mL basic fibroblast growth factor, 10 ng/mL insulin-like growth factor, 3 U/mL erythropoietin, 100 ng/mL stem cell factor, 100 ng/mL endothelial cell growth supplement, 1 ng/mL IL-3, 50 ng/mL IL-11, and 4.5 × 10−4 1-thioglycerol). After 12 to 15 days, the blast cells differentiated into nonadherent cells or adherent cells. These cells were harvested by gentle pipetting (nonadherent) or trypsin-ethylenediaminetetraacetic acid treatment (adherent).
Immunochemistry and LDL uptake
The nonadherent cells (putative hematopoietic lineage cells) were slide fixed using cytospin, stained with Giemsa-May-Grünwald, and immunolabeled with antibodies against CD4, CD8, CD13, CD16, CD45, or Mac-3. The adherent cells (putative vascular cells) were fixed and immunolabeled with antibodies against CD31 and factor VIII. To assess DiI-ac-low density lipoprotein (LDL) uptake, adherent cells were incubated with 10 μg/mL DiI-ac-LDL (Invitrogen) at 37°C for 6 hours, washed with phosphate-buffered saline twice, fixed, and examined under fluorescence microscopy.
RT-PCR
Both adherent and nonadherent cells were analyzed for mRNA expression of Flk-1, CD31, GATA1, SCL, β-H1 globin, and β-Major. Total RNA was extracted from the cells using the Trizol method (Invitrogen) according to the manufacturer's protocol. The RNA was treated with DNAse and then used for first-strand cDNA synthesis with Oligo18 and Superscript RT III (Invitrogen). The primers listed in Table 1 were used to amplify the cDNA.
Primers used for RT-PCR
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| EGFP | GAC GTA AAC GGC CAC AAG TT | GGG GTG TTC TGC TGG TAG TG |
| Flk-1 | CAC CTG GCA CTC TCC ACC TTC | GAT TTC ATC CCA CTA CCG AAG |
| CD31 | GTC ATG GCC ATG GTC GAG TA | CTC CTC GGC ATC TTG CTG AA |
| β-H1 | ACT CCC CAT GGA GTC AAA GA | CTC AAG GAG ACC TTT GCT CA |
| β-Major | CTG ACA GAT GCT CTC TTG GG | CAC AAA CCC CAG AAA CAG ACA |
| Scl | AGA GGG AGC CAG TAA CAG CA | AGT GGG ACT TAGGCT GCT CA |
| Gata-1 | TTG TGA GGC CAG ACA GTG TG | TTC CTC GTC TGG ATT CCA TC |
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| EGFP | GAC GTA AAC GGC CAC AAG TT | GGG GTG TTC TGC TGG TAG TG |
| Flk-1 | CAC CTG GCA CTC TCC ACC TTC | GAT TTC ATC CCA CTA CCG AAG |
| CD31 | GTC ATG GCC ATG GTC GAG TA | CTC CTC GGC ATC TTG CTG AA |
| β-H1 | ACT CCC CAT GGA GTC AAA GA | CTC AAG GAG ACC TTT GCT CA |
| β-Major | CTG ACA GAT GCT CTC TTG GG | CAC AAA CCC CAG AAA CAG ACA |
| Scl | AGA GGG AGC CAG TAA CAG CA | AGT GGG ACT TAGGCT GCT CA |
| Gata-1 | TTG TGA GGC CAG ACA GTG TG | TTC CTC GTC TGG ATT CCA TC |
Primary BM reconstitution
A single uterine CD34+/c-kit− cell isolated from a C57BL/6-Tg (ACTB-EGFP)1Osb/J mouse was cultured for 15 days. The resultant GFP+ blast colony was mixed with unfractionated, GFP− BM “helper cells” (1.5 × 106/mouse, collected from the tibias of female C57BL/6 mice). The mixture was injected into lethally irradiated (9.5 Gy γ-irradiation), female C57BL/6 mice (8-10 weeks old) through the tail vein. For the BM-uterine tracking study, the irradiated recipients were injected with unfractionated, GFP+ BM cells (BMCs; 3 × 106/mouse).
Secondary BM reconstitution
BMCs were isolated from chimeric (primary) recipients at 12 weeks or 12 months after the primary reconstitution. The isolated BMCs (3 × 106/mouse) were injected into a second set of lethally irradiated (9.5 Gy γ-irradiation) C57BL/6 mice through the tail vein. For the BM-uterine tracking study, the secondary recipients were injected with whole (unselected) uterine cells isolated from the primary recipient (1.5 × 106/mouse, combined with 1.5 × 106 GFP− C57BL/6 BMCs).
Colony-forming unit assay
BMCs were collected from chimeric mice at 12 weeks or 12 months after primary BM reconstitution. The cells (2 × 104/dish) were mixed with 1 mL MethoCult media (Stem Cell Technologies, catalog no. 03434) and plated into 35-mm dishes (media were distributed evenly with gentle tilting and rotation). The cultures were maintained in an incubator at 37°C, 5% CO2 in air, and more than or equal to 95% humidity. Colonies were quantified visually in a blinded fashion using a Nikon Ti-S phase contrast microscope.
Spleen colony assay
Spleens were collected at 9 days after secondary BM reconstitution (1 × 104 BMCs/mouse). GFP+ spleen colonies were identified and photographed under fluorescence microscopy.
Statistical analyses
Data are presented as mean plus or minus SE. Analyses were performed using SPSS software (Version 12.0), with the critical α level set at P less than .05. Multigroup comparisons were made using one-way analysis of variance. When F values were significant, differences between the groups were specified with Tukey multiple comparison post-tests.
Immunofluorescence
Images were viewed with a Nikon Ti-S Eclipse microscope with 10×/0.13, 20×/0.45, and 40×/0.60 air objective lenses. Images were captured with a Nikon Digital Sight DS-02 camera using NIS-Elements BR v3.0 and Adobe Illustrator C53 software.
Results
Lineage determination and blast colony formation from uterine cells
The uterus contains contractile myometrial tissue and extensively vascularized endometrial tissue that is cyclically shed and regenerated. We compared hematopoietic lineages in established hematopoietic tissue (blood and BM) and other contractile structures that rely on hematopoietic contributions for revascularization (uterus, heart, and skeletal muscle). Distribution assessment revealed that the highest population of CD34+ cells is maintained in the uterus (Figure 1A; P < .01); however, uterine tissue has a relatively modest population of c-kit+ cells that is less than half the size of the population identified in the BM (Figure 1B; P < .05). Uterine cells isolated from the CD34+/c-kit− fraction (∼ 8% of the total uterine cell population) yielded blast colonies at a formation rate of 0.14% plus or minus 0.02% (Figure 1C).
Blast colony formation by single uterine CD34+/c-kit− cells. Percentage of cells expressing CD34 (A) or c-kit+ (B) in each of multiple organs by flow cytometry. SKM indicates skeletal muscle. Uterine tissue contained the highest percentage of CD34+ cells. **P < .01 vs other organs. BM contained the highest percentage of c-kit+ cells. *P < .05 vs other organs (N = 6). (C) Morphology of a representative blast colony derived from a single CD34+ c-kit− uterine cell over 20 days. Representative micrographs illustrating clonal blast colony formation in a 1:1 suspension mixture of CD34+/c-kit− cells from GFP+ mice (green arrows) and RFP+ mice (red arrows). There was no aggregation; red and green cells formed separate blast colonies (D).
Blast colony formation by single uterine CD34+/c-kit− cells. Percentage of cells expressing CD34 (A) or c-kit+ (B) in each of multiple organs by flow cytometry. SKM indicates skeletal muscle. Uterine tissue contained the highest percentage of CD34+ cells. **P < .01 vs other organs. BM contained the highest percentage of c-kit+ cells. *P < .05 vs other organs (N = 6). (C) Morphology of a representative blast colony derived from a single CD34+ c-kit− uterine cell over 20 days. Representative micrographs illustrating clonal blast colony formation in a 1:1 suspension mixture of CD34+/c-kit− cells from GFP+ mice (green arrows) and RFP+ mice (red arrows). There was no aggregation; red and green cells formed separate blast colonies (D).
To confirm that cells were not aggregating to form colonies, we performed a dual fluorescent coculture of CD34+/c-kit− cells from RFP- and GFP-expressing mice in a 1:1 mixture. Aggregation would result in blast colocalization of RFP and GFP. All blast colonies derived were without green-red colocalization (Figure 1D); thus, aggregation was not a factor.
Multipotent differentiation of uterine blast colony cells
We cultured a putative clonogenic blast colony derived from the uterus (CD34+/c-kit−; Figure 2A) in blast cell differentiation media (CD34−/c-kit− cells did not form colonies). Two phenotypically distinct cell types emerged (Figure 2B): adherent (Figure 2C) and nonadherent (Figure 2D). Of 43 blast colonies examined, 79% produced both phenotypes, whereas 7% and 11% produced only one phenotype (adherent or nonadherent, respectively).
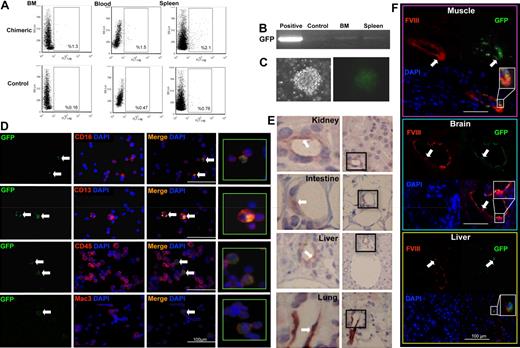
Uterine CD34+/c-kit− population exhibits hemangioblast-like potential in vitro. A single uterine blast (A) was plated in differentiation media in 1 well of a 96-well plate. Adherent cells (red; B-C) and nonadherent cells (blue; B,D) were observed after 12 days of culture. Nonadherent and adherent cells were collected separately for RT-PCR analysis of mRNA expression (Flk-1, CD31, β-H1, β-Major, GATA1, and SCL; GAPDH represents the housekeeping gene) (E). Representative micrographs illustrating expression of hematopoietic markers (CD45, Mac-3, CD13, CD16, CD4, and CD8) by the nonadherent cells (F, white arrows), and endothelial markers (CD31, factor VIII [FVIII]) and uptake of DiI-Ac-LDL by the adherent cells (G, white arrows). Rt- or Rb-IgG indicates rat or rabbit IgG negative control, respectively; DAPI (4,6-diamidino-2-phenylindole), nuclear stain. Tubular structures (H, white arrows) were formed by the adherent cells on Matrigel (original magnification ×100). Representative micrographs illustrating Giemsa-May-Grünwald–stained cytospin preparations of cells generated from the nonadherent uterine blast cells. Several different hematopoietic-like phenotypes were observed (I).
Uterine CD34+/c-kit− population exhibits hemangioblast-like potential in vitro. A single uterine blast (A) was plated in differentiation media in 1 well of a 96-well plate. Adherent cells (red; B-C) and nonadherent cells (blue; B,D) were observed after 12 days of culture. Nonadherent and adherent cells were collected separately for RT-PCR analysis of mRNA expression (Flk-1, CD31, β-H1, β-Major, GATA1, and SCL; GAPDH represents the housekeeping gene) (E). Representative micrographs illustrating expression of hematopoietic markers (CD45, Mac-3, CD13, CD16, CD4, and CD8) by the nonadherent cells (F, white arrows), and endothelial markers (CD31, factor VIII [FVIII]) and uptake of DiI-Ac-LDL by the adherent cells (G, white arrows). Rt- or Rb-IgG indicates rat or rabbit IgG negative control, respectively; DAPI (4,6-diamidino-2-phenylindole), nuclear stain. Tubular structures (H, white arrows) were formed by the adherent cells on Matrigel (original magnification ×100). Representative micrographs illustrating Giemsa-May-Grünwald–stained cytospin preparations of cells generated from the nonadherent uterine blast cells. Several different hematopoietic-like phenotypes were observed (I).
The nonadherent cells expressed genetic markers for GATA1, β–H1, and β-Major globin (erythroid markers; Figure 2E) and were positive for CD45 (pan leukocyte marker), Mac-3/CD16/CD13 (myeloid markers), and CD4/CD8 (markers suggesting possible lymphoid development; Figure 2F). Differentiation of this nonadherent population in vitro resulted in an array of hematopoietic morphologies identified with Giemsa-May-Grünwald stains (Figure 2I).
Adherent cells did not express the erythroid genetic markers GATA1, β–H1, or β-Major but had high expression of Flk-1 (KDR/VEGFR2/CD309) and CD31 (Figure 2E). Consistent with vascular cells, the adherent population expressed the endothelial cell proteins CD31 and factor VIII. These cells also demonstrated uptake of acetylated-LDL (Figure 2G) and spontaneous tube formation on Matrigel (Figure 2H). Together, these results strongly suggest that the adherent cells had an endothelial lineage. The in vitro bilineage differentiation of blast colonies derived from the adult CD34+/c-kit− uterine cells was similar to that reported for a subset of CD34+/c-kit− epiblast mesodermal germ layer stem cells previously established as mammalian embryonic hemangioblasts.4,5
Long-term self-renewal of uterine blast colony cells
Our in vitro experiments demonstrated phenotypic and functional bilineage hemangioblast-like differentiation from adult uterine CD34+/c-kit− cells. Next, we performed in vivo BM reconstitution experiments (Figure 3A) to examine the retention and renewal properties of the cells. In this model, a single GFP+ uterine CD34+/c-kit− blast colony (Figure 3B; ∼ 75 cells) was mixed with wild-type BM helper cells and then transplanted into a lethally irradiated wild-type recipient. GFP+ cells were identified in the hematopoietic compartments of the recipients 12 weeks or 12 months later. We obtained a reconstitution rate of approximately 90% (GFP+ cells were not detected in 3 of 32 reconstituted mice). At 12 weeks, uterine blast cell progeny were detected (by FACS analysis) in 1% to 2% of total cells in the recipient BM (1.2% ± 0.2%), blood (1.3% ± 0.2%), and spleen (2.2% ± 0.2%) (Figure 3C). At 12 months, GFP expression was similarly detected in 1.1% plus or minus 0.3% of cells in the BM, 1.0% plus or minus 0.3% cells in the blood, and 1.3% plus or minus 0.3% of cells in the spleen (Figure 4A). The presence of GFP mRNA was confirmed in BM and spleen at both 12 weeks (Figure 3D) and 12 months (Figure 4B) after reconstitution. BM isolates from the reconstituted mice exhibited a capacity for both wild-type and GFP+ hematopoietic colony formation at both time points (Figures 3E, 4C). These BM isolates contained GFP+ cells that expressed the same hematopoietic lineage markers (CD45, Mac-3, CD13, CD16) at both 12 weeks (Figure 3F) and 12 months (Figure 4D) we recorded in nonadherent cultures of uterine CD34+/c-kit− cells (Figure 2F). Vascular differentiation was confirmed by demonstrating the colocalization of GFP with factor VIII (an endothelial marker) after one year in the vascular structures of several organs (Figure 4E-F). These results show that uterine CD34+/c-kit− cells retain their capacity for bipotent differentiation and extensive engraftment in vivo.
BM reconstitution with uterine GFP+ blast: 12-week follow-up in vivo. (A) Schematic representation of the in vivo BM reconstitution. (B) A single, GFP+ CD34+/c-kit− uterine blast colony (mixed with wild-type [C57BL/6] BMCs) was used to reconstitute the BM of irradiated, C57BL/6 mice (N = 15). Representative scatter plots illustrating percentages of GFP+ cells in the BM, blood, or spleen of the chimeric recipients (C; by FACS; N = 5). RT-PCR confirmed GFP mRNA expression in the chimeric BM and spleen (D). Control indicates wild-type BMCs (negative control); and positive, GFP+ transgenic BMCs (positive control). Recipient BMCs were cultured in MethoCult media. GFP+ colony-forming units were observed at day 7 (E). Representative micrographs illustrating coexpression of GFP and hematopoietic markers (CD45, CD4, Mac-3, CD13, CD16, and CD8) in BM isolates from the recipients (F, white arrows). DAPI indicates nuclear stain.
BM reconstitution with uterine GFP+ blast: 12-week follow-up in vivo. (A) Schematic representation of the in vivo BM reconstitution. (B) A single, GFP+ CD34+/c-kit− uterine blast colony (mixed with wild-type [C57BL/6] BMCs) was used to reconstitute the BM of irradiated, C57BL/6 mice (N = 15). Representative scatter plots illustrating percentages of GFP+ cells in the BM, blood, or spleen of the chimeric recipients (C; by FACS; N = 5). RT-PCR confirmed GFP mRNA expression in the chimeric BM and spleen (D). Control indicates wild-type BMCs (negative control); and positive, GFP+ transgenic BMCs (positive control). Recipient BMCs were cultured in MethoCult media. GFP+ colony-forming units were observed at day 7 (E). Representative micrographs illustrating coexpression of GFP and hematopoietic markers (CD45, CD4, Mac-3, CD13, CD16, and CD8) in BM isolates from the recipients (F, white arrows). DAPI indicates nuclear stain.
BM reconstitution with uterine GFP+ blast: 12-month follow-up in vivo. Representative scatter plots illustrating percentages of GFP+ cells in the BM, blood, or spleen of the chimeric recipients (A; by FACS; N = 5). RT-PCR confirmed GFP mRNA expression in the chimeric BM and spleen (B). Control indicates wild-type BMCs (negative control); and positive, GFP+ transgenic BMCs (positive control). Recipient BMCs were cultured in MethoCult media. GFP+ colony-forming units were observed at day 7 (C). Representative micrographs illustrating coexpression of GFP and hematopoietic markers (CD16, CD13, CD45, and Mac-3) in BM isolates from the recipients (D, white arrows). DAPI indicates nuclear stain. Representative micrographs illustrating GFP expression (areas indicated in black boxes at right are enlarged at left, original magnification ×400) in formaldehyde-fixed tissue sections from multiple organs in the recipients (E, white arrows). Representative micrographs illustrating factor VIII and GFP expression (white arrows; colocalization and enlargement shown at bottom right) in the vasculature of frozen tissue sections from recipient somatic tissues (F).
BM reconstitution with uterine GFP+ blast: 12-month follow-up in vivo. Representative scatter plots illustrating percentages of GFP+ cells in the BM, blood, or spleen of the chimeric recipients (A; by FACS; N = 5). RT-PCR confirmed GFP mRNA expression in the chimeric BM and spleen (B). Control indicates wild-type BMCs (negative control); and positive, GFP+ transgenic BMCs (positive control). Recipient BMCs were cultured in MethoCult media. GFP+ colony-forming units were observed at day 7 (C). Representative micrographs illustrating coexpression of GFP and hematopoietic markers (CD16, CD13, CD45, and Mac-3) in BM isolates from the recipients (D, white arrows). DAPI indicates nuclear stain. Representative micrographs illustrating GFP expression (areas indicated in black boxes at right are enlarged at left, original magnification ×400) in formaldehyde-fixed tissue sections from multiple organs in the recipients (E, white arrows). Representative micrographs illustrating factor VIII and GFP expression (white arrows; colocalization and enlargement shown at bottom right) in the vasculature of frozen tissue sections from recipient somatic tissues (F).
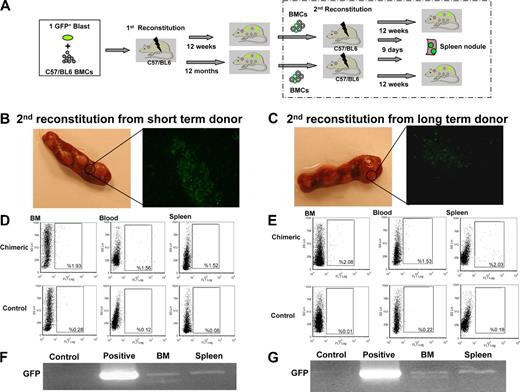
To establish long-term self-renewal by uterine CD34+/c-kit− cells, we reconstituted the BM of lethally irradiated wild-type mice with BMCs from primary recipients reconstituted either 12 weeks (short-term) or 12 months (long-term) earlier with a GFP+ uterine CD34+/c-kit− blast colony (secondary reconstitution; Figure 5A). Long-term self-renewal would be confirmed by the presence of GFP+ stem cells (of uterine hemangioblast origin) in the secondary BM recipients. After 9 days, we identified GFP+ cells in spleen nodes in the secondary recipients of BM obtained from short-term (Figure 5B) or long-term (Figure 5C) primary recipients. At 12 weeks, FACS analysis revealed GFP expression in 1% to 2% of cells in the BM, blood, and spleen (similar to the proportions measured in the primary recipients; Figure 5D-E), and reverse-transcribed polymerase chain reaction (RT-PCR) revealed GFP mRNA transcript expression in the BM and spleen (Figure 5F-G) of both groups of secondary recipients.
Long-term self-renewal of uterine blast colony cells. (A) Schematic representation of primary and secondary BM reconstitutions. BMCs were isolated from primary recipients at 12 weeks (short-term donor) or 12 months (long-term donor) after the primary reconstitution, and then injected into a second set of lethally irradiated wild-type (C57BL/6) mice through the tail vein. At 9 days after the second reconstitution, GFP+ nodules were observed in the spleens of the secondary recipients (B-C; n = 6 per group). At 12 weeks, GFP+ cells were detected in the BM, blood, and spleens of the secondary recipients (D-E; by FACS; n = 6 per group). RT-PCR confirmed GFP mRNA expression in the BM and spleen of the secondary recipients (F-G). Control indicates wild-type BMCs (negative control); and positive, GFP+ transgenic BMCs (positive control).
Long-term self-renewal of uterine blast colony cells. (A) Schematic representation of primary and secondary BM reconstitutions. BMCs were isolated from primary recipients at 12 weeks (short-term donor) or 12 months (long-term donor) after the primary reconstitution, and then injected into a second set of lethally irradiated wild-type (C57BL/6) mice through the tail vein. At 9 days after the second reconstitution, GFP+ nodules were observed in the spleens of the secondary recipients (B-C; n = 6 per group). At 12 weeks, GFP+ cells were detected in the BM, blood, and spleens of the secondary recipients (D-E; by FACS; n = 6 per group). RT-PCR confirmed GFP mRNA expression in the BM and spleen of the secondary recipients (F-G). Control indicates wild-type BMCs (negative control); and positive, GFP+ transgenic BMCs (positive control).
Adult uterine CD34+/c-kit− blast colony-forming cells exhibited expansion, bipotent differentiation, long-term retention, and self-renewal; therefore, they are true stem cells with hemangioblast characteristics. However, as the uterus is a blood-rich organ that has previously been shown to retain BMCs capable of in vitro transdifferentiation, it remains unclear whether these uterine resident hemangioblasts are itinerant BM-derived stem cells7 or germinal stem cells retained from embryonic development.
Extramedullary origin of the uterine hemangioblast
To determine the origin of the uterine hemangioblasts, we performed a BM-uterine tracking study (Figure 6). First, we reconstituted the BM of lethally irradiated wild-type mice with unfractionated BMCs from GFP+ donors. Six and 12 weeks later, we identified GFP+ cells in the uterine wall (Figure 6A-B). CD34+/c-kit− cells isolated from the recipient uterus did not generate GFP+ or GFP− blast colonies (Figure 6C), suggesting that the uterine hemangioblast is not a BM-derived cell and is not radioresistant. Alternatively, to account for the possibility of immunophenotypic drift and eliminate the possibility that we excluded a population of BMCs based on antigen expression, we reconstituted the BM of a second set of lethally irradiated wild-type mice with whole (unselected) uterine cells isolated from the primary recipient (combined with wild-type BM helper cells). Twelve weeks later, we identified no GFP+ cells in the BM, blood, or spleen of the secondary recipients (Figure 6D). This confirms that BM-derived cells in the uterus did not undergo self-renewal, and the BM is not the source of the uterine hemangioblasts.
Extramedullary origin of the uterine hemangioblast. BM-uterine tracking study. First, we reconstituted the BM of lethally irradiated wild-type (C57BL/6) mice with unfractionated BMCs from GFP+ donors (A). GFP+ cells were mobilized to the uterus after reconstitution (B; insets show GFP+ cells at higher magnification). CD34+/c-kit− BMCs were isolated from the recipient uterus and cultured in MethoCult media. No colony-forming units (GFP+ or GFP−) were observed at day 7 (C; insets show GFP+ cell at higher magnification). White arrows indicate GFP+ cells. We reconstituted the BM of a second set of lethally irradiated C57BL/6 mice with whole (unselected) uterine cells (UCs) isolated from the primary recipient (combined with C57BL/6 BM helper cells) (D). At 12 weeks after the secondary reconstitution, no GFP+ cells were detected in the BM, blood, or spleens of the secondary recipients (by FACS).
Extramedullary origin of the uterine hemangioblast. BM-uterine tracking study. First, we reconstituted the BM of lethally irradiated wild-type (C57BL/6) mice with unfractionated BMCs from GFP+ donors (A). GFP+ cells were mobilized to the uterus after reconstitution (B; insets show GFP+ cells at higher magnification). CD34+/c-kit− BMCs were isolated from the recipient uterus and cultured in MethoCult media. No colony-forming units (GFP+ or GFP−) were observed at day 7 (C; insets show GFP+ cell at higher magnification). White arrows indicate GFP+ cells. We reconstituted the BM of a second set of lethally irradiated C57BL/6 mice with whole (unselected) uterine cells (UCs) isolated from the primary recipient (combined with C57BL/6 BM helper cells) (D). At 12 weeks after the secondary reconstitution, no GFP+ cells were detected in the BM, blood, or spleens of the secondary recipients (by FACS).
Altogether, our findings identify an adult hemangioblast (CD34+/c-kit−) residing outside the BM, with a capacity for bilineage differentiation to hematopoietic and vascular cells. This clonogenic cell is capable of long-term self-renewal and hematopoietic reconstitution. To our knowledge, this work provides the first complete proof of an adult hemangioblast unrelated to the BM.
Discussion
This study identified blast colony-forming cells in the adult uterus that undergo bilineage differentition to both hematopoietic and vascular cells. We characterized the outgrowth and differentiation of these cells in parallel with observations that defined the hemangioblast in mice and humans.3,5 Through a series of serial transplantation experiments, we also established that these cells are capable of long-term retention, asymmetric division, and self-renewal. We concluded that uterine blast colony-forming cells are true stem cells. These uterine hemangioblasts are not of BM origin and may contribute to the unique functions of uterine tissue to create an environment that supports embryonic growth.
The close spatial and temporal emergence of blood and vasculature was the rationale for a common precursor cell,23,24 the hemangioblast.25 Studies attempting to characterize this cell were often plagued by technical limitations or challenged because of circumstantial evidence for gene targeting/expression, but the hemangioblast is now widely accepted as a component of mammalian development.3,5,26,27 In studies with mouse and human embryonic stem cells, the hemangioblast was defined as a blast colony-forming cell with bilineage commitment to a hematopoietic stem cell and an angioblast.28 Its phenotypic character was distinguished by Flk-1+ (KDR/VEGFR2/CD309) blast colony formation with divergent differentiation in culture to both nonadherent hematopoietic and adherent vascular lineages.3,5 Both emerging cell types expressed Flk-1 and CD31, but β-globin and GATA1 were expressed almost exclusively by the nonadherent cells. Expansion of the lineages further distinguished them as functionally vascular or hematopoietic. The current study documents similar phenotypic and functional patterns in a uterine-derived CD34+/c-kit− cell that forms blast colonies that subsequently produce both hematopoietic and vascular cells in vitro. Further, we performed an in vivo analysis of stem cell self-renewal.
We screened several tissues for clonogenic potential, including the blood, BM, heart, skeletal muscle, and uterus. The uterus had the highest potential for blast cell formation, and the in vitro characteristics of uterine CD34+/c-kit− cells were similar to those reported for embryonic-derived hemangioblasts. However, the acquisition of alternate cell characteristics and markers does not provide definitive proof of stem cell character. Indeed, many purported stem cells are incapable of long-term self-renewal. Till and McCulloch provided the first direct evidence of stemness in a series of experiments that involved clonogenic reconstitution of the BM29 and nodal spleen colony analysis using the double transplantation technique.30 Here, we performed this same evaluation of stemness by reconstituting the BM of a lethally irradiated recipient with a single, GFP+ uterine blast. To avoid the high mortality of single stem cell reconstitution, we combined the blast with helper BM cells from wild-type mice. Although this approach limited the total number of cells derived from a single cell after reconstitution, all animals survived and were available for study. We confirmed that a single blast reconstitution produced significant and consistent cellular expansion from the blast in vivo. Further, the turnover in cell progeny from the blast was stable for one year. Of 32 animals in the study, only 3 animals were GFP− (ie, failed to reconstitute). As further proof of blast stemness, secondary recipients were successfully reconstituted with BM from blast reconstituted (primary) recipients, including those assayed 12 months after transplantation. If the uterine blast was not derived from a true stem cell, then GFP+ cells would disappear from the circulation of the secondary recipients. However, we found that these animals had no proportional loss of blast progeny in the hematopoietic system. Previous studies have implied that adult hemangioblasts probably originate in the BM. Here, to establish that the uterine hemangioblast is a result of development and not of BM origin, we reconstituted the BM of wild-type mice with GFP+ BMCs and traced the integration of BM-derived cells in the uterus. The BM-derived uterine cells may have retained a capacity for multipotent differentiation, but not for self-renewal, and were thus not the source of the uterine hemangioblasts.
The start of embryonic hematopoiesis can be defined by the emergence of the hemangioblast in development.5 The posterior primitive streak of the embryo becomes the site of hematopoietic and vascular lineage commitment from the hemangioblast before blood islands form in the yolk sac.5 Subsequently, the aorta-gonad-mesonephros region houses hematopoietic stem cell development; and from there, hematopoietic stem cells are established in a liver niche that eventually shifts to the BM. A great deal of uncertainty surrounds these transitions, and the intrinsic/extrinsic mechanisms are the foci of many ongoing investigations.31 Despite the knowledge gaps from germination to adulthood, hematopoietic stem cells and vascular progenitors have been described in adult organs arising from the BM32-35 and circulation.36-38 That a hemangioblast persists in the adult in its true bilineage form is possible but uncertain. Also unknown is whether the spatial and phenotypic character of such a cell would match those of the embryonic hemangioblast. Defining adult stem/progenitor cells with hematopoietic and angiogenic potential in the adult is challenging because these cells would probably be spatially and temporally dynamic, and could be spread throughout differentiated tissue in various states of commitment. This diversity is revealed in the vast array of immunophenotypes and individual functions of cells, which may be required for niche maintenance and guided differentiation.
For nearly 80 years, we have had access to studies that describe cells in the circulation and BM with the capacity to differentiate into vascular cells and hematopoietic cells38 ; however, the search for the adult hemangioblast had not begun in earnest until much more recently, prompted by the description from Asahara et al6 of the endothelial progenitor cell and its angioblast character. The foundation for a BM-derived hemangioblast grew from the discovery of an adult lineage-depleted (Lin−) single cell (Sca-1+) that could reconstitute the hematopoietic system and undergo epithelial differentiation.7,8,10 However, the contributions of these cells to normal physiology (outside of injury and pathology) have been questioned.38,39 Uniquely, the current study presents evidence for an adult hemangioblast not associated with the BM that is a component of normal physiology, specifically, the estrous cycle. The possible existence of 2 sources of hemangioblasts in the adult raises many questions about hemangioblast compartmentalization from fetal to adult development. For example, it is unclear whether uterine hemangioblasts contribute to trophoblast or yolk sac formation. It is possible that hemangioblast outgrowth in these sites has a maternal contribution, whereas spontaneous hemangioblast emergence occurs fetally (in the liver). Our report may provide new insight into fetal-maternal association40 and may also have broad clinical implications for both sexes.41,42
In pregnancy, and well after birth, fetal cells that persist in maternal tissues respond to organ injury signals.43,44 Fetal-maternal microchimerism was not a factor in this study because we used only virgin mice, but important questions remain regarding menopause and routine hysterectomy in women. If the uterine hemangioblasts are itinerant (similar to BM cells), we may uncover an inadvertent handicap in women for cell-mobilized tissue repair. Our group used uterine myometrial cells for cell therapy in an ischemic cardiac injury model and recorded both capillary and extensive arteriogenic (rare with other donor cell types) neovascularization after therapy.45 Uterine hemangioblasts may be more accessible than BMCs,46 thus offering potential clinical benefits for treating ischemic diseases. However, although vascular growth is helpful in ischemic tissue repair, it can promote tumor growth in cancer. There is speculation that a uterine stem cell might be involved in endometriosis, uterine cancer, and pre-eclampsia in women, whereas the prostatic utricle, a uterine remnant in men, may be involved in prostatic cancer/hyperplasia and rare cases of male endometriosis.41,42,47,48
At a fundamental physiologic level, unanswered questions about the uterine hemangioblasts include the following: By what mechanisms do they maintain asymmetric division in the uterus? Are they itinerant cells? What is the nature of the endocrine milieu that regulates their normal functions? Future work will parallel the current study in humans. Our findings also present an opportunity to study stem cell niche character. Specifically, the anatomic location of the uterine hemangioblast niche remains to be established, as does the potential role of the BM stroma in niche support. In light of the routine turnover in the uterine tissue, this information might provide insight into stem cell niche maintenance, asymmetric division, and hemangioblast differentiation in vivo. It is noteworthy that some groups have identified stem/progenitor cells in blood menses,49,50 although it is unknown whether angioblasts or hemangioblasts could also be isolated from this source.
The current study sheds light on the fundamental nature of an adult hemangioblast stem cell by establishing the existence of an adult, non–BM-derived hemangioblast. This finding could improve our fundamental understanding of physiologic and pathologic conditions involving uterine tissue and contribute new insights into developmental stem cell compartmentalization. Our results may also have implications for the broad fields of developmental, vascular, hematologic, reproductive, and pathologic biology.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Heather McDonald Kinkaid for helpful discussion and editorial assistance.
This work was supported by the Canadian Institutes of Health Research (grant MOP86661; R.-K.L.). K.R.B. is a Heart and Stroke Foundation of Canada research fellow. R.-K.L. is a career investigator of the Heart and Stroke Foundation of Ontario and holds a Canada Research Chair in cardiac regeneration. A.K. holds the Epstein Chair in Cell Therapy and Transplantation at the University Health Network and the University of Toronto.
Authorship
Contribution: Z.S., Y.Z., S.F., R.D.W., A.K., and R.-K.L. conceived and designed the study; Z.S., Y.Z., K.R.B., J.W., and S.-H.L. collected and assembled the data; K.R.B., Z.S., Y.Z., R.D.W., A.K., and R.-K.L. analyzed and interpreted the data; and K.R.B., R.D.W., and R.-K.L. drafted the manuscript, critically revised the manuscript for intellectual content, and gave final approval of the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ren-Ke Li, MaRS Centre, Toronto Medical Discovery Tower, Rm no. 3-702, 101 College St, Toronto, ON, Canada M5G 1L7; e-mail: renkeli@uhnres.utoronto.ca.
REFERENCES
Author notes
Z.S. and Y.Z. contributed equally to this study.


![Figure 2. Uterine CD34+/c-kit− population exhibits hemangioblast-like potential in vitro. A single uterine blast (A) was plated in differentiation media in 1 well of a 96-well plate. Adherent cells (red; B-C) and nonadherent cells (blue; B,D) were observed after 12 days of culture. Nonadherent and adherent cells were collected separately for RT-PCR analysis of mRNA expression (Flk-1, CD31, β-H1, β-Major, GATA1, and SCL; GAPDH represents the housekeeping gene) (E). Representative micrographs illustrating expression of hematopoietic markers (CD45, Mac-3, CD13, CD16, CD4, and CD8) by the nonadherent cells (F, white arrows), and endothelial markers (CD31, factor VIII [FVIII]) and uptake of DiI-Ac-LDL by the adherent cells (G, white arrows). Rt- or Rb-IgG indicates rat or rabbit IgG negative control, respectively; DAPI (4,6-diamidino-2-phenylindole), nuclear stain. Tubular structures (H, white arrows) were formed by the adherent cells on Matrigel (original magnification ×100). Representative micrographs illustrating Giemsa-May-Grünwald–stained cytospin preparations of cells generated from the nonadherent uterine blast cells. Several different hematopoietic-like phenotypes were observed (I).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/16/10.1182_blood-2010-01-266882/4/m_zh89991058410002.jpeg?Expires=1767718313&Signature=TktxV-Jd6SsEnGtzD6Jnp4lxCmydWm5fZnzpsxuvoKNrXFHBhj-s9AczU-TuAUICtz2k0oqC4WRhJMuD4wN2zx1bqjO9RCN~z~YK83OhwkeQxrgKkqLGLqtCfUwGiGOKk6WeWPxAAUU-a91M6UXFJj2fPdIQOjSfgHxAtC8gMwz6NkXCQBfT-X1fxxmOGEAHHqA4dPHMTJdJIV7bK82yBfCRwJsxh-cq70ZU3cHFIpkPJSScnppFWlYUk-rQTncALkyxXgdXu9YqdyJCY6uuUx5CI9ZZEyqz~W2qQ-topJX6g1sY0CmVrzZUa9BbgKin-duFaxfx0NVNiVAOusLKgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. BM reconstitution with uterine GFP+ blast: 12-week follow-up in vivo. (A) Schematic representation of the in vivo BM reconstitution. (B) A single, GFP+ CD34+/c-kit− uterine blast colony (mixed with wild-type [C57BL/6] BMCs) was used to reconstitute the BM of irradiated, C57BL/6 mice (N = 15). Representative scatter plots illustrating percentages of GFP+ cells in the BM, blood, or spleen of the chimeric recipients (C; by FACS; N = 5). RT-PCR confirmed GFP mRNA expression in the chimeric BM and spleen (D). Control indicates wild-type BMCs (negative control); and positive, GFP+ transgenic BMCs (positive control). Recipient BMCs were cultured in MethoCult media. GFP+ colony-forming units were observed at day 7 (E). Representative micrographs illustrating coexpression of GFP and hematopoietic markers (CD45, CD4, Mac-3, CD13, CD16, and CD8) in BM isolates from the recipients (F, white arrows). DAPI indicates nuclear stain.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/16/10.1182_blood-2010-01-266882/4/m_zh89991058410003.jpeg?Expires=1767718313&Signature=qqlfDTU5tfMrNmTrDyQU~1iawaVbNUjb1S8yFeoVW9ARQTU~GT0CH~vlwbAnkxhLjybemJ16ffA6b0HseZL0R07BkE~YzS9AiugVYjMFrQYL4dE366~OAM58F6WeTjDDHH3PvwlG7ONYonECvPGqsm-VQyo7ob~rklvm3U51mqZficSdFmhZUnYS9f5I5mZKhlggj~XgrzVKPadErzAVsn3jx30siyqqR1VTe4qzAxZKdLjEnItflkj8dgg~sbAIqvIndR7HV~U-SAU0Cxm5t53i5yEbxSJQejUIQ6eixHDgJm3zeERbE-IEo2iB73y5cEsVu~cA00zFfIGSa3E2IA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal