Abstract
Long-lived plasma cells in the bone marrow produce memory antibodies that provide immune protection persisting for decades after infection or vaccination but can also contribute to autoimmune and allergic diseases. However, the composition of the microenvironmental niches that are important for the generation and maintenance of these cells is only poorly understood. Here, we demonstrate that, within the bone marrow, plasma cells interact with the platelet precursors (megakaryocytes), which produce the prominent plasma cell survival factors APRIL (a proliferation-inducing ligand) and IL-6 (interleukin-6). Accordingly, reduced numbers of immature and mature plasma cells are found in the bone marrow of mice deficient for the thrombopoietin receptor (c-mpl) that show impaired megakaryopoiesis. After immunization, accumulation of antigen-specific plasma cells in the bone marrow is disturbed in these mice. Vice versa, injection of thrombopoietin allows the accumulation and persistence of a larger number of plasma cells generated in the course of a specific immune response in wild-type mice. These results demonstrate that megakaryocytes constitute an important component of the niche for long-lived plasma cells in the bone marrow.
Introduction
Antibody-secreting plasma cells are found in many tissues. However, the plasma cells that provide antigen-specific systemic antibodies for up to decades after immunization or infection predominantly reside in the bone marrow.1-3 There are multiple lines of evidence that individual plasma cells can survive in humans and mice for many months at the least.4-7 These long-lived plasma cells are important for maintaining protective antibody memory. However, autoantibody-secreting long-lived plasma cells are refractory to conventional immunosuppressive therapy and therefore represent a therapeutic challenge in autoimmune diseases.8-10 Plasma cell survival is not cell-autonomous but depends on signals provided by their environment. The most potent plasma cell survival factors identified so far are a proliferation-inducing ligand (APRIL), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), stromal-derived factor-1α, and signals transduced via CD44.11-14 The bone marrow contains multiple microenvironmental niches that stimulate cellular proliferation, differentiation, and survival.15-20 Each niche seems to support specifically one or a few particular hematopoietic stem or precursor cells. In this way, the sizes of these populations are limited by the number of available niches.16,21 Similarly, competition for a limited number of survival niches may also control the turnover rate within the bone marrow plasma cell compartment.12,22-24 One or multiple niches may exist that have the capability to support the terminal differentiation and survival of bone marrow plasma cells.25
As indicated by strong colocalization between a particular subtype of stromal-derived factor-1α+ reticular stromal cells and immunoglobulin G+ (IgG+) bone marrow plasma cells, the former seems to be an important element of plasma cell niches in that tissue.26 However, in culture, bone marrow stromal cells support plasma cell survival only for a limited time,13 suggesting that additional cell types contribute to the formation of plasma cell niches.
In addition, it has been shown that macrophage-derived APRIL is required to support differentiation/survival of bone marrow plasma cells during early life, suggesting that factors supplied by other cell types are indeed essential for the function of plasma cell niches in vivo.27 So far, despite their importance, little is known about the constitution and function of such niches.
Like long-lived plasma cells, megakaryocytes are also predominantly found in the bone marrow.28 The most prominent function of megakaryocytes is the production and release of platelets. Megakaryocytes also can produce and secrete several cytokines, including the plasma cell growth factors APRIL and IL-6, as has been demonstrated in vitro.29-31 Megakaryopoiesis, the process of generating new megakaryocytes, is a continuous process tightly regulated by thrombopoietin (TPO) and its receptor c-mpl that is expressed on megakaryocyte lineage cells and platelets.28,32 c-mpl−/− mice display reduced megakaryocyte and platelet numbers, whereas injection of TPO increases both. Platelet counts in blood are also altered during severe infection and chronic inflammation, indicating a modulation of megakaryopoiesis under these conditions.33
Here we demonstrate that the megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow.
Methods
Mice, immunizations, TPO treatment, and BrdU feeding
Female BALB/c mice were bred at the animal facility of the German Arthritis Research Center (DRFZ Berlin) under specific pathogen–free conditions. Mice were intraperitoneally immunized with 100 μg of alum-precipitated ovalbumin (Ova) diluted in 100 μL of phosphate-buffered saline (PBS). For secondary immunization, mice received an intravenous injection of 100 μg of Ova in 100 μL of PBS 3 weeks later. Recombinant murine TPO (PeproTech) was administered by injections (intraperitoneally, 10 μg/kg) on 5 consecutive days. For primary and secondary immunizations, wild-type C57BL/6 and C57BL/6 c-mpl−/− mice were immunized by intraperitoneal injection of 50 μg of alum-precipitated 4-hydroxy-3-nitrophenylacetyl coupled to keyhole limpet hemocyanin (NP-KLH). To label proliferating cells, mice received 5-bromo-2′-deoxy-uridine (BrdU; 1 mg/mL; Sigma-Aldrich) with their drinking water, containing 1% glucose. The drinking water was changed every 3 days. Mice were killed by cervical dislocation. All animal experiments have been approved by the Institutional Review Board of the German Arthritis Research Center or the Walter and Eliza Hall Institute.
ELISPOT
Frequencies of NP-specific plasma cells were determined by ELISPOT as follows: single-cell suspensions were plated in 96-well ELISPOT plates (cellulose ester–based), coated with NP24 (splenic antibody-secreting cells [ASCs]) or NP13 (bone marrow ASCs), bovine serum albumin (BSA), and subsequently incubated for 20 hours at 37°C; 5% CO2. Then, plates were washed, anti-NP IgG1 was revealed with goat anti–mouse IgG1 conjugated to horseradish peroxidase (Southern Biotechnology Associates) and visualized by the subsequent addition of 3-aminoethyl carbazole. ASCs were counted using a dissecting microscope. For investigation of plasma cell survival in vitro, total ASCs were quantified after 3 days in culture by ELISPOT as described earlier.11 Plates were coated with a mixture of polyclonal goat anti–mouse IgA, IgG, and IgM (Southern Biotechnology).
Histology
Femurs were embedded in Tissue-Tek medium (Sakura Finetek), snap-frozen in liquid nitrogen, and stored at −80°C; 8-μm tissue sections were prepared with a microtome and fixed with acetone at −20°C for 10 minutes. Unspecific binding was blocked by preincubation with PBS/3% BSA and incubation with anti-CD16/CD32 (clone 2.4G2; made in-house). Staining with antibodies and secondary reagents was performed for 30 minutes at room temperature. The following antibodies and staining reagents were used: anti-Ig κ light chain-Cy5 (clone 187.1, made in-house), anti-Ig κ light chain-fluorescein isothiocyanate (FITC, clone 187.1; BD Biosciences), anti–IL-6-DIG (clone MP5-20F3, made in-house), anti–IL-4-DIG (clone 11B11, made in-house), anti–DIG-Alexa 546 (made in-house), anti-CD41 biotin (clone MWReg30; AbD Serotec), streptavidin–Alexa 546 (made in-house), antiendothelial-biotin (clone B78, made in-house), Ova (Sigma-Aldrich) conjugated to Cy5 (made in-house), polyclonal rabbit anti-APRIL (CSA-836; Stressgen), polyclonal rabbit-Ig (Z0494; Dako Cytomation), anti–rabbit Alexa 647 (A21245; Invitrogen), and Sytox-orange (Invitrogen). Sections were analyzed by confocal microscopy (Leica TCS-SL) and processed with the Leica Confocal Software (LCS Lite; Version 2.61 Build 1538).
PCR
Naive B cells (B220/IgD+) and plasma cells (CD138++) were sorted by fluorescence-activated cell sorting (FACS) using a FACSDiva (BD Biosciences). Megakaryocytes were generated from mouse fetal liver cells and concentrated by a BSA step gradient as recently described.34 RNA was isolated after a one-step preparation with Trizol (Invitrogen) and reverse transcribed to cDNA using an iScript kit (Bio-Rad). Lineage-specific expression patterns of the indicated genes were determined by standard polymerase chain reaction (PCR; 35 cycles). Primer sequences are available on request. For performing real-time PCR, mRNA was extracted using RNeasy columns from QIAGEN. Reverse transcription was performed with random hexanucleotides (Fermentas) using Moloney Murine Leukemia Virus reverse transcriptase (Promega) for 1 hour at 42°C. For each cDNA preparation, the amounts of cDNA specific for APRIL, IL-6, and reference genes (β-actin or β2-microglobulin) were assessed by real-time PCR on ABI 7900 using TaqMan chemistry (Applied Biosystems). The relative amounts of APRIL, IL-6, CD11b, and CD41 cDNA were calculated by referring to the amount of β-actin or β2-microglobulin cDNA. Probes for APRIL and IL-6 were detected via a 5′ label with 6-FAM phosphoramidite, whereas probes for β-actin or β2-microglobulin were 5′ labeled with VIC or Yakima Yellow, respectively (Applied Biosystems). TaqMan probes and primers for APRIL, IL-6, β-actin, and β2-microglobulin were designed using Primer Express computer software (Version 1.0; Applied Biosystems) and synthesized by Eurogenetec. For APRIL: forward primer 5′-CGAGTCTGGGACACTGGAATTT-3′; reverse primer 5′-AGATACCACCTGACCCATTGTGA-3′; probe 5′-CTGCTCTATAGTCAGGTCCTGTTTCATGATGTGAC-3′; IL-6: forward primer 5′-TCGGAGGCTTAATTACACATGTTC-3′; reverse primer 5′-AAGTGCATCATCGTTGTTCATACA-3′; probe 5′-CAGAATTCCCATTGCACAACTCTTTTCTCAT-3′; β-actin: forward primer 5′-CGTGAAAAGATGACCCAGATCA-3′; reverse primer 5′-TGGTACGACCAGAGGCATACAG-3′; probe 5′-TCAACACCCCAGCCATGTACGTAGCC-3′; β2-microglobulin: forward primer 5′-CATACGCCTGCAGAGTTAAGCA-3′; reverse primer 5′-ATCACATGTCTCGATCCCAGTAGA-3′; probe 5′-CAGTATGGCCGAGCCCAAGACCG CD11b and CD41 cDNA were quantified using iQ SYBR Green Supermix (Bio-Rad). Primer sequences for CD11b detection were obtained at Primer Bank1: ID 6680484a1. Primers for CD41 detection were a kind gift from Dr Stephanie Dumon; forward primer: 5′-TTTCTGCAGCCTAAGGGCC-3′; reverse primer: 5′-GGCAGCCACAGCAATATCATT-3′.
Identification of plasma cells on bone marrow tissue sections
Plasma cells identified by CD138++ expression produce distinguishably high levels of Ig κ light chains, as shown by intracellular staining by flow cytometry (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Based on this observation, plasma cells were detected on tissue sections by staining for Ig κ light chain (titrated concentrations). To confirm Ig κ staining for the identification of bone marrow plasma cells, Blimp-1–green fluorescence protein (GFP) reporter mice were analyzed. In these mice, mature and presumably long-lived bone marrow plasma cells are detectable by GFP expression.35 In Blimp-1-GFP reporter mice, all cells identified by bright expression for Ig κ were GFP+, confirming κ light chain as a marker for plasma cells on tissue sections. GFP+/κ+ plasma cells were also found to be in contact with megakaryocytes (supplemental Figure 1). Details of the protocol used for tissue preparation and fixation allowing detection of GFP-expressing cells are available on request.
Calculation of contact frequencies per cell surface area
Plasma cell contacts to B220+ B cells and Gr-1+ granulocytes and megakaryocytes are determined by confocal microscopy. B220+ B cells and Gr-1+ granulocytes are approximately 100-fold more abundant in bone marrow than megakaryocytes (ie, ∼ 20% B220+ B cells and 30% Gr-1+ granulocytes cells compared with 0.3% CD41+ megakaryocytes). In total numbers, this is approximately 20 million B cells, 30 million granulocytes, and 300 000 megakaryocytes in whole bone marrow. If these 3 distinct cell types make random contact to plasma cells, it could be assumed that the contact frequencies per total cell surface area are similar for all populations. The total cell surface area of a population is given by the surface area of a single cell multiplied by the total cell number of the population. Based on their diameters (B cells, 8 μm; granulocytes, 11 μm; megakaryocytes, 30 μm), the surface area of a single B cell, granulocyte, and megakaryocyte could be calculated as 201, 380, and 2827 μm2, respectively. Multiplied by the total cell number of the respective population, this results in the total surface area of the B-cell, granulocyte, and megakaryocte populations as 4021, 11 404, and 848 mm2, respectively. Division of the absolute cell contacts of these 3 cell types to plasma cells by the total population cell surface areas results in the contact frequency per cell surface area, which are 0.42 for B cells, 0.53 for granulocytes, and 3.5 for megakaryocytes.
Flow cytometry
Single-cell suspensions of spleen and bone marrow were filtered through a 70-μm cell strainer (BD Falcon) and washed twice with PBS/0.5% BSA. For analysis of megakaryocytes, cells were incubated with anti-CD16/CD32 (clone 2.4G2; made in-house) for 15 minutes on ice and subsequently fixed with 80% ethanol at −20°C for 30 minutes. Then, cells were treated with RNase A (Sigma-Aldrich) at 37°C for 30 minutes at a final concentration of 50 μg/mL. Finally, megakaryocytes were stained with either anti–CD41-FITC (clone MWReg30; AbD Serotec) or anti–CD61-FITC (clone 2C9.G2; BD Biosciences) and propidium iodide (Sigma-Aldrich) at room temperature for 15 minutes. For analysis of plasma cells, cell suspensions were incubated with anti–CD138-phycoerythrin (clone 281-2; BD Biosciences) for 15 minutes and washed in PBS/BSA. To detect intracellular Ig κ light chain and/or BrdU, cells were fixed with the BrdU-Flow Kit (BD Biosciences) according to the recommendation of the manufacturers and stained with anti-Ig κ light chain-Alexa 405 (clone 187.1, made in-house) and/or anti-BrdU-FITC (clone3D4; BD Biosciences). For detection of Ova-specific plasma cells, samples stained for surface expression of CD138 were fixed (according to the protocols described above in this section) and subsequently incubated with Ova-Cy5 together with anti-Ig κ light chain-Alexa 405. Analysis was performed by flow cytometry (BD Biosciences LSRII).
Cell count
Absolute cell numbers of spleen and bone marrow were assessed by automatic cell counting (Schaerfe CASY Cell Counter + Analyser Model TT). Absolute plasma cell and megakaryocyte numbers were calculated on the basis of the total cell numbers (by CASY) and the frequencies of these cell types in each organ (by FACS). For quantifying bone marrow, femur cells were counted and total bone marrow cell numbers were calculated on the basis that both femurs together contain approximately 12.6% of total bone marrow cells.36
Plasma cell culture
Cell preparation and culture were performed as described earlier.11 Briefly, after isolation by FACS, CD138++ plasma cells were cultured in 96-well plates in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal calf serum (Sigma-Aldrich). A total of 300 plasma cells per well (250 μL) were cultured at 37°C for 3 days in a humidified incubator. To some wells, IL-6 (10 ng/mL; R&D Systems), TPO (50 ng/mL; PeproTech), or both cytokines were added.
Statistic software
Computation and statistical analysis (unpaired t test, confidence interval 95%, P values) were done with the GraphPad Prism 4 software.
Results
Plasma cells colocalize and interact with megakaryocytes in the bone marrow
To identify the nature of bone marrow plasma cell niches, a long-lived “memory” plasma cell response was induced by secondary immunization with the protein antigen Ova.5 The environments of total and Ova-specific bone marrow plasma cells were investigated by confocal microscopy during the initial immune response (at day 6 and 9 after antigenic rechallenge when Ova-specific plasma cells have accumulated to peak numbers and drop to the plateau levels, respectively37 ) and during the memory phase at day 130. The environments of 150 to 200 individual Ova-specific plasma cells were analyzed at each time point. Plasma cells did not accumulate at particular sites but were scattered throughout the tissue (supplemental Figure 2). In keeping with previous findings by Tokoyoda et al,26 they were typically localized adjacent to fibroblast-like VCAM-1+ stromal cells (data not shown). At all times after immunization, approximately 30% of Ova-specific plasma cells were found in direct contact with large CD41+ megakaryocytes (Figure 1A). Of note, there was a broad variation in the contact frequencies between individual mice, ranging from 10% to 60% (data not shown). Megakaryocytes in contact with plasma cells displayed an advanced stage of maturation, as indicated by their size and ploidy. In common with Ova-specific plasma cells, on average 30% of total (Ig κ light chain positive) bone marrow plasma cells were also seen in contact with megakaryocytes (Figure 1B). Megakaryocytes, like Ova-specific plasma cells induced after immunization, were found mainly in the bone marrow. Both cell types were present in much smaller numbers in the spleen (Figure 2A; supplemental Figure 3). Even in bone marrow, the absolute numbers of each cell type were less than 300 000 (ie, < 0.5% of all nucleated cells).
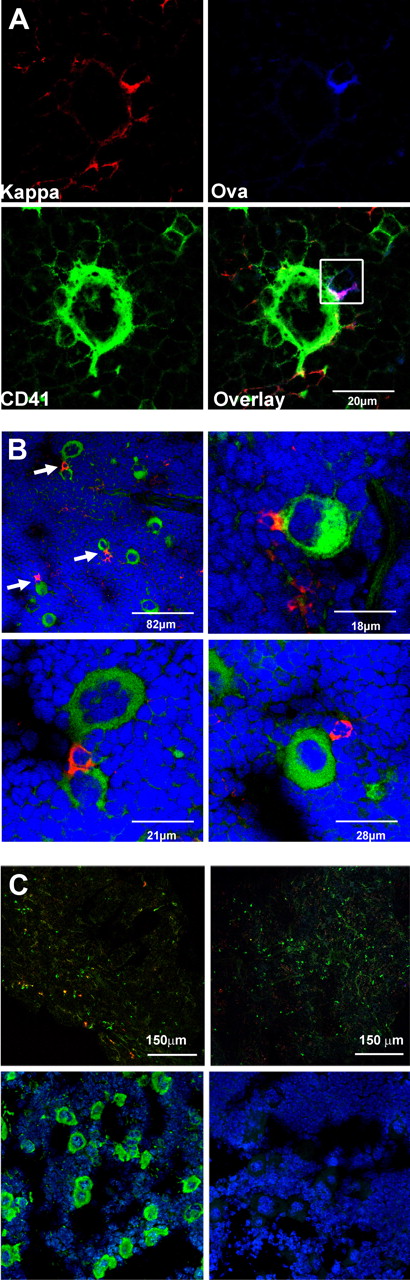
Bone marrow plasma cells are closely associated with megakaryocytes. (A) Cryosections of murine bone marrow (femur) after secondary immunization with Ova. Sections were stained for Ig κ light chain (top left, red), fluorochrome-coupled Ova (top right, blue), and the megakaryocyte marker CD41 (bottom left, green). Overlay of the 3 colors (bottom right) reveals contact between an Ova-specific plasma cell (Ig κ+/Ova binding, red and blue merges to pink) and a large CD41+ megakaryocyte. Analysis was performed with a Leica TCS-SL confocal microscope and processed with Leica software (LCS Lite Version 2.61 Build 1538) and Adobe Photoshop CS3. Approximately 30% of the Ova-specific plasma cells were found to colocalize with megakaryocytes during the whole period of observation (6-150 days after secondary immunization; in total, ∼ 500 Ova-specific plasma cells from 15 mice were analyzed using a 60×/0.135 NA oil objective). (B) Plasma cells and megakaryocytes are frequently found in close contact with each other. Ig κ light chain was used as a marker for plasma cells (“Methods”). Femurs of unimmunized mice were stained for Ig κ light chain (red) and the megakaryocyte marker CD41 (green); nuclei are stained with Sytox-orange (blue). Arrows indicate plasma cells in contact with polyploid CD41+ megakaryocytes (top left). Larger magnification images are shown in the top right and bottom panels (40×/0.95 NA oil objective). (C) Staining controls. (Top panels) Specificity of Ova staining was tested by blocking with unlabeled Ova. (Left section) Bone marrow sections were stained for Ig κ (green) and Ova (red). (A 40×/0.95 NA oil objective was used.) (Right section) No Ova-positive cells were detectable anymore after blockade with unlabeled protein. For that purpose, sections were preincubated with 100 excess unlabeled Ova and subsequently stained for Ig κ (green) and Ova (red). (Bottom panels) Bone marrow section stained for (left) nuclei (blue) and CD41 (green) or (right) rat IgG-isotype control.
Bone marrow plasma cells are closely associated with megakaryocytes. (A) Cryosections of murine bone marrow (femur) after secondary immunization with Ova. Sections were stained for Ig κ light chain (top left, red), fluorochrome-coupled Ova (top right, blue), and the megakaryocyte marker CD41 (bottom left, green). Overlay of the 3 colors (bottom right) reveals contact between an Ova-specific plasma cell (Ig κ+/Ova binding, red and blue merges to pink) and a large CD41+ megakaryocyte. Analysis was performed with a Leica TCS-SL confocal microscope and processed with Leica software (LCS Lite Version 2.61 Build 1538) and Adobe Photoshop CS3. Approximately 30% of the Ova-specific plasma cells were found to colocalize with megakaryocytes during the whole period of observation (6-150 days after secondary immunization; in total, ∼ 500 Ova-specific plasma cells from 15 mice were analyzed using a 60×/0.135 NA oil objective). (B) Plasma cells and megakaryocytes are frequently found in close contact with each other. Ig κ light chain was used as a marker for plasma cells (“Methods”). Femurs of unimmunized mice were stained for Ig κ light chain (red) and the megakaryocyte marker CD41 (green); nuclei are stained with Sytox-orange (blue). Arrows indicate plasma cells in contact with polyploid CD41+ megakaryocytes (top left). Larger magnification images are shown in the top right and bottom panels (40×/0.95 NA oil objective). (C) Staining controls. (Top panels) Specificity of Ova staining was tested by blocking with unlabeled Ova. (Left section) Bone marrow sections were stained for Ig κ (green) and Ova (red). (A 40×/0.95 NA oil objective was used.) (Right section) No Ova-positive cells were detectable anymore after blockade with unlabeled protein. For that purpose, sections were preincubated with 100 excess unlabeled Ova and subsequently stained for Ig κ (green) and Ova (red). (Bottom panels) Bone marrow section stained for (left) nuclei (blue) and CD41 (green) or (right) rat IgG-isotype control.
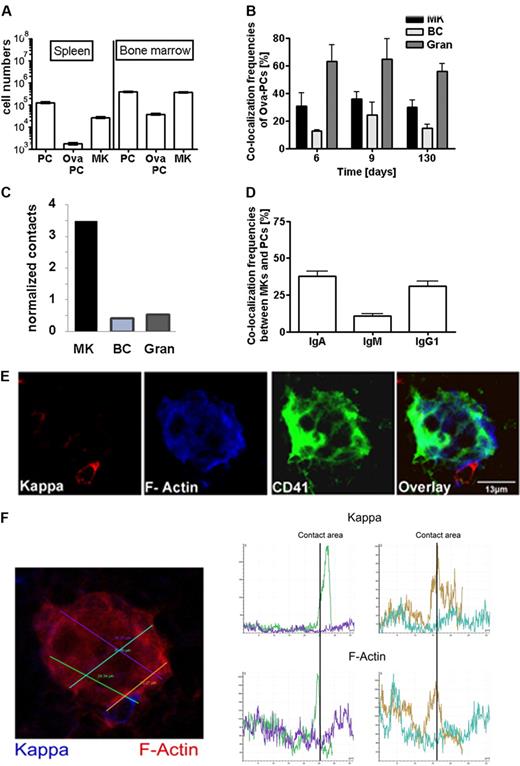
Immunization-induced plasma cells colocalize and interact with megakaryocytes. (A) Distribution of Ova-specific plasma cells and megakaryoctes in spleen and bone marrow. Numbers of total CD138+ plasma cells (PC), Ova-specific CD138+/intracellular Ova-binding plasma cells (Ova-PC), and CD61+ megakaryocytes (MK) were quantified 3 weeks after secondary immunization by flow cytometry (supplemental Figure 3). Absolute cell numbers were calculated from frequencies determined by flow cytometry and total cell numbers per organ as described in “Calculation of contact frequencies per cell surface area.” Data are mean ± SD for groups of 6 mice. (B) Colocalization frequencies between total plasma cells and CD41+ megakaryocytes (MK), B220+ (BC), or Gr-1+ (Gran) cells at days 6, 9, and 130 after secondary immunization. The environments of 150 to 200 plasma cells were analyzed at each time point. B220+ and Gr-1+ cells represent approximately 20% and 30% of total bone marrow cells, whereas megakaryocytes represent only approximately 0.3% of cells in this tissue. (C) Relative contacts between plasma cells and B cells (BC), granulocytes (Gran), or megakaryocytes (MK) normalized on the basis of the surface areas of the respective whole populations as calculated in “Calculation of contact frequencies per cell surface area.” (D) Colocalization frequencies between megakaryocytes and IgA, IgG1, or IgM expressing plasma cells as detected in unimmunized mice. (E) Bone marrow (femur) tissue section stained for CD41 (green), κ light chain (red), and f-actin (blue). (F) F-actin density was quantified along the plasma cell megakaryocyte contact areas using Leica Confocal software (LCS Lite Version 2.61 Build 1538). Histograms show intensity of Ig κ and f-actin staining along the 4 lines within and adjacent to a megakaryocyte indicated on the bone marrow section in corresponding colors (left). The green and brown lines cross the plasma cell megakaryocyte contact areas. Here, f-actin and Ig κ intensity reached a maximum. (Top histograms) Ig kappa. (Bottom histogram) f-actin.
Immunization-induced plasma cells colocalize and interact with megakaryocytes. (A) Distribution of Ova-specific plasma cells and megakaryoctes in spleen and bone marrow. Numbers of total CD138+ plasma cells (PC), Ova-specific CD138+/intracellular Ova-binding plasma cells (Ova-PC), and CD61+ megakaryocytes (MK) were quantified 3 weeks after secondary immunization by flow cytometry (supplemental Figure 3). Absolute cell numbers were calculated from frequencies determined by flow cytometry and total cell numbers per organ as described in “Calculation of contact frequencies per cell surface area.” Data are mean ± SD for groups of 6 mice. (B) Colocalization frequencies between total plasma cells and CD41+ megakaryocytes (MK), B220+ (BC), or Gr-1+ (Gran) cells at days 6, 9, and 130 after secondary immunization. The environments of 150 to 200 plasma cells were analyzed at each time point. B220+ and Gr-1+ cells represent approximately 20% and 30% of total bone marrow cells, whereas megakaryocytes represent only approximately 0.3% of cells in this tissue. (C) Relative contacts between plasma cells and B cells (BC), granulocytes (Gran), or megakaryocytes (MK) normalized on the basis of the surface areas of the respective whole populations as calculated in “Calculation of contact frequencies per cell surface area.” (D) Colocalization frequencies between megakaryocytes and IgA, IgG1, or IgM expressing plasma cells as detected in unimmunized mice. (E) Bone marrow (femur) tissue section stained for CD41 (green), κ light chain (red), and f-actin (blue). (F) F-actin density was quantified along the plasma cell megakaryocyte contact areas using Leica Confocal software (LCS Lite Version 2.61 Build 1538). Histograms show intensity of Ig κ and f-actin staining along the 4 lines within and adjacent to a megakaryocyte indicated on the bone marrow section in corresponding colors (left). The green and brown lines cross the plasma cell megakaryocyte contact areas. Here, f-actin and Ig κ intensity reached a maximum. (Top histograms) Ig kappa. (Bottom histogram) f-actin.
Plasma cell contacts to B220+ B cells and Gr-1+ granulocytes were also frequently found (Figure 2B). However, both cell types are approximately 100-fold more abundant in bone marrow than megakaryocytes (ie, ∼ 20% B220+ B cells and 30% Gr-1+ granulocytes cells compared with 0.3% CD41+ megakaryocytes). Normalized on population and individual cell sizes, the relative plasma cell contact frequencies were calculated to be 0.42 for B cells, 0.53 for granulocytes, and 3.5 for megakaryocytes (Figure 2C). This result is clearly different from what is expected if all 3 cell types make random contacts to plasma cells. Instead, this indicates that contacts between plasma cells and megakaryocytes were approximately 7-fold overrepresented, thus reflecting specific colocalization. Interestingly, bone marrow plasma cells expressing isotype switched antibodies of the IgA and IgG subclasses more frequently colocalized with megakaryocytes than plasma cells expressing IgM (Figure 2D).
To test directly for cell-cell interaction, f-actin expression was analyzed at the contact areas between plasma cells and megakaryocytes (Figure 2E). Quantitative analysis showed that f-actin expression peaked at these contact areas (Figure 2F), as characteristic for cell-cell junctions in-between interacting cells.38 In conclusion, class-switched plasma cells and megakaryocytes share a microenvironmental niche in the bone marrow and interact with each other.
Megakaryocytes are a local source for the plasma cell growth factors APRIL and IL-6
Multiple cytokines and contact-mediated signals are required for the terminal differentiation and maintenance of bone marrow plasma cells. Among the most important plasma cell growth and survival factors are IL-6, APRIL, and TNF-α.11,13,14,27 Although the main function of megakaryocytes is the production and release of platelets, there is multiple evidence that in vitro generated megakaryocytes can also produce and secrete several cytokines, including the 3 plasma cell survival factors IL-6, APRIL, and TNF-α.29-31 Here, we demonstrated that IL-6 is produced by all bone marrow megakaryocytes in vivo, as shown by flow cytometry and confocal microscopy (Figure 3). Moreover, comparing the expression of this cytokine in CD41+ and CD41− cells by flow cytometry indicates that CD41+ megakaryocytes constitute approximately one-third of all nucleated cells that constitutively express high levels of IL-6 in the bone marrow. This identifies megakaryocytes as a major source for IL-6 in this tissue. The production of high levels of IL-6 was confirmed by quantitative PCR. By this method, moderate levels of APRIL expression were also detected in megakaryocytes (Figure 3C). On tissue sections, megakaryocytes stained for both cytokines, although APRIL staining was not as bright as the IL-6 signals (Figure 3D; supplemental Figure 4). It has been shown that IL-6 very efficiently supports the survival of isolated plasma cells in culture11,13 and that APRIL is essential for establishing a long-lived plasma cell response in the bone marrow in vivo.11,13,14,27 The capacity to produce these cytokines, together with their physical contact to bone marrow plasma cells, suggests that megakaryocytes support the growth and survival of neighboring plasma cells, thus contributing to their microenvironmental niche.
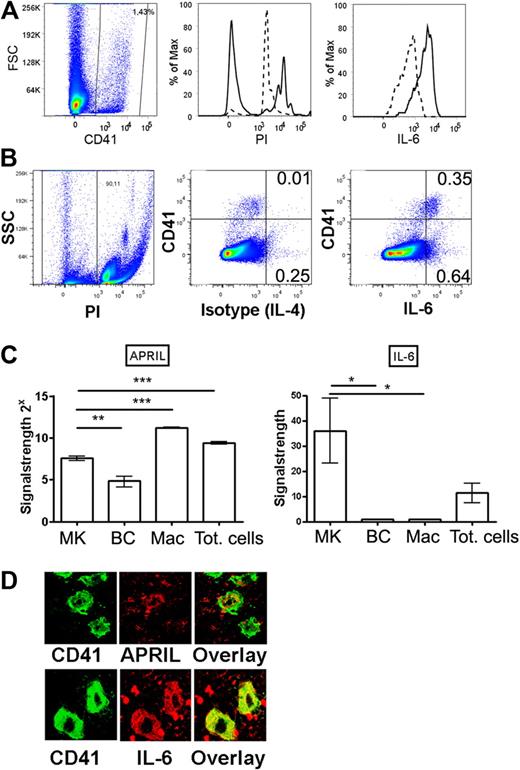
Megakaryocytes produce APRIL and IL-6 in vivo. (A) Bone marrow megakaryocytes were identified by flow cytometry according to CD41 staining (left) and propidium iodide incorporation (middle, as a measure for polyploidy). Propidium iodide content of total bone marrow cells (dashed line, 2n-stadium) was used as a standard. Polyploid CD41+ megakaryocytes (n > 2) were stained for IL-6 (solid line) or isotype control (IL-4, dashed line). (B) Dot plot showing IL-6 or isotype control (IL-4) versus CD41. (C) Megakaryocytes, B cells, and macrophage cells were isolated by FACS, and IL-6 and APRIL expression was determined by quantitative PCR as indicated. MK indicates CD41+ megakaryocyte; BC, B220+ B cell; Mac, CD11b+/F4/80+/Gr-1− macrophages; and Tot, total bone marrow cells. (D) Megakaryocytes were identified on bone marrow sections (femur) by CD41 staining (green) and counterstained for either APRIL or IL-6 (red) as indicated. Controls for IL-6 and APRIL stainings are shown in supplemental Figure 4.
Megakaryocytes produce APRIL and IL-6 in vivo. (A) Bone marrow megakaryocytes were identified by flow cytometry according to CD41 staining (left) and propidium iodide incorporation (middle, as a measure for polyploidy). Propidium iodide content of total bone marrow cells (dashed line, 2n-stadium) was used as a standard. Polyploid CD41+ megakaryocytes (n > 2) were stained for IL-6 (solid line) or isotype control (IL-4, dashed line). (B) Dot plot showing IL-6 or isotype control (IL-4) versus CD41. (C) Megakaryocytes, B cells, and macrophage cells were isolated by FACS, and IL-6 and APRIL expression was determined by quantitative PCR as indicated. MK indicates CD41+ megakaryocyte; BC, B220+ B cell; Mac, CD11b+/F4/80+/Gr-1− macrophages; and Tot, total bone marrow cells. (D) Megakaryocytes were identified on bone marrow sections (femur) by CD41 staining (green) and counterstained for either APRIL or IL-6 (red) as indicated. Controls for IL-6 and APRIL stainings are shown in supplemental Figure 4.
Formation of bone marrow plasma cell compartments is impaired in c-mpl−/− mice
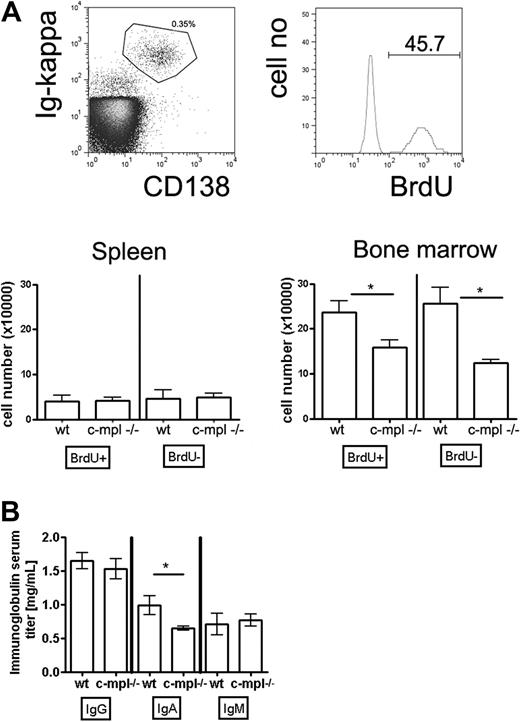
To address the question of whether megakaryocytes contribute to the function of a plasma cell niche as indicated by the experiments described in “Results” above, we analyzed plasma cell compartments in c-mpl−/− mice. c-mpl is the receptor for TPO, the major growth factor for megakaryocyte lineage cells, but is absent on mature leukocytes.28,32 Accordingly, it has been shown that megakaryocyte populations are 8-fold reduced in c-mpl−/− compared with wild-type mice, whereas populations of other mature hematopoietic cell types and total cell numbers in spleen and bone marrow are normal.28,32 In the present study, we further demonstrated that neither mature B cells nor plasma cells express c-mpl (Figure 4A). Consequently, isolated bone marrow plasma cells did not respond to TPO (Figure 4B). Thus, a direct effect of c-mpl/TPO-mediated signaling on plasma cells can be ruled out, making c-mpl−/− mice suitable for testing whether impaired megakaryopoiesis affects plasma cell responses. To distinguish mature nonproliferating plasma cells from proliferating precursors, mice were continuously fed BrdU with the drinking water for 3 weeks. This base analog is efficiently incorporated into the DNA of plasma cell precursors going through S phase of the cell cycle but leaves mature nonproliferating plasma cells unlabeled.9 In c-mpl−/− mice, the absolute numbers of immature and mature bone marrow plasma cells were reduced compared with wild-type mice (Figure 5A). In the spleen, a major site of B-cell activation and initiation of plasma cell growth, immature and mature plasma cell counts were normal (Figure 5A). These findings indicate that the formation of plasma cells in c-mpl−/− mice is normal, whereas the establishment of the bone marrow plasma cell compartment is disturbed. Although bone marrow is the major site for the persistence of long-lived plasma cells, it is only one organ among many others where IgG antibodies are produced. Therefore, a partial reduction of bone marrow plasma cells as detected in the c-mpl−/− mice did not result in a significant reduction of IgG serum antibodies. Interestingly, serum IgA was reduced in these mice (Figure 5B). Although the mucosa is the major site for the production of the dimeric form of IgA found on mucosal surfaces, it still needs to be elucidated whether monomeric IgA found in serum is maintained by plasma cells of mucosal tissues or of bone marrow.2 The reduction of serum IgA levels resulting from an impaired bone marrow plasma cell compartment in c-mpl mice may suggest that this organ is a major source for the production of monomeric IgA.
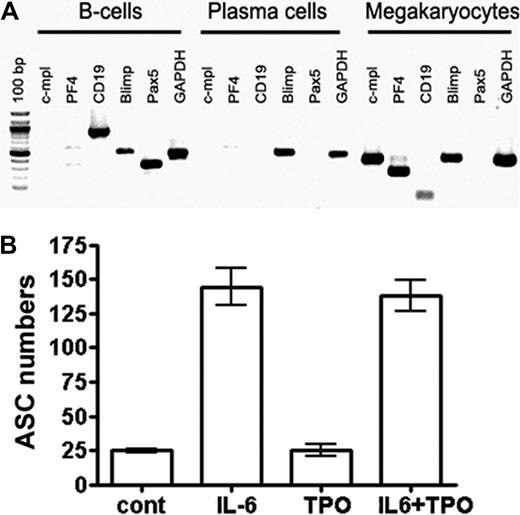
IL-6, but not TPO, directly stimulates plasma cells. (A) Neither B cells nor plasma cells express the TPO receptor c-mpl. B220+/IgD+ B cells and CD138++ plasma cells were isolated from bone marrow by FACS, and expression of the indicated genes was determined by reverse-transcribed PCR. In vitro–generated megakaryocytes were used as a high control for c-mpl expression. (B) Plasma cells do not respond to TPO. CD138++ plasma cells were isolated from bone marrow and cultured as described earlier.11 Total ASCs were quantified by ELISPOT. The percentage of plasma cells (ASCs) surviving for 3 days is shown when these cells were cultured: in the absence of specific stimulation, with either IL-6 or TPO alone, or with a combination of the 2 cytokines. Data are representative of 2 independent experiments. TPO was used in concentrations sufficient for the stimulation of megakaryopoiesis (supplemental Figure 8).
IL-6, but not TPO, directly stimulates plasma cells. (A) Neither B cells nor plasma cells express the TPO receptor c-mpl. B220+/IgD+ B cells and CD138++ plasma cells were isolated from bone marrow by FACS, and expression of the indicated genes was determined by reverse-transcribed PCR. In vitro–generated megakaryocytes were used as a high control for c-mpl expression. (B) Plasma cells do not respond to TPO. CD138++ plasma cells were isolated from bone marrow and cultured as described earlier.11 Total ASCs were quantified by ELISPOT. The percentage of plasma cells (ASCs) surviving for 3 days is shown when these cells were cultured: in the absence of specific stimulation, with either IL-6 or TPO alone, or with a combination of the 2 cytokines. Data are representative of 2 independent experiments. TPO was used in concentrations sufficient for the stimulation of megakaryopoiesis (supplemental Figure 8).
Immature and mature bone marrow plasma cell populations are disturbed in c-mpl−/− mice. Mice were continuously fed with BrdU in the drinking water for 3 weeks. Subsequently, CD138++/ intracellular κ light chain+ plasma cells were analyzed for BrdU content and quantified by flow cytometry. (A) Top panels: Flow cytometric identification of CD138+/Ig κ+ bone marrow plasma cells and analysis of BrdU incorporation. Bottom panels: Absolute numbers of BrdU+ and BrDU− plasma cells in spleen and bone marrow of wild-type and c-mpl−/− mice as indicated. (B) Ig serum levels of the IgG, IgA, and IgM isotypes in c-mpl−/− and wild-type mice as measured by enzyme-linked immunosorbent assay. Data are mean ± SD for absolute plasma cell numbers of groups of 5 mice (statistics: unpaired t test).
Immature and mature bone marrow plasma cell populations are disturbed in c-mpl−/− mice. Mice were continuously fed with BrdU in the drinking water for 3 weeks. Subsequently, CD138++/ intracellular κ light chain+ plasma cells were analyzed for BrdU content and quantified by flow cytometry. (A) Top panels: Flow cytometric identification of CD138+/Ig κ+ bone marrow plasma cells and analysis of BrdU incorporation. Bottom panels: Absolute numbers of BrdU+ and BrDU− plasma cells in spleen and bone marrow of wild-type and c-mpl−/− mice as indicated. (B) Ig serum levels of the IgG, IgA, and IgM isotypes in c-mpl−/− and wild-type mice as measured by enzyme-linked immunosorbent assay. Data are mean ± SD for absolute plasma cell numbers of groups of 5 mice (statistics: unpaired t test).
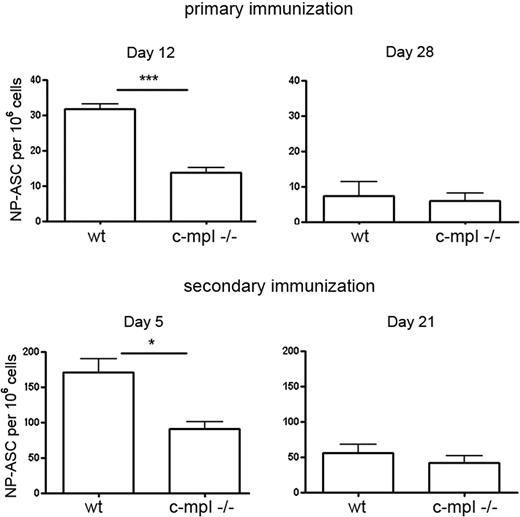
To further investigate the plasma cell response in c-mpl−/− mice, mice were immunized with the T-dependent antigen NP-KLH, inducing a predominant IgG1 response.35 At day 12 after primary and day 5 after secondary immunization, antigen-specific plasma cells formed in the spleen accumulated in the bone marrow. At these times, the frequencies of NP-specific IgG1 ASCs in the bone marrow of c-mpl−/− mice were reduced compared with control mice (Figure 6). The initiation of the NP-specific plasma cell response in the spleen was not affected (supplemental Figure 5). These results indicate that megakaryocyte lineage cells support the accumulation of early plasma cells into the bone marrow compartment. Later, NP-specific IgG1-ASC frequencies in the bone marrow of c-mpl−/− mice reached levels comparable with that in wild type (Figure 6).
Delayed bone marrow plasma cell response in c-mpl−/− mice. Wild-type and c-mpl−/− mice were immunized with NP-KLH that induces a predominant IgG1 response. NP-specific IgG1-ASCs in bone marrow were quantified at day 12 and day 28 after primary and day 5 and day 21 after secondary immunization by ELISPOT. Frequencies of NP-IgG1-ASCs in the spleen were normal in the c-mpl−/− mice (supplemental Figure 5). Data are mean ± SD for groups of 3 to 5 mice (statistics: unpaired t test).
Delayed bone marrow plasma cell response in c-mpl−/− mice. Wild-type and c-mpl−/− mice were immunized with NP-KLH that induces a predominant IgG1 response. NP-specific IgG1-ASCs in bone marrow were quantified at day 12 and day 28 after primary and day 5 and day 21 after secondary immunization by ELISPOT. Frequencies of NP-IgG1-ASCs in the spleen were normal in the c-mpl−/− mice (supplemental Figure 5). Data are mean ± SD for groups of 3 to 5 mice (statistics: unpaired t test).
Increased TPO levels support the formation and persistence of bone marrow plasma cells
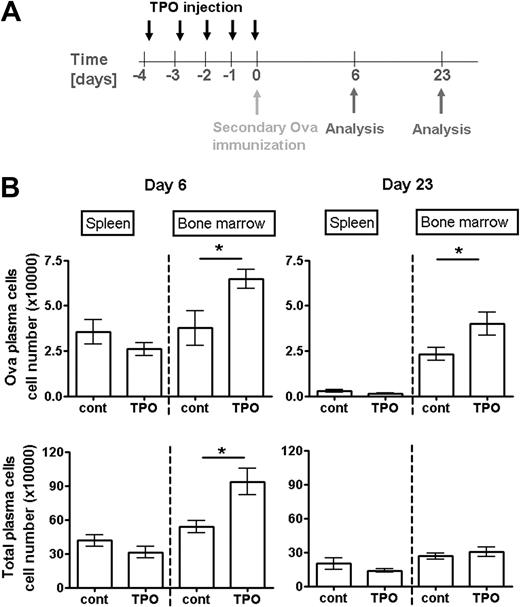
Systemic levels of TPO are the major regulator of megakaryopoiesis under physiologic conditions.28,32 To get further insight into the role of megakaryocytes for the regulation of plasma cell responses, we induced a transient increase in megakaryopoiesis by the injection of TPO.39 As described,40 this treatment led to the generation of new megakaryocytes and prolonged megakaryocyte maturation, hence to a transient increase in total megakaryocyte numbers lasting for approximately 10 days. Thereafter, megakaryocyte counts in the bone marrow dropped to homeostatic levels, mainly through the reduction of highly mature polyploid megakaryocytes, whereas newly formed cells of lower ploidy remained in this tissue (supplemental Figure 6). Four days after the initial TPO injection, a plasma cell response was induced by secondary immunization with Ova. Between day 3 and day 6 after Ova immunization, Ova-specific plasma cells, formed in the spleen, accumulate in the bone marrow.37 TPO injection had no effect on Ova-specific plasma cell numbers in the spleen at the peak response at day 6 after immunization (Figure 7). By contrast, Ova-specific bone marrow plasma cells reached approximately 2-fold higher numbers in TPO-treated mice than in controls (Figure 7). Three weeks later, when megakaryocyte and total plasma cell numbers were no longer augmented by immunization and TPO injection, Ova-specific plasma cells were still present in increased numbers in the bone marrow of TPO-treated mice (Figure 7). In contrast to megakaryocytes and plasma cells, other major hematopoietic bone marrow populations were not increased after TPO injection. Primitive hematopoietic cells (Lin−/c-kit+/Sca-1+), monoctye (CD11b+), and granulocyte (Gr-1+) lineage cells were rather diminished at day 4 or 7 after starting the TPO treatment (supplemental Figure 7). Because TPO does not directly stimulate plasma cell survival, these findings suggest that megakaryocyte lineage cells play an important role for establishing plasma cell populations in the bone marrow. Moreover, they directly demonstrate that a transient change in TPO levels modulates the formation and persistence of plasma cell memory.
Increased levels of systemic TPO support the accumulation and persistence of plasma cells formed in a specific immune response. (A) Scheme of TPO injection and immunization: Mice received TPO (in PBS) starting from day 4 before immunization as described in “Mice, immunizations, TPO treatment, and BrdU feeding.” Controls received PBS alone. (B) Absolute numbers of total (bottom panels) and Ova-specific (top panels) plasma cells in spleen and bone marrow after secondary immunization are shown at the peak of the anti-Ova response at day 6 (left panels) and day 23 (right panels) when plasma cell counts have reached their plateau. Ova-specific plasma cells were analyzed by flow cytometry. Data are mean ± SD for groups of 6 mice (statistics: unpaired t test).
Increased levels of systemic TPO support the accumulation and persistence of plasma cells formed in a specific immune response. (A) Scheme of TPO injection and immunization: Mice received TPO (in PBS) starting from day 4 before immunization as described in “Mice, immunizations, TPO treatment, and BrdU feeding.” Controls received PBS alone. (B) Absolute numbers of total (bottom panels) and Ova-specific (top panels) plasma cells in spleen and bone marrow after secondary immunization are shown at the peak of the anti-Ova response at day 6 (left panels) and day 23 (right panels) when plasma cell counts have reached their plateau. Ova-specific plasma cells were analyzed by flow cytometry. Data are mean ± SD for groups of 6 mice (statistics: unpaired t test).
Discussion
Our results demonstrate that bone marrow plasma cells share a microenvironmental niche with megakaryocytes, the latter producing important factors supporting plasma cell growth and survival. Moreover, increased megakaryopoiesis supports the accumulation and persistence of newly formed plasma cells, whereas c-mpl−/− mice, which exhibit reduced megakaryopoiesis, show an impaired plasma cell response in the bone marrow. Together, these results demonstrate that megakaryocytes constitute a functional component of a plasma cell niche in this tissue.
The finding that only approximately 30% of bone marrow plasma cells colocalize with megakaryocytes may suggest that the contact between plasma cells and megakaryocytes is transient. Alternatively, there might be niches in which megakaryocytes are substituted by other cell types. In accordance with the latter hypothesis, other bone marrow cells (eg, macrophages) can also produce plasma cell growth factors.27
c-mpl−/− mice display delayed plasma cell accumulation after immunization, whereas intensified megakaryopoiesis after TPO injection supports the accumulation of bone marrow plasma cells in wild-type mice. These findings suggest that megakaryocytes support robust plasma cell formation in this tissue. In accordance, Ova-specific plasma cells and megakaryocytes colocalize already early after immunization, when plasma cells accumulate in the bone marrow. APRIL has been shown to be essential for establishing bone marrow plasma cell responses14 ; it seems probable that megakaryocyte-derived APRIL increases the local concentration of this important factor. However, the production of additional plasma cell growth and survival factors, such as IL-6, or direct cell-cell contact may also play a role. Particularly megakaryocyte-derived IL-6 is probably of importance, as indicated by the finding that megakaryocytes constitute a major source for this cytokine in the bone marrow.
Five months after immunization, Ova-specific plasma cells were still in contact with megakaryocytes, in frequencies similar to those early after immunization. These plasma cells are mature and long-lived.5 Aside from promoting the survival of immature plasma cells, which is important for the initiation of an antibody response,27,41 the cytokines APRIL and IL-6 are potent survival factors for mature plasma cells.11,14 This suggests that megakaryocytes also contribute to the survival of plasma cells localized within the same microenvironment. However, in c-mpl−/− mice, NP-specific plasma cell counts normalize late after immunization, possibly indicating that either megakaryocytes play a minor role in maintaining the bone marrow plasma cell population or that the function of megakaryocytes could be mediated by alternative cell types. Alternatively, the small megakaryocyte population still present in c-mpl−/− bone marrow might be sufficient to support plasma cell accumulation, although less efficiently.
Megakaryopoiesis is a continuous process, indicating that megakaryocytes constitute a dynamic component of plasma cell niches. How can newly formed megakaryocytes migrate to this specific microenvironment? Earlier work by Tokoyoda et al showed that approximately 90% of IgG+ bone marrow plasma cells reside adjacent to a particular type of reticular stromal cell that expresses high levels of CXCL12,26 a chemokine important for the accumulation of plasma cells in bone marrow.37,42 Likewise, CXCL12 also defines the localization of megakaryocytes in this tissue.43 It seems plausible that the stromal cell described by Tokoyoda et al26 provides a meeting point for plasma cells and megakaryocytes, with the latter continuously being replaced by new ones.
Systemic TPO levels are the major regulator of megakaryopoiesis under physiologic conditions.28,32 After injection of TPO, newly formed plasma cells accumulated and persisted in larger numbers. Platelet counts in blood are altered during severe infection and chronic inflammation, indicating a modulation of megakaryopoiesis under these conditions.33 Together with the findings presented here, the following model could be proposed: alterations in megakaryopoiesis change the availability of megakaryocytes in their microenvironmental niche shared by bone marrow plasma cells. Because megakaryocytes provide an important local source for IL-6 and other cytokines that stimulate plasma cell differentiation and survival, the conditions for plasma cells accumulating in the bone marrow change when megakaryopoiesis is modified (eg, during infection/inflammation). Thus, the rate of megakaryopoiesis could serve as a sensor that allows adjusting the generation of bone marrow plasma cells in correspondence to the current systemic immune status. Consistent with this idea, severe infections are very potent, not only in inducing initial antibody responses but also in maintaining antibody memory.44
Together with previous findings,27,45 our results demonstrate that various hematopoietic cell types, in addition to fibroblast-like cells, contribute to bone marrow niches. The identification of megakaryocytes as a component of a plasma cell niche in bone marrow establishes a novel link between plasma cell memory and megakaryopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mahmood Khan for his excellent help in establishing bone marrow tissue sections, Silke Fleischhauer for performing PCR analysis of c-mpl, Warren Alexander for providing c-mpl−/− mice, Stephen Nutt for providing Blimp-1-GFP reporter mice, and Raif Geha for providing APRIL−/− mice.
This work was supported by the Deutsche Forschungs Gemeinschaft (grants MA 2273/4-2 and MA 2273/5-1) and the Excellence Cluster “Inflammation at Interfaces.” E.M and I.C.M.M. were supported by the British Medical Research Council (program grant).
Authorship
Contribution: I.C.M.M., D.M.T., and R.A.M. designed research; O.W., K.M., E.M., D.Z., H.K., K.R., M.S., C.D., H.S., and D.M.W. performed research; O.W., R.A.M., E.M., and D.M.T. analyzed data; and O.W., I.C.M.M., and R.A.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rudolf A. Manz, University of Lübeck, Institute for Systemic Inflammation Research, ISEF, Ratzeburger Allee 160, 23583 Lübeck, Germany; e-mail: Rudolf.Manz@uk-sh.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal