Abstract
To explore whether and how T cells can affect myelopoiesis, we investigated myeloid differentiation in a model for T cell-mediated immune activation. We found that CD70-transgenic (CD70TG) mice, which have elevated numbers of interferon-γ (IFN-γ)–producing effector T cells in the periphery and bone marrow, are almost devoid of eosinophilic granulocytes. Induction of allergic airway inflammation in these mice failed to induce eosinophilia as well as airway hyperresponsiveness. CD70TG mice also have strongly reduced numbers of eosinophil lineage-committed progenitors, whereas granulocyte/macrophage progenitors from these mice are unable to generate eosinophils in vitro. We found that granulocyte/macrophage progenitors express IFN-γR1 and that IFN-γ is sufficient to inhibit eosinophil differentiation of both murine and human progenitor cells in vitro. We demonstrate that inhibition of eosinophil development in CD70TG mice is IFN-γ–dependent and that T cell–derived IFN-γ is sufficient to inhibit eosinophil formation in vivo. Finally, we found that IFN-γ produced on anti-CD40 treatment and during viral infection can also suppress eosinophil formation in wild-type mice. These data demonstrate that IFN-γ inhibits the differentiation of myeloid progenitors to eosinophils, indicating that the adaptive immune system plays an important role in orchestrating the formation of the appropriate type of myeloid cells during immune activation.
Introduction
Differentiation of hematopoietic progenitor cells to all separate lineages of mature red and white blood cells is tightly controlled by cytokines locally produced by stromal cells in the bone marrow. However, this process can also be influenced by bone marrow external factors. In particular, T cells have been implicated in steady-state hematopoiesis, as T cell–deficient mice have impaired maturation and accumulation of myeloid progenitors in the bone marrow, which can be restored by reconstitution with CD4+ T cells.1 The bone marrow harbors a relatively large number of memory T cells, and it also recruits antigen-specific effector T cells during the course of an immune response (reviewed by Di Rosa and Pabst2 ). We have previously postulated that activated lymphocytes could influence the function of hematopoietic progenitor cells through direct cell-cell contact,3 but it is also clear from a variety of disease models that T cells can modulate myelopoiesis during immune activation via production of cytokines, such as interleukin-3 (IL-3), IL-5, IL-17, oncostatin M, or granulocyte-macrophage colony-stimulating factor (GM-CSF) (reviewed by Dent and Kaplan4 ). These data imply that the adaptive immune system is able to directly modulate the formation of new blood cells during immune activation by the migration of activated T cells to the bone marrow.
The ability of activated T cells to modulate the formation of myeloid cells is particularly relevant in asthma, as the characteristic occurrence of eosinophilia in this disease is accompanied by an increase of IL-5-producing T helper 2 (Th2) cells in the bone marrow.5,6 Moreover, induction of Th1 responses before the induction of asthma decreases the levels of IL-5 in the lung and reduces local eosinophilia.7,8 It has therefore been proposed that IL-5 production by Th2 cells induces eosinophil accumulation and that Th1 cells merely inhibit lung eosinophilia by antagonizing the formation of Th2 cells. However, it is not clear from these studies whether Th1 cells can also directly affect the formation of eosinophils in the bone marrow. This is important because the prototypical Th1 cytokine interferon-γ (IFN-γ) can directly affect proliferation of hematopoietic progenitor cells and their differentiation toward myeloid cells.9-11 Because eosinophils play an important role both in allergic diseases and during helminth infections, it is important to understand how eosinophil formation is regulated. Therefore, we investigated whether and how IFN-γ–producing T cells affect eosinophil formation in vivo.
Myeloid cells in the bone marrow originate from a well-defined population of precursor cells, known as the common myeloid progenitor (CMP), which can give rise to monocytes, mast cells, and granulocytes via the intermediate granulocyte/macrophage progenitor (GMP) and to platelets and erythrocytes via the megakaryocyte/erythrocyte progenitor.12 GMPs give rise to eosinophilic granulocytes through an intermediate eosinophil lineage-committed progenitor (EoP) in mice,13 whereas the human EoP is derived from the CMP or its upstream multipotent progenitor.14 Expression of the IL-5 receptor on EoPs is a result of commitment of GMPs to the eosinophil lineage, which means that IL-5 can support eosinophil development from EoPs rather than instruct GMPs to commit to the eosinophil lineage.13
To study how eosinophil development in the bone marrow is regulated by activated T cells, we made use of a mouse model in which the formation of effector T cells is enhanced because of overexpression of the costimulatory ligand CD70. Under normal circumstances, CD70 is only transiently expressed on activated DCs, T cells, and B cells, but constitutive expression of CD70 on either of these cell types induces strong type 1 effector T-cell formation because of enhanced costimulation through its receptor CD27.15-18 CD70-transgenic (CD70TG) mice develop high numbers of IFN-γ–producing effector CD4 and CD8 T cells, which is dependent on CD27 expression and T-cell receptor stimulation.15,19 Although the antigens driving this T-cell activation are not known, CD70TG mice mount a protective T-cell response against a challenge with influenza virus or tumor cells.20 NK cell numbers are strongly reduced in these mice, and the remaining NK cells produce less IFN-γ than wild-type (WT) mice.21 Here we describe that CD27-mediated T-cell activation has a strong and specific impact on myelopoiesis, as CD70TG mice are almost devoid of eosinophils, whereas the formation of neutrophils and monocytes is actually increased. Detailed analysis revealed that this defect in eosinophil formation can be fully attributed to the increased production of IFN-γ by effector T cells in these mice. We show that IFN-γ is sufficient to block eosinophil development in vitro and in vivo by affecting the formation of EoPs from GMPs, which demonstrates that IFN-γ is inhibitive for eosinophil development. These data reveal that type 1 effector T cells can directly regulate hematopoiesis by influencing the differentiation capacity of specific myeloid progenitor cells.
Methods
Mice
WT, IFN-γ−/−, and CD70TG15 C57BL/6 and BALB/c mice were housed at the animal research institute of the Academic Medical Center under specific pathogen-free conditions. C57BL/6 mice were used for all experiments, except for the ovalbumin (OVA)-induced allergic asthma model, in which BALB/c mice were used. Therefore, CD70TG C57BL/6 mice were backcrossed 10 times to BALB/c. Animal experiments were approved by the Animal Ethics Committee and performed in accordance with institutional and national guidelines.
Flow cytometry and cell sorting
Single-cell suspensions were obtained by mincing the organ through 40-μm cell strainers. Erythrocytes were lysed with an ammonium chloride solution. Purification of CMPs and GMPs was based on described methods.12 Briefly, c-Kit+ cells were enriched using anti-c-Kit microbeads (Miltenyi Biotec) and MACS MS-columns (Miltenyi Biotec). Enriched cells were incubated with antibodies for CD34, CD16/32, and c-Kit, and progenitors were sorted on a FACSAria (BD Biosciences). Sca-1 could not be included in our identification of myeloid progenitors because of a systemic IFN-γ–mediated up-regulation of Sca-1 in CD70TG mice.3,15 Although this implies that the CMP and GMP fraction in our experiments also contains some Sca-1+ stem/progenitor cells (∼ 10%), this did not affect our functional analyses because c-Kit+Sca-1+ progenitors do not contribute significantly to the outgrowth of eosinophils in our culture system (data not shown).
For identification of progenitor cells by flow cytometry, cells were stained with a lineage cocktail of unlabeled or biotin-conjugated antibodies directed against CD4 (GK1.5), CD8α (53-6.7), B220 (RA3-6B2), CD11b (M1/70), Gr1 (RB6-8C5), Ter119 (Ly-76), and IL-7Rα (B12-1). For identification of eosinophil progenitors, anti-CD11b was excluded from the lineage definition. The antibodies used were CD4-fluorescein isothiocyanate (FITC) (GK1.5), CD45.1-phycoerythrin (PE; A-20), CD44-PE (IM7), CD44-Alexa Fluor 700 (IM7), CD3ϵ-allophycocyanin (APC; 145-2C11), CD4-PE-Cy5.5 (RM4-5), CD62L-APC, CD62L-PE-Cy7 (MEL-14), CD8-APC-Cy7 (53-6.7; BioLegend), IFN-γ-APC (XMG1.2), CD34-FITC (RAM34), F4/80-FITC (BM8), Gr1-FITC, Gr1-PE (RA3-6B2; both from BD PharMingen), CD115-biotin (AFS98), Siglec-F-PE (E50-2440; BD PharMingen), CCR3-AlexaFluor 647 (BD PharMingen), CD16/32-PE-Cy7 (93), CD11b-APC (M1/70), CD11b-AlexaFluor 750 (M1/70), c-Kit-AlexaFluor 750 (2B8), unlabeled IL-5Rα (H7; Wako Pure Chemicals), and CD119-biotin (BioLegend). Biotin-conjugated and unlabeled antibodies were visualized by streptavidin-PE-Cy7, streptavidin-PE, streptavidin-APC, or antirat AlexaFluor 633 (Invitrogen). Where possible, cells were stained in the presence of anti-CD16/CD32 block (2.4G2; purified from hybridoma supernatant). Intracellular cytokine staining was performed as described previously.18 All antibodies and secondary reagents were obtained from eBioscience, unless otherwise specified. Flow cytometric analyses were performed on FACSCalibur or FACSCanto (BD Biosciences), and data were analyzed using FlowJo software Version 8.8.7 (TreeStar).
Culture of murine progenitors
Sorted CMPs and GMPs were cultured for 9 days at 37°C in a humidified incubator at 5% CO2 in 96-well plates in Iscove modified Dulbecco medium (Lonza) containing 10% fetal calf serum at a density of 5000 or 1000 cells per well. For eosinophil differentiation, medium contained IL-5 and stem cell factor (SCF). For differentiation toward monocytes/macrophages, neutrophils, and eosinophils, medium contained IL-5, GM-CSF, and SCF. Differentiation of GMPs toward eosinophil progenitors was assessed after 3 days of culture in IL-3, IL-5, GM-CSF, and SCF. IFN-γ was added to cultures when indicated. Concentrations used were: IL-5 (40 ng/mL), SCF (5 ng/mL), GM-CSF (2 ng/mL), IL-3 (5 ng/mL), and IFN-γ (20 or 100 ng/mL). All cytokines were obtained from PeproTech.
Culture of human CD34+ and TF-1 cells
Human eosinophils were differentiated from CD34+ cells purified from umbilical cord blood and analyzed as previously described.22 TF-1 cells were cultured with IL-5 (100pM; PeproTech) in the presence or absence of IFN-γ (50 ng/mL; PeproTech). After 2 days, cells were stained with a PE-labeled antibody against IL-5Rα or an isotype control (BD PharMingen).
Quantitative real-time PCR
RNA was extracted using TRIzol (Invitrogen) and reverse transcribed to cDNA using random hexamers and Superscript II reverse transcriptase (Roche Diagnostics). Quantitative real-time polymerase chain reaction (PCR) was performed in duplicate using Express SYBR GreenER (Invitrogen) on the StepOnePlus RT-PCR system (Applied Biosystems). Data were normalized using 18S rRNA as a reference gene. Primer sequences available on request.
Allergic asthma model
WT and CD70TG BALB/c mice were sensitized with intraperitoneal injections of 20 μg of OVA with alum (200 μg) at days 0 and 14. Mice were intranasally challenged with 50 μL of OVA (2 mg/mL) on days 28, 29, and 30. Mice receiving intraperitoneal injections with alum and intranasal challenge with phosphate-buffered saline (PBS) were used as controls. On day 31, airway responsiveness to inhaled metacholine was measured using whole body plethysmography. Responsiveness to aerosolized PBS was used to set a baseline value, followed by increasing concentrations of aerosolized metacholine. Enhanced pause (PenH) values were measured after each metacholine challenge.
Adoptive transfer, LCMV infection, and anti-CD40 injection
T cells were obtained from lymph nodes and spleens of WT (CD45.1+) and IFN-γ−/− mice by incubating single-cell suspensions with CD4 and CD8 microbeads (Miltenyi Biotec) and sorting by MACS-positive bead selection with LS columns (Miltenyi Biotec). Cells were washed and resuspended in PBS, and 9 × 106 cells in 200 μL PBS were injected intravenously into CD27−/− and CD70TGxCD27−/− recipient mice. Purity of isolated T cells was more than 95% as determined by flow cytometric analysis. Mice were killed and analyzed 3 weeks after transfer of T cells. For lymphocytic choriomeningitis virus (LCMV) infection, mice were infected intraperitoneally with 1.5 × 105 PFU of LCMV-Armstrong and analyzed 8 days after infection. An agonistic antibody to CD40 (100 μg, clone FGK-45) or a rat control antibody (100 μg, GL113) was injected intraperitoneally at days 1 and 3, and mice were analyzed at day 5.
Results
CD70TG mice lack eosinophils and have increased numbers of neutrophils and monocytes
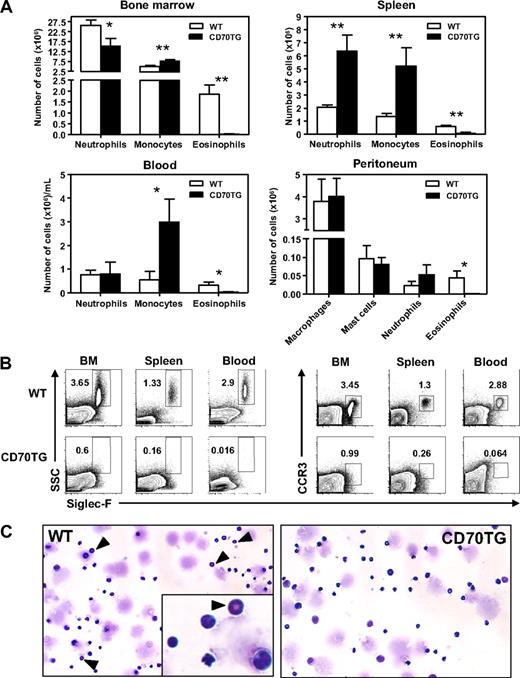
To examine the influence of CD27-driven T-cell activation on myelopoiesis, we analyzed the presence of myeloid cells in bone marrow, blood, and spleen of WT and B cell-specific CD70TG mice. In all these compartments, we found that CD70TG mice were almost completely devoid of eosinophils, based on their characteristic phenotype of Siglec-F+ and sideward scatter (SSC)hi23 (Figure 1A-B). On the other hand, the numbers of neutrophils and monocytes were in most cases increased (Figure 1A). In the periphery, neutrophil and monocyte numbers were increased in the spleen, and a strong increase of monocytes was observed in blood (Figure 1A). Eosinophils were also absent from the peritoneal cavity, which normally serves as a reservoir for eosinophils,24 whereas no differences were found for macrophages, mast cells, and neutrophils at this site (Figure 1A). As eosinophils lose their typical high SSC profile when they degranulate, we also stained for other eosinophil-related markers,25,26 such as CCR3 (Figure 1B), FIRE, CD125 (IL-5Rα), F4/80, Gr1, or CD16/32 (data not shown). This analysis confirmed that eosinophils are virtually absent in CD70TG mice, which was also found in a second, independent founder line (data not shown) and corroborated by May-Grünwald staining of bone marrow smears (Figure 1C). This demonstrates that CD27-mediated immune activation has a particular impact on the myeloid compartment, as it inhibits the formation of eosinophils, whereas it increases the numbers of monocytes and neutrophils.
CD70TG mice lack eosinophils and have increased numbers of neutrophils and monocytes. (A) Absolute numbers of neutrophils (CD11b+, Gr1+, CD115−, F4/80−), monocytes (CD11b+, CD115+, F4/80+), and eosinophils (SSChi, Siglec-F+) in bone marrow (2 femurs and 2 tibiae), spleen, and blood and of macrophages (CD11b+, F4/80+, Gr1−), mast cells (c-Kit+, FcϵR1+), neutrophils, and eosinophils in the peritoneum. (B) The presence of eosinophils was determined by analyzing expression of Siglec-F on SSChi and on CCR3+ cells (percentages are shown). (C) May-Grünwald-Giemsa staining of bone marrow smears from WT and CD70TG mice. ▴ indicates eosinophils. Images were taken using an Olympus BX51 microscope (10×/0.3 NA objective) equipped with a mounted Olympus DP70 digital camera and ACDSee 5.0 software for image acquisition. Data are mean ± SD for 3 individual mice per group. Results are representative of 3 independent experiments. *P < .05; **P < .01.
CD70TG mice lack eosinophils and have increased numbers of neutrophils and monocytes. (A) Absolute numbers of neutrophils (CD11b+, Gr1+, CD115−, F4/80−), monocytes (CD11b+, CD115+, F4/80+), and eosinophils (SSChi, Siglec-F+) in bone marrow (2 femurs and 2 tibiae), spleen, and blood and of macrophages (CD11b+, F4/80+, Gr1−), mast cells (c-Kit+, FcϵR1+), neutrophils, and eosinophils in the peritoneum. (B) The presence of eosinophils was determined by analyzing expression of Siglec-F on SSChi and on CCR3+ cells (percentages are shown). (C) May-Grünwald-Giemsa staining of bone marrow smears from WT and CD70TG mice. ▴ indicates eosinophils. Images were taken using an Olympus BX51 microscope (10×/0.3 NA objective) equipped with a mounted Olympus DP70 digital camera and ACDSee 5.0 software for image acquisition. Data are mean ± SD for 3 individual mice per group. Results are representative of 3 independent experiments. *P < .05; **P < .01.
CD70TG mice fail to produce eosinophils during experimental asthma
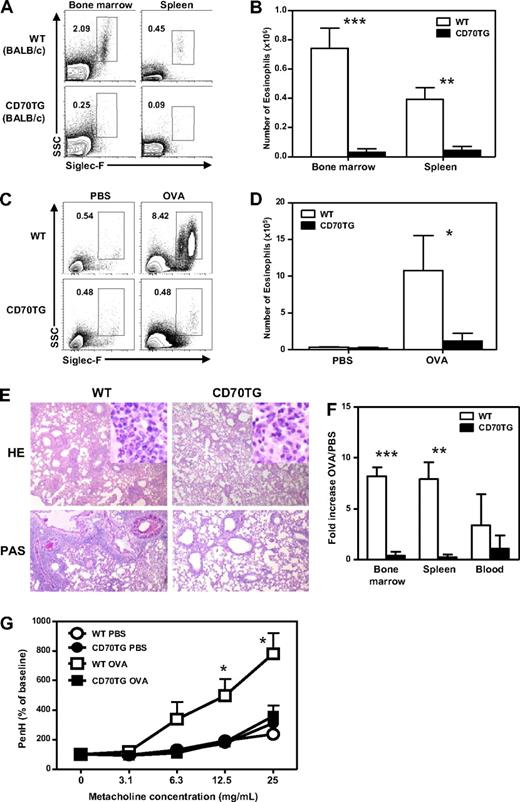
To test whether eosinophil production could be induced in CD70TG mice, we made use of an OVA-induced asthma model, as this treatment typically enhances eosinophil production in the bone marrow and induces eosinophilia in blood, lung, and spleen.6,27-29 Because this model works most effectively in BALB/c mice, we used CD70TG mice that were backcrossed on a BALB/c background; importantly, the decrease in eosinophil numbers in CD70TG mice is not dependent on their genetic background (Figure 2A-B). WT and CD70TG BALB/c mice were sensitized (day 0 and 14) with OVA/alum and intranasally challenged with OVA at days 28, 29, and 30. This treatment provoked a robust influx of eosinophils in the lungs of WT mice, but not of CD70TG mice, as judged by flow cytometric analysis (Figure 2C-D). Histology on the lungs of CD70TG mice confirmed the almost complete absence of eosinophils and showed a strong reduction in perivascular or peribronchial infiltrates and mucus production compared with WT mice (Figure 2E). Analysis of bone marrow, spleen, and blood also showed that eosinophil numbers were systemically increased in OVA-challenged WT mice, but not in CD70TG mice (Figure 2F). Finally, and in line with the absence of eosinophilia in the lung, we found that CD70TG mice lacked the induction of airway hyperresponsiveness, as assessed by a challenge with increasing doses of metacholine (Figure 2G). These data demonstrate that CD70TG mice not only have strongly reduced numbers of eosinophils but are also unable to generate them under strongly stimulating pathophysiologic conditions in vivo.
CD70 mice fail to produce eosinophils during experimental asthma and lack airway hyperresponsiveness. (A) Representative plots of Siglec-F staining on bone marrow and spleens of WT BALB/c and CD70TG BALB/c mice showing eosinophils as SSChiSiglec-F+ cells (percentages of eosinophils of all cells are shown) and (B) absolute numbers of eosinophils in the bone marrow and spleens of these mice. (C) WT BALB/c and CD70TG BALC/c mice were sensitized and challenged with OVA or treated with PBS as control. Contour plots display Siglec-F expression on eosinophils in the lung gated on CD4− cells (percentages are shown) from PBS- and OVA-treated WT and CD70TG mice. (D) Absolute numbers of eosinophils in the lung were calculated. (E) Representative hematoxylin and eosin and periodic acid–Schiff staining on tissue slides of lungs from OVA-treated WT and CD70TG mice. Images were taken using an Olympus BX51 microscope (4×/0.3 NA objective) equipped with a mounted Olympus DP70 digital camera and ACDSee 5.0 software for image acquisition. (F) Eosinophil numbers in PBS- and OVA-treated mice from both groups were determined in bone marrow, spleen, and blood. Fold increase in eosinophil numbers compared with PBS-treated mice is shown. (G) Airway hyperresponsiveness to increasing concentrations of metacholine was measured in PBS- and OVA-treated WT and CD70TG mice. Data are from 5 mice per group for OVA-treated animals and 3 mice per group for PBS-treated animals. An identical experiment with comparable results was performed in WT and CD70TG mice on C57BL/6 background. Data are mean ± SD. *P < .05; **P < .01; ***P < .001. (D) *Difference between OVA-treated WT and CD70TG mice.
CD70 mice fail to produce eosinophils during experimental asthma and lack airway hyperresponsiveness. (A) Representative plots of Siglec-F staining on bone marrow and spleens of WT BALB/c and CD70TG BALB/c mice showing eosinophils as SSChiSiglec-F+ cells (percentages of eosinophils of all cells are shown) and (B) absolute numbers of eosinophils in the bone marrow and spleens of these mice. (C) WT BALB/c and CD70TG BALC/c mice were sensitized and challenged with OVA or treated with PBS as control. Contour plots display Siglec-F expression on eosinophils in the lung gated on CD4− cells (percentages are shown) from PBS- and OVA-treated WT and CD70TG mice. (D) Absolute numbers of eosinophils in the lung were calculated. (E) Representative hematoxylin and eosin and periodic acid–Schiff staining on tissue slides of lungs from OVA-treated WT and CD70TG mice. Images were taken using an Olympus BX51 microscope (4×/0.3 NA objective) equipped with a mounted Olympus DP70 digital camera and ACDSee 5.0 software for image acquisition. (F) Eosinophil numbers in PBS- and OVA-treated mice from both groups were determined in bone marrow, spleen, and blood. Fold increase in eosinophil numbers compared with PBS-treated mice is shown. (G) Airway hyperresponsiveness to increasing concentrations of metacholine was measured in PBS- and OVA-treated WT and CD70TG mice. Data are from 5 mice per group for OVA-treated animals and 3 mice per group for PBS-treated animals. An identical experiment with comparable results was performed in WT and CD70TG mice on C57BL/6 background. Data are mean ± SD. *P < .05; **P < .01; ***P < .001. (D) *Difference between OVA-treated WT and CD70TG mice.
CD70TG mice lack eosinophil-specific progenitors and are unable to generate eosinophils from GMPs
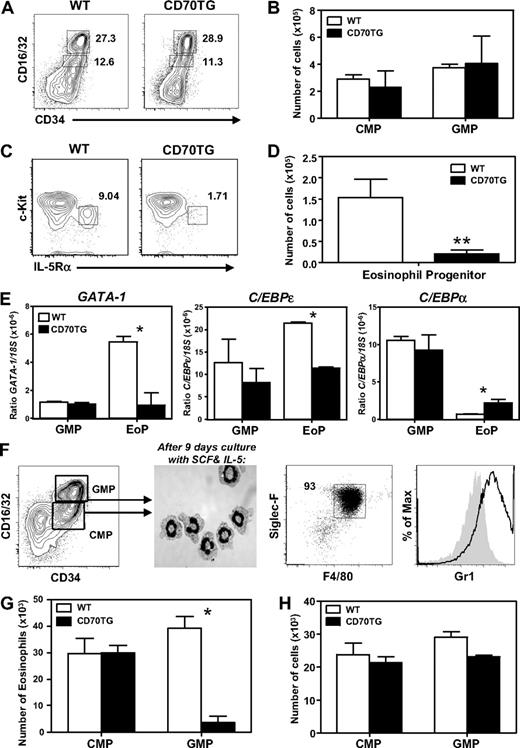
To determine whether the defect in eosinophil development could be explained at the level of progenitor cells, the presence of various precursor subsets was examined in the bone marrow of CD70TG and WT mice. Absolute numbers of CMPs (Lineage−c-Kit+CD16/32intCD34+) and GMPs (Lineage−c-Kit+CD16/32hiCD34+) was not different between CD70TG and WT mice (Figure 3A-B; see “Flow cytometry and cell sorting” for more information on the phenotypic definition of these cells). However, we did observe a dramatic decrease in the numbers of EoPs (Lineage−c-KitlowCD16/32hiCD34+IL-5Rα+) in the bone marrow of CD70TG mice (Figure 3C-D), which indicates a block in the commitment of GMPs to the eosinophil lineage. This was supported by quantitative PCR analysis of key transcription factors in GMPs and EoPs, as we found that GATA-1 was not induced in the few EoPs of CD70TG mice compared with GMPs (Figure 3E), which is a key event in eosinophil development.13 C/EBPϵ expression was also reduced in EoPs of CD70TG compared with WT mice, whereas the reverse was true for C/EBPα expression (Figure 3E). GATA-2 and PU.1 were not differentially expressed in EoPs from CD70TG mice (data not shown). These data indicate CD70TG mice not only have a strong reduction of EoPs, but that the remaining cells lack the required transcription factors for eosinophil development.
CD70TG mice lack EoPs and CD70TG GMPs are unable to generate eosinophils. (A) Representative contour plot of staining for CMPs (CD16/32intCD34+) and GMPs (CD16/32hiCD34+) within the Lin−c-Kit+ compartment in bone marrow of WT and CD70TG mice (percentages are shown). (B) Absolute numbers of CMPs and GMPs present in 2 femurs and 2 tibiae per mouse. (C) Representative contour plot of staining for EoPs (c-KitlowIL-5Rα+) within the Lineage−CD34+CD16/32hi compartment of WT and CD70TG mice (percentages are shown). (D) Absolute numbers of EoPs. (E) Amount of mRNA of GATA1, C/EBPϵ, and C/EBPα relative to 18S in GMPs and EoPs from WT and CD70TG mice. (F) When CMPs or GMPs (left) are sorted and cultured for 9 days with SCF and IL-5, they develop into a near homogeneous population of eosinophils, as determined by May-Grunwald Giemsa staining on cytospins (middle) and flow cytometric analysis for expression of Siglec-F, F4/80, and Gr1 (right). Filled histogram shows fluorescence-minus-one control staining. (G) Absolute number of eosinophils derived from 1.0 × 104 CMPs or GMPs from WT and CD70TG mice cultured for 9 days with SCF and IL-5. (H) Absolute number of cells derived from 2.5 × 103 CMPs or GMPs from WT and CD70TG mice cultured for 9 days with SCF, IL-5, and GM-CSF. Data are mean ± SD for 3 individual mice per group (B,D) and for 2 or 3 mice (E). (G-H) Data are mean ± SD for 2 cultures per condition. (B,D,G) Data are representative of 3 independent experiments. (H) Data are representative of 2 independent experiments. *P < .05; **P < .01.
CD70TG mice lack EoPs and CD70TG GMPs are unable to generate eosinophils. (A) Representative contour plot of staining for CMPs (CD16/32intCD34+) and GMPs (CD16/32hiCD34+) within the Lin−c-Kit+ compartment in bone marrow of WT and CD70TG mice (percentages are shown). (B) Absolute numbers of CMPs and GMPs present in 2 femurs and 2 tibiae per mouse. (C) Representative contour plot of staining for EoPs (c-KitlowIL-5Rα+) within the Lineage−CD34+CD16/32hi compartment of WT and CD70TG mice (percentages are shown). (D) Absolute numbers of EoPs. (E) Amount of mRNA of GATA1, C/EBPϵ, and C/EBPα relative to 18S in GMPs and EoPs from WT and CD70TG mice. (F) When CMPs or GMPs (left) are sorted and cultured for 9 days with SCF and IL-5, they develop into a near homogeneous population of eosinophils, as determined by May-Grunwald Giemsa staining on cytospins (middle) and flow cytometric analysis for expression of Siglec-F, F4/80, and Gr1 (right). Filled histogram shows fluorescence-minus-one control staining. (G) Absolute number of eosinophils derived from 1.0 × 104 CMPs or GMPs from WT and CD70TG mice cultured for 9 days with SCF and IL-5. (H) Absolute number of cells derived from 2.5 × 103 CMPs or GMPs from WT and CD70TG mice cultured for 9 days with SCF, IL-5, and GM-CSF. Data are mean ± SD for 3 individual mice per group (B,D) and for 2 or 3 mice (E). (G-H) Data are mean ± SD for 2 cultures per condition. (B,D,G) Data are representative of 3 independent experiments. (H) Data are representative of 2 independent experiments. *P < .05; **P < .01.
To test the capacity of myeloid progenitors from CD70TG mice to generate eosinophils in vitro, CMPs or GMPs were sorted and cultured for 9 days with SCF and IL-5. This induced the development of eosinophils that contain eosinophilic granules and a characteristic donut-shaped nucleus, as observed by cytology (Figure 3F). Flow cytometric analysis confirmed the development of eosinophils, as these cells were F4/80+, Siglec-F+, and Gr1+ (Figure 3F). We found that the formation of eosinophils from CD70TG GMPs was dramatically reduced compared with WT controls, whereas CMPs from CD70TG mice did not show this defect (Figure 3G). CD70TG GMPs were not deficient in generating other types of myeloid cells in vitro, as these cells were fully capable of generating neutrophils and monocytes when GM-CSF was added to the cultures (Figure 3H). These experiments indicate that differentiation toward the eosinophilic, but not other myeloid lineages, is specifically inhibited in CD70TG GMPs.
Development of eosinophils from myeloid progenitors is inhibited by IFN-γ
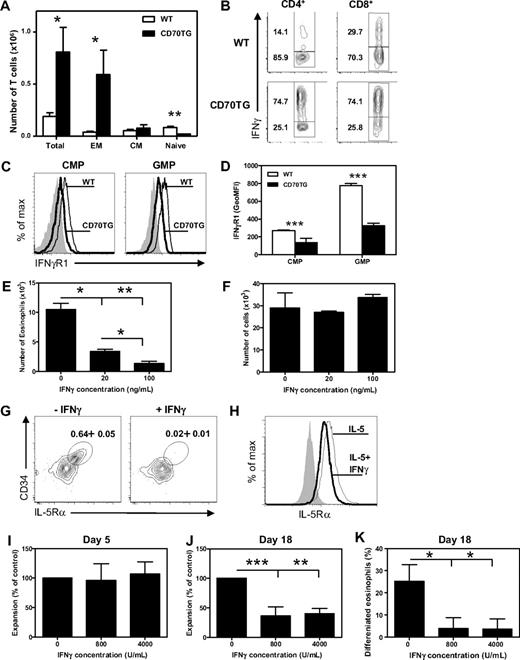
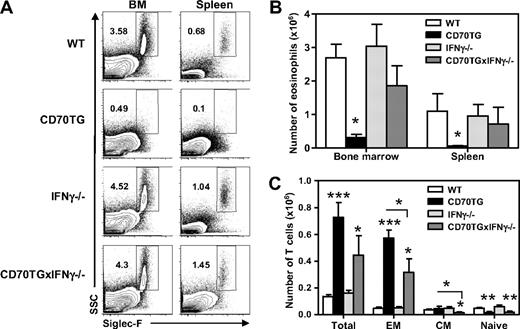
CD70TG mice have an accumulation of T cells in the bone marrow, which are mainly effector memory-like (CD44+CD62L−) T cells (Figure 4A) and which are potent producers of the typical Th1 cytokine IFN-γ (Figure 4B). To address the possibility that IFN-γ can directly inhibit the development of eosinophils from myeloid progenitors, we analyzed the expression of the ligand-binding chain of the IFN-γ receptor (IFN-γR1) on CMPs and GMPs by flow cytometry. Expression of IFN-γR1 could be detected on both CMPs and GMPs, with the highest expression on GMPs. Expression of IFN-γR1 was down-regulated on CMPs and GMPs of CD70TG mice (Figure 4C-D), indicative of recent signaling through this receptor.30
Development of eosinophils from myeloid progenitors is inhibited by IFN-γ. (A) Absolute numbers of total T cells and T-cell subsets in the bone marrow (1 femur and 1 tibia) of WT and CD70TG mice. EM indicates effector memory; and CM, central memory. (B) Intracellular expression of IFN-γ in CD3+CD4+ gated and CD3+CD8+ gated T cells from bone marrow of WT and CD70TG mice on phorbol myristate acetate and ionomycin stimulation (percentages are shown). (C) Expression of IFN-γR1 on CMPs and GMPs of WT (thin line) and CD70TG mice (bold line). Filled graph shows fluorescence-minus-one control staining. (D) Expression of IFN-γR1 on CMPs and GMPs as the average geometric mean fluorescence intensity (GeoMFI). (E) Number of eosinophils generated from 5.0 × 103 WT CMPs after 9 days of culture with SCF and IL-5 with or without addition of various concentrations of IFN-γ. (F) Number of cells generated from 2.5 × 103 WT CMPs after 9 days of culture with SCF, IL-5, and GM-CSF with or without addition of various concentrations of IFN-γ. (G) Representative plot of staining for CD34+ IL-5Rα+ EoPs gated on the Lineage−CD16/32+c-Kitlow compartment after a 3-day culture of GMPs in the presence of SCF, IL-3, IL-5, and GM-CSF with or without the addition of IFN-γ (data indicate percentage of EoPs from total cells). (H) Flow cytometric analysis of IL-5Rα expression on TF1 cells cultured with IL-5 in the presence (thick line) or absence (thin line) of IFN-γ. Isotype control is shown in shaded gray. (I-K) Human CD34+ progenitor cells were cultured for 3 days in the presence of SCF, FLT-3, GM-CSF, IL-3, and IL-5 followed by culture for 15 days in IL-3 and IL-5. Expansion of progenitor cells at day 5 (I) and day 18 (J) in cultures in the presence of IFN-γ, expressed as percentage of expansion in the cultures without IFN-γ. (K) Percentage of differentiated eosinophils after 18 days of culture analyzed after May-Grünwald Giemsa staining of cytospins. (A,D) Data are the mean of 3 mice per group. (E-F) Data are mean ± SD for 2 cultures per condition. (H) Data are mean ± SD for 4 cultures per condition. (A-H) Data are representative of at least 2 independent experiments. (I-K) Data are the mean of 3 independent experiments from different donors. Data are mean ± SD. *P < .05; **P < .01; ***P < .001.
Development of eosinophils from myeloid progenitors is inhibited by IFN-γ. (A) Absolute numbers of total T cells and T-cell subsets in the bone marrow (1 femur and 1 tibia) of WT and CD70TG mice. EM indicates effector memory; and CM, central memory. (B) Intracellular expression of IFN-γ in CD3+CD4+ gated and CD3+CD8+ gated T cells from bone marrow of WT and CD70TG mice on phorbol myristate acetate and ionomycin stimulation (percentages are shown). (C) Expression of IFN-γR1 on CMPs and GMPs of WT (thin line) and CD70TG mice (bold line). Filled graph shows fluorescence-minus-one control staining. (D) Expression of IFN-γR1 on CMPs and GMPs as the average geometric mean fluorescence intensity (GeoMFI). (E) Number of eosinophils generated from 5.0 × 103 WT CMPs after 9 days of culture with SCF and IL-5 with or without addition of various concentrations of IFN-γ. (F) Number of cells generated from 2.5 × 103 WT CMPs after 9 days of culture with SCF, IL-5, and GM-CSF with or without addition of various concentrations of IFN-γ. (G) Representative plot of staining for CD34+ IL-5Rα+ EoPs gated on the Lineage−CD16/32+c-Kitlow compartment after a 3-day culture of GMPs in the presence of SCF, IL-3, IL-5, and GM-CSF with or without the addition of IFN-γ (data indicate percentage of EoPs from total cells). (H) Flow cytometric analysis of IL-5Rα expression on TF1 cells cultured with IL-5 in the presence (thick line) or absence (thin line) of IFN-γ. Isotype control is shown in shaded gray. (I-K) Human CD34+ progenitor cells were cultured for 3 days in the presence of SCF, FLT-3, GM-CSF, IL-3, and IL-5 followed by culture for 15 days in IL-3 and IL-5. Expansion of progenitor cells at day 5 (I) and day 18 (J) in cultures in the presence of IFN-γ, expressed as percentage of expansion in the cultures without IFN-γ. (K) Percentage of differentiated eosinophils after 18 days of culture analyzed after May-Grünwald Giemsa staining of cytospins. (A,D) Data are the mean of 3 mice per group. (E-F) Data are mean ± SD for 2 cultures per condition. (H) Data are mean ± SD for 4 cultures per condition. (A-H) Data are representative of at least 2 independent experiments. (I-K) Data are the mean of 3 independent experiments from different donors. Data are mean ± SD. *P < .05; **P < .01; ***P < .001.
To determine whether IFN-γ can directly block development of eosinophils, we analyzed the effect of IFN-γ on the outgrowth of eosinophils from purified WT progenitors. Indeed, the addition of IFN-γ to WT CMPs (Figure 4E) as well as GMPs (data not shown) severely reduced their ability to generate eosinophils in a dose-dependent manner. This was not the result of a generally inhibitory effect of IFN-γ on the differentiation capacity of these precursors, as neutrophils and monocytes developed normally when GM-CSF was added to these cultures (Figure 4F). Together with the observation that CD70TG GMPs, but not CMPs, are impaired in generating eosinophils in vitro, we postulate that at least GMPs are sensitive to the inhibitive effect of IFN-γ on eosinophil differentiation; the observed impact of IFN-γ on WT CMPs is probably because CMPs first give rise to GMPs before differentiating to eosinophils in these cultures.
The strongly reduced numbers of EoPs, but not GMPs, in CD70TG mice indicate that IFN-γ specifically blocks the differentiation of GMPs to this downstream eosinophil-specific progenitor. To test this hypothesis, WT GMPs were cultured with SCF, IL-3, IL-5, and GM-CSF with or without IFN-γ. After 3 days in the absence of IFN-γ, we found expression of IL-5Rα on a small fraction of the undifferentiated cells, which were phenotypically similar to the EoP (Figure 4G). Strikingly, the addition of IFN-γ inhibited the appearance of IL-5Rα+ cells in these cultures and reduced the fraction of EoP-like cells by 95% (Figure 4G). These observations indicate that IFN-γ can inhibit the outgrowth of eosinophils from myeloid progenitor cells by preventing the development of GMPs to EoPs. To test whether IFN-γ can directly affect the expression of the IL-5R, we cultured the IL-5-responsive erythroleukemic cell line TF-1 with IL-5 in the presence or absence of IFN-γ. We found that IFN-γ indeed inhibited the surface expression of the IL-5Rα by 30% (Figure 4H), but not the mRNA expression (data no shown). In accordance, this treatment inhibited the IL-5-dependent proliferation of these cells, but not the GM-CSF-dependent proliferation (data not shown).
Finally, to determine whether IFN-γ could also inhibit eosinophil development from human hematopoietic progenitors, cord blood-derived CD34+ cells were differentiated to eosinophils in the absence or presence of IFN-γ. Cells were expanded for 3 days in the presence of SCF, FLT-3, GM-CSF, IL-3, and IL-5 and subsequently cultured for another 15 days in the presence of IL-3 and IL-5 alone to induce eosinophil differentiation.22 Expansion of CD34+ progenitor cells during the first period of culture was not affected by the presence of IFN-γ (Figure 4I). However, after 18 days of culture, both the cellular expansion (Figure 4J) and the eosinophil differentiation (Figure 4K) were strongly reduced by IFN-γ. This demonstrates that IFN-γ does not inhibit the expansion of early hematopoietic stem and progenitor cells, but rather the differentiation and expansion of more restricted eosinophil precursors.
Reduced eosinophil development during CD70-mediated immune activation is IFN-γ dependent
Thus far, we have shown that CD70TG mice are almost devoid of eosinophils and that IFN-γ is sufficient to impair eosinophil development in vitro. To establish whether the block in eosinophil differentiation in CD70TG mice is a direct consequence of the increased IFN-γ production, we backcrossed CD70TG mice on an IFN-γ-deficient background. Indeed, eosinophil differentiation was largely restored in CD70TGxIFN-γ−/− mice, as absolute eosinophil numbers in bone marrow and spleen were comparable with WT and IFN-γ−/− mice (Figure 5A-B). Although less pronounced than in CD70TG mice, CD70TGxIFN-γ−/− mice still had an accumulation of effector memory-like T cells in the bone marrow compared with control mice (Figure 5C). Yet, the restoration of eosinophil differentiation in these mice indicates that the inhibition in eosinophil formation in CD70TG mice can be attributed to the enhanced production of IFN-γ.
Reduced eosinophil development during CD70-mediated immune activation is IFN-γ dependent. (A) Representative plots of Siglec-F staining on bone marrow and spleens of WT, CD70TG, IFN-γ−/−, and CD70TGx IFN-γ−/− mice showing eosinophils as SSChiSiglec-F+ cells (percentages are shown). (B) Absolute numbers of eosinophils in the bone marrow (2 femurs and 2 tibiae) and spleens of these mice. (C) Absolute numbers of total T cells and T-cell subsets (percentages are shown) in the bone marrow of WT, CD70TG, IFN-γ−/−, and CD70TGx IFN-γ−/− mice. EM indicates effector memory; and CM, central memory. (B-C) Data are the mean of 3 individual mice per group. Data are mean ± SD. Experiments were performed at least 3 times with similar results. *P < .05; **P < .01; ***P < .001.
Reduced eosinophil development during CD70-mediated immune activation is IFN-γ dependent. (A) Representative plots of Siglec-F staining on bone marrow and spleens of WT, CD70TG, IFN-γ−/−, and CD70TGx IFN-γ−/− mice showing eosinophils as SSChiSiglec-F+ cells (percentages are shown). (B) Absolute numbers of eosinophils in the bone marrow (2 femurs and 2 tibiae) and spleens of these mice. (C) Absolute numbers of total T cells and T-cell subsets (percentages are shown) in the bone marrow of WT, CD70TG, IFN-γ−/−, and CD70TGx IFN-γ−/− mice. EM indicates effector memory; and CM, central memory. (B-C) Data are the mean of 3 individual mice per group. Data are mean ± SD. Experiments were performed at least 3 times with similar results. *P < .05; **P < .01; ***P < .001.
IFN-γ–producing T cells in the bone marrow can block eosinophil development
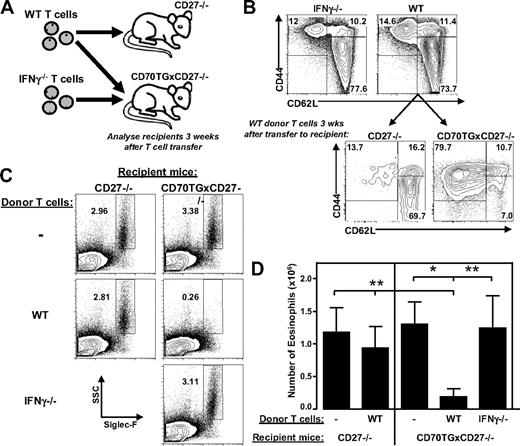
To determine whether IFN-γ derived from T cells is sufficient to induce the observed block in eosinophil development, we used a previously described adoptive transfer model of T cells to CD70TG mice that are deficient for CD27. CD27−/− mice have normal myeloid differentiation and because CD27 and CD70 are a unique receptor-ligand pair, the strong phenotype of CD70TG mice is completely nullified when backcrossed on a CD27−/− background.15 Transfer of T cells to these CD70TGxCD27−/− mice is sufficient to induce activation of the donor T cells and results in CD27-dependent, IFN-γ-mediated depletion of B cells in the bone marrow. To assess whether this treatment also affects eosinophil formation, we transferred T cells to CD70TGxCD27−/− mice (Figure 6A), which differentiated to effector cells and accumulated in the bone marrow (Figure 6B). Although CD70TGxCD27−/− mice have normal numbers of eosinophils, the transfer of WT T cells is sufficient to significantly inhibit eosinophil differentiation in the bone marrow within a period of 3 weeks (Figure 6C-D). These effects could be attributed to CD70-mediated activation of the donor T cells, as transfer of WT T cells to CD27−/− mice did not lead to effector T-cell formation (Figure 6B), nor did it inhibit eosinophil development (Figure 6C-D). Importantly, the observed block in eosinophil differentiation in CD70TGxCD27−/− recipient mice was fully dependent on the production of IFN-γ by the donor T cells because eosinophil formation was not affected when IFN-γ−/− T cells were transferred (Figure 6C-D). Neutrophil numbers did not change in the bone marrow of CD70TGxCD27−/− mice receiving WT T cells, whereas the number of monocytes was slightly increased (data not shown). These results demonstrate that T cell-derived IFN-γ is sufficient to specifically block the development of eosinophils in vivo.
T cell-derived IFN-γ is sufficient to block differentiation of eosinophils. (A) Schematic overview of the experiment. (B) Distribution of subsets of donor T cells derived from WT (CD45.1+) and IFN-γ−/− mice (upper panel) and phenotype of transferred CD45.1+ WT T cells in the bone marrow of CD27−/− and CD70TGxCD27−/− host mice (lower panel; gated on CD45.1+ cells). CD44+CD62L− indicates effector memory; CD44+CD62L+, central memory; and CD44−CD62L+, naive. (C) Representative plots of Siglec-F staining of bone marrow of CD27−/− and CD70TGxCD27−/− host mice injected with PBS, WT, or IFN-γ−/− T cells (9 × 106 cells/mouse) showing eosinophils as SSChiSiglec-F+ cells (percentages are shown). (D) Absolute numbers of eosinophils in bone marrow (one femur and one tibia) of host mice after injection with PBS or WT or IFN-γ−/− T cells. (B-C) Data are representative of 3 mice per group are representative of 2 independent experiments. (D) Data are representative of 5 individual mice per group and are representative of 2 independent experiments. Data are mean ± SD. *P < .05; **P < .01.
T cell-derived IFN-γ is sufficient to block differentiation of eosinophils. (A) Schematic overview of the experiment. (B) Distribution of subsets of donor T cells derived from WT (CD45.1+) and IFN-γ−/− mice (upper panel) and phenotype of transferred CD45.1+ WT T cells in the bone marrow of CD27−/− and CD70TGxCD27−/− host mice (lower panel; gated on CD45.1+ cells). CD44+CD62L− indicates effector memory; CD44+CD62L+, central memory; and CD44−CD62L+, naive. (C) Representative plots of Siglec-F staining of bone marrow of CD27−/− and CD70TGxCD27−/− host mice injected with PBS, WT, or IFN-γ−/− T cells (9 × 106 cells/mouse) showing eosinophils as SSChiSiglec-F+ cells (percentages are shown). (D) Absolute numbers of eosinophils in bone marrow (one femur and one tibia) of host mice after injection with PBS or WT or IFN-γ−/− T cells. (B-C) Data are representative of 3 mice per group are representative of 2 independent experiments. (D) Data are representative of 5 individual mice per group and are representative of 2 independent experiments. Data are mean ± SD. *P < .05; **P < .01.
IFN-γ produced during immune activation in WT mice also suppresses eosinophil development
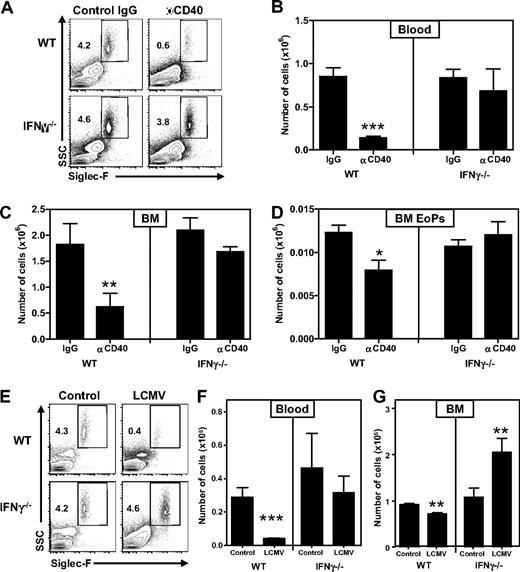
To test whether IFN-γ can also suppress development of eosinophils in vivo in a nontransgenic setting, we treated WT and IFN-γ−/− mice with an agonistic anti-CD40 antibody, which is frequently used to boost immunizations and increases the number of IFN-γ–producing T cells.31,32 Indeed, we found that this treatment up-regulated the expression of IFN-γ-responsive molecules, such as MHC-II and Sca-1 on a large proportion of bone marrow cells in WT mice, but not IFN-γ−/− mice3,15 (and data not shown). Importantly, anti-CD40–treated WT mice displayed a strong decrease in eosinophil numbers in both bone marrow and blood, whereas IFN-γ−/− mice were not affected (Figure 7A-C). In addition, EoP numbers were also decreased in an IFN-γ–dependent manner (Figure 7D), indicating that IFN-γ also impaired development from bone marrow progenitors in this model.
IFN-γ produced on immune activation in WT mice suppresses eosinophil development. (A) Representative plots of Siglec-F staining on blood of WT and IFN-γ−/− mice, showing eosinophils as SSChiSiglec-F+ cells (percentages are shown) 4 days after the first injection of αCD40 or control IgG. Numbers of eosinophils in (B) blood and (C) bone marrow (total from 2 femurs and 2 tibiae). (D) Numbers of EoPs in the bone marrow of these mice. (E) Representative plots of Siglec-F staining on blood of WT and IFN-γ−/− mice showing eosinophils as SSChiSiglec-F+ cells (percentages are shown) 8 days after infection with LCMV-Armstrong. Numbers of eosinophils in (F) blood and (G) bone marrow (total from one femur and one tibia). (A-D) Data are from 5 mice per group. (E-G) Data are from 5 or 6 mice per infected group and 3 or 4 mice per control group. Both experiments have been performed twice with comparable outcome. Data are mean ± SEM. *P < .05; **P < .01; ***P < .001.
IFN-γ produced on immune activation in WT mice suppresses eosinophil development. (A) Representative plots of Siglec-F staining on blood of WT and IFN-γ−/− mice, showing eosinophils as SSChiSiglec-F+ cells (percentages are shown) 4 days after the first injection of αCD40 or control IgG. Numbers of eosinophils in (B) blood and (C) bone marrow (total from 2 femurs and 2 tibiae). (D) Numbers of EoPs in the bone marrow of these mice. (E) Representative plots of Siglec-F staining on blood of WT and IFN-γ−/− mice showing eosinophils as SSChiSiglec-F+ cells (percentages are shown) 8 days after infection with LCMV-Armstrong. Numbers of eosinophils in (F) blood and (G) bone marrow (total from one femur and one tibia). (A-D) Data are from 5 mice per group. (E-G) Data are from 5 or 6 mice per infected group and 3 or 4 mice per control group. Both experiments have been performed twice with comparable outcome. Data are mean ± SEM. *P < .05; **P < .01; ***P < .001.
Finally, to test whether IFN-γ produced in response to a natural infection also inhibits eosinophil development, we infected WT and IFN-γ−/− mice with LCMV-Armstrong. Analysis 8 days after infection revealed that eosinophil numbers were strongly reduced in peripheral blood and to a lesser extent also in bone marrow of WT mice (Figure 7E-G). In contrast, eosinophils were not changed in peripheral blood and even increased in the bone marrow of IFN-γ−/− mice. These data demonstrate that IFN-γ also negatively affects the development of eosinophils during the course of an antiviral immune response.
Discussion
In this report, we show that T-cell activation and, in particular, the ensuing IFN-γ production can inhibit eosinophil differentiation in vivo and thereby modulate myelopoiesis in a very specific manner. Impaired eosinophil production was found in 2 independent B cell–specific as well as a T cell–specific CD70TG founder line and, in either case, dependent on CD27 and IFN-γ (data not shown). These findings demonstrate that the block in eosinophil differentiation in CD70TG mice is a direct result of enhanced IFN-γ production in these mice and not the result of an intrinsic impairment in eosinophils or their precursors, nor to enhanced CD27 triggering on early hematopoietic progenitor cells in these mice.3 Serum levels of IFN-γ are below detection limit in CD70TG mice, although these mice express high levels of MHC-II and Sca-1 and gradually lose their B cells, which are known consequences of IFN-γ triggering and indeed do not occur in CD70TG*IFN-γ−/− mice.15 Moreover, our in vitro experiments in which IFN-γ is added to purified progenitor cells demonstrate that IFN-γ can directly inhibit the development of both murine and human eosinophils (Figure 4). This is corroborated by the fact that IFN-γ induced by anti-CD40 treatment or LCMV infection strongly decreased eosinophil numbers in WT mice (Figure 7) but also in CD27−/− mice (data not shown), which demonstrates that CD27 triggering is sufficient, but not required for IFN-γ-mediated inhibition of eosinophil development.
The eosinophil defect in CD70TG mice could be traced back to a strong decrease in the number of IL-5Rα+ eosinophil progenitors in the bone marrow. Moreover, IFN-γ could inhibit formation of these EoPs from GMPs and down-regulate IL-5Rα expression on TF-1 cells, which could indicate that the eosinophil defect is the result of a direct effect of IFN-γ on the expression of the IL-5R. However, IL-5Rα expression is not a cause, but rather a consequence, of commitment of myeloid progenitors to the eosinophil lineage through the expression of GATA-1 and GATA-2.13 We observed that the remaining EoPs from CD70TG mice lacked GATA-1 expression, indicating that IFN-γ actually blocks the commitment of GMPs to the eosinophilic lineage by inhibiting the induction of GATA-1. This will make these cells unable to up-regulate essential genes, such as the IL-5R, to respond to environmental cues and differentiate to eosinophils. We did not observe induction of apoptosis of GMPs by IFN-γ, but it is most probable that IFN-γ will reduce the viability of such precursors when they do not receive the appropriate signals to differentiate further. The finding that addition of GM-CSF to IFN-γ–treated GMPs enabled normal differentiation to monocytes and neutrophils supports this view (Figure 4F). Furthermore, the paradoxical finding that CD70TG CMPs were able to generate eosinophils, but WT CMPs incubated with IFN-γ were not, is most probable because GMPs derived from CD70TG CMPs were not exposed to IFN-γ in these cultures, whereas CMP-derived WT GMPs were. GMPs are probably also more sensitive to IFN-γ, as they have higher expression of the IFN-γR (Figure 4C). Whether IFN-γ does indeed inhibit the up-regulation and/or function of GATA-1 in GMPs and by what molecular mechanism is currently under investigation.
An important question is why the production of IFN-γ inhibits eosinophil formation. Considering the fact that CD70TG mice not only have strongly reduced eosinophil numbers but also increased monocyte numbers, we speculate that IFN-γ inhibits eosinophil development at the benefit of other myeloid cells. This would be highly relevant during viral infections, where monocytes rather than eosinophils are required to combat the infection. Indeed, we found that IFN-γ produced during LCMV infection inhibits the numbers of eosinophils in bone marrow and blood (Figure 7). The increase in eosinophils in bone marrow of LCMV-infected IFN-γ−/− mice is probably the result of a more general increase in myelopoiesis induced by the infection, in which eosinophil formation is not inhibited because of the absence of IFN-γ. This is supported by the finding that large numbers of eosinophils accumulate in the lungs of IFN-γR-deficient mice on infection with respiratory syncytial virus33 and in the brains of IFN-γ-deficient mice on infection with Borna disease virus,34 but not in WT controls. Because virus-specific CD8 T cells migrate to the bone marrow during the course of a viral infection with LCMV in mice,35 simian immunodeficiency virus in monkeys,36 and HIV in humans,37 we postulate that these cells actively inhibit the local development of eosinophils through the production of IFN-γ and thereby balance myeloid output from the bone marrow. Furthermore, studies in which mice were treated with IL-128 or challenged with murine cytomegalovirus7 before induction of an allergic airway response, showed that Th1 induction reduced the ensuing airway eosinophilia. IL-12 treatment also prevented systemic eosinophilia induced either by challenging sensitized mice with an allergen38 or by infecting mice intraperitoneally with a parasite,39 which is in both cases IFN-γ dependent. Altogether, these data support the hypothesis that IFN-γ–producing T cells that develop during antiviral responses can actively prevent the development of those myeloid cells that are not required and possibly even counterproductive to eradicate the virus.
IL-5 plays a crucial role in the development of eosinophils, as IL-5 overexpression is sufficient to increase eosinophil formation in the bone marrow and induce eosinophilia in blood.40,41 Conversely, IL-5 deficiency decreases eosinophil production in the bone marrow and prevents induction of eosinophilia on allergen challenge or parasite infection.42,43 Importantly, CD70TG mice have no defect in IL-5 production and even have more serum IL-5 (62.8 ± 14.3 pg/mL; n = 3) than WT controls (< 10 pg/mL; n = 3). Thus, our data demonstrate, for the first time, that IFN-γ can, even in the presence of sufficient levels of IL-5, directly inhibit eosinophil development, both in vitro and in vivo. A regulating role for IFN-γ, rather than IL-5 in the development of eosinophilia and asthma, is supported by studies in humans. Smart et al44 showed that both patients with ongoing, severe asthma and patients with resolved asthma had increased house dust mite-induced IL-5 production. Whereas IFN-γ responses were decreased in patients with persistent asthma, IFN-γ production was back to normal in subjects with resolved disease, demonstrating that normalization of IFN-γ responses was associated with resolution of asthma.44 This finding is supported by other studies demonstrating decreased IFN-γ production in response to rhinovirus or house dust mite and an inverse correlation between IFN-γ levels and asthma severity.45-47 In addition, a genetic functional polymorphism in the promoter of suppressor of cytokine signaling 1 (SOCS1), a negative regulator of IFN-γ signaling, resulting in increased levels of SOCS1 protein, was associated with adult asthma.48 Mice deficient in SOCS1 tend to have reduced numbers of eosinophils in the blood, which is restored to normal in SOCS1-deficient mice on an IFN-γ−/− background.49 Collectively, these studies corroborate our findings that increased IFN-γ signaling is negatively associated with asthma development and severity by affecting eosinophil formation.
Taken together, we have demonstrated that IFN-γ is a potent inhibitive cytokine in eosinophil differentiation, even in the presence of the eosinophil-permissive cytokine IL-5. As such, IFN-γ-producing T cells are important modulators of myeloid output of the bone marrow, as they specifically limit the generation of eosinophils by inhibiting the commitment of myeloid precursor cells to the eosinophil lineage.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sten Libregts, Cláudia Brandão Silva, and Ronald van Olffen for technical assistance, Berend Hooibrink for cell sorting, the staff of the animal facility of the AMC for excellent animal care, Prof Dr Rene van Lier for stimulating discussions, and Drs Esther Nolte-‘t Hoen, Kris Reedquist, Christian Geest, and Prof Dr Rene van Lier for critical reading of the manuscript.
This work was supported by The Netherlands Organization of Scientific Research (VENI grant 91676137, M.B.; and VIDI grant 91776310, M.A.N.).
Authorship
Contribution: A.M.d.B. designed and performed experiments, analyzed the data, and wrote the paper; M.B., K.F.v.d.S., and K.P.J.M.v.G. performed experiments and analyzed the data; L.B. provided essential reagents; and M.A.N. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martijn A. Nolte, Department of Experimental Immunology, Academic Medical Center, Meibergdreef 9, 1105AZ Amsterdam, The Netherlands; e-mail: m.a.nolte@amc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal