Abstract
Killer-cell immunoglobulin-like receptor (KIR) genes form a diverse, immunogenetic system. Group A and B KIR haplotypes have distinctive centromeric (Cen) and telomeric (Tel) gene-content motifs. Aiming to develop a donor selection strategy to improve transplant outcome, we compared the contribution of these motifs to the clinical benefit conferred by B haplotype donors. We KIR genotyped donors from 1409 unrelated transplants for acute myelogenous leukemia (AML; n = 1086) and acute lymphoblastic leukemia (ALL; n = 323). Donor KIR genotype influenced transplantation outcome for AML but not ALL. Compared with A haplotype motifs, centromeric and telomeric B motifs both contributed to relapse protection and improved survival, but Cen-B homozygosity had the strongest independent effect. With Cen-B/B homozygous donors the cumulative incidence of relapse was 15.4% compared with 36.5% for Cen-A/A donors (relative risk of relapse 0.34; 95% confidence interval 0.2-0.57; P < .001). Overall, significantly reduced relapse was achieved with donors having 2 or more B gene-content motifs (relative risk 0.64; 95% confidence interval 0.48-0.86; P = .003) for both HLA-matched and mismatched transplants. KIR genotyping of several best HLA-matched potential unrelated donors should substantially increase the frequency of transplants by using grafts with favorable KIR gene content. Adopting this practice could result in superior disease-free survival for patients with AML.
Introduction
Acute myelogenous leukemia (AML) is the most common form of adult acute leukemia, with approximately 12 000 cases diagnosed annually in the United States.1 For patients with high-risk or recurrent disease, hematopoietic cell transplantation (HCT) offers a potential cure.2,3 Successful allogeneic HCT depends upon the elimination of leukemic cells by the combination of chemotherapy, radiotherapy, and T cell–mediated graft-versus-leukemia (GVL) reaction, with reconstitution of the patient's ablated hematopoietic system by donor stem cells. Although matching for human leukocyte antigen (HLA) class I and II alleles is the most important criterion for unrelated donor selection, consideration of other factors, such as donor sex, parity, cytomegalovirus serostatus, and age, also can improve transplant outcome.4-6
Natural killer (NK) cells were discovered for their capacity to kill cancer cells,7 and later were shown to be an essential element of innate immunity.8 Like killer CD8 T cells, the development and function of NK cells are controlled by NK-cell receptors that recognize HLA class I.9 Among these ligand:receptor interactions, some are conserved, like that of HLA-E with the CD94:NKG2A NK-cell receptor,10 but others are variable. Extreme in this regard are the polymorphic killer-cell immunoglobulin-like receptors (KIRs)11,12 that recognize polymorphic epitopes of HLA-A, B, and C, called KIR ligands.13 As a consequence of this genetic variation, donor-derived NK cells can mediate beneficial GVL reactions in HCT. Such beneficial NK-cell alloreactivity, which can be predicted from the differences in KIR ligands between donor and recipient based on their HLA class I type,14 was first described for HLA haploidentical transplantation by the use of an extensively T cell–depleted graft15 and later investigated in other transplantation settings.16,17 These studies did not consider a role for KIR gene variability, which in its extent and functional importance approaches that of HLA class I.18
Because HLA and KIR segregate independently on different chromosomes, only a minority of HLA-matched transplants are KIR matched. Thus, 25% of HLA-matched siblings are KIR identical, and unrelated HLA-matched donors are rarely KIR identical.19 This situation facilitated a retrospective analysis to examine the impact of donor and recipient KIR genotypes on transplant outcome. The simplest genetic distinction is the division of KIR haplotypes into groups A and B according to gene content.12,20 KIR A haplotypes have simple, fixed gene content; B haplotypes have variable gene content and 1 or more of the B-specific genes: KIR2DS1, 2, 3, 5, KIR2DL2, and KIR2DL5. When this distinction is used, all individuals can be assigned to the A/A genotype (homozygous for A haplotypes) or the B/x genotype (having 1 or 2 B haplotypes). Previously, we showed that the outcome of unrelated donor transplantation for AML was significantly improved with B/x donors compared with A/A donors, whereas recipient KIR genotype had no effect.21 Similar effects have been reported by other investigators in unrelated donor and sibling donor settings.22,23 Because KIR B haplotypes are present in approximately two-thirds of unrelated registry donors, interventions that merely increase the probability of selecting KIR B donors are unlikely to affect survival because most donors already have this characteristic by chance. Further, the specific genetic mechanism for the protective effect of B haplotype donors, perhaps attributable to the presence or absence of individual or groups of inhibitory or activating KIR, remains unknown. This analysis was designed, from understanding of the organization of the KIR locus, to identify which particular B-specific genes improve the therapeutic effect of transplantation for AML. The results point to a clinically applicable donor selection strategy that could improve the success of transplantation.
Methods
Characteristics of the study cohort and clinical database
We studied 1409 patients who received myeloablative, T-cell–replete, unrelated donor (URD) transplantation as treatment for either AML (n = 1086) or ALL (n = 323). Transplants were facilitated by the National Marrow Donor Program (NMDP) between 1988 and 2006 (Table 1). For each donor and recipient, a DNA sample was obtained from the Research Sample Repository of the NMDP. Outcome data were obtained from the Center for International Blood and Marrow Transplant Research. Complete high-resolution HLA matching data at HLA-A, B, C, DRB1, and DQB1 were obtained from the NMDP retrospective typing program. Samples and clinical data were obtained after informed consent in accordance with the Declaration of Helsinki and approval from the NMDP and University of Minnesota Institutional Review Boards.
Demographics of the transplanted leukemia patients
| Variable . | ALL . | AML . | ||
|---|---|---|---|---|
| Total (n = 323) . | Total (n = 1086) . | Donor with KIR B–content score 0 or 1 (n = 748) . | Donor with KIR B–content score ≥ 2 (n = 338) . | |
| Median age, y (range) | 18.5 (8-55) | 38.9 (1-70) | 39.0 (1-70) | 38.6 (1-68) |
| Race | ||||
| White | 261 (81) | 757 (70) | 512 (68) | 245 (72) |
| Nonwhite | 62 (19) | 329 (30) | 236 (32) | 93 (28) |
| Karnofsky score | ||||
| 90-100 | 232 (72) | 666 (61) | 460 (62) | 206 (61) |
| 10-80 | 91 (28) | 329 (30) | 227 (30) | 102 (30) |
| Unknown | 91 (8) | 61 (8) | 30 (9) | |
| Disease status when transplanted* | ||||
| Early | 80 (25) | 290 (27) | 200 (27) | 90 (27) |
| Intermediate | 159 (49) | 349 (32) | 232 (31) | 117 (35) |
| Advanced | 84 (26) | 447 (41) | 316 (42) | 131 (39) |
| Disease risk by cytogenetics | ||||
| Low | 0 (0) | 120 (11) | 75 (10) | 45 (13) |
| Intermediate | 133 (41) | 471 (43) | 341 (46) | 130 (38) |
| High | 68 (21) | 232 (21) | 164 (22) | 68 (20) |
| Unknown | 122 (38) | 263 (24) | 168 (22) | 95 (28) |
| Donor:recipient match for HLA-A, B, C, DRB1, and DQB1 | ||||
| 10/10 allele matched | 148 (46) | 539 (50) | 374 (50) | 165 (49) |
| 9/10 | 60 (19) | 301 (28) | 212 (28) | 89 (26) |
| 8/10 | 55 (17) | 158 (15) | 106 (14) | 52 (15) |
| Less than 8/10 | 60 (19) | 88 (8) | 56 (8) | 32 (10) |
| CMV serostatus | ||||
| Donor −/recipient − | 127 (39) | 331 (30) | 233 (31) | 98 (29) |
| Donor +/recipient − | 80 (25) | 144 (13) | 92 (12) | 52 (15) |
| Donor + or −/recipient + | 111 (34) | 578 (53) | 398 (53) | 180 (53) |
| Data missing | 5 (2) | 33 (3) | 25 (3) | 9 (3) |
| Graft type | ||||
| Bone marrow | 301 (93) | 641 (59) | 429 (57) | 212 (63) |
| Peripheral blood progenitor cells | 22 (7) | 445 (41) | 319 (43) | 126 (37) |
| Variable . | ALL . | AML . | ||
|---|---|---|---|---|
| Total (n = 323) . | Total (n = 1086) . | Donor with KIR B–content score 0 or 1 (n = 748) . | Donor with KIR B–content score ≥ 2 (n = 338) . | |
| Median age, y (range) | 18.5 (8-55) | 38.9 (1-70) | 39.0 (1-70) | 38.6 (1-68) |
| Race | ||||
| White | 261 (81) | 757 (70) | 512 (68) | 245 (72) |
| Nonwhite | 62 (19) | 329 (30) | 236 (32) | 93 (28) |
| Karnofsky score | ||||
| 90-100 | 232 (72) | 666 (61) | 460 (62) | 206 (61) |
| 10-80 | 91 (28) | 329 (30) | 227 (30) | 102 (30) |
| Unknown | 91 (8) | 61 (8) | 30 (9) | |
| Disease status when transplanted* | ||||
| Early | 80 (25) | 290 (27) | 200 (27) | 90 (27) |
| Intermediate | 159 (49) | 349 (32) | 232 (31) | 117 (35) |
| Advanced | 84 (26) | 447 (41) | 316 (42) | 131 (39) |
| Disease risk by cytogenetics | ||||
| Low | 0 (0) | 120 (11) | 75 (10) | 45 (13) |
| Intermediate | 133 (41) | 471 (43) | 341 (46) | 130 (38) |
| High | 68 (21) | 232 (21) | 164 (22) | 68 (20) |
| Unknown | 122 (38) | 263 (24) | 168 (22) | 95 (28) |
| Donor:recipient match for HLA-A, B, C, DRB1, and DQB1 | ||||
| 10/10 allele matched | 148 (46) | 539 (50) | 374 (50) | 165 (49) |
| 9/10 | 60 (19) | 301 (28) | 212 (28) | 89 (26) |
| 8/10 | 55 (17) | 158 (15) | 106 (14) | 52 (15) |
| Less than 8/10 | 60 (19) | 88 (8) | 56 (8) | 32 (10) |
| CMV serostatus | ||||
| Donor −/recipient − | 127 (39) | 331 (30) | 233 (31) | 98 (29) |
| Donor +/recipient − | 80 (25) | 144 (13) | 92 (12) | 52 (15) |
| Donor + or −/recipient + | 111 (34) | 578 (53) | 398 (53) | 180 (53) |
| Data missing | 5 (2) | 33 (3) | 25 (3) | 9 (3) |
| Graft type | ||||
| Bone marrow | 301 (93) | 641 (59) | 429 (57) | 212 (63) |
| Peripheral blood progenitor cells | 22 (7) | 445 (41) | 319 (43) | 126 (37) |
Values are n (%) unless otherwise noted.
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia; and CMV, cytomegalovirus.
Disease status was defined as early (first complete remission), intermediate (second or higher complete remission), and advanced (first relapse, second or higher relapse, primary induction failure).
KIR genotyping and haplotype group assignment
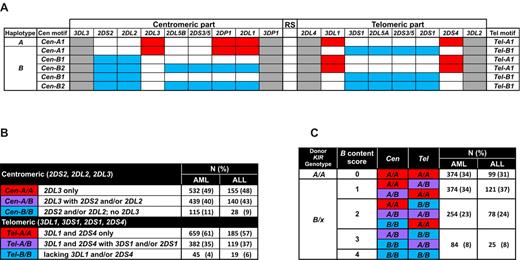
The presence or absence of 15 KIR genes (KIR3DL3, KIR2DS2, KIR2DL2, KIR2DL3, KIR2DL5A/B, KIR2DS3/2DS5, KIR2DP1, KIR2DL1, KIR3DP1, KIR2DL4, KIR3DL1, KIR3DS1, KIR2DS1, KIR2DS4, and KIR3DL2) was determined by the use of high-throughput analysis of single-nucleotide polymorphisms by mass spectrometry as described24 and applied.21 Donors were assigned the B/x or A/A genotype as defined previously.21,25 Genotypes for the centromeric (Cen) and telomeric (Tel) parts of the KIR locus were assigned according to the presence or absence of one or more B haplotype-defining KIR genes (Figure 1A). Thus, Cen-A1 and Tel-A1 are the centromeric and telomeric motifs, respectively, of the canonical A KIR haplotype; Cen-B1 and Cen-B2 are alternative centromeric motifs of common B KIR haplotypes, and Tel-B1 is the common centromeric motif of B haplotypes.26,27 For much of this analysis, Cen-B1 and Cen-B2 are grouped together as Cen-B, whereas Cen-A1 is shortened to Cen-A and Tel-A1 to Tel-A (Figure 1B).
We defined the KIR B–content score for each donor's KIR genotype as the number of centromeric and telomeric gene-content motifs containing B haplotype–defining genes. Permissible values for the KIR B–content score are 0, 1, 2, 3, and 4 (Figure 1C). A calculator for classification of the donor KIR B status (best, better, neutral) may be found at http://www.ebi.ac.uk/ipd/kir/.
Statistical analysis
Six measures of transplant outcome were considered: overall survival (OS) and disease-free survival (DFS), relapse, treatment-related mortality (TRM), and the incidence of both acute graft versus host disease (GVHD; grade II-IV) and chronic GVHD. OS and DFS were evaluated by the use of Kaplan-Meier curves; other outcomes were evaluated by the use of the cumulative incidence function. Unadjusted comparisons between KIR genotypes were made with the use of the log rank test on either the hazard rates for OS and DFS or the crude hazard rates for relapse, TRM, and chronic GVHD. For acute GVHD at 3 months, we used the pseudo-observation approach,28 which reduces to a logistic regression model when there is no censoring. In this transplant cohort the completeness of follow-up at 3 years was more than 99%, and 90% of the events had occurred.
Cox proportional hazards models29 were used to adjust for important clinical factors, including KIR genotype, HLA match, time from diagnosis to transplant, disease status at time of transplant, cytogenetic risk group (good, intermediate, poor), graft source, patient age, race, sex match, and Karnofsky performance score (KPS). Forward stepwise regression modeling was used to determine which factors required adjustment in each model on the basis of a significance level of 5%. All models included the A/B KIR genotype. Models for DFS and relapse in AML also included disease status, KPS, time from diagnosis to HCT, and, for DFS, high-resolution HLA matching and age required adjustment. Models for DFS and relapse in ALL included disease status, KPS, and graft source, and the DFS model required adjustment for age. Proportional hazards were checked in a time-dependent covariate model and graphically. No significant interactions were detected between the donor KIR genotype groups and any covariates for the outcomes of interest.
Results
Characteristics of the transplant recipients and donors studied
We studied 1409 myeloablative, T cell–replete URD transplants given to AML (n = 1086) or ALL (n = 323) patients performed between 1988 and 2006 (Table 1). The transplant recipients included patients with early, intermediate, and advanced disease. Approximately one-half of the donor-recipient pairs (AML: 50%, ALL: 46%) were 10/10 HLA-allele matched at HLA-A, B, C, DRB1, and DQB1, and one-half had some HLA mismatch. Transplant donors were typed for presence and absence of individual KIR genes (Figure 1A). From the genotypes we determined whether each donor was of A/A or B/x genotype. For the B/x donors we further determined whether their B haplotype genes were in the centromeric or telomeric part of the KIR locus, or in both (Figure 1B). From these data we calculated the KIR B–content score for each donor, which gives the total number of centromeric and telomeric motifs containing B haplotype genes (Figure 1C). There was no significant difference in the frequencies of KIR genes, haplotypes, or motifs between either the cohorts of ALL or AML transplant donors or with the white population, to which 72% of the donors belong.
The KIR locus comprises centromeric (Cen) and telomeric (Tel) gene content motifs. (A) The organization of genes in the KIR locus. The centromeric and telomeric regions are separated by a unique recombination site (RS) sequence that can function to reassort the centromeric and telomeric gene motifs. The gene content of the common motifs is shown. The conserved framework genes are shaded gray, B haplotype genes are blue, and A haplotype genes are red. (B) Groups used for the Cen and Tel analysis on the basis of the content of the inhibitory (L-long) or activating (S-short) KIR genes, and their frequencies among donors in the AML and ALL cohorts. (C) KIR B–content score and the frequency of donors in each group. The KIR B–content score is calculated by adding the number of Cen-B and/or Tel-B motifs in each genotype.
The KIR locus comprises centromeric (Cen) and telomeric (Tel) gene content motifs. (A) The organization of genes in the KIR locus. The centromeric and telomeric regions are separated by a unique recombination site (RS) sequence that can function to reassort the centromeric and telomeric gene motifs. The gene content of the common motifs is shown. The conserved framework genes are shaded gray, B haplotype genes are blue, and A haplotype genes are red. (B) Groups used for the Cen and Tel analysis on the basis of the content of the inhibitory (L-long) or activating (S-short) KIR genes, and their frequencies among donors in the AML and ALL cohorts. (C) KIR B–content score and the frequency of donors in each group. The KIR B–content score is calculated by adding the number of Cen-B and/or Tel-B motifs in each genotype.
Centromeric KIR genes of donor B haplotypes reduce relapse and improve survival of transplanted AML patients
By using multivariate models we analyzed the effect of donor KIR genotype on critical clinical outcomes after URD HCT for AML. As we reported previously,21 a protective effect of donor B/x genotype was observed for the cohort of AML patients (DFS: donor KIR B/x vs A/A: relative risk of relapse [RR] 0.85; 95% confidence interval [95% CI] 0.73-0.99; P = .04) but not for the ALL patients. This result indicates that one or more of the KIR genes or alleles restricted to group B haplotypes is associated with the protective effect in AML. The logical first step to identify the protective gene was to address the inherent heterogeneity within the group of transplant donors having the B/x genotype.
Three conserved, framework regions divide the KIR locus into similarly sized centromeric and telomeric segments that differ in gene content.20,27 As shown in Figure 1A, the A haplotype has invariant gene-content, comprising a centromeric A motif (Cen-A) and a telomeric A motif (Tel-A). In contrast, B haplotypes are of 3 distinct types: 1 combining a centromeric B motif (Cen-B) with a telomeric B motif (Tel-B); 1 combining Cen-B with Tel-A; and 1 combining Cen-A with Tel-B. These differences allowed us to compare the role of the centromeric and telomeric segments in the protective effect of donor B/x genotype in AML HCT (Table 2).
Multivariate analysis of relapse and disease-free survival
| . | AML patient transplantations . | ||||||
|---|---|---|---|---|---|---|---|
| n . | Relapse . | Disease-free survival . | |||||
| RR of relapse . | 95% CI . | P . | RR of relapse or death . | 95% CI . | P . | ||
| Donor KIR: A/A and B/x genotypes | |||||||
| Donor KIR genotype | |||||||
| KIR-A/A | 362 | 1.00 | 1.00 | ||||
| KIR-B/x | 696 | 0.72 | 0.58-0.89 | .003 | 0.85 | 0.73-0.99 | .04 |
| Donor KIR: centromeric and telomeric genotypes | |||||||
| Donor Cen genotype | |||||||
| Cen-A/A | 516 | 1.00 | 1.00 | ||||
| Cen-A/B | 427 | 0.87 | 0.70-1.09 | .20 | 0.94 | 0.81-1.10 | .45 |
| Cen-B/B | 115 | 0.34 | 0.20-0.57 | < .001 | 0.72 | 0.55-0.93 | .01 |
| Cen-B/A vs Cen-B/B | < .001 | .04 | |||||
| Donor Tel genotype | |||||||
| Tel-A/A | 643 | 1.00 | 1.00 | ||||
| Tel-A/B | 371 | 0.70 | 0.56-0.89 | .003 | 0.87 | 0.74-1.02 | .08 |
| Tel-B/B | 44 | 0.52 | 0.26-1.06 | .07 | 0.82 | 0.55-1.20 | .32 |
| Tel-A/B vs Tel-B/B | .45 | .77 | |||||
| Donor KIR B–content score | |||||||
| Donor KIR B motifs | |||||||
| 0 | 364 | 1.00 | 1.00 | ||||
| 1 | 366 | 0.93 | 0.73-1.19 | .56 | 0.94 | 0.79-1.11 | .45 |
| 2 | 242 | 0.54 | 0.39-0.74 | < .001 | 0.77 | 0.63-0.94 | .01 |
| 3 or 4 | 86 | 0.44 | 0.27-0.73 | < .001 | 0.76 | 0.56-1.01 | .06 |
| Donor KIR: B–content group | |||||||
| KIR B = 0 or 1 | 730 | 1.00 | 1.00 | ||||
| B ≥ 2 (Cen-A/x, Tel-B/x) | 213 | 0.64 | 0.48-0.86 | .003 | 0.84 | 0.70-1.01 | .07 |
| B ≥ 2 (Cen-B/B, Tel-x/x) | 115 | 0.33 | 0.20-0.55 | < .001 | 0.70 | 0.55-0.90 | .007 |
| Donor KIR genotype and HLA match | |||||||
| HLA-matched | |||||||
| B = 0 or 1 | 374 | 1.00 | 1.00 | ||||
| B ≥ 2 | 165 | 0.52 | 0.36-0.75 | < .001 | 0.80 | 0.62-1.02 | .07 |
| HLA-mismatched | |||||||
| B = 0 or 1 | 374 | 1.00 | 1.00 | ||||
| B ≥ 2 | 173 | 0.52 | 0.35-0.75 | < .001 | 0.78 | 0.63-0.97 | .03 |
| Disease status and HLA match | |||||||
| Disease status | |||||||
| Early | 286 | 1.00 | 1.00 | ||||
| Intermediate | 341 | 1.51 | 1.01-2.26 | .044 | 1.47 | 1.13-1.90 | .005 |
| Advanced | 431 | 2.99 | 2.25-3.98 | < .001 | 2.38 | 1.94-2.92 | < .001 |
| HLA match | |||||||
| 10/10 | 539 | 1.00 | 1.0 | ||||
| Less than 10/10 | 547 | 0.94 | 0.88-1.17 | .57 | 1.30 | 1.12-1.51 | < .001 |
| . | AML patient transplantations . | ||||||
|---|---|---|---|---|---|---|---|
| n . | Relapse . | Disease-free survival . | |||||
| RR of relapse . | 95% CI . | P . | RR of relapse or death . | 95% CI . | P . | ||
| Donor KIR: A/A and B/x genotypes | |||||||
| Donor KIR genotype | |||||||
| KIR-A/A | 362 | 1.00 | 1.00 | ||||
| KIR-B/x | 696 | 0.72 | 0.58-0.89 | .003 | 0.85 | 0.73-0.99 | .04 |
| Donor KIR: centromeric and telomeric genotypes | |||||||
| Donor Cen genotype | |||||||
| Cen-A/A | 516 | 1.00 | 1.00 | ||||
| Cen-A/B | 427 | 0.87 | 0.70-1.09 | .20 | 0.94 | 0.81-1.10 | .45 |
| Cen-B/B | 115 | 0.34 | 0.20-0.57 | < .001 | 0.72 | 0.55-0.93 | .01 |
| Cen-B/A vs Cen-B/B | < .001 | .04 | |||||
| Donor Tel genotype | |||||||
| Tel-A/A | 643 | 1.00 | 1.00 | ||||
| Tel-A/B | 371 | 0.70 | 0.56-0.89 | .003 | 0.87 | 0.74-1.02 | .08 |
| Tel-B/B | 44 | 0.52 | 0.26-1.06 | .07 | 0.82 | 0.55-1.20 | .32 |
| Tel-A/B vs Tel-B/B | .45 | .77 | |||||
| Donor KIR B–content score | |||||||
| Donor KIR B motifs | |||||||
| 0 | 364 | 1.00 | 1.00 | ||||
| 1 | 366 | 0.93 | 0.73-1.19 | .56 | 0.94 | 0.79-1.11 | .45 |
| 2 | 242 | 0.54 | 0.39-0.74 | < .001 | 0.77 | 0.63-0.94 | .01 |
| 3 or 4 | 86 | 0.44 | 0.27-0.73 | < .001 | 0.76 | 0.56-1.01 | .06 |
| Donor KIR: B–content group | |||||||
| KIR B = 0 or 1 | 730 | 1.00 | 1.00 | ||||
| B ≥ 2 (Cen-A/x, Tel-B/x) | 213 | 0.64 | 0.48-0.86 | .003 | 0.84 | 0.70-1.01 | .07 |
| B ≥ 2 (Cen-B/B, Tel-x/x) | 115 | 0.33 | 0.20-0.55 | < .001 | 0.70 | 0.55-0.90 | .007 |
| Donor KIR genotype and HLA match | |||||||
| HLA-matched | |||||||
| B = 0 or 1 | 374 | 1.00 | 1.00 | ||||
| B ≥ 2 | 165 | 0.52 | 0.36-0.75 | < .001 | 0.80 | 0.62-1.02 | .07 |
| HLA-mismatched | |||||||
| B = 0 or 1 | 374 | 1.00 | 1.00 | ||||
| B ≥ 2 | 173 | 0.52 | 0.35-0.75 | < .001 | 0.78 | 0.63-0.97 | .03 |
| Disease status and HLA match | |||||||
| Disease status | |||||||
| Early | 286 | 1.00 | 1.00 | ||||
| Intermediate | 341 | 1.51 | 1.01-2.26 | .044 | 1.47 | 1.13-1.90 | .005 |
| Advanced | 431 | 2.99 | 2.25-3.98 | < .001 | 2.38 | 1.94-2.92 | < .001 |
| HLA match | |||||||
| 10/10 | 539 | 1.00 | 1.0 | ||||
| Less than 10/10 | 547 | 0.94 | 0.88-1.17 | .57 | 1.30 | 1.12-1.51 | < .001 |
AML indicates acute myelogenous leukemia; 95% CI, 95% confidence interval; HLA, human leukocyte antigen; and RR, relative risk.
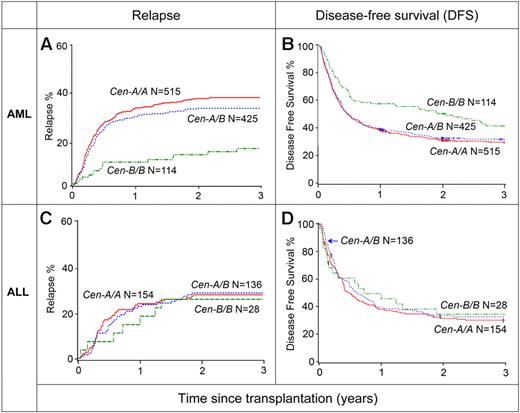
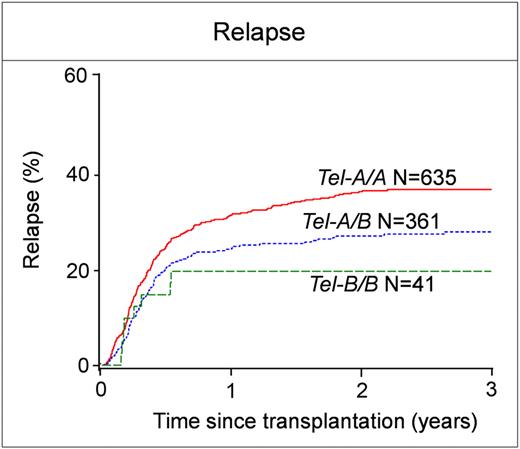
The analysis showed that the B haplotype genes of the centromeric region had a stronger effect in improving the outcome of transplantation than those of the telomeric region. For AML, donors having B genes in the centromeric region of both KIR haplotypes (Cen-B/B genotype) were associated with a striking decrease in the incidence of relapse compared with either Cen-A/A or Cen-A/B donors (Cen-B/B vs Cen-A/A: RR 0.34; 95% CI 0.2-0.57; P < .001); with absolute relapse rates of only 15.4% (Cen-B/B) versus 36.5% (Cen-A/A; Figure 2A; Table 2). The advantage of a Cen-B/B donor also was seen in improved OS (data not shown) and DFS (Cen-B/B vs Cen-A/A: RR of relapse or death 0.72; 95% CI 0.55-0.93; P = .01; Figure 2B). A partial contribution of B haplotype genes in the telomeric regions is also suggested by the reduced relapse associated with donors having Tel-A/B or Tel-B/B genotype, compared with Tel-A/A (Figure 3; Table 2). However, the effect did not result in significantly increased DFS (Table 2). Donor KIR genotype did not have any significant effect on rates of TRM, grade II-IV or III-IV acute GVHD or chronic GVHD (data not shown). No protection was observed for ALL patients, for whom relapse (Cen-B/B vs Cen-A/A: RR 0.97; 95% CI 0.43-2.16; P < .94; Figure 2C), DFS (Cen-B/B vs Cen-A/A: RR 0.99; 95% CI 0.61-1.62; P < .97; Figure 2D), and all other outcomes of transplantation (data not shown) were unaffected by the KIR genes of the transplant donor for ALL. These negative results with ALL emphasize the specificity and importance of the AML effect.
Specific reduction in relapse and improvement in DFS from donors with Cen-B/B after transplantation for AML but not ALL. Donors were assigned Cen-A/A, Cen-A/B, and Cen-B/B genotypes. Top, the incidence of relapse (A) and probability of DFS (B) for AML patients. Bottom, the incidence of relapse (C) and probability of DFS (D) are shown for ALL patients on the basis of their donor Cen genotype group.
Specific reduction in relapse and improvement in DFS from donors with Cen-B/B after transplantation for AML but not ALL. Donors were assigned Cen-A/A, Cen-A/B, and Cen-B/B genotypes. Top, the incidence of relapse (A) and probability of DFS (B) for AML patients. Bottom, the incidence of relapse (C) and probability of DFS (D) are shown for ALL patients on the basis of their donor Cen genotype group.
Donors with Tel-B/B contribute to reduction in relapse after transplantation for AML. Donors were assigned Tel-A/A, Tel-A/B, and Tel-B/B genotypes. The incidence of relapse is shown for the respective groups.
Donors with Tel-B/B contribute to reduction in relapse after transplantation for AML. Donors were assigned Tel-A/A, Tel-A/B, and Tel-B/B genotypes. The incidence of relapse is shown for the respective groups.
Donors having KIR B–content scores of 2 or greater protect against relapse and improve DFS
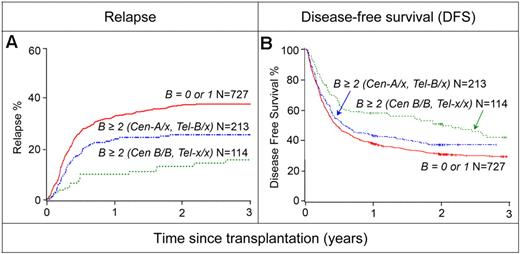
We next examined how the number of KIR B gene motifs, irrespective of their centromeric or telomeric origin, influenced relapse and DFS (Table 2). Although there was little difference in outcome when donors had a KIR B–content score (the total number of B-associated domains) of 0 or 1, donors with a score of 2 or greater provided significantly better relapse protection. Within this KIR B–content score group (ie, > 2), the reduction in relapse (Figure 4A) and the increase of DFS (Figure 4B) were greater when the donor was homozygous for Cen-B (relapse: RR 0.33; 95% CI 0.20-0.55; P < .001 and DFS: RR 0.70; 95% CI 0.55-0.90; P = .007), further demonstrating the major role played by centromeric region B genes. However, not all of the favorable effect is determined by Cen-B homozygosity because donors having a KIR B–content score of 2 or greater and at least 1 copy of Cen-A also achieved significant protection against relapse (RR 0.64; 95% CI 0.48-0.86; P = .003). As shown in Figure 4A, the cumulative incidence curves for relapse for these donors lie between and are clearly separated from the curves for donors with a KIR B–content score of 0 or 1 and Cen-B homozygous donors. This led to improved DFS (Figure 4B). A similar beneficial effect of donor KIR B–content scores 2 or greater was observed for OS (Figure 5E-F). Further subsetting the Cen-B/B donors on the basis of gene content into Cen B1/B1 and Cen B2/x groups revealed similar levels of relapse protection, with 10.8% versus 18.5% relapse rates, respectively. This finding suggests that relapse protection is associated with 2DL2/2DS2 (present in both groups) rather than the presence of 2DL5 and/or 2DS3/5 (only in B2; Figure 1A). Furthermore, compared with donors with Cen-A/A, Tel-A/A, a decreased relative risk of relapse is observed in both the Cen-B/B, Tel-A/A (0.23, P = .001, n = 52) and Cen-A/A, Tel-B/B (0.43, P = n.s., n = 14) donor groups, suggesting that telomeric KIR B genes also contribute to the overall relapse protection and survival benefit associated with KIR B/x donors.
Protection against relapse and improved DFS on the basis of donor KIR B–content groups in AML. The AML cohort was evaluated for relapse (A) and DFS (B) on the basis of donor KIR B content by use of the indicated groups. On the basis of this analysis, donor KIR B–content groups are divided as: (1) “best” with a KIR B–content score of more than 2 where the KIR haplotype is Cen-B/B, Tel-x/x (defined as 2DL3 absent, 2DS2 and/or 2DL2 present); (2) “better” with a KIR B–content score of more than 2 and the KIR haplotype is Cen-A/x, Tel-B/x (defined as 2DL3 present, 2DS2 and/or 2DL2 present, 3DS1 and/or 2DS1 present or 2DL3 present, 2DS2 and/or 2DL2 absent, and 3DS1 and/or 2DS1 present); or (3) “neutral” with a KIR B–content score of 0 or 1 (defined as 2DL3 present, 2DS2 and/or 2DL2 absent, 3DL1 and 2DS4 present).
Protection against relapse and improved DFS on the basis of donor KIR B–content groups in AML. The AML cohort was evaluated for relapse (A) and DFS (B) on the basis of donor KIR B content by use of the indicated groups. On the basis of this analysis, donor KIR B–content groups are divided as: (1) “best” with a KIR B–content score of more than 2 where the KIR haplotype is Cen-B/B, Tel-x/x (defined as 2DL3 absent, 2DS2 and/or 2DL2 present); (2) “better” with a KIR B–content score of more than 2 and the KIR haplotype is Cen-A/x, Tel-B/x (defined as 2DL3 present, 2DS2 and/or 2DL2 present, 3DS1 and/or 2DS1 present or 2DL3 present, 2DS2 and/or 2DL2 absent, and 3DS1 and/or 2DS1 present); or (3) “neutral” with a KIR B–content score of 0 or 1 (defined as 2DL3 present, 2DS2 and/or 2DL2 absent, 3DL1 and 2DS4 present).
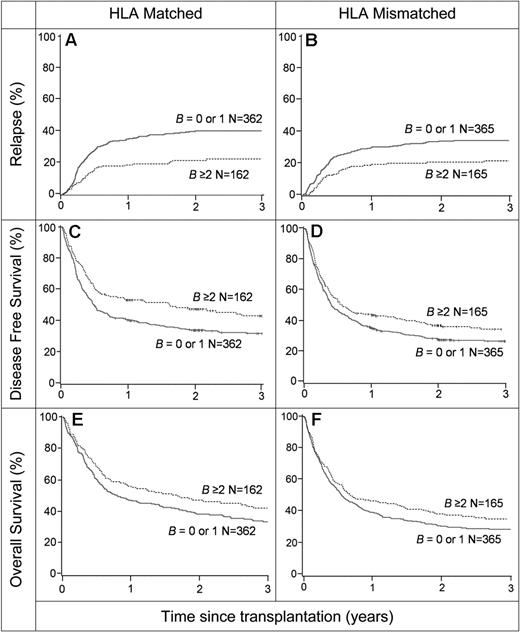
Selection of donors with KIR B–content scores of 2 of greater has significant potential to improve the outcome of HLA-matched and HLA-mismatched HCT. For patients transplanted for AML, the incidence of relapse (A-B), the probability of DFS (C-D) and the probability of overall survival (E-F) are shown. Panels A, C, and E show the data from HLA-matched transplants, and panels B, D, and F show the data from HLA-mismatched transplants. Comparison is made between donors with a KIR B–content score of 0 or 1 (374 matched and 374 mismatched) and donors with a KIR B–content score of 2 or greater (162 matched and 165 mismatched).
Selection of donors with KIR B–content scores of 2 of greater has significant potential to improve the outcome of HLA-matched and HLA-mismatched HCT. For patients transplanted for AML, the incidence of relapse (A-B), the probability of DFS (C-D) and the probability of overall survival (E-F) are shown. Panels A, C, and E show the data from HLA-matched transplants, and panels B, D, and F show the data from HLA-mismatched transplants. Comparison is made between donors with a KIR B–content score of 0 or 1 (374 matched and 374 mismatched) and donors with a KIR B–content score of 2 or greater (162 matched and 165 mismatched).
Donor selection for favorable KIR-B gene motifs improves outcomes in HLA-matched or HLA-mismatched URD HCT for AML
Our study shows that the outcome of allogeneic transplantation for AML is influenced by the donor's KIR genotype in a manner that appears insensitive to the HLA type of either donor or recipient. Benefit accrues with increasing number of B haplotype genes, with the centromeric genes exerting a greater effect than the telomeric genes. These results imply that the success of transplantation for AML could be improved by selecting donors on the basis of their KIR type as well as their HLA match by aiming to obtain the best HLA-matched donors that have KIR B–content score 2 or greater.
To evaluate the potential impact of such donor selection on URD HCT, we compared the outcome for transplants where the donor KIR B–content score was 0 or 1 with transplants in which the donor KIR B–content score was 2 or greater (Figure 5). This retrospective analysis also compared the effects of donor KIR B content for transplants that were fully HLA matched and transplants that were HLA mismatched. Significant and similar reductions in relapse (Figure 5A-B), along with improved DFS (Figure 5C-D) and OS (Figure 5E-F) were conferred by a donor KIR B–content score of 2 or greater for HLA-matched (Figure 5A,C,E) and mismatched (Figure 5B,D,F) transplants. Selecting for transplant donors with a KIR B–content score of 2 or greater has potential to reduce the risk of relapse by 50% and the relative risk of relapse or death by 20% (Table 2).
Discussion
For patients undergoing URD transplantation, the preferred donor is matched at the allele level for HLA class I and II.4,5 Even then, the risk of relapse is high (21%-43% for early or advanced AML), and DFS rates of 13% to 38% are the norm.30 Here, we investigated the contribution of a second immunogenetic system, the KIR gene family,18 on the outcome of transplantation for AML. Of the 2 KIR haplotype groups, donor B haplotypes yield significantly superior protection against relapse and improved DFS compared with donor A haplotypes. Clinical benefit increases with the number of B-specific gene content motifs, particularly with homozygosity for those in the centromeric part of the KIR locus (Cen-B/B). Similar benefits were observed for patients receiving fully HLA-matched or partly mismatched grafts. Emphasizing the specificity of the effect, donor KIR genotype was not correlated with the outcome of transplantation for ALL.
The clinical benefit conferred by Cen-B/B could be caused by presence of KIR2DS2 and KIR2DL2, absence of KIR2DL3, or by the combination of these 2 factors. A possible advantage of the activating KIR2DS2, which has no detectable avidity for HLA class I,31,32 is that it may recognize a different type of ligand present on AML but not ALL cells. A possible advantage to KIR2DL2 is that it binds both the C1 and C2 epitopes of HLA-C with greater avidity than KIR2DL3.33 Because NK-cell education through interaction between MHC class I ligands and cognate inhibitory receptors determines the strength of NK-cell responsiveness,34,35 the stronger avidity of KIR2DL2 than KIR2DL3 could educate NK cells that are more effective at eliminating residual AML cells.
The fact that Cen-B heterozygosity has a beneficial effect much less than one-half of that achieved by Cen-B homozygosity (Figure 2A-B) raises the possibility that a negative effect caused by the KIR2DL3 component of Cen-A is the causative mechanism rather than a positive effect caused by the KIR2DS2 and/or KIR2L2 of Cen-B/B. In the setting of acute hepatitis C virus infection, 2 copies of Cen-A were necessary to achieve a strong improvement in the response to infection.36 Here, we find the opposite effect, namely that complete absence of Cen-A is required for strong improvement in the response to AML.
We should also consider the possibility that the clinical benefit associated with Cen-B/B homozygosity is not necessarily attributable to KIR2DS2, KIR2DL2, or KIR2DL3. The strong linkage disequilibrium between KIR2DS2 and KIR2DL212 extends into the 3′ exons of the highly polymorphic framework gene, KIR3DL3 for which ligands and functions are unknown.37,38 Consequently, genetic analysis alone is unlikely to distinguish the contribution of individual Cen-B genes. Functional studies will be required to define the mechanism by which NK cells reconstituting from the transplanted hematopoietic stem cells of Cen-B/B donors protect against relapse in AML.
NK cells are the first lymphocyte population to expand after HCT and engraftment with alloreactive NK cells has many potential benefits, including (1) decreased rates of GVHD,39 (2) decreased rates of graft rejection mediated by NK lysis of host T cells, (3) decreased relapse,40 (4) improved engraftment mediated by NK-cell release of hematopoietic cytokines,41 and (5) enhanced immune reconstitution and decreased infectious complications mediated by NK-cell antiviral activity. After haploidentical transplantation for AML, beneficial alloreactive NK cells that reduce relapse are generated when the HLA class I type of the donor includes a KIR ligand that the recipient lacks.15 This observation stimulated many studies that examined the effect of HLA mismatches that involved KIR ligands in transplant outcome.16,17,42,43 Additional reports demonstrate an association between KIR ligand mismatch and favorable clinical outcomes in myeloid malignancies, especially when T cells are depleted in vivo with antithymocyte globulin,16 whereas others have found no benefit.44 Beneficial effects from KIR-ligand mismatch have not been seen in the T cell–replete setting.45,46
Here, in contrast, we have focused on donor KIR gene variability, demonstrating a clinically significant influence on the success of transplantation for AML. Previously we have shown that the use of donors with KIR B haplotypes, who express more activating KIR, was associated with significant improvements in overall and relapse-free survival after T cell–replete, unrelated donor HCT for AML, with more than a 30% better relative risk in both these end points.21 The benefit of donor KIR B haplotypes also has been observed for T cell–replete transplants from HLA-matched sibling donors,47 an effect reported in other settings.22,23 The authors of numerous other studies have reported varied effects of activating KIR on outcomes after various types of HCT, including increased rates of acute GVHD,25,48,49 or protection against acute GVHD.50 KIR B haplotypes are present in two-thirds of unrelated donors. Therefore, identification of the specific beneficial gene content is necessary to select which of those KIR B donors will provide the most relapse protection and to develop a donor selection strategy that translates into survival benefit.
Our results show how KIR genotyping could be used to supplement HLA typing to improve transplant outcome for AML by allowing the selection of donors with favorable KIR types. On the basis of gene content alone we can sort donors into 3 groups by their composition of their KIR B haplotype-defining genes. The donor KIR B–content groups are easily defined as (1) “best,” (2) “better,” or (3) “neutral” when the specific definitions applied in Figure 4 are used. On the basis of the KIR gene frequencies in this sample from the NMDP donor registry, we estimate that by KIR genotyping 3 of the best HLA-matched donors the ability to select donors with favorable KIR B genotypes (KIR B–content score ≥ 2) will be increased from the current, random rate of 31% to a rate of 67%. Similarly, the availability of Cen-B/B donors for selection would increase from 11% to 31%. This could be achieved by adding KIR genotyping to the confirmatory HLA genotyping now performed on donor DNA samples. Inexpensive KIR genotyping methods are available in many HLA typing laboratories, and the necessary KIR typing could be completed using the sample sent for confirmatory typing. Thus, the addition of KIR genotyping would not cause any delays in the search time (formal search to transplant), which currently takes a median time of 12 weeks (95% CI 6-23 weeks). Overall, a 22% decrease in relapse is predicted by applying this donor selection strategy.
This analysis, determined by current understanding of the structure of the KIR gene locus, has identified donors with the Cen-B genes as conferring the most relapse protection and survival benefit. We have developed a simple algorithm on the basis of donor KIR B gene content that can be used today to identify unrelated donors who will provide the most protection against AML relapse in T cell–replete transplants. A prospective clinical trial selecting the best HLA-matched donors with favorable KIR B genotypes will begin next year.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank James Robinson of the Anthony Nolan Research Institute and University College London for developing the online KIR haplotype calculator.
This work was supported by National Institutes of Health/NCI grant P01 111412. The Center for International Blood and Marrow Transplant Research is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); 2 grants, N00014-06-1-0704 and N00014-08-1-0058, from the Office of Naval Research; and others. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
National Institutes of Health
Authorship
Contribution: S.C., D.J.W., L.A.G., P.P., and J.S.M. designed this study, and analyzed data; J.P.K., T.W., and C.T.L. performed the biostatistical analysis for this study; S.S. and M.D.H. identified DNA samples, patient cohorts, and provided clinical data integration; S.G.E.M. and D.G. interpreted data and were involved in analysis; M.L. and E.T. performed all KIR genotyping and were involved in data analysis; and all authors wrote the manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, MD, Professor of Medicine, University of Minnesota Cancer Center, MMC 806, Division of Hematology, Oncology, and Transplantation, Harvard Street at East River Rd, Minneapolis, MN 55455; e-mail: mille011@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal