Abstract
To determine the role of vascular endothelial growth factor (Vegf) in embryonic erythroid development we have deleted or overexpressed Vegf specifically in the erythroid lineage using the EpoR-iCre transgenic line in combination with Cre/loxP conditional gain and loss of function Vegf alleles. ROSA26 promoter-based expression of the Vegf164 isoform in the early erythroid lineage resulted in a differentiation block of primitive erythroid progenitor (EryP) development and a partial block in definitive erythropoiesis between the erythroid burst-forming unit and erythroid colony-forming unit stages. Decreased mRNA expression levels of the key erythroid transcription factor Gata1 were causally linked to this phenotype. Conditional deletion of Vegf within the erythroid lineage was associated with increased Gata1 levels and increased erythroid differentiation. Expression of a ROSA26-based GATA2 transgene rescued Gata1 mRNA levels and target genes and restored erythroid differentiation in our Vegf gain of function model. These results demonstrate that Vegf modulates Gata1 expression levels in vivo and provides new molecular insight into Vegf's ability to modulate erythropoiesis.
Introduction
Erythrocytes or red blood cells (RBCs) fulfill the essential functions of facilitating gas exchange in the lungs and meeting tissue oxygen demands. They are continuously renewed from undifferentiated, self-generating cells throughout life in a tightly controlled growth process termed erythropoiesis. This process is part of the larger hematopoietic program that is defined by the production of all blood cells from hematopoietic stem cells (HSCs). These cells originate from different embryonic sites during development and are generated in close association with the vascular endothelium. The first hematopoietic cells detected during mouse embryonic development are the primitive erythroid cells of the yolk sac (YS) that are produced from precursors present in this tissue between embryonic days E7.0 and E8.25 and enter the circulation at E9.0. The definitive progenitors, that contain cells from the YS and the aorta-gonado-mesonephros (AGM) region,1 reach the liver at E10.5 and give rise to the first definitive progenitors which subsequently colonize the bone marrow and spleen in the adult mouse.2
Vascular endothelial growth factor (Vegf) is a secreted growth factor that mediates its biologic effects predominately by binding to either one of two transmembrane tyrosine kinase receptors, Vegfr-1(Flt1) and Vegfr-2(Flk1 or Kdr) as well as to the coreceptor Neuropilin1 (Nrp1). VEGF/VEGFR signaling pathways are essential for vascular and hematopoietic cell development. Indeed, heterozygous Vegf (+/−) deficient embryos are devoid of most endothelial and hematopoietic cells in the blood islands and die around E10 as a consequence of severe cardiovascular abnormalities.3,4 Gain of function studies showed that ubiquitous inducible expression of VEGF during early development leads to a paucity of mature RBCs.5
During erythropoiesis in the developing YS Vegf is expressed in the YS mesoderm and extraembryonic visceral endoderm.6 Using diploid and tetraploid embryo complementation approaches in conjunction with a Vegf hypomorphic allele, the importance of VEGF from the extraembryonic visceral endoderm in blood island formation has previously been demonstrated.7 In addition to these sources, VEGF is also expressed in committed erythroid progenitors and proerythroblasts.8,9 The Vegf receptor Flk1 is required during embryonic and adult hematopoiesis.10,11 Molecular synergism exists between Flk1 and the transcriptional regulators Tal1 and Runx1 for hematopoietic cell fate decisions from mesodermal progenitors.12 Flk1−/− embryonic stem (ES) cells do not contribute to primitive hematopoiesis in chimeric YSs or definitive hematopoiesis in adult chimeras due to a failure of Flk1 null hemangioblast progenitors to migrate into the primitive streak region of the embryo and further participate in vessel and hematopoietic cell development.10 In addition, Flk1-deficient immature erythroid progenitors derived from mouse ES cell cultures showed enhanced spontaneous differentiation resulting in a strong accumulation of mature, hemoglobinized erythrocytes.13 Previous loss of function studies in the adult have demonstrated a cell autonomous autocrine role of Vegf in both HSC14 and endothelial survival.15 Antiangiogenic tumor therapies aimed at Vegf inhibition (using either a Vegf-TRAP that sequesters Vegf protein, DC101-a monoclonal anti-Flk1 antibody, or ZD4190- a VEGFR inhibitor) results in enhanced terminal erythroid differentiation and increased hematocrit.16 The cell autonomous role of Vegf during erythropoiesis is still poorly understood because of its pleiotropic effects and the lethality observed during gestation associated with even mild alterations in Vegf levels.3,4,17
The hematopoietic factors Gata1 and Gata218 as well as several other transcriptional regulators, such as Fog1 (Friend of Gata1 or Zfpm1) or Tal1 (Scl), are essential for normal erythropoiesis (for review see Tsiftsoglou19 ). Gata2 is crucial for the maintenance and proliferation of immature hematopoietic progenitors,20 whereas Gata1 is essential for the survival of erythroid progenitors as well as the terminal differentiation of erythroid cells.21 Gata2 knockout mice die around E10.5 due to a severe reduction of primitive erythroid cells and Gata2−/− ES cells fail to contribute to the production of all hematopoietic lineages in chimeric mice, indicating that Gata2 also plays an important role in definitive hematopoiesis.20 Absence of Gata1 expression results in proerythroblast apoptosis22,23 and is embryonic lethal between day E10.5 and E11.524 whereas overexpression of Gata1 leads to embryonic lethality around E12.5 to E13.5 because of a block in erythroid terminal differentiation.25 These two transcription factors do not work independently and cross-regulatory mechanisms exist by which Gata1 controls the expression of Gata2 and vice versa,18,26,27 via the binding to several Gata sites present in the promoter regions of these genes. This cross-regulation between Gata1 and Gata2 allows controlled progression of erythroid progenitor differentiation between erythroid burst-forming unit (BFU-E) and erythroid colony-forming unit (CFU-E) stages.27
To elucidate the autocrine role of Vegf during erythropoiesis we have specifically modulated its expression in the erythroid lineage in vivo by intercrossing the erythroid-specific iCre line (EpoR-iCre28 ) with Vegf conditional loss and gain of function mouse lines. We observed that alterations of Vegf levels during development specifically within erythroid cells significantly influences erythropoietic lineage development by modulating Gata1 expression levels and Gata1 target genes. Our findings present novel cellular and molecular understanding concerning Vegf's ability to modulate erythropoiesis in the developing mouse embryo.
Methods
Generation of transgenic mice and experimental set-up
The pROSA26-VEGF16429 and pROSA26-hGata230 DNA constructs used to conditionally target the murine Vegf164 isoform and human GATA2 cDNA to the ROSA26 locus were described previously.29,30 The Flk1 (flox); Vegf (flox); Flk1 (LacZ), as well as EpoR-iCre, tet(o)-VEGF164 and ROSA26-rtTA mouse lines; combined here to generate triple transgenic +VEGF164Eryth-dox mice, have all been previously described.5,28,31-33 Controls for +VEGF164Eryth-dox embryos were double transgenic littermates (EpoR-iCre+; ROSA26-Flox/Stop-rtTA-EGFP or ROSA26-Flox/Stop-rtTA-EGFP; tet(o)-VEGF164). Doxycycline (Sigma-Aldrich) was supplemented at 1 mg/mL in the drinking water (changed daily) for 2 days. All experiments performed on mice were approved by the animal ethical committee of Ghent University.
Molecular analysis
YSs were dissected and snap-frozen in liquid nitrogen, and processed according to standard protocols. RNA was extracted with RNeasy Plus mini kit columns (QIAGEN), treated with DNaseI at 37°C for 10 minutes and purified using RNeasy clean up mini column (QIAGEN). cDNA synthesis (Roche) was performed starting from equal amounts of RNA. Quantitative reverse-transcription–polymerase chain reaction (qRT-PCR) was performed on a LightCycler 480 system (Roche) using the SYBR Green I Master kit (Applied Biosystems). Gene expression was normalized using β-actin and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as controls. Primers used are indicated in supplemental Table 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Enzyme-linked immunosorbent assay
Vegf levels were determined by the Quantikine ELISA kit (R&D) according to manufacturer's protocol. Protein extracts were obtained from individual YSs and fetal livers after 3 cycles of freezing and thawing in phosphate-buffered saline (PBS) and several passages through a 25G needle.
In vitro hematopoietic progenitor assays
For primitive erythroid (EryP) colony assays, E8.5 whole embryos were treated with trypsin (Invitrogen) for 5 minutes at 37°C, and washed with 5% fetal calf serum (FCS)–PBS. Cells were plated in 1% methylcellulose/Iscove Modified Dulbecco Medium supplemented with 10% plasma-derived serum (Animal Technologies), 5% CD hybridoma medium (Invitrogen), 12.5 μg/mL ascorbic acid (Sigma-Aldrich), 2 mM l-glutamine, 10 μg/mL transferrin (Sigma-Aldrich), 450 μM MTG (Sigma-Aldrich), 5 ng/mL Stem Cell Factor, and 4 U/mL erythropoietin (R&D Systems). EryP colonies were blindly counted after 3 days of culture. Definitive erythroid colony assays were performed on E10.5 YSs after digestion with 0.1% collagenase (Sigma-Aldrich) in PBS, 10% FCS for 20 minutes at 37°C. The total numbers of dissociated cells from the YS, fetal livers, and embryos of the different genotypes were not significantly different from control littermates (data not shown). Cells were plated at 1 × 105 cells/plate for YS or 2 × 104 cells/plate for fetal livers, in 1% methylcellulose (StemCell Technologies) containing Epo only (MethoCult M3334) or containing complete recombinant cytokines (MethoCult GF M3434) for the detection and quantification of CFU-E and BFU-E, respectively. Methylcellulose medium was supplemented with extra erythropoietin (100 U/mL final) where indicated. After 3 (CFU-E) and 7 (BFU-E) days colonies were scored under a microscope. The results are expressed as a percentage of the control's absolute number of colonies per organ.
Paraffin histology
Dissected samples were fixed overnight in 4% paraformaldehyde at 4°C, processed for paraffin embedding, and sectioned at 5 μm. Sections were stained with hematoxylin and eosin (HE). Additional paraffin sections were used for immunohistochemistry (IHC) with the following antibodies: Cd31 (Pecam-1, dilution 1:500; #550274; BD Pharmingen), anti–cleaved Caspase 3 (Asp175; dilution 1:500; #9661; Cell Signaling Technology). LacZ-stainings were done as previously described.29 Briefly, embryos were fixed at 4°C in 2% formaldehyde, whole-mount X-gal stained overnight at 37°C, post fixed in 4% paraformaldehyde, paraffin-embedded and sectioned (6-7 μm). Sections were counterstained with eosin.
Flow cytometric analysis
For flow cytometry (FACS), cells from E10.5 YS were stained with PECAM-PE-Cy7 (dilution 1:200; 25-0311-81; eBioscience). Cells were analyzed by ARIA II and FloJo Version 8.86 software (Becton Dickinson). Dead cells were determined by DAPI staining.
Imaging
Embryos were imaged on a Leica MS5 (Leica Microsystems) stereomicroscope. Digital images were acquired using a Canon PowerShot S70 camera and Canon ZoomBrowser EX Version 5.0 software (Canon). Hematoxylin eosin staining and immunohistochemistry were imaged using a SNAPCOOL camera (Roper Scientific) mounted on an Olympus Bx51 microscope (Olympus), with Plan Olympus 20×/0.40 or 40×/0.65 lens and RSImage Version 1.9.2 software (Roper Scientific).
Statistical analysis
Data were expressed as mean plus or minus SEM. Comparison between 2 data groups was done by the 2-sided Student t test.
Results
Effect of erythroid-specific Vegf expression modulation
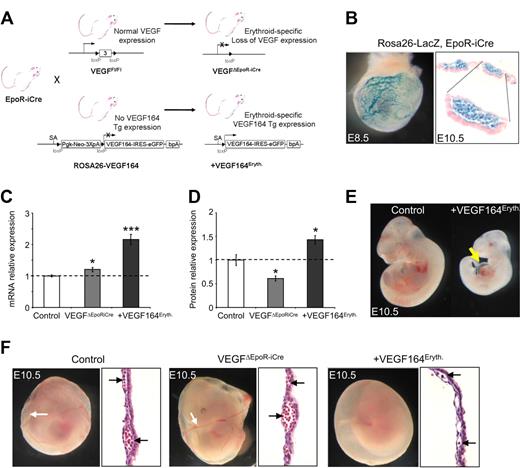
To specifically address the role of Vegf in erythropoiesis, we inter-crossed conditional gain of function ROSA26-VEGF164 mice29 or mice carrying the conditional loss of function Vegf allele31 with the erythroid-specific EpoR-iCre mouse line where the iCre transgene was specifically targeted to the endogenous erythropoietin receptor (Epor) locus28 (Figure 1A). Epor expression has previously been observed in E8.0 blood island primitive erythroid progenitors (EryP)34 and increases during definitive erythropoiesis at the BFU-E stage reaching maximal levels at the CFU-E/ proerythroblast stage. Upon maturation to the late erythroblast stage, Epor expression declines.35 ROSA26-LacZ reporter analysis confirmed the EpoR-iCre33 activity specifically in erythroid cells in YS vasculature and blood islands at E8.5 and E10.5 (Figure 1B). Therefore, the in vivo studies have been performed at a point in erythroid differentiation when Vegf is normally expressed within the BFU-E population until the mature erythroblast stage.8,9
Erythroid-specific Vegf expression modulation. (A) Strategy used to conditionally express the Vegf164 isoform from the ROSA26 locus or to delete all vascular endothelial growth factor (Vegf) isoforms specifically in the erythroid lineage. (B) ROSA26-LacZ reporter analysis of whole-mount X-gal stained embryonic day E8.5 embryo and sections from X-Gal stained blood island of E10.5 yolk sac (magnification: top right panel, 200×; bottom right panel, 400×) showing X-gal staining limited to circulating erythroid-lineage cells. (C) Relative Vegf mRNA levels measured by quantitative reverse-transcription–polymerase chain reaction (qRT-PCR) in E10.5 yolk sacs. (D) Vegf protein levels measured by enzyme-linked immunosorbent assay (ELISA) in E10.5 yolk sac. Bars represent mean ± SEM; *P < .05, ***P < .001. (E) Constitutive erythroid Vegf overexpression in +Vegf164Eryth mice leads to heart edema (arrow) at E10.5. (F) Embryonic phenotypes of erythroid-specific VEGF164 expression modulation. Control embryo (left), VegfΔEpoR-iCre embryo (middle), +VEGF164Eryth embryo (right). Erythroid Vegf overexpression leads to red blood cell (RBC) deficiency in yolk sacs by E10.5 compared with control and VEGFΔEpoR-iCre embryos (black arrows). Sections of E10.5 yolk sac are represented and black arrows show circulating cells (magnification, 200×).
Erythroid-specific Vegf expression modulation. (A) Strategy used to conditionally express the Vegf164 isoform from the ROSA26 locus or to delete all vascular endothelial growth factor (Vegf) isoforms specifically in the erythroid lineage. (B) ROSA26-LacZ reporter analysis of whole-mount X-gal stained embryonic day E8.5 embryo and sections from X-Gal stained blood island of E10.5 yolk sac (magnification: top right panel, 200×; bottom right panel, 400×) showing X-gal staining limited to circulating erythroid-lineage cells. (C) Relative Vegf mRNA levels measured by quantitative reverse-transcription–polymerase chain reaction (qRT-PCR) in E10.5 yolk sacs. (D) Vegf protein levels measured by enzyme-linked immunosorbent assay (ELISA) in E10.5 yolk sac. Bars represent mean ± SEM; *P < .05, ***P < .001. (E) Constitutive erythroid Vegf overexpression in +Vegf164Eryth mice leads to heart edema (arrow) at E10.5. (F) Embryonic phenotypes of erythroid-specific VEGF164 expression modulation. Control embryo (left), VegfΔEpoR-iCre embryo (middle), +VEGF164Eryth embryo (right). Erythroid Vegf overexpression leads to red blood cell (RBC) deficiency in yolk sacs by E10.5 compared with control and VEGFΔEpoR-iCre embryos (black arrows). Sections of E10.5 yolk sac are represented and black arrows show circulating cells (magnification, 200×).
Compared with control littermates, mutant EpoR-iCre+; ROSA26-VEGF164 (designated +VEGF164Eryth) embryos showed a 2-fold increased Vegf mRNA (Figure 1C) and 40% increase in Vegf protein levels (Figure 1D) in their YSs. In this system, erythroid-specific overexpression of the VEGF164 isoform led to embryonic lethality around E10.5. Before this stage, the embryos showed some mild heart edema along with growth retardation (Figure 1E) and a dramatic reduction of RBC development in the YS (Figure 1F), with the vasculature and blood islands nearly devoid of RBCs (Figure 1F). Whole mount Pecam and X-gal staining (after breeding in the Flk1-lacZ reporter allele for in situ blood vessel visualization) revealed relatively normal cephalic vascular development in the embryo and proper but somewhat delayed vascular remodeling and maturation of the YS vasculature. Flow cytometric analysis demonstrated overall normal numbers of Pecam positive cells in the YS, compared with control littermates (supplemental Figure 1).
Conditional erythroid-specific deletion of VEGF (EpoR-iCre+, VEGFfl/fl31 embryos, designated VEGFΔEpoR-iCre) led to a 30% decrease in Vegf protein levels (Figure 1D) in the YS and a mild but significant (P < .05) 20% increase in Vegf mRNA levels that may be indicative of compensatory up-regulation of Vegf expression in other YS tissues (Figure 1C). VEGFΔEpoR-iCre embryos showed no obvious phenotype, no signs of RBC deficiency in the blood islands or lethality compared with control littermates or +VEGF164Eryth embryos. The VEGFΔEpoR-iCre mice survived to adulthood at the expected Mendelian numbers (Figure 1F and supplemental Table 1). In addition, Pecam and Caspase-3 immunostaining were performed on E9.5 YSs and revealed no major alteration of the vasculature or increased apoptosis (Figure 2A) in either +VEGF164Eryth or VEGFΔEpoR-iCre embryos. Global qRT-PCR analysis for key genes previously implicated in blood island development36 at E8.5 showed no (Fgf2, Fgfr1, Fgfr2, Bmp4, Bmpr1) or significant (Ihh, Ptch, Vegf, Flk1) changes in expression in either +VEGF164Eryth or VEGFΔEpoR-iCre embryos (Figure 2B). All together, these results imply that erythroid Vegf is able to modulate erythroid development.
Effect of erythroid Vegf overexpression on vasculature and environment. (A) E9.5 yolk sac Pecam (left) vessel and Caspase-3 (right) apoptosis immunohistochemistry staining analysis (magnifications, 200×). (B) Relative mRNA levels of key growth factor receptors and ligands measured by q-RT-PCR in E8.5 yolk sacs. Bars represent mean ± SEM; *P < .05, **P < .01.
Effect of erythroid Vegf overexpression on vasculature and environment. (A) E9.5 yolk sac Pecam (left) vessel and Caspase-3 (right) apoptosis immunohistochemistry staining analysis (magnifications, 200×). (B) Relative mRNA levels of key growth factor receptors and ligands measured by q-RT-PCR in E8.5 yolk sacs. Bars represent mean ± SEM; *P < .05, **P < .01.
Vegf modulates yolk sac erythropoiesis
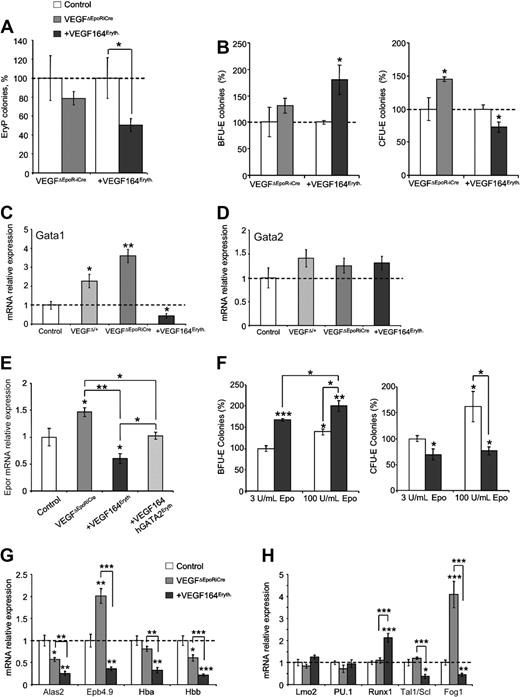
As erythroid-specific Vegf overexpression resulted in embryonic lethality around E11, defective primitive and/or definitive erythropoiesis could have contributed to the observed lethality at this stage.24,37 Therefore, we first investigated if the alterations of Vegf levels had an impact on primitive erythropoiesis. The number of EryP colonies derived from +VEGF164Eryth embryos was decreased by 50% to 60% whereas VEGFΔEpoR-iCre embryos did not show significant differences at E8.5. (Figure 3A).
Erythroid Vegf modulates yolk sac erythropoiesis. (A) Primitive erythroid (EryP) colonies from whole E8.5 control (▭), +VEGF164Eryth ( ) and VEGFΔEpoR-iCre (
) and VEGFΔEpoR-iCre ( ) embryos measured by methylcellulose assay. Results are given as percentage of control absolute number of colonies (100%). (B) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭+ - - -), +VEGF164Eryth (
) embryos measured by methylcellulose assay. Results are given as percentage of control absolute number of colonies (100%). (B) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭+ - - -), +VEGF164Eryth ( ) and VEGFΔEpoR-iCre (
) and VEGFΔEpoR-iCre ( ) yolk sac measured by methylcellulose assays. Cells (1 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (C-D) Gata1 (C) and Gata2 (D) relative mRNA levels measured by qRT-PCR in E10.5 yolk sac. (E) Expression of Epor mRNA in E10.5 yolk sac from control, VEGFΔEpoRiCre, +VEGF164Eryth, and +VEGF164/hGata2Eryth embryos. Relative Epor mRNA levels were measured by qRT-PCR. (F) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭) and +VEGF164Eryth (
) yolk sac measured by methylcellulose assays. Cells (1 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (C-D) Gata1 (C) and Gata2 (D) relative mRNA levels measured by qRT-PCR in E10.5 yolk sac. (E) Expression of Epor mRNA in E10.5 yolk sac from control, VEGFΔEpoRiCre, +VEGF164Eryth, and +VEGF164/hGata2Eryth embryos. Relative Epor mRNA levels were measured by qRT-PCR. (F) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭) and +VEGF164Eryth ( ) yolk sac measured by methylcellulose assays in 2 concentrations of Epo (3 and 100 U/mL). Cells (2 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (G-H) Relative E10.5 yolk sac mRNA levels measured by qRT-PCR of (G) Gata1 target genes Alas2, Epb4.9, Hba and Hbb and (H) transcription factors Lmo2, PU.1, Runx1, Tal1 and Fog1. Bars in panels A through H represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
) yolk sac measured by methylcellulose assays in 2 concentrations of Epo (3 and 100 U/mL). Cells (2 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (G-H) Relative E10.5 yolk sac mRNA levels measured by qRT-PCR of (G) Gata1 target genes Alas2, Epb4.9, Hba and Hbb and (H) transcription factors Lmo2, PU.1, Runx1, Tal1 and Fog1. Bars in panels A through H represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
Erythroid Vegf modulates yolk sac erythropoiesis. (A) Primitive erythroid (EryP) colonies from whole E8.5 control (▭), +VEGF164Eryth ( ) and VEGFΔEpoR-iCre (
) and VEGFΔEpoR-iCre ( ) embryos measured by methylcellulose assay. Results are given as percentage of control absolute number of colonies (100%). (B) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭+ - - -), +VEGF164Eryth (
) embryos measured by methylcellulose assay. Results are given as percentage of control absolute number of colonies (100%). (B) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭+ - - -), +VEGF164Eryth ( ) and VEGFΔEpoR-iCre (
) and VEGFΔEpoR-iCre ( ) yolk sac measured by methylcellulose assays. Cells (1 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (C-D) Gata1 (C) and Gata2 (D) relative mRNA levels measured by qRT-PCR in E10.5 yolk sac. (E) Expression of Epor mRNA in E10.5 yolk sac from control, VEGFΔEpoRiCre, +VEGF164Eryth, and +VEGF164/hGata2Eryth embryos. Relative Epor mRNA levels were measured by qRT-PCR. (F) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭) and +VEGF164Eryth (
) yolk sac measured by methylcellulose assays. Cells (1 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (C-D) Gata1 (C) and Gata2 (D) relative mRNA levels measured by qRT-PCR in E10.5 yolk sac. (E) Expression of Epor mRNA in E10.5 yolk sac from control, VEGFΔEpoRiCre, +VEGF164Eryth, and +VEGF164/hGata2Eryth embryos. Relative Epor mRNA levels were measured by qRT-PCR. (F) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭) and +VEGF164Eryth ( ) yolk sac measured by methylcellulose assays in 2 concentrations of Epo (3 and 100 U/mL). Cells (2 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (G-H) Relative E10.5 yolk sac mRNA levels measured by qRT-PCR of (G) Gata1 target genes Alas2, Epb4.9, Hba and Hbb and (H) transcription factors Lmo2, PU.1, Runx1, Tal1 and Fog1. Bars in panels A through H represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
) yolk sac measured by methylcellulose assays in 2 concentrations of Epo (3 and 100 U/mL). Cells (2 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (G-H) Relative E10.5 yolk sac mRNA levels measured by qRT-PCR of (G) Gata1 target genes Alas2, Epb4.9, Hba and Hbb and (H) transcription factors Lmo2, PU.1, Runx1, Tal1 and Fog1. Bars in panels A through H represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
To address the role of Vegf in definitive embryonic erythropoiesis, erythroid progenitors were quantified in vitro. Methylcellulose assays conducted on E10.5 YSs showed that erythroid-specific Vegf overexpression leads to a 70% increase of BFU-E and 20% decrease in CFU-E colony numbers (Figure 3B). These results demonstrate that Vegf overexpression has negative effects on definitive erythropoiesis, in particular at the BFU-E and CFU-E transition stages. In contrast, erythroid-specific deletion of Vegf leads to a 50% increase in CFU-E numbers (Figure 3B).
To explain these effects on embryonic definitive erythropoiesis observed in +VEGF164Eryth and VEGFΔEpoR-iCre YSs, expression analysis of key erythroid transcription factors was performed. qRT-PCR analysis conducted on E10.5 YSs demonstrated a decrease in Gata1 mRNA expression in +VEGF164Eryth gain of function embryos and a reciprocal dosage-dependent increase in Gata1 mRNA expression in heterozygous VEGFΔEpoR-iCre/+ and homozygous VEGFΔEpoR-iCre loss of function YSs (Figure 3C). Gata2 mRNA levels were not affected by erythroid-specific Vegf overexpression or deletion at E10.5 (Figure 3D).
Interestingly, the RNA expression of the Gata1 target gene, Epor, is increased and decreased in VEGFΔEpoR-iCre/+ and +VEGF164Eryth YSs, respectively (Figure 3E). In addition, the transition block observed in +VEGF164Eryth YS cannot be overcome by the addition of an excess of Epo (100 U/mL instead of 3 U/mL) in methylcellulose assays (Figure 3F). Indeed, extra Epo induces 40% and 30% increase BFU-E formation in controls and +VEGF164Eryth YSs, respectively. However, when 60% increased CFU-E formation was observed in controls, no increase or rescue was detected in mutant YS cultures. These results indicate that despite altered Epor expression VEGF modulated expression of this key receptor is not sufficient to explain the Vegf induced block in erythroid differentiation and that Gata1 or other Gata1 target genes may be involved in this VEGF-induced differentiation block.
Indeed, as shown in Figure 3G, other Gata1 target genes involved in erythroid differentiation, the heme synthesis enzyme (Alas2), the erythroid structural protein (Epb4.9) and globins (Hba and Hbb), were also down-regulated in E10.5 +VEGF164Eryth YSs.38 In comparison, Epb4.9 was up-regulated in VEGFΔEpoR-iCre YSs whereas Alas2, Hba and Hbb showed normal or decreased expression (40% decreased expression for Alas2 and Hbb; Figure 3G). Similar reciprocal alterations were found for Fog1 in +VEGF164Eryth and VEGFΔEpoR-iCre YSs which is a potential transcriptional target of Gata119 (Figure 3H). Other key transcription factors were also modulated by Vegf overexpression in the erythroid lineage. Runx1 and Tal1 expression were increased and decreased, respectively, in +VEGF164Eryth YSs, but not in VEGFΔEpoR-iCre samples (Figure 3H). Taken together, these data suggest that Vegf affects erythroid differentiation mainly through modulation of Gata1 and downstream target gene expression.
Erythroid deletion of Flk1 cannot rescue Vegf-induced erythropoiesis block
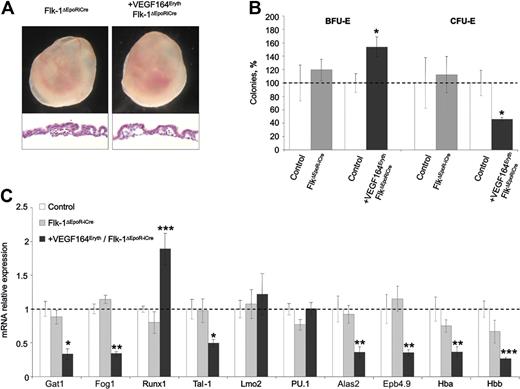
Previous in vitro erythroid differentiation assays performed on Flk1-deficient ES cells resulted in increased differentiation with a strong accumulation of mature hemoglobinized erythrocytes.13 These results were consistent with the observed increase in CFU-E colonies obtained from the VEGFΔEpoR-iCre loss of function YSs and implied that Vegf could be directly signaling through Flk1 present on erythroblast progenitors. To determine whether this was the case we tried to compensate for the Vegf-induced erythroid differentiation block using a conditionally deleted Flk1 allele32 within the erythroid lineage. In comparison to control littermates, erythroid-specific deletion of Flk1 did not lead to any obvious embryonic phenotypes or lethality and these mice could survive to adulthood (supplemental Table 1). Erythroid-specific deletion of Flk1 in +VEGF164Eryth embryos (hereafter referred to as +VEGF164Eryth, Flk1ΔEpoR-iCre embryos) could not rescue the Vegf-induced block in erythroid development and embryonic lethality (Figure 4A and supplemental Table 1). Specifically, in vitro quantification of erythroid progenitors revealed that Flk1 deletion could not rescue the erythroid differentiation block induced by Vegf expression (Figure 4B). Moreover, qRT-PCR from E10.5 YSs showed a decrease in Gata1, Fog1 and Gata1 target genes mRNA levels comparable with the decrease observed in +VEGF164Eryth YSs (Figure 4C). Taken together, these results suggest that the effects of autocrine Vegf on erythropoiesis are not mediated by Flk1 signaling on erythroid cells.
Deletion of Flk-1 in the erythroid lineage cannot rescue the +Vegf164Eryth embryonic lethal phenotype. (A) Images of E10.5 embryos in yolk sac. Yolk sacs of +Vegf164Eryth/Flk-1ΔEpoRiCre embryos were paler and vessels containing red blood cells were absent. Yolk sac section shows decreased circulating cells in blood island, compared with Flk-1ΔEpoRiCre embryos (magnification, 200×). (B) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 yolk sac; control (▭), +Vegf164Eryth/Flk-1ΔEpoRiCre ( ), and Flk-1ΔEpoRiCre (
), and Flk-1ΔEpoRiCre ( ) yolk sac measured by methylcellulose assay. Cells (1 × 105) were plated and results are given as percentage to control absolute number of colonies (100%). (C) Relative mRNA expression levels of erythropoietic transcription factors and Gata1 target genes. Bars represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
) yolk sac measured by methylcellulose assay. Cells (1 × 105) were plated and results are given as percentage to control absolute number of colonies (100%). (C) Relative mRNA expression levels of erythropoietic transcription factors and Gata1 target genes. Bars represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
Deletion of Flk-1 in the erythroid lineage cannot rescue the +Vegf164Eryth embryonic lethal phenotype. (A) Images of E10.5 embryos in yolk sac. Yolk sacs of +Vegf164Eryth/Flk-1ΔEpoRiCre embryos were paler and vessels containing red blood cells were absent. Yolk sac section shows decreased circulating cells in blood island, compared with Flk-1ΔEpoRiCre embryos (magnification, 200×). (B) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 yolk sac; control (▭), +Vegf164Eryth/Flk-1ΔEpoRiCre ( ), and Flk-1ΔEpoRiCre (
), and Flk-1ΔEpoRiCre ( ) yolk sac measured by methylcellulose assay. Cells (1 × 105) were plated and results are given as percentage to control absolute number of colonies (100%). (C) Relative mRNA expression levels of erythropoietic transcription factors and Gata1 target genes. Bars represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
) yolk sac measured by methylcellulose assay. Cells (1 × 105) were plated and results are given as percentage to control absolute number of colonies (100%). (C) Relative mRNA expression levels of erythropoietic transcription factors and Gata1 target genes. Bars represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
Vegf affects definitive fetal liver erythropoiesis
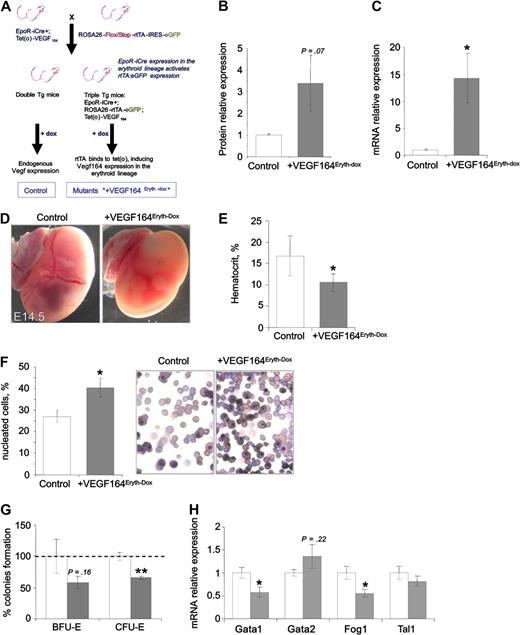
To circumvent the early embryonic lethality associated with constitutive erythroid-specific Vegf expression and to investigate the role of Vegf in other hematopoietic organs during embryonic definitive erythropoiesis, we have developed a doxycyclin-tet(o)–inducible system that allows both temporal and cell-specific control of Vegf164 expression by combining EpoR-iCre with conditional ROSA26-rtTA-IRES-eGFP5 and tet(o)-VEGF16439 transgenic alleles. To induce the expression of the transgene, triple transgenic EpoR-iCreTg/+, ROSA-rtTATg/+, tet(o)-VEGF164Tg/+ embryos (designated +VEGF164Eryth-Dox mice) and littermate controls were treated with doxycycline (via the drinking water given to the pregnant dams) 2 days before dissection (Figure 5A). In E14.5 fetal livers, treatment with doxycycline results in 10 and 3.5 time increase of Vegf mRNA (Figure 5B) and protein (Figure 5C), respectively. During fetal liver erythropoiesis, these levels of Vegf164 induction dramatically altered erythroid development with mutant/compound offspring showing an absence of RBCs at E14.5 (Figure 5D), accompanied by a 30% decrease in hematocrit (Figure 5E) and a 30% increase in the number of nucleated RBCs (consisting mainly of primitive erythroid cells and definitive nucleated precursors abnormally released to the circulation) in the peripheral blood at E14.5 (Figure 5F). In addition, in vitro quantification of E14.5 fetal liver erythroid progenitors showed a nonsignificant decrease in BFU-E colonies (40%, p = .29) but a significant decrease in the total number of CFU-E colonies (35%, P < .01; Figure 5G) suggesting that the block in erythropoiesis occurs between the BFU-E and CFU-E stages of erythroid development. We also found normal Gata2 levels and decreased Gata1 and Fog1 mRNA expression in the fetal liver in the +VEGF164Eryth-dox embryos in accordance with the constitutively expressing +VEGF164Eryth embryos (Figure 5H). However, the level of expression of Tal1 mRNA is not affected at this fetal liver stage (Figure 5H). Furthermore, no significant changes in Epo or Gata4 levels were observed that have previously been implicated in altered fetal liver erythropoiesis40 (supplemental Figure 2). These data show that similar to what we observed in the +VEGF164Eryth YSs, Vegf has negative effects on fetal liver erythropoiesis.
Erythroid-specific Vegf164 overexpression affects fetal liver erythropoiesis. (A) Breeding scheme to generate +VEGF164Eryth-Dox embryos expressing the inducible transcriptional activator rtTA and EGFP constitutively in erythroid cell lineages. Only upon doxycycline administration is Vegf overexpressed via the activation of tet(o)-responsive element by rtTA. Control embryos were littermates lacking the tet(o)-Vegf164 or the EpoR-iCre transgene but receiving doxycycline. (B-C) Relative Vegf mRNA levels measured by qRT-PCR (B) and protein levels measured by ELISA (C) in E14.5 fetal livers after 2 days of doxycycline treatment. (D-F) Doxycycline-induced Vegf164 expression after 2 days of treatment leads to (D) decreased RBCs in the yolk sac, (E) decreased hematocrit, and (F) increased numbers of nucleated RBC progenitors in peripheral blood smears (magnification, 400×) in E14.5 embryos. (G) Definitive erythroid colonies (BFU-E and CFU-E) from E14.5 fetal livers of control and +VEGF164Eryth-Dox measured by methylcellulose assay after 2 days of doxycycline treatment. Cells (2 × 104) were plated and results are given as percentage to control absolute number of colonies (100%). (H) Relative Gata1, Gata2, Fog1, and Tal1 mRNA levels measured by qRT-PCR in E14.5 fetal livers. Bars in panels B and C and E through H represent mean ± SEM; *P < .05, **P < .01.
Erythroid-specific Vegf164 overexpression affects fetal liver erythropoiesis. (A) Breeding scheme to generate +VEGF164Eryth-Dox embryos expressing the inducible transcriptional activator rtTA and EGFP constitutively in erythroid cell lineages. Only upon doxycycline administration is Vegf overexpressed via the activation of tet(o)-responsive element by rtTA. Control embryos were littermates lacking the tet(o)-Vegf164 or the EpoR-iCre transgene but receiving doxycycline. (B-C) Relative Vegf mRNA levels measured by qRT-PCR (B) and protein levels measured by ELISA (C) in E14.5 fetal livers after 2 days of doxycycline treatment. (D-F) Doxycycline-induced Vegf164 expression after 2 days of treatment leads to (D) decreased RBCs in the yolk sac, (E) decreased hematocrit, and (F) increased numbers of nucleated RBC progenitors in peripheral blood smears (magnification, 400×) in E14.5 embryos. (G) Definitive erythroid colonies (BFU-E and CFU-E) from E14.5 fetal livers of control and +VEGF164Eryth-Dox measured by methylcellulose assay after 2 days of doxycycline treatment. Cells (2 × 104) were plated and results are given as percentage to control absolute number of colonies (100%). (H) Relative Gata1, Gata2, Fog1, and Tal1 mRNA levels measured by qRT-PCR in E14.5 fetal livers. Bars in panels B and C and E through H represent mean ± SEM; *P < .05, **P < .01.
Increased expression of Gata2 partially rescues the Vegf-induced erythropoiesis block
Vegf overexpression in the erythroid lineage leads to decreased Gata1 expression (Figure 3C). Given that Gata2 acts as an inducer of Gata1 which is necessary for the BFU-E to CFU-E differentiation process18,26,27 and because Gata2 expression under the control of the Gata1 promoter can rescue the embryonic lethality caused by Gata1 deletion,41 we next tried to rescue the constitutive erythroid-specific Vegf gain of function phenotype through the use of mouse lines conditionally expressing the human Gata2 gene in erythroid cells (EpoR-iCre+/−, ROSA26-hGata2+/−, designated +hGata2Eryth mice). The +hGata2Eryth mice were born at the expected Mendelian ratios and survived to adulthood without any obvious phenotype (supplemental Table 1 and supplemental Figure 3A). As expected, +hGata2Eryth embryos displayed a 60% increase in Gata1 expression (supplemental Figure 3D) without affecting Vegf mRNA expression (supplemental Figure 3B). However, hGata2 expression did not significantly affect the number of primitive and definitive erythroid progenitors in colony-forming cell assays (supplemental Figure 3C).
Interestingly, when Vegf164 and Gata2 were cooverexpressed in the erythroid lineage in EpoR-iCre+/−, ROSA26+VEGF164/hGata2 embryos (designated +VEGF164/hGata2Eryth embryos) the embryonic lethality observed in +VEGF164Eryth embryos was delayed from E10.5 to E12.5 (supplemental Table 1) and up until E10.5 +VEGF164/hGata2Eryth mutants were barely distinguishable (Figure 6A) from control embryos, although the level of Vegf protein expression in these E10.5 embryos is 70% higher than in the control littermates (Figure 6B). This delayed lethality was associated with a rescue of the number of EryP in vitro (Figure 6C). Furthermore, the changes observed in Ihh/Ptch and Flk1 expression in E8.5 +VEGF164Eryth mutants was normalized in +VEGF164/hGATA2Eryth embryos (supplemental Figure 4). In addition, definitive erythropoiesis was also modulated by the hGata2 transgene expression in +VEGF164/hGata2Eryth YSs. Compared with +VEGF164Eryth mutants, the number of definitive progenitors in +VEGF164/hGata2Eryth YSs was rescued and the number of BFU-E and CFU-E colonies was comparable with controls (Figure 6D). Moreover, the levels of Gata1, Fog1, Runx1, Tal1, and Gata1 target genes were also normalized in +VEGF164/hGata2Eryth YSs (Figure 6E-G). These results demonstrate that erythroid hGata2 expression rescues the VEGF-induced erythropoiesis block. It is likely that restored Gata1 expression levels is involved in this rescue.
Rescue of erythropoiesis by ROSA26-based hGata2 expression. (A) Phenotypes of +Vegf164/hGata2Eryth embryos. White arrows show RBCs in E10.5 yolk sac blood vessels. Sections of E10.5 yolk sac are represented and black arrows show circulating RBCs cells in yolk sac vasculature (magnification, 200×). (B) Vegf relative protein levels measured by ELISA in E10.5 yolk sac. (C) Primitive erythroid colonies (EryP) from E8.5 whole control (- - -), +Vegf164Eryth ( ), and +Vegf164/hGata2Eryth (
), and +Vegf164/hGata2Eryth ( ) embryos measured by methylcellulose assays. Results are given as percentage of control absolute number of colonies (100%). (D) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭), +VEGF164Eryth (
) embryos measured by methylcellulose assays. Results are given as percentage of control absolute number of colonies (100%). (D) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭), +VEGF164Eryth ( ), and +Vegf164/hGata2Eryth (
), and +Vegf164/hGata2Eryth ( ) yolk sac measured by methylcellulose assay. Cells (1 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (E-G) E10.5 yolk sac relative mRNA levels measured by qRT-PCR of (E) Gata1, (F) Gata1 target genes Alas2, Epb4.9, Hba and Hbb, and (G) transcription factors LMO2, PU.1, RUNX1, TAL1, and FOG1. Bars in panels B through H represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
) yolk sac measured by methylcellulose assay. Cells (1 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (E-G) E10.5 yolk sac relative mRNA levels measured by qRT-PCR of (E) Gata1, (F) Gata1 target genes Alas2, Epb4.9, Hba and Hbb, and (G) transcription factors LMO2, PU.1, RUNX1, TAL1, and FOG1. Bars in panels B through H represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
Rescue of erythropoiesis by ROSA26-based hGata2 expression. (A) Phenotypes of +Vegf164/hGata2Eryth embryos. White arrows show RBCs in E10.5 yolk sac blood vessels. Sections of E10.5 yolk sac are represented and black arrows show circulating RBCs cells in yolk sac vasculature (magnification, 200×). (B) Vegf relative protein levels measured by ELISA in E10.5 yolk sac. (C) Primitive erythroid colonies (EryP) from E8.5 whole control (- - -), +Vegf164Eryth ( ), and +Vegf164/hGata2Eryth (
), and +Vegf164/hGata2Eryth ( ) embryos measured by methylcellulose assays. Results are given as percentage of control absolute number of colonies (100%). (D) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭), +VEGF164Eryth (
) embryos measured by methylcellulose assays. Results are given as percentage of control absolute number of colonies (100%). (D) Definitive erythroid colonies (BFU-E and CFU-E) from E10.5 control (▭), +VEGF164Eryth ( ), and +Vegf164/hGata2Eryth (
), and +Vegf164/hGata2Eryth ( ) yolk sac measured by methylcellulose assay. Cells (1 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (E-G) E10.5 yolk sac relative mRNA levels measured by qRT-PCR of (E) Gata1, (F) Gata1 target genes Alas2, Epb4.9, Hba and Hbb, and (G) transcription factors LMO2, PU.1, RUNX1, TAL1, and FOG1. Bars in panels B through H represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
) yolk sac measured by methylcellulose assay. Cells (1 × 105) were plated and results are given as percentage of control absolute number of colonies (100%). (E-G) E10.5 yolk sac relative mRNA levels measured by qRT-PCR of (E) Gata1, (F) Gata1 target genes Alas2, Epb4.9, Hba and Hbb, and (G) transcription factors LMO2, PU.1, RUNX1, TAL1, and FOG1. Bars in panels B through H represent mean ± SEM; *P < .05, **P < .01, ***P < .001.
The +VEGF164/hGata2Eryth embryos still died during development at E12.5 and are most probably succumbing to lethality associated with dilated cardiomyopathy and cardiac cushion and septal defects that have been described previously3,4,17 as these embryos showed a similar dilated atrium and thinner walled myocardium in both ventricles, although less severe, to the +VEGF164Eryth embryos (supplemental Figure 5A). In addition, the expression of Bmp4 mRNA, which is essential for proper cardiac formation,42 is decreased in +VEGF164Eryth and +Vegf164/hGata2Eryth E11.5 hearts (supplemental Figure 5B).
Discussion
In this study we have genetically probed the influence of loss of Vegf and Flk1 as well as gain of Vegf164 on erythroid lineage differentiation in vivo. Vegf31 and Flk132 were conditionally deleted in progenitors of erythroid cells using the EpoR-iCre line.28 To complement these studies the Vegf16429,30 isoform was also overexpressed either constitutively or inducibly in these precursors.
Erythroid-specific Vegf modulation revealed an important role of this growth factor during primitive as well as definitive embryonic erythropoiesis. Overexpression of Vegf during the early stages of erythropoiesis resulted in a reduction of erythroid cells within the blood islands of the YS. This erythropoiesis defect in +Vegf164Eryth embryos was associated with growth retardation and death by E10.5. We found that Vegf overexpression blocks the differentiation potential of both primitive and definitive erythroid progenitors, between the BFU-E and CFU-E stages. This erythropoiesis block was associated with decreased expression of Gata1, Fog1 and their target genes involved in erythroid differentiation. Moreover, deletion of Vegf in the same lineage led to a dosage-dependent increase in expression of some of these genes and increased erythroid progenitor differentiation in YS colony forming assays. Several studies have shown that mutant mice lacking Gata1 exhibit no functional primitive erythrocytes and die around E10.5 to E11.5,24,37 indicating that intact primitive erythrocytes are essential for survival of the embryo after E11.5. Similar to these mutants, +VEGF164Eryth embryos die around this stage with defects in primitive as well as definitive erythroid differentiation. Taken together, these data suggest a functional link between erythroid Vegf levels and Gata1 expression. Moreover, after 2 days of Vegf164 induction, all the +VEGF164Eryth-Dox embryos showed a reduced number of erythrocytes in the embryo associated with a similar block of erythroid differentiation in the fetal liver as observed in E10.5 +VEGF164Eryth YSs. This block in fetal liver erythropoiesis also correlated with decreased Gata1 and Fog1 expression, demonstrating that the negative effects of Vegf on erythropoiesis are not limited to the YS. In accordance, similar Gata1-related erythroid development blocks have been observed in Xenopus embryos injected with Vegf.43 Thus, increased Vegf expression has an evolutionary conserved negative effect on erythropoiesis. However, the effects of Vegf are not limited to the modulation of Gata1 expression, as other hematopoietic transcription factors such as Runx1 and Tal1 were also modulated by erythroid Vegf overexpression. Indeed, Runx1 and Tal1 expression levels, respectively, were increased and decreased in +VEGF164Eryth embryos, but remained unchanged in VEGFΔEpoR-iCre and +VEGF164/hGata2Eryth YSs. These transcription factors could also be implicated in the negative effects of Vegf overexpression on erythroid differentiation and the specific alterations in some but not all Gata1 target genes examined. Additional studies are required to establish the putative role that these transcription factors play in the Vegf-induced erythropoietic defects.
Recently, Ferreira et al demonstrated that the regulation of Gata factor levels is more important than their identity by rescuing the embryonic lethality induced by Gata1 deletion with a Gata2 transgene expressed under the control of the Gata1 promoter.41 In addition, several studies have shown that Gata2 is responsible for the induction of Gata1 at the BFU-E stage to allow the differentiation into CFU-E progenitors.18,26,27 Accordingly, we found that the Gata1 expression levels in +hGata2Eryth YS was increased. In addition, Suzuki et al have shown that Gata1.05 cells, which express Gata1 at approximately 5% of the control level throughout hematopoietic development,44 failed to differentiate to the CFU-E stage and are blocked at the BFU-E stage.27 Therefore, to overcome the decreased Gata1 levels observed in +Vegf164Eryth YSs and to attempt to rescue the erythroid differentiation block induced by Vegf overexpression, we expressed hGata2 specifically in the erythroid lineage of +Vegf164Eryth embryos. In E10.5 YSs of these double mutant embryos, the level of Gata1 expression is comparable with that observed in control embryos. Interestingly, colony forming cell assays performed on +VEGF164/hGata2Eryth YS derived progenitors showed a rescue in the differentiation block of both primitive and definitive erythroid progenitors induced by Vegf overexpression. This suggests that the negative effects of Vegf on erythroid differentiation are exerted through down-regulation of Gata1 expression.
Specific Vegf loss of function experiments during primitive and definitive erythropoiesis showed normal and increased erythroid progenitor differentiation, respectively. VEGFΔEpoR-iCre loss of function mice survived to adulthood without any obvious phenotypical abnormalities. We can therefore assume that Vegf from other sources such as the YS extraembryonic visceral endoderm7 can act on erythroid cells in a paracrine manner and that autocrine sources of Vegf from the erythroid cells are not absolutely required for normal erythropoiesis but need to be tightly controlled and may serve to fine tune erythroid cell development. Accordingly, a recent publication showed that the absence of visceral endoderm in differentiating embryoid bodies (EBs) does not prevent emergence of definitive progenitors from EBs when supplemented with Vegf.45
To determine the cell autonomous effects of autocrine Vegf levels on the proliferative and differentiation abilities of erythroid progenitor cells, Flk1 was specifically deleted in the erythroid lineage at the beginning of progenitor differentiation. Loss of Flk1 did not lead to any obvious effects on Gata1 mRNA expression or any changes in erythroid progenitor differentiation. Previously we have demonstrated that deletion of Flk1 within osteochondroprogenitors could partially rescue Vegf-mediated excessive bone formation.29 Considering the fact that erythroid-specific Flk1 deletion cannot rescue Vegf induced decreases in Gata1 mRNA expression, erythropoiesis block or lethality, our data suggest that Flk1 does not mediate Vegf's effects on this lineage. Furthermore, this receptor is not necessarily required in a cell-autonomous manner for erythroid differentiation itself. Thus, Flk1 action is most probably limited to ensure that mesodermal progenitors are placed in the right environment to respond to appropriate signals for hematopoiesis10 and for generation of committed erythroid progenitors.46 Our data are consistent with previous reports that demonstrated the presence of definitive erythrocytes in Flk1−/− EBs.47 Previous research implies that Flt1 expression in contrast to Flk1 increases with maturation of hematopoietic cells48 and is expressed at the BFU-E stage whereas Flk1 is not.49 In addition, deletion of the tyrosine kinase domain of Flt1 results in a decreased number of BFU-E progenitors.50 Therefore, we propose that this receptor may be responsible for the cell autonomous effects of Vegf on erythroid differentiation. Our in vivo data go slightly against previous studies showing that upon addition of exogenous Vegf to differentiating embryoid bodies, the amount of erythroid colonies is increased and that those progenitors appear to have a greater self-renewal capacity. However, the effect of Vegf on erythroid cell proliferation and differentiation was obtained when the medium was enriched with additional cytokines such as BMP4 that was not used in these studies, which is a potent inducer of Flk1 and Flk1+ mesodermal progenitors.46 As well, in both HSC14 and mature vascular endothelium of the mouse15 the importance of autocrine Vegf signaling is essential for cell survival through signaling pathways distinct from those elicited by non-cell autonomous paracrine stimulation by Vegf. Here we propose that within the erythroid lineage autocrine Vegf is tightly controlled and may serve to fine tune erythroid development through alterations in Gata1 levels and Gata1 target genes (Figure 7). This may elicit different responses in erythroid development than exogenously added or paracrine Vegf.
Model of Vegf regulation of embryonic erythroid development through Gata1 modulation. Development of erythropoiesis profiles are shown from progenitor cells to the terminally differentiated erythrocytes or RBCs (gray bars). Alteration of erythroid Vegf levels lead to cell intrinsic effects on Gata1 expression levels and key transcriptional targets. Expression of Gata2 transgene specifically in erythroid cells can overcome both the decrease of Gata1 expression and the block in erythroid cell differentiation, induced by Vegf overexpression. Modulation of Vegf levels within the erythroid lineage can also modulate the environment and indirectly affect developmental erythropoiesis through the altered secretion of growth factors such as paracrine Vegf, Ihh, Epo, and potentially other as yet unidentified factors.
Model of Vegf regulation of embryonic erythroid development through Gata1 modulation. Development of erythropoiesis profiles are shown from progenitor cells to the terminally differentiated erythrocytes or RBCs (gray bars). Alteration of erythroid Vegf levels lead to cell intrinsic effects on Gata1 expression levels and key transcriptional targets. Expression of Gata2 transgene specifically in erythroid cells can overcome both the decrease of Gata1 expression and the block in erythroid cell differentiation, induced by Vegf overexpression. Modulation of Vegf levels within the erythroid lineage can also modulate the environment and indirectly affect developmental erythropoiesis through the altered secretion of growth factors such as paracrine Vegf, Ihh, Epo, and potentially other as yet unidentified factors.
In addition, our studies demonstrate that increased Vegf emanating from the erythroid progenitors results in minor but significant changes in the expression of Ihh/PTCH expression during primitive erythropoiesis at E8.5, delays in vessel maturation in the developing YS and significant cardiovascular developmental alterations at E10.5. In the rescued +VEGF164/hGATA2Eryth embryos, Ihh/Ptch expression was normalized at E8.5 but heart malformations were still observed at E11.5, although not as dramatic in the +VEGF164Eyrth embryos. This suggests that restored eythropoiesis and/or normalized Ihh/Ptch expression in the YS environment in +VEGF164/hGata2Eryth embryos could partially ameliorate this secondary defect at E10.5 and E11.5 (Figure 1E and supplemental Figure 5). The cardiovascular defects and timing of lethality at E12.5 associated with mild erythroid Vegf overexpression during development are consistent with previous findings showing that a mild 2- to 3-fold increase in Vegf levels in hypermorphic embryos can lead to cardiovascular defects similar to those reported here.3,4,17 In addition, we have shown that Bmp4 levels are significantly down-regulated in the VEGF164Eryth and +VEGF164/hGATA2Eryth hearts and may contribute to the cardiac cushion, septation, and myocardial defects observed in this study.42 Overall, these data suggest that modulation of Vegf levels specifically within the erythroid lineage may indirectly modulate the environment in a tissue-specific fashion and these alterations may indirectly contribute synergistically with the observed autocrine defects in Gata1 expression and its targets that leads to the erythropoiesis defects observed in this study (Figure 7).
In summary, we have demonstrated that altered autocrine Vegf levels can affect erythroid differentiation through the modulation of expression of the key hematopoietic transcription factor Gata1. Additional studies will be necessary to further delineate the functional role of Vegf in its ability to modulate Gata1 levels and their effects on physiologic erythropoiesis in the adult as well as alterations seen during anti-Vegf tumor therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was partially supported by the Belgium Federation Against Cancer (Stichting tegen Kanker) grant #203-2008 to J.J.H.. L.G. and S.P. were supported by the Netherlands Organization for Scientific Research (NWO, grants # 700.55.006, DN 82-294 and 863.09.012).
Authorship
Contribution: B.D. and J.J.H. designed research; B.D., J.K., S.G., M.F.G., S.B., K.H., K.D., O.N., and M.N. performed research; B.D., L.G., H.H., S.G., S.P., and J.J.H. analyzed data; B.D. and J.J.H. wrote the manuscript; and N.F., U.K., B.N.L., and A.N. provided essential mouse lines and/or infrastructural support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.K. is Institute of Pathology, University Clinic Carl Gustav Carus, Technical University, Dresden, Germany. The current affiliation for O.N. is Laboratory of Pediatric Hepatology & Cell Therapy, Université Catholique de Louvain, Brussels, Belgium. The current affiliation for L.G. is Department of Blood Cell Research, Sanquin Research, Amsterdam, The Netherlands.
Correspondence: Dr Jody J. Haigh, VIB, Department for Molecular Biomedical Research, Vascular Cell Biology Unit, Technologiepark 927, B-9052 Ghent, Zwijnaarde, Belgium; e-mail address: jody.haigh@dmbr.vib-UGent.be.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal