Abstract
Hemophagocytic lymphohistiocytosis (HLH) is an often-fatal hyperinflammatory syndrome characterized by fever, hepatosplenomegaly, cytopenia, and in some cases hemophagocytosis. Here, we describe the mutation analysis, clinical presentation, and functional analysis of natural killer (NK) cells in patients with mutations in STXBP2 encoding Munc18-2, recently associated with familial HLH type 5. The disease severity among 11 persons studied here was highly variable and, accordingly, age at diagnosis ranged from 2 months to 17 years. Remarkably, in addition to typical manifestations of familial HLH (FHL), the clinical findings included colitis, bleeding disorders, and hypogammaglobulinemia in approximately one-third of the patients. Laboratory analysis revealed impairment of NK-cell degranulation and cytotoxic capacity. Interleukin-2 stimulation of lymphocytes in vitro rescued the NK cell–associated functional defects. In conclusion, familial HLH type 5 is associated with a spectrum of clinical symptoms, which may be a reflection of impaired expression and function of Munc18-2 also in cells other than cytotoxic lymphocytes. Mutations in STXBP2 should thus also be considered in patients with clinical manifestations other than those typically associated with HLH.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory syndrome with high mortality.1-3 According to the HLH-2004 study, HLH diagnosis requires fulfillment of 5 of 8 of the following criteria: fever, splenomegaly, bicytopenia, high triglycerides/low fibrinogen, hemophagocytosis, high ferritin, low natural killer (NK)–cell cytotoxicity, and high soluble CD25.4 Diagnosis of primary, familial HLH (FHL) can also be based on the identification of mutations in genes previously associated with HLH. Typically, FHL patients have an early onset and a severe course of disease. Notably, many FHL patients are affected by meningo-encephalitis causing neurologic alterations.5 Hematopoietic stem cell transplantation (HSCT) is currently the only available curative option for FHL patients. Secondary HLH typically has a later onset and is associated with severe infections, rheumatic disorders, or malignancies.6
A number of genes are associated with development of HLH. Autosomal recessive mutations in PRF1, UNC13D, and STX11, encoding perforin, Munc13-4, and syntaxin-11, are causative of FHL type 2 (FHL2), FHL3, and FHL4, respectively.7-9 Recently, 2 groups independently described mutations in STXBP2, encoding Munc18-2, as the cause of familial HLH type 5 (FHL5).10,11 Other autosomal recessive immunodeficiencies associated with the development of HLH are Griscelli syndrome type 2 (GS2) and Chediak-Higashi syndrome type 1 (CHS1), caused by mutations in RAB27A and LYST, respectively.12,13 Moreover, patients with Hermansky-Pudlak syndrome type 2 (HPS2), caused by mutations in AP3B1, may also present with HLH.14 In addition to immunodeficiency, a common denominator of GS2, CHS1, and HPS2 is partial albinism. Patients with X-linked lymphoproliferative syndrome 1 and 2 (XLP1, XLP2), caused by mutations in SH2D1A or XIAP, respectively, also frequently present with HLH, besides lymphomas or hypogammaglobulinemia.15,16
The increasing spectrum of genes associated with development of HLH has provided insight into the pathophysiology of this disease. The aforementioned genes encode proteins that facilitate cellular cytotoxicity mediated by cytotoxic T cells and NK cells.7,8,12,17 In animal models, impairment in cytotoxic function of lymphocytes predisposes to development of disproportionately aggressive immune responses similar to those seen in HLH patients.18-20 Furthermore, results with animal models indicate that pathogenic triggers are required for the development of hypercytokinemia and T-cell expansions. Hemophagocytosis is considered to develop secondary to elevated IFN-γ and is not necessarily observed at early stages in disease. Notably, the expression of gene products associated with HLH is not restricted to cytotoxic lymphocytes. Rather, expression of several of these proteins can be detected in cells of other hematopoietic lineages and even in nonhematopoietic tissues. Thus, although pathogenesis is closely associated with impaired perforin-mediated cytotoxicity, mutations may have ramifications beyond those caused by defects in lymphocyte cytotoxicity.
There is currently limited information regarding the clinical presentation of patients with biallelic mutations in STXBP2. Here we describe 11 persons diagnosed with FHL5: 9 with biallelic mutations in STXBP2 and 2 siblings whose DNA was not available for genetic analysis. We provide a detailed case report of a 17-year-old adolescent patient who illustrates several interesting features of FHL5 and also highlights the difficulties in diagnosis of late-onset primary immunodeficiencies and dilemmas faced in regards to treatment. Notably, observations from this patient and data from other FHL5 patients suggest that, in addition to typical HLH-associated symptoms, FHL5 patients may also present with colitis, bleeding disorders, and hypogammaglobulinemia.
Methods
Patients and controls
Eight families with biallelic mutations in STXBP2, including 3 families with 2 affected siblings, were identified in a cohort of patients without identifiable mutations in the other FHL-associated genes, PRF1, UNC13D, and STX11. Healthy blood donors (n = 95) in Sweden were used as controls. The studies were approved by the ethics committee at the Karolinska Institutet. Patient or parental consent was obtained for all patients in accordance with the Declaration of Helsinki.
Sequencing analyses
Genomic DNA was isolated from peripheral blood or cultured fibroblasts according to standard procedures. Specific primers were used for polymerase chain reaction (PCR) amplifications of exons 1 to 19 and exon/intron boundaries of STXBP2. Direct sequencing was performed according to standard procedures (BigDye, Version 3.1; Applied Biosystems), and reactions were subsequently analyzed by capillary electrophoresis (ABI 3730 Genetic Analyzer; Applied Biosystems). Data were analyzed using SeqScape software (Version 2.5; Applied Biosystems) and a reference sequence template (NCBI accession no. NM_006949.2). Primers, PCR conditions, and sequencing reaction conditions are available on request.
Analysis of cytotoxic lymphocyte function
NK cell–mediated lysis of K562 target cells (ATCC) was assessed using a standard 4-hour 51Cr-release assay, as previously described.21 Freshly isolated peripheral blood mononuclear cells (PBMCs) or PBMCs cultured for 48 to 72 hours with 400 IU/mL interleukin-2 (IL-2; Chiron) were used as effector cells and NK-cell activity was calculated as lytic units (LU) at 25% lysis. According to the HLH-2004 guidelines, NK-cell activity less than 10 LU is one of the 8 diagnostic criteria for HLH.4 NK-cell degranulation was assessed by flow cytometry quantifying induction of CD107a surface expression (ΔCD107a) on CD3−CD56+ gated cells in response to incubation with K562 target cells, as previously described.17
Results
STXBP2 mutations in patients diagnosed with HLH
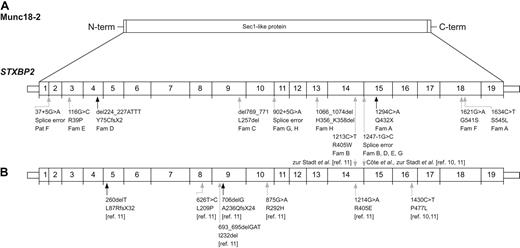
Patients from 8 unrelated families carried 11 different mutations, 9 of which were novel (Table 1; Figure 1A). In total, 4 different missense mutations, one nonsense mutation, one out-of-frame deletion, 2 in-frame deletions, and 3 mutations affecting splice sites were identified. One of the splice site mutations, c.1247-1G>C, was identified in 4 of 8 families of white and Asian origin. This mutation was also described in 9 of 22 families reported by Côte et al10 and zur Stadt et al11 (Figure 1B). Thus, this STXBP2 mutation is found in 43% of the families so far reported with FHL5. DNA from healthy adult blood donors (190 alleles) was analyzed and found negative for the novel nontruncating STXBP2 mutations.
Clinical and laboratory findings in patients with FHL5
| . | Patient A . | Patient B:1 . | Patient B:2 . | Patient C . | Patient D . | Patient E . | Patient F . | Patient G:1 . | Patient G:2 . | Patient H:1 . | Patient H:2 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic origin | Russia | Pakistan | Pakistan | Pakistan | The Netherlands | The Netherlands | Norway | Denmark | Denmark | Denmark | Denmark |
| Familial disease | No | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | |
| Parental consanguinity | No | Yes | Yes | Yes | No | No | No | No | No | No | |
| Sex | Female | Female | Male | Female | Female | Male | Female | Female | Male | Male | Female |
| Allele 1 | c.1294C>A p.Gln432X | c.1213C>T p.Arg405Trp | c.del769_771 p.Leu257del | c.del224_227ATTT p.Tyr75CysfsX2 | c.116G>C p.Arg39Pro | c.37+5G>A splice error? | c.902+5G>A splice error? | c.902+5G>A splice error? | c.902+5G>A splice error? | ||
| Allele 2 | c.1634C>T p.Ser545Leu | c.1247-G>C splice error | c.del769_771 p.Leu257del | c.1247-1G>C splice error | c.1247-G>C splice error | c.1621G>A p.Gly541Ser | c.1247-1G>C splice error | c.1066_1074del; p.His356_Lys358del | c.1066_1074del; p.His356_Lys358del | ||
| Age at onset, HLH | 17 y | 4 y | 4 mo | 6 mo | 8.5 mo | 19 mo | 11 mo | 7 y | 12 y | 2 mo | Not applicable |
| Age at diagnosis, HLH | 17 y | 9 y | 4 y | 6 mo | 9 mo | 19 mo | 11 mo | 7 y (postmortem) | 12 y | 3.5 mo | 6 mo |
| Fever | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Splenomegaly | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Hepatomegaly | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No |
| Hemoglobin, g/L | 138 | 72 | 79 | 67 | 108 | 48 | 71 | 80 | 76 | 94 | 114 |
| Neutrophils, 109/L | 8.2 | 2.7 | 0.9 | 1.0 | 0.8 | 0 | 0.4 | 0.2 | 0.5 | 1.1 | 1.3 |
| Platelets, 109/L | 76 | 15 | 27 | 7 | 91 | 18 | 17 | 40 | 25 | 37 | 280 |
| Triglycerides,mM | 2.2 | 3.1 | 4.19 | 5.4 | 4.2 | 6.3 | 4.4 | Elevated | 5.6 | 3.2 | 1.4 |
| Fibrinogen, g/L | 1.3 | 0.9 | 1.5 | 1.8 | 1.1 | 1 | 1.6 | Decreased | 5 | < 0.7 | < 0.7 |
| Hemophagocytosis | No | No | No | Yes (BM) | Yes (spleen) | Yes (BM) | No | Yes (BM) | Yes (BM) | Yes (ascites and BM) | No |
| Ferritin, μg/L | 3101 | 3615 | 256 | 884 | 6100 | 35866 | 848 | 1559 | 2000 | 15 | |
| sCD25, U/mL | > 2400 | 3400 | . | ||||||||
| Liver transaminases | Elevated | Elevated | Elevated | Elevated | Elevated | Elevated | Elevated | Elevated | Elevated | Normal | Elevated |
| NK-cell activity* | Defective | Defective | ND | Defective | Defective | Defective | Defective | Defective | Defective | Defective | |
| NK-cell degranulation | Defective | Defective | ND | Defective | Defective | Defective | Defective | Defective | Defective | ||
| Neurologic manifestations† | No | Yes | Yes | No | No | Yes | Yes | Uncertain‡ | Yes | Yes | No |
| EBV infection† | Yes | Yes | Yes | No | No | Yes | No | No | No | No | No |
| Hypogammaglobulinemia† | Yes | Yes | Yes | No | No | No | No | No | No | No | Yes |
| Treatment active disease | HLH-94, rituximab | HLH-2004 | Steroids | HLH-2004 | HLH-2004 | HLH-2004 | HLH-2004 | No | HLH-94 | HLH-2004 | No |
| Remission at 2 mo | Yes | Yes | No | Yes | Yes | No | No | Yes | Not applicable | ||
| Age at HSCT | Not done | Not done | Not done | Yes | 14 mo | 22/28 mo | 19/24 mo | No | 13 y | 7.5 mo | Donor search started |
| Outcome and follow-up | Alive 2 y after onset | Alive 7 y after onset | Dead 4 y after onset | Alive 11 mo after HSCT | Alive 4 y after HSCT | Dead 4 mo after 2nd HSCT | Alive 2 y after 2nd HSCT | Dead 3 mo after onset | Alive 8 y after HSCT | Alive 1 mo after HSCT | Alive |
| . | Patient A . | Patient B:1 . | Patient B:2 . | Patient C . | Patient D . | Patient E . | Patient F . | Patient G:1 . | Patient G:2 . | Patient H:1 . | Patient H:2 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic origin | Russia | Pakistan | Pakistan | Pakistan | The Netherlands | The Netherlands | Norway | Denmark | Denmark | Denmark | Denmark |
| Familial disease | No | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | |
| Parental consanguinity | No | Yes | Yes | Yes | No | No | No | No | No | No | |
| Sex | Female | Female | Male | Female | Female | Male | Female | Female | Male | Male | Female |
| Allele 1 | c.1294C>A p.Gln432X | c.1213C>T p.Arg405Trp | c.del769_771 p.Leu257del | c.del224_227ATTT p.Tyr75CysfsX2 | c.116G>C p.Arg39Pro | c.37+5G>A splice error? | c.902+5G>A splice error? | c.902+5G>A splice error? | c.902+5G>A splice error? | ||
| Allele 2 | c.1634C>T p.Ser545Leu | c.1247-G>C splice error | c.del769_771 p.Leu257del | c.1247-1G>C splice error | c.1247-G>C splice error | c.1621G>A p.Gly541Ser | c.1247-1G>C splice error | c.1066_1074del; p.His356_Lys358del | c.1066_1074del; p.His356_Lys358del | ||
| Age at onset, HLH | 17 y | 4 y | 4 mo | 6 mo | 8.5 mo | 19 mo | 11 mo | 7 y | 12 y | 2 mo | Not applicable |
| Age at diagnosis, HLH | 17 y | 9 y | 4 y | 6 mo | 9 mo | 19 mo | 11 mo | 7 y (postmortem) | 12 y | 3.5 mo | 6 mo |
| Fever | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Splenomegaly | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Hepatomegaly | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No |
| Hemoglobin, g/L | 138 | 72 | 79 | 67 | 108 | 48 | 71 | 80 | 76 | 94 | 114 |
| Neutrophils, 109/L | 8.2 | 2.7 | 0.9 | 1.0 | 0.8 | 0 | 0.4 | 0.2 | 0.5 | 1.1 | 1.3 |
| Platelets, 109/L | 76 | 15 | 27 | 7 | 91 | 18 | 17 | 40 | 25 | 37 | 280 |
| Triglycerides,mM | 2.2 | 3.1 | 4.19 | 5.4 | 4.2 | 6.3 | 4.4 | Elevated | 5.6 | 3.2 | 1.4 |
| Fibrinogen, g/L | 1.3 | 0.9 | 1.5 | 1.8 | 1.1 | 1 | 1.6 | Decreased | 5 | < 0.7 | < 0.7 |
| Hemophagocytosis | No | No | No | Yes (BM) | Yes (spleen) | Yes (BM) | No | Yes (BM) | Yes (BM) | Yes (ascites and BM) | No |
| Ferritin, μg/L | 3101 | 3615 | 256 | 884 | 6100 | 35866 | 848 | 1559 | 2000 | 15 | |
| sCD25, U/mL | > 2400 | 3400 | . | ||||||||
| Liver transaminases | Elevated | Elevated | Elevated | Elevated | Elevated | Elevated | Elevated | Elevated | Elevated | Normal | Elevated |
| NK-cell activity* | Defective | Defective | ND | Defective | Defective | Defective | Defective | Defective | Defective | Defective | |
| NK-cell degranulation | Defective | Defective | ND | Defective | Defective | Defective | Defective | Defective | Defective | ||
| Neurologic manifestations† | No | Yes | Yes | No | No | Yes | Yes | Uncertain‡ | Yes | Yes | No |
| EBV infection† | Yes | Yes | Yes | No | No | Yes | No | No | No | No | No |
| Hypogammaglobulinemia† | Yes | Yes | Yes | No | No | No | No | No | No | No | Yes |
| Treatment active disease | HLH-94, rituximab | HLH-2004 | Steroids | HLH-2004 | HLH-2004 | HLH-2004 | HLH-2004 | No | HLH-94 | HLH-2004 | No |
| Remission at 2 mo | Yes | Yes | No | Yes | Yes | No | No | Yes | Not applicable | ||
| Age at HSCT | Not done | Not done | Not done | Yes | 14 mo | 22/28 mo | 19/24 mo | No | 13 y | 7.5 mo | Donor search started |
| Outcome and follow-up | Alive 2 y after onset | Alive 7 y after onset | Dead 4 y after onset | Alive 11 mo after HSCT | Alive 4 y after HSCT | Dead 4 mo after 2nd HSCT | Alive 2 y after 2nd HSCT | Dead 3 mo after onset | Alive 8 y after HSCT | Alive 1 mo after HSCT | Alive |
BM indicates bone marrow.
Defective: ≤ 10 LU.
Reported at some point during the course of the disease.
Hearing deficiency of unknown cause.
Location of STXBP2 mutations relative to exon structure. (A) Sites of mutations described in this report. (B) Sites of previously reported mutations. Exon borders are indicated. Black arrows indicate disruptive nonsense and small deletion mutations; and gray arrows, missense mutations and splice-site mutations.
Location of STXBP2 mutations relative to exon structure. (A) Sites of mutations described in this report. (B) Sites of previously reported mutations. Exon borders are indicated. Black arrows indicate disruptive nonsense and small deletion mutations; and gray arrows, missense mutations and splice-site mutations.
Case report of patient A
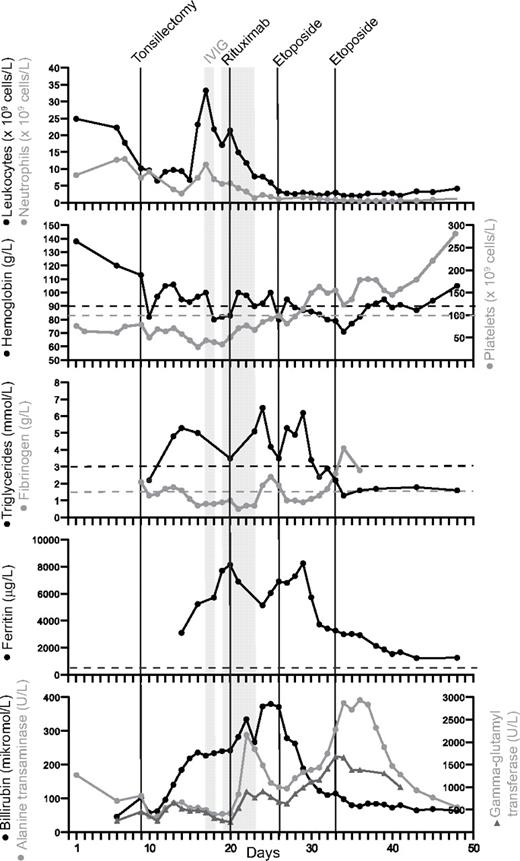
A 17-year-old previously healthy girl of Russian origin (patient A) was admitted with a 3-day history of fever, abdominal pain, and sore throat. C-reactive protein and aspartate transanimase/alanine transaminase were elevated (Figure 2). Heterophile antibody titers for Epstein-Barr virus (EBV) were detected. Platelets were decreased and leukocytes were elevated, whereas hemoglobin was within the normal reference interval (Figure 2). Tonsillectomy and abrasio of the adenoid were performed 9 days after admission because of increasing respiratory difficulties. Severe bleeding necessitated a second surgery procedure the same day. Extubation after surgery was not possible, and the patient was transferred to the intensive care unit (ICU). Despite treatment with antibiotics (cefuroxime), antivirals (aciclovir), and corticosteroids, her vital parameters worsened. Because of severe bleeding and a low platelet count, she received platelet infusions, coagulation factors, fibrinogen concentrate, and intravenous immunoglobulins (IVIG; day 17, 0.17 g/kg; days 19-22, 0.40 g/kg per day). Because of renal failure, the patient was treated with continuous renal replacement therapy. One course of rituximab was administered (day 20, 412 mg/m2) because of a high EBV viral load (43 000 EBV copies/mL blood detected by PCR). In this setting, rituximab appeared to ameliorate parameters of EBV-induced HLH disease severity, including platelets and ferritin.
Clinical and laboratory parameters of index case (patient A). Body temperature and laboratory parameters included in the diagnostic criteria for hemophagocytic lymphohistiocytosis (HLH) according to the Histiocyte Society (panels 1-5) and liver parameters (panel 6) are charted for the first 50 days after admission. Black lines indicate tonsillectomy, treatment with rituximab (day 20, 412 mg/m2), and 2 doses of etoposide (days 26 and 33, 100 mg/m2). The shaded area represents days with administrated intravenous immunoglobulin (IVIG; day 17 and days 19-22; 0.17 g/kg and 0.40 g/kg per day, respectively).
Clinical and laboratory parameters of index case (patient A). Body temperature and laboratory parameters included in the diagnostic criteria for hemophagocytic lymphohistiocytosis (HLH) according to the Histiocyte Society (panels 1-5) and liver parameters (panel 6) are charted for the first 50 days after admission. Black lines indicate tonsillectomy, treatment with rituximab (day 20, 412 mg/m2), and 2 doses of etoposide (days 26 and 33, 100 mg/m2). The shaded area represents days with administrated intravenous immunoglobulin (IVIG; day 17 and days 19-22; 0.17 g/kg and 0.40 g/kg per day, respectively).
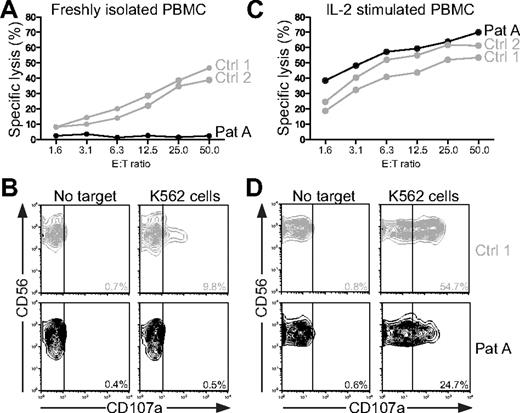
In regards to a diagnosis of HLH, the patient fulfilled 4 of the 8 criteria, namely, fever, splenomegaly, low fibrinogen, and elevated ferritin. Bone marrow aspirate did not reveal hemophagocytosis. As for bicytopenia, only the platelet count was low at admission; anemia developed later but was attributed to severe bleeding. Hence, samples were sent for NK-cell functional analysis on the 23rd day of hospitalization. Pathologic NK cell–mediated cytotoxicity substantiated the diagnosis of HLH (Figure 3A, LU were calculated for patient A, 3 LU; healthy controls, 227 and 179 LU, respectively). Defective degranulation by freshly isolated NK cells indicated a congenital defect in cytotoxic lymphocyte function (Figure 3B, induced CD107a surface expression, ΔCD107a, was determined for patient A, 0.1%; healthy controls, 9.1% and 6.9%, respectively). Notably, a rapidly reversible restoration of cytotoxicity and degranulation after IL-2 stimulation of NK cells was observed (Figure 3C-D). Thus, with fulfillment of at least 5 HLH criteria, treatment was started according to the HLH-94 protocol.22 Etoposide was administered day 26 at a reduced dosage because of the patient's age (100 mg/m2 instead of 150 mg/m2). In addition, the patient received betamethasone (10 mg/m2) as a substitute for dexamethasone, which was not readily available. After HLH-94 treatment, laboratory and clinical parameters gradually improved with more stable circulation and reduced edema (Figure 2; and data not shown). The second dose of etoposide was postponed to week 2 because of the prompt response to treatment. The patient was extubated at day 35 after admission and discharged on day 55. Thus, although IVIG and rituximab at least transiently reduced leukocytosis and improved platelet and ferritin values, etoposide might be required for more long-term immune suppression and clinical remission.
NK-cell cytotoxicity and degranulation in the index case (patient A). (A-B) Resting peripheral blood mononuclear cells (PBMCs) or (C-D) PBMCs stimulated for 72 hours with IL-2 from patient A and 2 healthy adults were evaluated for cytotoxicity and degranulation toward K562 cells. (A,C) Plots show specific lysis for different effector cell to target cell (E:T) ratios in a 4-hour 51Cr-release assay. (B,D) After 2 hours of incubation at 37°C, the cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a monoclonal antibodies. Lymphocytes were gated on forward/side scatter plots, followed by gating on CD3 versus CD56 plots. Plots show CD56 versus CD107a expression on CD3−CD56+ natural killer (NK) cells. The percentage of CD107a+ cells is indicated. NK-cell cytotoxicity and degranulation were analyzed repeatedly in the patient. One representative experiment is shown.
NK-cell cytotoxicity and degranulation in the index case (patient A). (A-B) Resting peripheral blood mononuclear cells (PBMCs) or (C-D) PBMCs stimulated for 72 hours with IL-2 from patient A and 2 healthy adults were evaluated for cytotoxicity and degranulation toward K562 cells. (A,C) Plots show specific lysis for different effector cell to target cell (E:T) ratios in a 4-hour 51Cr-release assay. (B,D) After 2 hours of incubation at 37°C, the cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a monoclonal antibodies. Lymphocytes were gated on forward/side scatter plots, followed by gating on CD3 versus CD56 plots. Plots show CD56 versus CD107a expression on CD3−CD56+ natural killer (NK) cells. The percentage of CD107a+ cells is indicated. NK-cell cytotoxicity and degranulation were analyzed repeatedly in the patient. One representative experiment is shown.
During the following months, doses of corticosteroids were slowly tapered, whereas IVIG was administrated every second week because of persistent hypogammaglobulinemia. Still, the patient had recurrent episodes of diarrhea, vomiting, and abdominal pain. Fecal calprotectin was moderately elevated (128 mg/kg). Finally, the patient's severe vomiting and diarrhea instigated rehospitalization 2 months after initial discharge. Calicivirus infection was confirmed by real-time PCR, but the infection cleared within one week of presentation. Colonoscopy revealed diffuse inflammation from cecum to the sigmoid colon, and a diagnosis of inflammatory bowel disease was considered. The colitis improved with oral mesalazin treatment.
According to the parents, the patient tends to develop ecchymosis and has protracted bleeding from wounds, corroborating clinical notes regarding bleeding disorders during her surgery. In retrospect, the patient might have had an earlier episode of HLH at the age of 6 years, when she was hospitalized in Russia with a high fever and symptoms of gastroenteritis. Culture did not identify any causative pathogen. Two years after HLH-94 treatment, patient A is doing well and is a high achiever at school. IVIG is administered on a regular basis because of low immunoglobulins despite being 2 years past a single course of rituximab. At the last follow-up, the lymphocyte profile revealed 90% CD3+ T cells (4.15 × 109 cells/L; 26% CD4+ T cells, 61% CD8+ T cells), 6% CD3−CD56+ NK cells (0.25 × 109 cells/L), and 4% CD19+ B cells (0.19 × 109 cells/L). Thus, this index patient demonstrated several features not commonly associated with other types of FHL, such as colitis, bleeding disorders, and hypogammaglobulinemia.
Characteristics of FHL5 patient cohort
The age at onset of HLH in our cohort of FHL5 patients varied from 2 months to 17 years, with a median onset of 15 months (Table 1; mean age, 54 months; n = 10). If patients previously reported by zur Stadt et al11 are included, the median age at onset is 9 months (range, 1.5 months to 17 years; mean, 30 months; n = 23). Apart from patient B:2 (who fulfilled 4 of 6 criteria evaluated) and patient H:2, a twin sibling with a confirmed molecular diagnosis of FHL5 at 6 months of age but without symptoms of HLH (presently 8 months of age), the other 9 patients fulfilled the diagnostic criteria for HLH (Table 1).4
To date, 8 of the 11 patients are still alive. Two of the patients (patients B:2 and G:2) died before HSCT, whereas patient E died of a reactivated adenovirus infection after a haploidentical paternal transplantation. Of the 8 patients still alive, 5 have been successfully transplanted. Patient A has not received a transplantation but is doing well 2 years past the HLH-94 treatment without any ongoing therapy except IVIG. Patient B:1 developed EBV-induced HLH at 4 years of age and has since then presented with intermittent fever, hepatosplenomegaly, and elevated liver transaminases in addition to chronic diarrhea, a bleeding disorder, and hypogammaglobulinemia. The patient is now 9 years old and recently was treated successfully with the HLH-2004 protocol after the diagnosis of FHL5 was confirmed. Of note, Patient B:1 reportedly also has a growth hormone deficiency. Patient C received a HLA-identical sibling HSCT and now has chronic graft versus host disease causing a malabsorption syndrome with cachexia. Patient D is doing well after a cord blood HSCT. Patient F first received a haploidentical paternal HSCT but on relapse received a second transplantation from an unrelated donor. Notably, the mother of patient F had a history of a miscarriage at week 22 of pregnancy with fetal malformations, including hepatosplenomegaly. Patient G:1 died 3 months after onset of disease and was diagnosed with HLH postmortem. Patient G:2 is doing well after a matched unrelated donor HSCT. Patient H:1 presented, at 2 months of age, with HLH that resolved without treatment, but the patient relapsed one month later, required treatment, and has now undergone HSCT. Interestingly, a twin sister carrying the same mutations (patient H:2), has to date not developed HLH and has successfully cleared a rotavirus infection that elicited increases of alanine transaminase (466 U/L) as well as neutropenia (0.72 × 109 cells/L) lasting 2 days.
Neurologic manifestations
Neurologic symptoms during the course of the disease were observed in approximately 50% of the FHL5 patients reported here. Three weeks after initial presentation, patient B:1 was admitted with convulsions and hypotonia. Analysis of the cerebrospinal fluid (CSF) revealed no cellular infiltrate, but a computed tomography of the brain showed a hypodense area in the occipital lobe. Symptoms subsequently resolved. The brother of patient B:1, patient B:2, presented with facial palsy that resolved within one week. Magnetic resonance imaging (MRI) of the brain revealed hyperintensities distributed throughout the cerebellum and cerebrum, suggestive of a demyelinating process. Five months later, he presented with left eye strabismus, in addition to bilateral chorioretinitis. Patient E was irritable without other abnormal neurologic symptoms, and brain MRI performed one week after the HLH diagnosis showed triventricular hydrocephalus without aqueduct stenosis. Analysis of the CSF revealed no cellular infiltrate but was performed a week after treatment according to the HLH-2004 protocol was started. At admission, patient F was hypotonic with vertical nystagmus and later developed ataxia and convergent strabismus. MRI showed abnormalities in cerebellum and in the supratentorial white matter in both hemispheres. The CSF cell count was elevated (72 × 106 cells/L). On presentation of HLH, patient G:1 developed a hearing deficiency of unknown cause. However, a computed tomography scan of the brain and evaluation of CSF cell count were normal. Patient G:2 did not present with neurologic symptoms, but the CSF cell count was elevated (17 × 106 cells/L). Patient H:1 was reported irritable at admission and the CSF protein was elevated (0.78 g/L), whereas the cell count was normal.
Gastrointestinal symptoms
The observation of an inflammatory bowel-like disease in the index patient (patient A) prompted a review of the other patients for gastrointestinal symptoms. Former histories reported that patient B:1 had chronic diarrhea, cytomegalovirus colitis, and Giardia enteritis; similarly, patient B:2 had chronic diarrhea. Relatively minor gastrointestinal abnormalities were noted in 2 other patients assessed here. Patient C displayed a severe gastroesophagal reflux and a failure to thrive. However, when treatment according to the HLH-2004 protocol began, the vomiting ceased and the patient recovered in weight. Patient E presented with a failure to thrive and inflammation of the lips and oral mucosa. Furthermore, patient G:1 was affected by recurrent abdominal pain. A brother (patient G:2) also had severe abdominal pain and loss of appetite. It should be noted that no gastrointestinal symptoms have been reported for patient G:2 8 years after transplantation. In summary, severe gastrointestinal symptoms may present before typical clinical manifestations of HLH.
Bleeding disorders
As outlined in the case report of index patient A, the patient displayed ecchymosis and a tendency for severe, prolonged bleeding after superficial cuts. The 2 affected siblings in family B also had bleeding disorders. Patient B:1 proved to be thrombocytopenic, along with a prolonged coagulation profile, bloody pleural effusions, and petechiae. Patient B:2 was similarly thrombocytopenic, had a prolonged coagulation profile, and developed petechiae. No obvious bleeding disorders were reported in the remaining patients.
Hypogammaglobulinemia
After EBV-induced HLH, which included treatment with rituximab, patient A developed hypogammaglobulinemia. Because of this, the patient has been administered IVIG. 2 years after treatment with rituximab, IgA and IgM levels are still low (IgA < 0.1 g/L; IgM 0.1 g/L). IgG levels are normal (IgG 7.0 g/L) but reflect the regularly administered substitution. Hypogammaglobulinemia is not common after a single cycle of rituximab but may occur transiently after repeated administration of rituximab.23 Remarkably, the immunoglobulin levels of patient B:1 were normal during the initial EBV-induced HLH (IgG 7.4 g/L; IgA 1.2 g/L; IgM 2.6 g/L) but decreased 3 months later (IgG 2.0 g/L; IgA < 0.1 g/L; IgM < 0.1 g/L). An affected brother demonstrated a similar course displaying normal immunoglobulin levels initially (IgG 5.3 g/L; IgA < 1.2 g/L; IgM 1.0 g/L) but progressed to hypogammaglobulinemia after an episode of EBV-induced HLH (IgG 2.4 g/L; IgA < 0.1 g/L; IgM 0.3 g/L). Importantly, the patients in family B were not treated with rituximab. Importantly, patient H:2, who has not yet presented with HLH, was reported with a hypogammaglobulinemia (IgG 2.4 g/L; IgA 0.1 g/L; IgM 0.3 g/L), whereas her brother, patient H:1, remains normal in immunoglobulin levels. Thus, hypogammaglobulinemia can present in patients before typical clinical manifestations of HLH.
Cytotoxic lymphocyte function
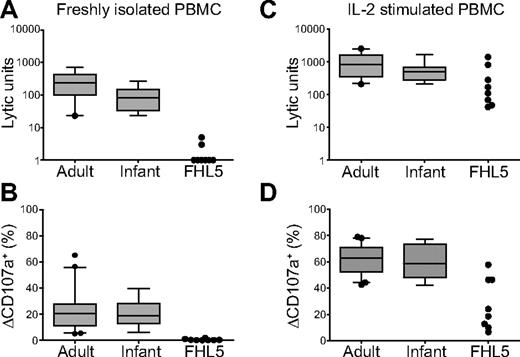
Decreased NK-cell activity, typically assessed by quantifying lysis of K562 target cells using freshly isolated PBMCs as effector cells, is a diagnostic criterion for HLH.4 A value of less than or equal to 10 LU is indicative of HLH. With freshly isolated PBMCs, NK cell–mediated lysis of K562 target cells was below the limit set as pathologic in all patients tested (n = 9; Table 1; Figure 4A). Degranulation by freshly isolated NK cells was also assessed in 8 of 11 patients. Degranulation by CD3−CD56+ NK cells in response to K562 cells was less than 2% in all FHL5 patients tested (FHL5 patients: mean ± SD = 0.4% ± 0.6%; range, 0.0%–1.8%, n = 8; Figure 4B). Remarkably, on stimulation with IL-2 for 48 to 72 hours, K562 cell lysis and degranulation increased markedly (Figure 4C-D), as previously reported for FHL4 patients.17 These results are in agreement with the findings of Côte et al10 and zur Stadt et al,11 describing defective NK cell–mediated cytotoxicity in FHL5 patients.
NK-cell cytotoxicity and degranulation in FHL5 patients. (A-B) Resting PBMCs or (C-D) PBMCs stimulated for 72 hours with IL-2 from healthy adult and infant donors or FHL5 patients were evaluated for cytotoxicity and degranulation toward K562 cells. (A,C) NK-cell cytotoxicity evaluated in a 4-hour 51Cr-release assay. Plots show LU calculated at 25% lysis, with boxes indicating 25th, 50th, and 75th percentiles and error bars representing SD. For FHL5 patients, each point represents one person. (B,D) NK-cell degranulation evaluated in a 2-hour flow cytometric assay. The cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a monoclonal antibodies. Lymphocytes were gated on forward/side scatter plots, followed by gating on CD3 versus CD56 plots. Plots show induced CD107a surface expression (ΔCD107a+) on CD3−CD56+ NK cells, with boxes indicating 25th, 50th, and 75th percentiles and error bars representing SD. For FHL5 patients, each point represents 1 person and is representative for at least 2 independent experiments.
NK-cell cytotoxicity and degranulation in FHL5 patients. (A-B) Resting PBMCs or (C-D) PBMCs stimulated for 72 hours with IL-2 from healthy adult and infant donors or FHL5 patients were evaluated for cytotoxicity and degranulation toward K562 cells. (A,C) NK-cell cytotoxicity evaluated in a 4-hour 51Cr-release assay. Plots show LU calculated at 25% lysis, with boxes indicating 25th, 50th, and 75th percentiles and error bars representing SD. For FHL5 patients, each point represents one person. (B,D) NK-cell degranulation evaluated in a 2-hour flow cytometric assay. The cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a monoclonal antibodies. Lymphocytes were gated on forward/side scatter plots, followed by gating on CD3 versus CD56 plots. Plots show induced CD107a surface expression (ΔCD107a+) on CD3−CD56+ NK cells, with boxes indicating 25th, 50th, and 75th percentiles and error bars representing SD. For FHL5 patients, each point represents 1 person and is representative for at least 2 independent experiments.
Discussion
We here describe the clinical presentation of 11 patients diagnosed with FHL5, recently associated with mutations in STXBP2.10,11 The presentation can be diverse, from a typical FHL phenotype with onset in infancy (< 3 years of age) to a more diffuse clinical presentation of patients in childhood or adolescence with colitis, bleeding disorders, and hypogammaglobulinemia.
The index patients in this study presented at the age of 17 years and almost died at an ICU before HLH was suspected and confirmed. In settings of life-threatening infections, immunosuppressive treatment including chemotherapy is not an obvious choice and must be carefully considered. In this case, studies of cytotoxic lymphocyte function clearly indicated congenital defects in lymphocyte cytotoxic function, warranting HLH immunochemotherapy. Genetic studies subsequently confirmed that the patient carries heterozygous compound mutations in STXBP2. Thus, our results underscore how even adolescent patients, whether or not admitted to ICU, may have a severe primary immunodeficiency and urgently need meticulous evaluation of immune function to facilitate rapid diagnosis and treatment.
Including the index patient, 4 of 10 patients in our cohort were 3 years or older at onset of HLH and median age at onset was 15 months (9 months including the patients in the study by zur Stadt et al11 ). By comparison, the median age at diagnosis of FHL2, FHL3, FHL4, and GS2 patients has been reported to be 2.3, 6.2, 14.4, and 6.1 months of age, respectively.24,25 In terms of age at onset, FHL5 thus most closely resembles that of FHL4. The age at onset, both among different subtypes of FHL and within patients with mutations of different severity in any given subtype, appears to correlate with the degree of defect in NK-cell cytotoxicity.17,26,27 The earliest age at onset and most severe defects in NK-cell cytotoxicity are associated with FHL2.24 FHL3 and GS2 patients display similarly severe defects in NK-cell cytotoxic activity in vitro, with slight restoration of cytotoxicity on IL-2 stimulation, whereas NK cells from FHL4 patients gain robust degranulation and cytotoxic responses on IL-2 stimulation.17,28 The level of NK-cell activity in FHL5 patients with and without IL-2 stimulation therefore resembles that of FHL4 patients, consistent with the direct binding between Munc18-2 and syntaxin-11.10,11 However, patients within different FHL subtypes can vary in their onset and presentation of disease depending on mutation severity and environmental factors. Still, the absence of degranulation and cytotoxicity by freshly isolated cytotoxic lymphocytes in the index patient was astonishing considering the late presentation of disease and resolution of many past infections. In this regard, FHL5 patients highlight the immune system's resilience and the contribution to immunity of a cytokine-induced pathway for degranulation and cytotoxicity. This pathway, which is independent of Munc18-2 and, as previously reported, syntaxin-11, allows some function of stimulated NK cells in vitro and may be a factor in the later onset of FHL4 and FHL5.17
Interestingly, the clinical data suggest that FHL5 might be associated with gastrointestinal symptoms, bleeding disorders, and development of hypogammaglobulinemia. Manifestations of colitis in some of these patients might be explained by impaired immunity and a general hyperinflammatory condition, or could reflect the expression and function of Munc18-2 in cell types other than cytotoxic lymphocytes. As the gastrointestinal tract is the largest lymphoid organ in the body, many immunodeficiencies, including Wiskott-Aldrich syndrome and severe combined immunodeficiency, are associated with gastrointestinal diseases.29 On the other hand, gastrointestinal symptoms are not a common feature of FHL2, FHL3, or GS2 patients, suggesting that the pathology of FHL does not necessarily lead to gastrointestinal disease, even in the more severe FHL subtypes.24,25 Furthermore, in a model of colitis-associated colon cancer, inflammation and cancer decreased in perforin-deficient mice.30 The increased susceptibility of FHL5 patients to gastrointestinal infections may alternatively be explained by impairments in immunity other than those caused by defective lymphocyte cytotoxicity. Besides cytotoxic lymphocytes, mast cell and neutrophil exocytosis may require Munc18-2.31,32 Likewise, Munc13-4 and Rab27a have also been implicated in mast cell and neutrophil exocytosis.33 Therefore, impairments of mast cell and neutrophil functions may not be unique to FHL5 patients. Notably, the initial reports that cloned and characterized mammalian Munc18-2 proteins described widespread expression in epithelial tissues, such as the kidney and intestines, with localization to the apical surface of the plasma membrane.34-36 Thus, Munc18-2 in gastrointestinal epithelial cells might act to maintain epithelial integrity, but more patients and mechanistic studies are required to determine the cause of gastrointestinal symptoms. Whereas we have highlighted that HLH patients might first present with colitis, James et al have described 7 patients with inflammatory bowel disease that developed HLH-like disease, including hemophagocytosis and had a 29% rate of mortality.37 Notably, infections with herpes viruses precipitated disease in the majority of these patients. Thus, inflammatory bowel disease with ensuing HLH could be associated with STXBP2 mutations. Our findings should precipitate further research on the link between defective secretory mechanisms and inflammatory bowel disorders. In this context, defects in the secretory pathway are a proposed cause of Crohn disease.38 In regards to bleeding disorders, patient A and the 2 siblings in family B displayed clinical symptoms consistent with abnormal bleeding. Whereas disorders of coagulation have been associated with delayed bleeding, deep dissecting hematomas, and hemarthrosis, disorders of platelets rather cause petechiae, superficial ecchymoses, and prolonged bleeding from superficial cuts,39 much like the symptoms observed in some of our patients. Disorders of platelets are a feature of CHS1 and HPS2 because of defects in dense granule biogenesis.40,41 Results from one of several studies on the role of multiple Munc18 isoforms in platelet function42,43 suggested the participation of Munc18-3 in platelet granule exocytosis.43 Munc13-4 and Rab27a are also implicated in platelet granule exocytosis,44 but bleeding disorders have not been noted in FHL3 or GS2 patients. Although assessment of platelet function is warranted in FHL5 patients, only 27% of our patients had clinically notable bleeding disorders. Unexpectedly, we report hypogammaglobulinemia in 36% of the patients, typically after EBV-induced HLH episodes. Hypogammaglobulinemia is not a common feature of HLH45 but affects approximately 30% of XLP1 and XLP2 patients and develops progressively either before or after EBV infection.16,46 In XLP1 patients, a deficiency in NKT cell development was suggested as a cause of hypogammaglobulinemia,47 but recent evidence pointed to defective interactions between CD4+ T cells and B cells as contributors to the decline of B-cell memory.48 However, how the loss of Munc18-2 might contribute to the demise of humoral immunity in FHL5 and XLP2 patients, respectively, is not clear. In summary, mutations in STXBP2 appear to display a discrete spectrum of symptoms, some of which may have an impact beyond the realm of pediatric hematology. Colitis, bleeding disorders, and hypogammaglobulinemia may indicate STXBP2 mutations before onset of HLH.
With increased knowledge of the molecular pathogenesis of HLH, cases previously assumed to be secondary in nature can now be explained by congenital defects, as is increasingly the case for many immunodeficiency syndromes.49 Being able to identify and subcategorize late-onset patients with mutations in HLH-associated genes, with decreased clinical penetrance and milder or atypical presentations, raises many questions regarding treatment. Notably, treatment of our index patient with rituximab appeared to at least transiently ameliorate disease severity, in line with successful rituximab therapy of EBV-induced lymphoproliferation in XLP1 patients.50 However, in EBV-HLH, it remains unclear whether EBV resides in B cells or in T and NK cells, and usage of rituximab in this setting needs caution. Currently, in the HLH-2004 treatment protocol, when a genetic cause of the disease is molecularly confirmed, it is recommended to proceed to HSCT.4 In our cohort of FHL5 patients, 3 of 11 patients are still alive without a HSCT: one without symptoms and the others 2 and 6 years after onset of symptoms, respectively. In the previously described FHL5 patient cohorts, recurrent disease with spontaneous remission or response to steroids has been reported in 3 of 22 patients.10,11 HSCT for FHL is a high-risk procedure associated with significant posttransplant morbidity and mortality,22 but if HSCT is postponed this must be balanced with the continuous risk of HLH relapse, including the risk of developing neurologic abnormalities. Moreover, patients carrying biallelic PRF1 mutations associated with residual protein function indicate an elevated risk of developing hematologic malignancies,27 and it is possible that patients with biallelic mutations in other FHL-associated genes could also have an increased risk of malignant transformation. Thus, a possibly increased prospective risk of malignancies, combined with heightened susceptibility to severe viral infections, risk for HLH relapse, and risk for neurologic damage must be taken into account if HSCT is postponed.
In conclusion, knowledge about HLH and the diverse clinical presentation is important for clinicians working not only within pediatric hematology, but also for general hematologists, infectious diseases specialists, gastroenterologists, and intensivists. A correct diagnosis with adequate treatment can be life-saving in these patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for generous participation in the present studies, Sigrid Sahlén and Edvard Nordenskjöld for technical assistance, and Hans-Gustaf Ljunggren for insightful discussions.
This work was supported by the Swedish Children's Cancer Foundation, the Swedish Research Council, the Cancer and Allergy Foundation of Sweden, the Swedish Cancer Foundation, the Mary Béve Foundation, the Märta and Gunnar V Philipson Foundation, the David and Astrid Hageléns Foundation, and the Stockholm County Council (ALF project). M.M. was supported by an MD/PhD student scholarship awarded by the Board of Postgraduate Studies at Karolinska Institutet.
National Institutes of Health
Authorship
Contribution: M.M. designed research and performed genetic analysis, analyzed and interpreted data, and drafted the manuscript; M.E. performed genetic analysis and interpreted data; W.A.-H., B.G., and H. Hjelmqvist cared for patients, provided clinical data, and interpreted findings; S.C.C.C. and S.M.W. performed evaluations of NK-cell activity and interpreted data; F.A., W.A.-A., J.J.B., H. Hasle, M.I., B.L., and J.M.v.d.B. cared for patients and provided clinical data; M.N. designed research and contributed to drafting the manuscript; and Y.T.B. and J.-I.H. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie Meeths, Childhood Cancer Research Unit, CMM L8:02, Karolinska University Hospital, SE-171 76 Stockholm, Sweden; e-mail: marie.meeths@ki.se.
References
Author notes
Y.T.B. and J.-I.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal