Abstract
Lymphadenopathy in children for which no infectious or malignant cause can be ascertained constitutes a challenging diagnostic dilemma. Autoimmune lymphoproliferative syndrome (ALPS) is a human genetic disorder of lymphocyte apoptosis resulting in an accumulation of lymphocytes and childhood onset chronic lymphadenopathy, splenomegaly, multilineage cytopenias, and an increased risk of B-cell lymphoma. In 1999, investigators at the National Institutes of Health (NIH) suggested criteria to establish the diagnosis of ALPS. Since then, with approximately 500 patients with ALPS studied worldwide, significant advances in our understanding of the disease have prompted the need for revisions to the existing diagnostic criteria and classification scheme. The rationale and recommendations outlined here stem from an international workshop held at NIH on September 21 and 22, 2009, attended by investigators from the United States, Europe, and Australia engaged in clinical and basic science research on ALPS and related disorders. It is hoped that harmonizing the diagnosis and classification of ALPS will foster collaborative research and better understanding of the pathogenesis of autoimmune cytopenias and B-cell lymphomas.
Introduction
Lymphadenopathy in children with no known infectious or malignant cause constitutes a challenging diagnostic dilemma. A recently described entity that defines some children with previously unexplained lymphadenopathy is the autoimmune lymphoproliferative syndrome (ALPS).1,2 The clinical antecedents to ALPS entail various syndromes of familial chronic nonmalignant lymphadenopathy and splenomegaly, including pseudomononucleosis, pseudolymphoma, and the Canale-Smith syndrome.3-5 In 1992, Sneller et al6 recognized that these entities resembled 2 related mouse strains with lymphoproliferative phenotypes known as lpr (lymphoproliferation) and gld (generalized lymphoproliferative disease). Earlier that same year the molecular defect of the lpr mouse was shown to be a loss of function mutation in a “death receptor” gene that is a member of the tumor necrosis factor receptor superfamily, FAS/CD95/APO-1/TNFRSF6.7 Subsequently, this association was validated in humans when the underlying defect in 2 series of patients with a lymphoproliferative disorder was determined to be a failure of lymphocyte apoptosis because of a mutation in FAS.1,2 The mutations resulted in the accumulation of proliferating lymphocytes with childhood onset chronic lymphadenopathy, splenomegaly, multilineage cytopenias secondary to sequestration and autoimmune destruction, and an increased risk of B-cell lymphoma.6,8,9 Laboratory findings included polyclonal hypergammaglobulinemia and expansion of a unique population of circulating T-cell receptor αβ (TCRαβ)+B220+CD4−CD8− T lymphocytes, referred to as TCRαβ+ double-negative T (TCRαβ+-DNT) cells throughout this article.8,9
Most patients with ALPS harbor heterozygous germline mutations in FAS, inherited in an autosomal dominant fashion.10,11 Interestingly, somatic FAS mutations are the second most common genetic cause of ALPS.12,13 In addition, mutations in the genes encoding FAS ligand, caspase 10, caspase 8, and neuroblastoma RAS (NRAS) have been identified in a minority of patients with ALPS and related disorders.14-19 Approximately one-third of patients with ALPS have yet unidentified genetic defects.
In 1999, investigators at the National Institutes of Health (NIH) suggested a triad of criteria to establish the diagnosis of ALPS (Table 1).20,21 Since then, important advances have been made in our understanding of the disease. Here, we would like to propose several revisions to the current diagnostic criteria and classification system. The recommendations stem from a workshop held at the NIH in the fall of 2009 attended by investigators from the United States, Europe, and Australia engaged in clinical and basic science research that pertains to ALPS and related disorders. The changes proposed follow the deliberations at the meeting, leading to a consensus after further teleconferences and electronic communications among the coauthors of this document. It is hoped that these modifications will harmonize and simplify the diagnosis and classification of ALPS, facilitating collaboration and data exchange between different clinicians and research centers across the globe. A scientific summary of the meeting proceedings has been published elsewhere.22
Diagnostic criteria for ALPS as defined in 1999
| Required criteria |
| Chronic nonmalignant lymphadenopathy and/or splenomegaly |
| Increased peripheral CD3+TCRαβ+CD4−CD8− (DNT) cells |
| Lymphocyte apoptosis defect |
| Supporting criteria |
| Family history of ALPS |
| Characteristic histopathology |
| Autoimmune manifestations |
| Required criteria |
| Chronic nonmalignant lymphadenopathy and/or splenomegaly |
| Increased peripheral CD3+TCRαβ+CD4−CD8− (DNT) cells |
| Lymphocyte apoptosis defect |
| Supporting criteria |
| Family history of ALPS |
| Characteristic histopathology |
| Autoimmune manifestations |
Modifications to the diagnostic criteria of ALPS
Rationale
Reevaluation of the currently used ALPS diagnostic criteria (Table 1) suggested several potential problems hindering its widespread use:
The lymphocyte apoptosis assay, currently an absolute requirement for diagnosis, is resource-intensive to perform, available only in selected centers, and may be unable to identify patients with somatic FAS or germline FASLG mutations; moreover, methodology is not standardized among centers, often leading to varying results.
The current definition does not incorporate genetic information or other biomarkers that have recently been shown to predict ALPS.
Evaluation of a large number of control samples in different centers suggests that a diagnostic cutoff for TCRαβ+DNT cells of 1% of total lymphocytes does not always accurately predict ALPS.
Histopathologic findings, highly characteristic of ALPS in some cases, and compatible family history are not currently used for diagnosis.
Recommendations
This revision divides diagnostic criteria for ALPS into 2 required and 6 accessory criteria (Table 2). Required criteria include the presence of lymphadenopathy and/or splenomegaly, and elevated TCRαβ+-DNT cells. Accessory criteria are subdivided into primary, which include an abnormal lymphocyte apoptosis assay and the presence of pathogenic mutations in genes of the FAS pathway; and secondary, which include the presence of elevated circulating biomarkers, characteristic histopathology, the combined presence of autoimmune cytopenias, polyclonal hypergammaglobulinemia, and family history compatible with ALPS. These criteria are discussed in further detail.
Revised diagnostic criteria for ALPS
| Required |
| 1. Chronic (> 6 months), nonmalignant, noninfectious lymphadenopathy or splenomegaly or both |
| 2. Elevated CD3+TCRαβ+CD4−CD8− DNT cells (≥ 1.5% of total lymphocytes or 2.5% of CD3+ lymphocytes) in the setting of normal or elevated lymphocyte counts |
| Accessory |
| Primary |
| 1. Defective lymphocyte apoptosis (in 2 separate assays) |
| 2. Somatic or germline pathogenic mutation in FAS, FASLG, or CASP10 |
| Secondary |
| 1. Elevated plasma sFASL levels (>200 pg/mL) OR elevated plasma interleukin-10 levels (>20 pg/mL) OR elevated serum or plasma vitamin B12 levels (> 1500 ng/L) OR elevated plasma interleukin-18 levels > 500 pg/mL |
| 2. Typical immunohistological findings as reviewed by an experienced hematopathologist |
| 3. Autoimmune cytopenias (hemolytic anemia, thrombocytopenia, or neutropenia) AND elevated immunoglobulin G levels (polyclonal hypergammaglobulinemia) |
| 4. Family history of a nonmalignant/noninfectious lymphoproliferation with or without autoimmunity |
| Required |
| 1. Chronic (> 6 months), nonmalignant, noninfectious lymphadenopathy or splenomegaly or both |
| 2. Elevated CD3+TCRαβ+CD4−CD8− DNT cells (≥ 1.5% of total lymphocytes or 2.5% of CD3+ lymphocytes) in the setting of normal or elevated lymphocyte counts |
| Accessory |
| Primary |
| 1. Defective lymphocyte apoptosis (in 2 separate assays) |
| 2. Somatic or germline pathogenic mutation in FAS, FASLG, or CASP10 |
| Secondary |
| 1. Elevated plasma sFASL levels (>200 pg/mL) OR elevated plasma interleukin-10 levels (>20 pg/mL) OR elevated serum or plasma vitamin B12 levels (> 1500 ng/L) OR elevated plasma interleukin-18 levels > 500 pg/mL |
| 2. Typical immunohistological findings as reviewed by an experienced hematopathologist |
| 3. Autoimmune cytopenias (hemolytic anemia, thrombocytopenia, or neutropenia) AND elevated immunoglobulin G levels (polyclonal hypergammaglobulinemia) |
| 4. Family history of a nonmalignant/noninfectious lymphoproliferation with or without autoimmunity |
A definitive diagnosis is based on the presence of both required criteria plus one primary accessory criterion. A probable diagnosis is based on the presence of both required criteria plus one secondary accessory criterion.
For a definitive ALPS diagnosis a patient has to meet both required criteria and one of the primary accessory criteria (Table 2). A probable ALPS diagnosis can be entertained by the presence of the required criteria and any one of the secondary accessory criteria. From a clinical perspective, patients with probable ALPS should be treated and monitored in the same way as patients with a definitive diagnosis, but physicians are advised to pursue a genetic or apoptosis assay–based diagnostic workup whenever possible.
There is an absolute requirement for the presence of lymphadenopathy and/or splenomegaly persistent for more than 6 months. If isolated, the lymphadenopathy has to affect at least 2 nodal chains. Neoplastic and infectious causes must be excluded. In many cases associated hepatomegaly may also be present, but in isolation it is not a diagnostic criterion.23 The lymphadenopathy in ALPS typically fluctuates and involves the cervical, axillary, and inguinal chains, although mesenteric, retroperitoneal, pelvic, and mediastinal lymph node expansions are also often noted by imaging studies.23
The second required ALPS criterion is the presence of elevated circulating TCRαβ+-DNT cells, a hallmark of this disease.6 This population must be clearly distinguished from TCRγδ+-DNT cells by costaining with TCRαβ+-directed antibodies. Rare conditions unrelated to ALPS may present with an expansion of natural killer T cells, which can be CD3+TCRαβ+CD4−CD8−, and these can be distinguished from ALPS-specific DNTs by costaining with natural killer T markers such as CD16, CD56, Vα24Vβ11, or α-GalCer-CD1d tetramers; however, we do not recommend such extended staining routinely in ALPS investigation. The staining protocol currently used by the NIH Immunology Service can be found in supplementary File 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
A review of data, including pediatric controls, gathered from different centers showed that TCRαβ+-DNT levels between 1.0% and 1.5% of total lymphocytes may be observed in healthy persons or as a reactive phenomenon in conditions such as systemic lupus erythematosus; hence, their presence as an isolated finding should not prompt screening for ALPS24-26 (J.J.B., Division of Bone Marrow Transplantation and Immune Deficiency, Cincinnati Children's Hospital Medical Center, oral communication, September 22, 2009). As a consequence of these observations, the percentage of TCRαβ+-DNT cells required for a diagnosis has been revised to be greater than or equal to 1.5% of total lymphocytes (or 2.5% of T lymphocytes), in the setting of normal or elevated lymphocyte counts. The presence of lymphopenia invalidates this criterion, because its effect on the relative distribution of TCRαβ+-DNT cells is unknown. Absolute counts of TCRαβ+-DNT cells will vary by age. Elevations of TCRαβ+-DNT cells above 3% of the total lymphocytes (or > 5% of T-lymphocyte cells) are rarely, if ever, seen in conditions other than ALPS and are therefore essentially pathognomonic for this disease.24-27 Each testing laboratory should ideally develop its own reference ranges, adjusted for age, and provide both percentage and absolute numbers of this lymphocyte subset in the patient report.
Primary accessory criteria include an abnormal lymphocyte apoptosis assay (Table 2). This test is no longer considered essential for the diagnosis of ALPS, because patients with somatic FAS mutations and germline FASLG mutations typically are found to have normal FAS-induced apoptosis assays.12,14,18 However, the presence of a reproducible apoptotic defect in patients who fulfill the required criteria is enough for a diagnosis of ALPS. Given the high interlaboratory variability in the protocol used for this assay, a repeat assay for confirmation is now required. Acceptable apoptosis tests in cultures of activated primary T cells include direct FAS activation with the use of cross-linked agonistic antibodies or recombinant Fas ligand or TCR restimulation. Detailed description of the protocols used for apoptosis detection and measurement can be found in a recent related publication.28 Patient results must be compared with healthy controls set up in parallel, and a test is considered abnormal if the patient's cells show consistently 50% or less of the cell death observed in the control. In addition, shipped samples should be accompanied by a shipping control.
The demonstration of germline or somatic deleterious mutations in FAS, FASLG, or CASP10 is now considered a diagnostic criterion. Patients with germline CASP8 and somatic NRAS mutations are now classified separately (Table 2). Gene sequencing is generally available by selected commercial laboratories; however, because polymorphisms in FAS are not uncommon, a diagnostic mutation should be based on prior identification of the mutation linked to a diagnosis of ALPS or a proven functional consequence of the change in association with a new mutation. Existing databases of pathogenic FAS mutations are publicly available and can be used for diagnostic help (National Center for Biotechnology Information NIH ALPS Web site, http://www3.niaid.nih.gov/topics/ALPS/). However, isolated discovery of a heterozygous Fas mutation in a healthy relative of a patient with ALPS is of clinically uncertain significance at this time.
On the basis of recent data, the presence of elevated TCRαβ+-DNT cells coupled to high serum or plasma levels of either interleukin-10, interleukin-18, soluble FAS ligand, or vitamin B12 can accurately predict the presence of germline or somatic FAS mutations.24-27 These biomarkers can predict a FAS mutation with a posttest probability that ranges from 85% to 97%, depending on the biomarker used and the number of TCRαβ+-DNT cells.24-27 Given this high specificity, these biomarkers were also incorporated in the diagnostic criteria, and their use should greatly facilitate the diagnosis in settings without access to advanced genetic analysis or functional testing.
Two common presenting features of ALPS, autoimmune cytopenias and hypergammaglobulinemia, are now incorporated as diagnostic criteria. A recent publication suggests that their presence in patients with lymphoproliferation and elevated TCRαβ+-DNT cells indicates a high likelihood of ALPS, and these patients should be referred for further testing.24 Although autoimmune manifestations of ALPS are typically limited to hematopoietic elements that lead to multilineage cytopenias, occasionally other organs, including liver and kidneys, may also be affected.29
Lymph node pathologic findings initially described by Lim et al30 are characteristic of ALPS and are included as a secondary accessory diagnostic criterion. These findings include paracortical expansion due to infiltration by polyclonal TCRαβ+-DNT cells accompanied by follicular hyperplasia and polyclonal plasmacytosis.30 Marked TCRαβ+-DNT cell infiltration in some cases can lead to architectural effacement of lymph nodes with infiltration of bone marrow and spleen, leading in some instances to an erroneous diagnosis of peripheral T-cell lymphoma. The diagnostic workup should include flow cytometric or immunohistochemical evaluation of T-cells for CD3, CD4, CD8, CD57, CD45RO, and CD45RA with the use of standardized laboratory methods.31 Using flow cytometry αβ and γδ T cells should be distinguished, with gating for CD4 and CD8 performed on the respective populations. In addition, polymerase chain reaction studies of TCR gene rearrangement should indicate the absence of a clonal T-cell population in ALPS.
The final secondary accessory criterion is a positive family history for nonmalignant and noninfectious lymphadenopathy/splenomegaly with or without autoimmunity, because many patients with ALPS have family members with similar clinical histories.
Modifications to the classification of ALPS and related disorders
Rationale
The molecular classification of ALPS has seen many recent additions over time,14-19 leading to a somewhat chaotic nomenclature (Table 3). An ideal classification system should not only standardize the nomenclature among different centers but also easily accommodate future discoveries. This revision introduces extensive modifications to the previously used classification of ALPS and related disorders, as summarized here (Table 3).
Revised classification of ALPS
| Previous nomenclature . | Revised nomenclature . | Gene . | Definition . |
|---|---|---|---|
| ALPS type 0 | ALPS-FAS | FAS | Patients fulfill ALPS diagnostic criteria and have germline homozygous mutations in FAS. |
| ALPS type Ia | ALPS-FAS | FAS | Patients fulfill ALPS diagnostic criteria and have germline heterozygous mutations in FAS. |
| ALPS type Im | ALPS-sFAS | FAS | Patients fulfill ALPS diagnostic criteria and have somatic mutations in FAS. |
| ALPS type Ib | ALPS-FASLG | FASLG | Patients fulfill ALPS diagnostic criteria and have germline mutations in FAS ligand. |
| ALPS type IIa | ALPS-CASP10 | CASP10 | Patients fulfill ALPS diagnostic criteria and have germline mutations in caspase 10. |
| ALPS type III | ALPS-U | Unknown | Patients meet ALPS diagnostic criteria; however, genetic defect is undetermined (no FAS, FASL, or CASP10 defect). |
| Previous nomenclature . | Revised nomenclature . | Gene . | Definition . |
|---|---|---|---|
| ALPS type 0 | ALPS-FAS | FAS | Patients fulfill ALPS diagnostic criteria and have germline homozygous mutations in FAS. |
| ALPS type Ia | ALPS-FAS | FAS | Patients fulfill ALPS diagnostic criteria and have germline heterozygous mutations in FAS. |
| ALPS type Im | ALPS-sFAS | FAS | Patients fulfill ALPS diagnostic criteria and have somatic mutations in FAS. |
| ALPS type Ib | ALPS-FASLG | FASLG | Patients fulfill ALPS diagnostic criteria and have germline mutations in FAS ligand. |
| ALPS type IIa | ALPS-CASP10 | CASP10 | Patients fulfill ALPS diagnostic criteria and have germline mutations in caspase 10. |
| ALPS type III | ALPS-U | Unknown | Patients meet ALPS diagnostic criteria; however, genetic defect is undetermined (no FAS, FASL, or CASP10 defect). |
Recommendations
Classification of ALPS.
For simplicity, numbers should no longer be used when classifying ALPS according to the genetic defect (Table 3). Patients harboring germline homozygous, or heterozygous mutations in FAS, previously classified as ALPS type 0 and Ia, respectively, are now unified under ALPS-FAS. Similarly, patients with somatic FAS mutations should be classified as ALPS-sFAS; patients harboring Fas ligand mutations should be classified as ALPS-FASLG; and patients with caspase-10 mutations classified as ALPS-CASP10.
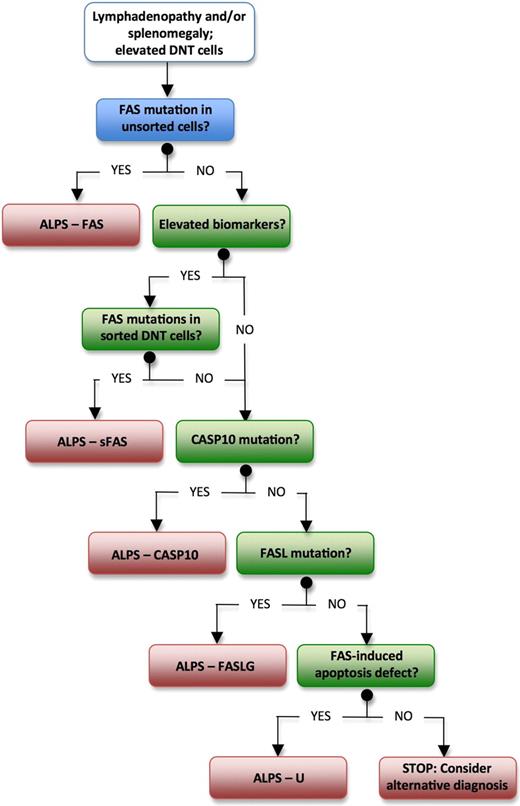
Patients who fulfill diagnostic criteria for ALPS but in whom no genetic diagnosis can be determined should now be classified as ALPS-U (undetermined), instead of ALPS type III. We expect new genetic defects to be discovered in this group of patients with further research. Despite the lack of a genetic diagnosis, our current understanding is that this group of patients has a clinical course that is similar to other patients with ALPS, except that there is no evidence yet of an increased incidence of lymphoma (V.K.R., unpublished observations, September 22, 2009). The diagnostic flow chart shown in Figure 1 should help the readers to navigate among different ALPS subtypes in their clinical practice.
Suggested algorithm for diagnostic workup for patients with suspected ALPS. ALPS indicates autoimmune lymphoproliferative syndrome; and DNT, double-negative T lymphocytes.
Suggested algorithm for diagnostic workup for patients with suspected ALPS. ALPS indicates autoimmune lymphoproliferative syndrome; and DNT, double-negative T lymphocytes.
Classification of ALPS-related disorders.
Patients with mutations in the gene encoding for caspase-8 (CASP8) present with a syndrome of lymphadenopathy and splenomegaly, marginal elevation of DNTs, and defective FAS-induced lymphocyte apoptosis and were previously classified as ALPS type IIb.15 However, in contrast to other ALPS cases, these patients also show defective T-, B-, and NK-cell activation, with consequent recurrent bacterial and viral infections.15,32 Given the distinct phenotype, the previously defined term caspase-8 deficiency state is now included to describe this disorder (Table 4).29
Revised classification of ALPS-related disorders
| Previous nomenclature . | Revised nomenclature . | Gene . | Definition . |
|---|---|---|---|
| ALPS type IIb | CEDS | CASP8 | Patients present with lymphadenopathy and/or splenomegaly, marginal DNT elevation, recurrent infections, and germline mutations in caspase 8. |
| ALPS type IV | RALD | NRAS | Patients present with autoimmunity, lymphadenopathy and/or splenomegaly, elevated or normal DNTs, and somatic mutations in NRAS. |
| DALD | DALD | Unknown | Patients present with autoimmunity, lymphadenopathy and/or splenomegaly, normal DNTs, and defective in vitro FAS-mediated apoptosis. |
| XLP1 | XLP1 | SH2D1A | Patients present with fulminant Epstein-Barr virus infection, hypogammaglobulinemia, or lymphoma. |
| Previous nomenclature . | Revised nomenclature . | Gene . | Definition . |
|---|---|---|---|
| ALPS type IIb | CEDS | CASP8 | Patients present with lymphadenopathy and/or splenomegaly, marginal DNT elevation, recurrent infections, and germline mutations in caspase 8. |
| ALPS type IV | RALD | NRAS | Patients present with autoimmunity, lymphadenopathy and/or splenomegaly, elevated or normal DNTs, and somatic mutations in NRAS. |
| DALD | DALD | Unknown | Patients present with autoimmunity, lymphadenopathy and/or splenomegaly, normal DNTs, and defective in vitro FAS-mediated apoptosis. |
| XLP1 | XLP1 | SH2D1A | Patients present with fulminant Epstein-Barr virus infection, hypogammaglobulinemia, or lymphoma. |
CEDS, caspase 8 deficiency state; RALD, RAS-associated autoimmune leukoproliferative disease; DALD, Dianzani autoimmune lymphoproliferative disease; and XLP1, X-linked lymphoproliferative syndrome.
The clinical syndrome of autoimmune phenomena, lymphocyte accumulation, and somatic mutations in NRAS, previously designated ALPS type IV, is now reclassified under a new nosologic entity termed RALD, for RAS-associated autoimmune leukoproliferative disease (Table 4).19 The main rationale for this change was the recognition of 2 additional patients with somatic NRAS mutations who did not show elevated TCRαβ+-DNT cells (J.B.O. and J.J.B., unpublished observations, September 22, 2009). In addition, these patients presented with atypical features such as elevations in cells of myeloid origin (monocytosis and granulocytosis) and showed partial overlap with juvenile myelomonocytic leukemia as well as lymph node histopathology not typical of ALPS (J.B.O., unpublished observations, September 22, 2009).
No nomenclature modifications are suggested for the ALPS-related clinical syndrome known as Dianzani autoimmune lymphoproliferative disease,33 characterized by autoimmunity, lymphadenopathy and/or splenomegaly, and defective in vitro Fas-mediated lymphocyte apoptosis, without elevation in TCRαβ+-DNT cells. The genetic defect is not known, but an inherited component is suggested on the basis of the defective FAS function displayed by relatives of these patients. Patients may display a wide range of autoimmune manifestations, and an increased risk of cancer has been reported.34,35 Finally, the X-linked lymphoproliferative disease (XLP1), a genetic immunodeficiency caused by mutations or deletions in the SH2D1A gene, can be included in the spectrum of ALPS-like disorders. These patients frequently display defective apoptosis in response to TCR restimulation, and this pathway appears to be essential for constraining effector T-cell expansion and preventing immunotoxicity.36,37
Conclusion
The modifications in the diagnostic criteria and classification system introduced here should facilitate diagnosis in locations without access to advanced testing, streamline diagnostic workup of patients, standardize nomenclature among different centers, and allow easy inclusion of newly discovered genetic defects resulting in classical ALPS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the participants of the NIH ALPS workshop, conducted on September 21-22, 2009, which led to further discussions and deliberations leading to the preparation of this document. We also thank Ugo Ramenghi, Andrew Snow, and Michael Sneller for reviewing this manuscript.
This research was supported by the Intramural Research Program of the NIH (National Cancer Institute, National Institute of Allergy and Infectious Diseases, Clinical Center), and funding for the ALPS Workshop was provided by NIH Office of the Rare Diseases and Division of Intramural Research, NIAID.
National Institutes of Health
Authorship
Contribution: J.B.O. and V.K.R. wrote the manuscript; and J.J.B., U.D., T.A.F., E.S.J., M.J.L, F.R.-L., R.M.S., H.C.S., and D.T.T. discussed the content and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: V. Koneti Rao, ALPS Unit, LCID, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; e-mail: krao@niaid.nih.gov; or Joao B. Oliveira, Department of Laboratory Medicine, National Institutes of Health, Bethesda, MD 20892; e-mail: oliveirajb@cc.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal