Abstract
The mechanisms responsible for the brief life span of blood platelets have been a subject of speculation since the 1950s. The most popular hypothesis to date has been the “multiple-hit” model, whereby damage inflicted by external “hits” triggers recognition and clearance by the reticuloendothelial system. Recently, it was demonstrated that platelets contain an apoptotic pathway that mediates their survival in vivo. Using a novel labeling technique to measure population and cohort survival in mice carrying mutations in this pathway, combined with mathematical modeling, we have studied the internal and external control of platelet fate. Our results cast doubt on the veracity of the multiple-hit model. An alternative model, under which platelets are born with an internal “timer,” provides a more parsimonious interpretation of the data. Thus, at steady state, platelet senescence is probably the product of internal processes rather than external hits.
Introduction
Platelets are small anucleate blood cells whose primary function is to facilitate clot formation at sites of vascular injury. They are the progeny of megakaryocytes, large polyploid cells that develop in the bone marrow and release platelets into the circulation. Although a small fraction of human platelets fulfill their raison d'être by being consumed in blood clots,1 the majority become senescent and are cleared by the reticuloendothelial system in the liver and spleen after approximately 10 days in the circulation. This finite existence was first noted in the 1950s when platelet transfusions were given to leukemia patients.2 The most popular hypothesis to explain platelet senescence at steady state has been the “multiple-hit” model proposed by Mustard et al in 1966.3 In this model, platelets accumulate damage over time via “hits” inflicted by the circulatory environment. Each platelet is able to endure a certain number of hits before being recognized and cleared by the reticuloendothelial system. In a groundbreaking series of papers, Murphy and Francis formulated the multiple-hit model into a mathematical form.4-6 The 2 mechanistic assumptions of the model, that (1) platelets can withstand a fixed number of hits and (2) hits occur at a constant rate, lead to a gamma distribution of life span. The multiple-hit model provides an elegant explanation for why platelets age; however, the precise nature of the hits and the veracity of the model have never been established. New light on the problem came from recent work demonstrating that platelets contain an apoptotic pathway that controls life span at steady state.7 The key components of the pathway are the Bcl-2 family proteins Bcl-xL and Bak. The balance between them dictates whether a platelet lives or dies: mutations in prosurvival Bcl-xL reduce life span in vivo, whereas mutations in prodeath Bak extend it. Transfusion experiments demonstrated that these effects are cell-intrinsic, so it is clear that the transition from life to death is mediated by an internal mechanism. However, the signal that initiates the transition is unknown. Do multiple external hits instruct a platelet to die? Or does the platelet contain an internal timer that triggers entry into apoptotic death?
Methods
The materials and methods used in this study are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All animal experiments complied with the regulatory standards of, and were approved by, the Walter and Eliza Hall Institute Animal Ethics Committee.
Results and discussion
We asked whether the multiple-hit model is consistent with what is now known at the molecular level about the control of platelet life span by apoptotic proteins. Ostensibly, the rate of hits is an extrinsic parameter, determined by the environment, whereas the number of hits platelets can withstand is a cell-intrinsic parameter. Hence, if the multiple-hit model were to hold true, we expected to find that fewer hits are required to kill Bcl-xL mutant platelets and more to kill Bak mutants, thereby explaining their shorter or longer life spans, respectively. To test this prediction, we developed a new method of double-labeling platelets that allowed us to simultaneously follow the in vivo survival of both the entire population of platelets, and a young cohort born within a 24-hour period (supplemental Figure 1). The method involves first injecting a fluorescent label specific for platelets, X488 (Emfret Analytics) followed by the standard technique of in vivo biotinylation 24 hours later.8 Regular blood sampling and flow cytometric analysis allowed the generation of simultaneous “population” (X488+) and “cohort” (X488−, biotin+) survival curves. As expected,9 the cohort exhibited a delay before clearance, relative to the population, demonstrating the age-dependent likelihood of clearance (supplemental Figure 1B).
Figure 1A through C illustrates fits of the multiple-hit model to double-labeling data from wild-type and mutant mice. The 2 parameters governing the multiple-hit model (the rate of hits and the number of hits required for clearance) were extracted from the fits and plotted (Figure 1D). Although small differences were found in the number of hits, the results did not follow the expected pattern (ie, Bcl-x+/Plt20 platelets, which have a reduced life span, appear to be able to withstand slightly more hits than wild-type). Instead, we found that under this model, it is primarily the rate of hits that leads to the differences in mean life span observed between the genotypes. This result is reinforced in Figure 1E. Here the population survival curves for the 3 genotypes are seen to almost perfectly overlay when time is scaled by mean life span for each genotype (extracted from the best fit). If the number of hits were different, but not the rate of hits, the survival curves would take different shapes and could not be overlaid in this manner. We would instead see the situation depicted in Figure 1F, where with more hits the linear part of the curve extends for longer, or with fewer hits the “tail” becomes more marked. Thus, although both the rate of hits and the number of hits can affect mean life span, only the number of hits affects the coefficient of variation and hence the shape of the curve when scaled by mean life span. The 2 parameters can therefore be distinguished by curve fitting.
Steady-state platelet survival data in mutant mice cast doubt on the multiple-hit model. Best fits (minimized sum of squared residuals) for populations and cohorts of platelets in (A) Bcl-x+/Plt20, (B) wild-type, and (C) Bak−/− mice. Data points are the mean of replicates (4 for Bcl-x+/Plt20, 6 for wild-type and Bak−/−), and error bars represent one SD. Arrows indicate the time of biotin injection to establish the cohort label (24 hours). The efficiency of labeling (∼ 90% for X488 and ∼ 60% for biotin) and the half-life of biotin were accounted for in the fitting procedure (“Methods” and supplemental data). (D) Monte-Carlo bootstrap resampling was performed for each genotype to generate estimates of the best-fit model parameters: hit rate, τ−1, and number of hits, k (20 samples; horizontal line and error bars represent median and interquartile range). The differences between any 2 genotypes are statistically significant (P < .001, 2-tailed unpaired t test) for either parameter; however, the magnitudes of the differences are clearly greater for the rate of hits. (E) Model fits to population survival data with label scaled by the initial percentage and time scaled by mean life span extracted from the best fits (curves overlay, same shape). (F) Expected (theoretical) curves if the rate of hits were the same in Bcl-x+/Plt20 or Bak−/− mice as in wild-type, and the decreased or increased mean life span were instead the result of differences in the number of hits that could be endured (note the shape change). Numerical values for the best-fit model parameters are reported in supplemental Table 1.
Steady-state platelet survival data in mutant mice cast doubt on the multiple-hit model. Best fits (minimized sum of squared residuals) for populations and cohorts of platelets in (A) Bcl-x+/Plt20, (B) wild-type, and (C) Bak−/− mice. Data points are the mean of replicates (4 for Bcl-x+/Plt20, 6 for wild-type and Bak−/−), and error bars represent one SD. Arrows indicate the time of biotin injection to establish the cohort label (24 hours). The efficiency of labeling (∼ 90% for X488 and ∼ 60% for biotin) and the half-life of biotin were accounted for in the fitting procedure (“Methods” and supplemental data). (D) Monte-Carlo bootstrap resampling was performed for each genotype to generate estimates of the best-fit model parameters: hit rate, τ−1, and number of hits, k (20 samples; horizontal line and error bars represent median and interquartile range). The differences between any 2 genotypes are statistically significant (P < .001, 2-tailed unpaired t test) for either parameter; however, the magnitudes of the differences are clearly greater for the rate of hits. (E) Model fits to population survival data with label scaled by the initial percentage and time scaled by mean life span extracted from the best fits (curves overlay, same shape). (F) Expected (theoretical) curves if the rate of hits were the same in Bcl-x+/Plt20 or Bak−/− mice as in wild-type, and the decreased or increased mean life span were instead the result of differences in the number of hits that could be endured (note the shape change). Numerical values for the best-fit model parameters are reported in supplemental Table 1.
This result suggested that the multiple-hit model as stated cannot explain platelet senescence. The mutations in Bcl-xL and Bak cause platelet-intrinsic changes in life span. It seems unlikely that they could affect either the circulatory environment, or, given their roles as regulators of apoptosis, the rate at which platelets experience hits. We therefore assessed the age-dependent decline in mean platelet size, a phenomenon that, although contentious, has been ascribed to the effect of mechanical hits.10-18 If the rate of hits were truly different between genotypes, then one should expect to find marked differences in the rate of decline in size of mutant platelets. We found this was not the case (supplemental Figure 2A). To test the hypothesis that the spleen plays a major role in inflicting hits,19 we examined platelet life span in mice that had been splenectomized. We found that splenectomy had negligible effect on the survival of platelets (supplemental Figure 2B-D), consistent with some,20,21 but not all,19,22 previous reports. Thus, several lines of evidence cast doubt on the multiple-hit model.
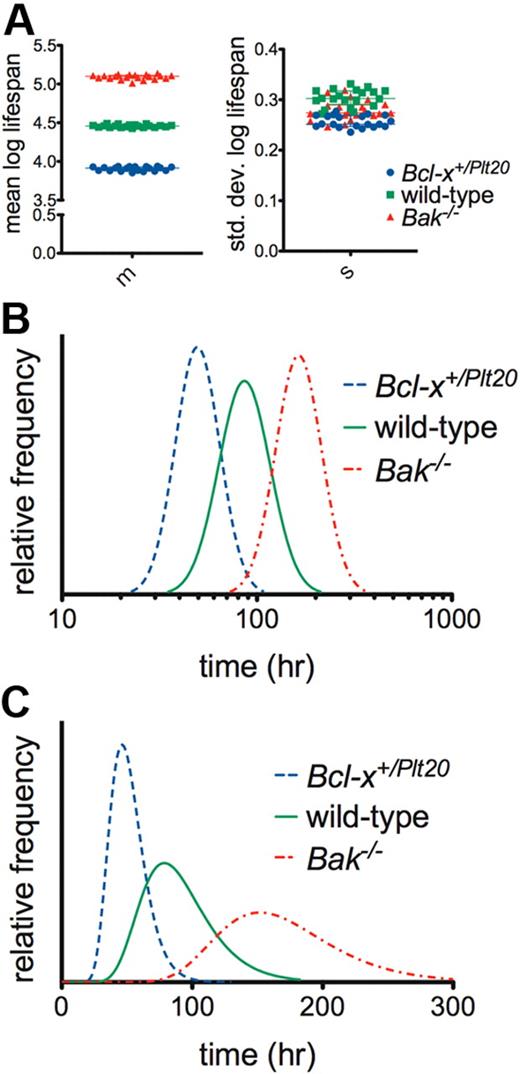
We therefore propose an alternative model for platelet senescence whereby platelets are born with an internal “timer,” key components of which include Bcl-xL and Bak. We assumed, based on empirical measurements in other systems,23,24 that the pattern of variation in life span conforms to a skewed right distribution and adopted the log-normal as the exemplar (ie, normally distributed when plotted on a log scale). We refer to this as the log-normal-senescent (LS) model.4,25,26 It is apparent from Figure 2 that the LS model offers a natural interpretation of the differences between the genotypes: the mean log life span is altered, but not the SD of log life span (Figure 2A). This result is most strikingly illustrated in Figure 2B, where comparing the genotypes on a log scale, the underlying life span distribution shifts to the right with little change in shape, suggesting that the mutations simply alter the timescale of a conserved mechanism of clearance (this quantitative relationship is not obvious on a linear scale, Figure 2C).
The log-normal senescent model indicates that platelet life span is internally controlled. (A) Monte Carlo bootstrap resampling was performed to generate estimates of the best-fit model parameters for wild-type, Bcl-x+/Plt20, and Bak−/− mice. m indicates mean log life span; and s, SD of log life span (20 samples; horizontal line and error bars represent median and interquartile range). The quality of fits was very similar to those obtained with the multiple-hit model (supplemental Figure 3). The differences between any 2 genotypes are statistically significant (P < .001, 2-tailed unpaired t test) for either parameter; however, the magnitudes of the differences are clearly greater in the mean of log life span. (B) Best-fit log-normal life span distributions with time on a log scale. (C) Best-fit log-normal life span distributions with time on a linear scale. Numerical values for the best-fit model parameters are reported in supplemental Table 2.
The log-normal senescent model indicates that platelet life span is internally controlled. (A) Monte Carlo bootstrap resampling was performed to generate estimates of the best-fit model parameters for wild-type, Bcl-x+/Plt20, and Bak−/− mice. m indicates mean log life span; and s, SD of log life span (20 samples; horizontal line and error bars represent median and interquartile range). The quality of fits was very similar to those obtained with the multiple-hit model (supplemental Figure 3). The differences between any 2 genotypes are statistically significant (P < .001, 2-tailed unpaired t test) for either parameter; however, the magnitudes of the differences are clearly greater in the mean of log life span. (B) Best-fit log-normal life span distributions with time on a log scale. (C) Best-fit log-normal life span distributions with time on a linear scale. Numerical values for the best-fit model parameters are reported in supplemental Table 2.
When taken together, the following observations are strongly suggestive that senescent platelet death is regulated by an internal timing mechanism rather than multiple hits: the quantitative influence of cell-intrinsic apoptotic proteins on life span, the logical inconsistency of kinetic data with the multiple-hit model, and, finally, the effective, natural reinterpretation of the kinetic data by the LS model, obviating the need for the abstract notion of a hit. If the latter is correct, it would follow that Bcl-x and Bak mutant platelets removed from the circulatory environment (the purported source of hits) should exhibit differential rates of apoptotic behavior when stored in vitro, and this is indeed the case (supplemental Figure 4).
The nature of the timing mechanism remains to be elucidated. It has been previously suggested that simple degradation of Bcl-xL might function as a “molecular clock.”7 Under this model, a decline in Bcl-xL levels relative to Bak would eventually trigger Bak activation and entry into apoptosis. The same proteins that regulate death would serve a dual purpose as key components of the clock. However, compelling evidence of time-dependent Bcl-xL degradation in platelets has yet to emerge. A distinct possibility is that the timing mechanism is more complex and that upstream pathways may actively signal the apoptotic machinery to initiate platelet death.27 Perhaps there is indeed a role for the environment, not in inflicting damage on platelets via hits, but in modulating signaling pathways that converge on and influence the internal timer. It may be that mutations in Bcl-x and Bak affect the way platelets react to their environment. A more thorough knowledge of platelet biochemistry and more sophisticated mathematical models that can accommodate the interleaving kinetics of alternative platelet fates will be required before platelet senescence can be completely understood.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rachael Lane, Jason Corbin, and Robyn Sutherland for excellent technical assistance; Kelly Trueman, Shauna Ross, Chris Evans, and Jaclyn Gilbert for expert animal husbandry; and Ken Duffy, Miles Davenport, David Huang, and Chloé James for discussions.
This work was supported by the Australian National Health and Medical Research Council (project grants 516725, 575535; fellowships, M.R.D., B.T.K., and P.D.H.; and Independent Research Institutes Infrastructure Support Scheme Grant 361646), the Sylvia and Charles Viertel Charitable Foundation (fellowship; B.T.K.), the Leukemia & Lymphoma Society (fellowship; E.C.J.), the Australian Cancer Research Fund, and the Victorian State Government Operational Infrastructure (support grant).
Authorship
Contribution: M.R.D., E.C.J., P.D.H., and B.T.K. designed research and analyzed results and wrote the paper; and M.R.D., E.C.J., and K.J.H. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benjamin T. Kile, Molecular Medicine Division, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville 3052, Victoria, Australia; e-mail: kile@wehi.edu.au.
References
Author notes
M.R.D. and E.C.J. contributed equally to this study and are joint first authors.
P.D.H. and B.T.K. contributed equally to this study and are joint senior authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal