Abstract
Stem cells must proliferate and differentiate to generate the lineages that shape mature organs; understanding these 2 processes and their interaction is one of the central themes in current biomedicine. An intriguing aspect is asymmetric division, by which 2 daughter cells with different fates are generated. Several cell fate determinants participate in asymmetric division, with the endocytic adaptor Numb as the best-known example. Here, we have explored the role of asymmetric division in thymocyte development, visualizing the differential segregation of Numb and pre-TCR in thymic precursors. Analysis of mice where Numb had been inhibited by expressing a dominant negative revealed enhanced pre–T-cell receptor (TCR) signaling and a smaller thymus. Conversely, Numb overexpression resulted in loss of asymmetric division and a larger thymus. The conclusion is that Numb determines the levels of pre-TCR signaling in dividing thymocytes and, ultimately, the size of the pool from which mature T lymphocytes are selected.
Introduction
The mammalian thymus contains different cell types originated from initially equivalent precursors that undergo several rounds of division. To ensure correct numbers of mature lineage-specific cells in adult organs, precursors divide asymmetrically, generating 2 sister cells with different fates.1,2 One of the “cell fate determinants” asymmetrically segregated is Numb, a plasma membrane–associated protein that contains a phosphotyrosine binding (PTB) domain and antagonizes Notch signaling. Numb function was first described in Drosophila sensory organ precursors,3 where loss of Numb resulted in both daughter cells adopting the fate of the cell that normally inherits Notch, whereas the opposite was caused by Numb overexpression.4-6 In mammalians, asymmetric division has been studied most extensively during neurogenesis.7 Deletion of Numb and its homologue Numblike resulted in loss of neural progenitors and block of neurogenesis,8 as a result of progenitor overdifferentiation and death of young neurons. In other systems, Numb binds to integrins9 and Src family kinases,10 mediates receptor internalization,11,12 and participates in clathrin-dependent endosomal association.13 Thus, Numb plays multiple and important roles in development and signaling; however, little is known about its role in the thymus.
T lymphocytes develop from precursors that undergo a series of cell fate decisions, resulting in differentiation into single positive (SP) thymocytes, the immediate precursors of fully functional CD4 and CD8 T lymphocytes.14,15 The initial CD4−CD8− double negative (DN) stages are influenced by signals from the pre–T-cell receptor (TCR)16-18 and Notch.19,20 Only DN thymocytes receiving proper pre-TCR and Notch signals are able to evolve into the CD4+CD8+ double positive (DP) αβ lineage stage. Pre-TCR internalization and degradation is very important for correct signaling.21-23 As a consequence of pre-TCR signaling, DN thymocytes proliferate, enabling normal numbers of DP thymocytes in the adult thymus.24-26
Both Numb and its homologue Numblike are expressed in hemopoietic stem cells27 and lymphoid tissues,10,28,29 also Numb colocalizes with the TCR complex in mature T cells30 and segregates asymmetrically in hemopoietic stem cells31 and mature T lymphocytes.32 Moreover, Numb overexpression in thymocytes results in the reduction of Notch activity.29 These data strongly suggest that Numb may play a role during the DN stage in the thymus.
Here, we have shown that DN thymocytes asymmetrically segregate Numb, but not the pre-TCR, during cell division. Our analysis of thymic development in transgenic mice overexpressing dominant-negative or full-length Numb has shown that Numb inhibition enhanced the pre-TCR pathway and resulted in a smaller thymus, whereas Numb overexpression resulted in less signaling, loss of asymmetric division, and a larger thymus. These results show that Numb helps modulate pre-TCR signaling in DN thymocytes to direct cell fate and regulate thymus cellularity.
Methods
Mice
Five-day embryos or 4- to 12-week-old mice were used for experiments with fetal and adult thymus, respectively. All procedures involving mice were performed in agreement with the Spanish Science Council Ethical Committee directives.
Antibodies
The following antibodies were from BD: FITC anti-CD25 (7D4), -CD4 (L3T4), -CD8 (53-6.7), -CD5 (53-7.3), –annexin V; phycoerythrin anti-TCRβ (H57-597), Lin [CD4 (RM4-5), CD8 (53-6.7), CD3 (145-2C11), CD11b (M1/70), NK1.1 (PK136), TCRβ (H57-597), TCRγδ (GL3), B220 (RA3-6B2)] CyC anti-CD44 (IM7); biotinylated anti-CD4 (RM4-5), CD8 (53-6.7), pre-Tα (2F5), Fc block (2.4G2), immunoglobulin G1 (IgG1; A85-1), and biotinylated Myc (9E10; Upstate Biotechnology). Biotinylated antibodies were revealed with streptavidin-allophycocyanin (eBioscience). FITC anti-cytokeratin was from Sigma-Aldrich. Lin mixture contains anti-B220, CD11b, natural killer, γδTCR, CD3, TCRβ, CD4, and CD8.
Western blotting
Thymocytes (1 × 107) were lysed in 100 μL of NP40 lysis buffer (Sigma) supplemented with protease inhibitor cocktail (Sigma) and 1mM phenylmethylsulfonyl fluoride for 30 minutes on ice. Lysates were run on a NuPAGE 4% to 12% Bis-Tris Gel (Invitrogen) and transferred to nitrocellulose. Membrane was blotted with primary antibodies, washed, incubated in secondary antibodies coupled to horseradish peroxidase (Santa Cruz Biotechnology), and detected with enhanced chemiluminescence (Bio-Rad).
Flow cytometry
Thymocytes were washed twice with phosphate-buffered saline (PBS) and stained at 4°C with antibodies. For intracellular staining, Cytofix/Cytoperm (BD) was used. Analysis was performed on a FACSCalibur (Becton Dickinson). Files were analyzed with FlowJo Version 4.6.2 software (TreeStar Inc).
Cell-cycle analysis
Thymocytes were surface-stained, washed twice with PBS, permeabilized and stained with 7-amino-actinomycin D in PBS containing 0.03% saponin.
OP9/OP9DL cocultures
Fetal thymocytes were cultured onto OP9/OP9DL monolayers for 6 days at 37°C, in the presence of 5 ng/mL interleukin-7 (IL-7; R&D Systems). All cocultures were maintained in complete α-modified essential medium (Gibco) supplemented with 10% fetal bovine serum and antibiotics (Gibco).
Immunofluorescence and confocal microscopy
Thymi were fixed with a 4% paraformaldehyde solution in PBS, treated with a 20% sucrose solution in PBS, immersed in OCT (Tissue Tek), and frozen with a mixture of 2-methylbutane (Sigma) and dry ice. Sections (10 μm) were fixed, permeabilized with Triton-X100, and stained in a solution containing 5% normal goat serum and 3% bovine serum albumin. Sections were mounted with ProLong Antifade (Molecular Probes). Antibodies used for immunofluorescence were anti-Numb (Santa Cruz Biotechnology), anti–c-Cbl (Santa Cruz Biotechnology), anti-CD25 (BD), rabbit anti-Ubiquitin (Sigma), and TOPRO-3 iodide (Molecular Probes). The secondary antibodies were anti–rabbit Alexa-647, anti–rabbit Alexa-488, anti–mouse Alexa 488, anti–mouse Alexa 555 (Molecular Probes). With the use of a Leica TCS SP5 confocal microscope and either 20× or 40× objectives, images were collected at 8-bit depth, with a resolution of 1024 × 1024 pixels. Images were processed using LAS AF (Leica Microsystems) and Adobe Photoshop 7.0 software.
Quantification of fluorescence staining in z-stacks of dividing cells
The software LAS AF (Leica Microsystem) was used to analyze fluorescent images. Inside each picture, one gate for each daughter cell was drawn, with the use of CD25 as a marker, and threshold intensities of total pixels were determined for Numb. The positively staining pixels on each daughter cell were expressed as a percentage of the total pixels in the sum of the 2 gates, for each picture. This was performed for all pictures of each z-stack. As a result, each daughter cell was associated with a percentage of positive pixels that corresponds to the percentage of inherited Numb.
RNA analysis
Total RNA was extracted with RNeasy Kit (QIAGEN). Reverse transcription–polymerase chain reaction (RT-PCR) was carried out with an I Script cDNA Synthesis Kit (Bio-Rad). The resulting cDNA pool was amplified by 40 cycles of PCR. RT-PCR primers were mouse 18S (5′ sense, CGGCTACCACATCCAAGGAA; 3′ antisense, GCTGGAATTACCGCGGCT), Hes1 (5′ sense, GCCAGTGTCAACACGACACCGG; 3′ antisense, TCACCTCGTTCATGCACTCG). Fold differences were calculated after normalizing cDNA levels of 18S transcripts.
Statistical analysis
Statistical comparisons were made with the Student t test.
Results
DN thymocytes segregate asymmetrically Numb, but not pre-TCR, during cell division
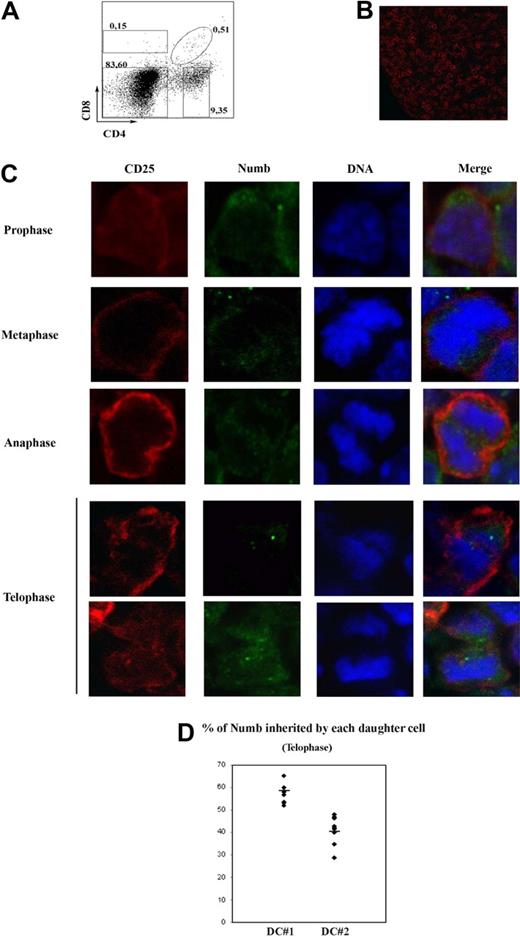
To explore asymmetric division in thymocytes, we focused on fetal day 15 thymus, when most cells are at the DN stage, as evidenced by flow cytometry (Figure 1A) and immunofluorescence (high frequency of CD25+ cells; Figure 1B). CD25 was used to detect dividing cells because it is only expressed on DN2 and DN3 thymocytes and is not polarized or asymmetrically segregated during signaling and cell division (unpublished observations, September 2007). We analyzed frozen sections of day 15 fetal thymi by immunofluorescence, using TOPRO (to detect DNA), CD25, and Numb. We localized high numbers of CD25+ dividing cells at all stages of mitosis (Figure 1C). Because DN2 thymocytes do not divide and DN4 thymocytes do not express CD25, these cells were at the DN3 stage. We observed Numb polarization in 43% of CD25+ premitotic DN3 cells. In dividing DN3 thymocytes, Numb was polarized at the prophase and metaphase stages (Figure 1C top). At anaphase, Numb was not polarized or asymmetrically localized. However, at telophase, Numb was inherited exclusively by one of the daughter cells in approximately 30% of the cases and equally segregated into both daughter cells in the rest of the cases (Figure 1C bottom). To further ascertain this fact, we quantified Numb at telophase (see “Quantification of fluorescent staining in z-stacks of dividing cells”). For each telophase, “dividing cell no. 1” (DC#1) was the daughter cell with more Numb, and “dividing cell no. 2” (DC#2) was the daughter cell with less Numb. We observed a high degree of asymmetry in 6 of the 10 analyzed z-stacks (Figure 1D; Table 1, see column “DC#1/DC#2”). In addition, average standard deviation of DC#1 and DC#2 in Figure 1D was high (10.31). Because Numb is a marker of asymmetric division, these data show that a subset of developing thymocytes divides asymmetrically.
Asymmetric Numb segregation in dividing thymocytes. (A) CD4 and CD8 expression in fetal thymocytes of WT mice, analyzed by flow cytometry. Numbers represent percentages of CD85P; DP, CD45P, and DN subpopulations (clockwise from top left). (B) Confocal image of a 15-day fetal frozen WT thymus stained with CD25. (C) Confocal images of mitotic WT fetal thymocytes stained with antibodies against Numb (green), CD25 (red), and treated with TOPRO-3 iodide (blue). The images are representative of at least 3 independent experiments. (D) Percentage of Numb protein contained in each daughter cell at the telophase stage. Quantification of Numb signal was performed as indicated in “Quantification of fluorescent staining in z-stacks of dividing cells.” DC #1 and #2 represent the daughter cells that inherited more and less Numb on each z-stack, respectively. A total of 10 telophases were analyzed. Average standard deviation for DC#1 and DC#2 = 10.31. Horizontal lines represent avarage values.
Asymmetric Numb segregation in dividing thymocytes. (A) CD4 and CD8 expression in fetal thymocytes of WT mice, analyzed by flow cytometry. Numbers represent percentages of CD85P; DP, CD45P, and DN subpopulations (clockwise from top left). (B) Confocal image of a 15-day fetal frozen WT thymus stained with CD25. (C) Confocal images of mitotic WT fetal thymocytes stained with antibodies against Numb (green), CD25 (red), and treated with TOPRO-3 iodide (blue). The images are representative of at least 3 independent experiments. (D) Percentage of Numb protein contained in each daughter cell at the telophase stage. Quantification of Numb signal was performed as indicated in “Quantification of fluorescent staining in z-stacks of dividing cells.” DC #1 and #2 represent the daughter cells that inherited more and less Numb on each z-stack, respectively. A total of 10 telophases were analyzed. Average standard deviation for DC#1 and DC#2 = 10.31. Horizontal lines represent avarage values.
Percentages of Numb in each daughter cell at telophase (WT)
| WT telophase . | ||
|---|---|---|
| DC#1 . | DC#2 . | DC#1/DC#2 . |
| 71.36 | 28.64 | 2.49 |
| 65.14 | 34.86 | 1.86 |
| 60.00 | 40.00 | 1.50 |
| 58.49 | 41.51 | 1.40 |
| 58.00 | 42.00 | 1.38 |
| 57.00 | 43.00 | 1.32 |
| 53.60 | 46.39 | 1.15 |
| 53.44 | 46.56 | 1.15 |
| 53.24 | 46.76 | 1.13 |
| 51.96 | 48.04 | 1.08 |
| WT telophase . | ||
|---|---|---|
| DC#1 . | DC#2 . | DC#1/DC#2 . |
| 71.36 | 28.64 | 2.49 |
| 65.14 | 34.86 | 1.86 |
| 60.00 | 40.00 | 1.50 |
| 58.49 | 41.51 | 1.40 |
| 58.00 | 42.00 | 1.38 |
| 57.00 | 43.00 | 1.32 |
| 53.60 | 46.39 | 1.15 |
| 53.44 | 46.56 | 1.15 |
| 53.24 | 46.76 | 1.13 |
| 51.96 | 48.04 | 1.08 |
DC indicates daughter cell.
Numb is colocalized with the TCR machinery in mature lymphocytes30-32 ; therefore, we wanted to investigate how Numb localizes relative to the pre-TCR in DN thymocytes. We analyzed by confocal microscopy pre-TCR localization in dividing fetal thymocytes, and we did not find asymmetric pre-TCR segregation: all the telophases showed uniform membrane (including the nascent membrane) and cytoplasmic pre-TCR localization (Figure 2A). The same result was observed on quantification of Numb signal (Figure 2B). Accordingly, average standard deviation of DC#1 and DC#2 was significantly lower than that of Numb in the wild-type (WT) cells (4.81 vs 10.31). To exclude the possibility that the observed pre-TCR staining on sections was unspecific, we stained fetal thymus sections with the use of an isotype control and observed no unspecific staining (data not shown). We also stained adult thymus sections with the use of anti–pre-Tα, CD4, and cytokeratin (expressed on thymic stromal cells; see Canelles et al33 ). If the anti–pre-Tα antibody is specific, there should be no staining on thymocytes in the adult cortex (DPs) or medulla (SPs). Indeed, we could not detect pre-TCR staining on CD4+ thymocytes (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article); there was some degree of staining, but it always correlated with cytokeratin (supplemental Figure 1A-B). Because cytokeratin staining in day 15 fetal thymus is absent (unpublished observations, November 2007), the observed pre-TCR staining must be specific.
Symmetric pre-TCR segregation in dividing thymocytes. (A) Confocal images of WT fetal mitotic thymocytes at the telophase stage stained with antibodies against CD25 (red), pre-TCR (green), and treated with TOPRO-3 iodide (blue). (B) Percentage of pre-TCR protein contained in each daughter cell at the telophase stage. Quantification of Numb signal was performed as indicated above. A total of 10 telophases were analyzed. Average standard deviation for DC#1 and DC#2 = 4.81. (C) Confocal images of WT fetal mitotic thymocytes at the prophase (top) and metaphase (bottom) stages stained with antibodies against pre-TCR (red), Numb (green), and treated with TOPRO-3 iodide (blue). (D) Confocal images of mitotic Rag2−/− thymocytes in prophase, metaphase, anaphase, and telophase stages stained with antibodies against CD25 (red), Numb (green), and treated with TOPRO-3 iodide (blue). The images are representative of at least 3 independent experiments. (E) Percentage of Numb protein contained in each daughter cell at the telophase stage of Rag2−/− thymocytes. Quantification of Numb signal was performed as indicated above. A total of 6 telophases were analyzed. Average standard deviation for DC#1 and DC#2 = 3.79. Horizontal lines in panels B and E represent average values.
Symmetric pre-TCR segregation in dividing thymocytes. (A) Confocal images of WT fetal mitotic thymocytes at the telophase stage stained with antibodies against CD25 (red), pre-TCR (green), and treated with TOPRO-3 iodide (blue). (B) Percentage of pre-TCR protein contained in each daughter cell at the telophase stage. Quantification of Numb signal was performed as indicated above. A total of 10 telophases were analyzed. Average standard deviation for DC#1 and DC#2 = 4.81. (C) Confocal images of WT fetal mitotic thymocytes at the prophase (top) and metaphase (bottom) stages stained with antibodies against pre-TCR (red), Numb (green), and treated with TOPRO-3 iodide (blue). (D) Confocal images of mitotic Rag2−/− thymocytes in prophase, metaphase, anaphase, and telophase stages stained with antibodies against CD25 (red), Numb (green), and treated with TOPRO-3 iodide (blue). The images are representative of at least 3 independent experiments. (E) Percentage of Numb protein contained in each daughter cell at the telophase stage of Rag2−/− thymocytes. Quantification of Numb signal was performed as indicated above. A total of 6 telophases were analyzed. Average standard deviation for DC#1 and DC#2 = 3.79. Horizontal lines in panels B and E represent average values.
To study pre-TCR localization during the cell cycle, we analyzed dividing fetal thymocytes at prophase, observing that pre-TCR is always polarized at prophase and metaphase, similar to Numb (Figure 2C). Probably, Numb polarization during prophase in thymocytes is related to signaling. In this case, thymocytes not receiving pre-TCR signals would not polarize Numb during prophase. To address this question, we analyzed Numb localization in dividing Rag−/− thymocytes, and we found that Numb is neither polarized nor asymmetrically segregated in dividing Rag−/− thymocytes (Figure 2D-E). Accordingly, the average standard deviation of DC#1 and DC#2 is significantly lower than in the WT (3.79 vs 10.31). This indicates that Numb may be needed for pre-TCR signaling and asymmetric division.
Functional Numb levels in thymocytes determine adult thymus size
To further investigate the role of Numb in thymocyte development, we used mice that expressed either a dominant-negative Numb (dnNb TG mice) or full-length Numb (Numb TG mice) under the human CD2 promoter. DnNb TG mice express the Numb PTB domain, whereas Numb TG mice overexpress the full-length p71 Numb isoform. A truncated protein similar to dnNb has a dominant-negative effect in neural cells.10
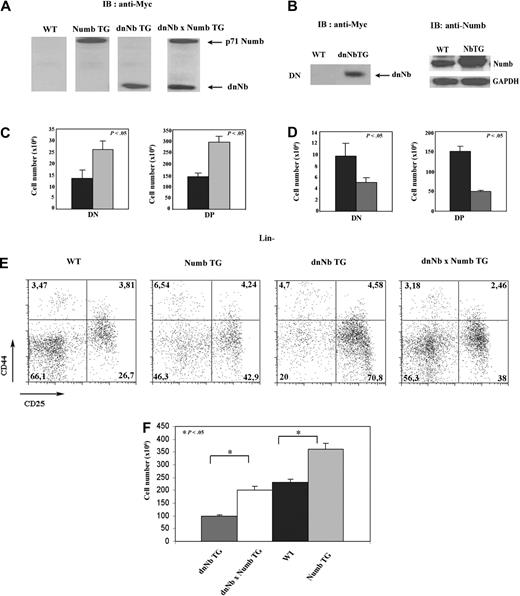
Both dnNb and Numb constructs contained the human Myc tag; therefore, we used an anti-hMyc antibody to detect by Western blotting transgenic proteins. We found that, although there was no detectable hMyc epitope in WT mice, both Numb and dnNb TG thymocytes expressed large amounts of transgenic protein, and these could be distinguished by their size, because dnNb is a truncated product (Figure 3A). Taking advantage of the size difference, we verified that dnNb × Numb TG mice expressed both transgenic proteins (Figure 3A). In addition, we performed Western blotting to detect dnNb and Numb in DN thymocytes of dnNb and Numb TG mice, respectively, and could confirm the expression of high quantities of the transgenic proteins in this subset of both transgenic mice (Figure 3B).
Levels of functional Numb correlate with thymus cellularity. (A) Western blot analysis, using an anti-Myc tag antibody, of dnNb and p71 Numb expression in thymus of WT, Numb TG, dnNb TG, and dnNb × Numb TG mice. (B) Western blot analysis, using an anti-Myc tag (left) or anti-Numb (right) antibody, of dnNb and Numb expression in DN thymocytes of WT, dnNb, and Numb TG mice. (C) Absolute numbers of DN and DP cell subsets in thymus of WT (black) and Numb TG (gray) mice; N = 5 (WT), 7 (Numb TG). (D) Absolute numbers of DN and DP cell subsets in thymus of WT (black) and dnNb TG (gray) mice; N = 5 (WT), 6 (dnNb TG). (E) CD25 and CD44 expression in Lin− thymocytes of 4-week-old WT, Numb TG, dnNb TG, and dnNb × Numb TG mice. Quadrants represent DN1, DN2, DN3, and DN4 subpopulations (clockwise from top left to bottom left). (F) Total cell numbers in dnNb TG (gray), dnNb × Numb TG (white), WT (black), and Numb TG (light gray) adult thymi; N = 12 (Numb TG), 7 (dnNb × Numb TG), 12 (WT), 12 (Numb TG). Means ± SD of at least 3 independent experiments are plotted.
Levels of functional Numb correlate with thymus cellularity. (A) Western blot analysis, using an anti-Myc tag antibody, of dnNb and p71 Numb expression in thymus of WT, Numb TG, dnNb TG, and dnNb × Numb TG mice. (B) Western blot analysis, using an anti-Myc tag (left) or anti-Numb (right) antibody, of dnNb and Numb expression in DN thymocytes of WT, dnNb, and Numb TG mice. (C) Absolute numbers of DN and DP cell subsets in thymus of WT (black) and Numb TG (gray) mice; N = 5 (WT), 7 (Numb TG). (D) Absolute numbers of DN and DP cell subsets in thymus of WT (black) and dnNb TG (gray) mice; N = 5 (WT), 6 (dnNb TG). (E) CD25 and CD44 expression in Lin− thymocytes of 4-week-old WT, Numb TG, dnNb TG, and dnNb × Numb TG mice. Quadrants represent DN1, DN2, DN3, and DN4 subpopulations (clockwise from top left to bottom left). (F) Total cell numbers in dnNb TG (gray), dnNb × Numb TG (white), WT (black), and Numb TG (light gray) adult thymi; N = 12 (Numb TG), 7 (dnNb × Numb TG), 12 (WT), 12 (Numb TG). Means ± SD of at least 3 independent experiments are plotted.
We observed in Numb TG mice, compared with WT mice, an increase in cellularity of both the DN and the DP compartments (Figure 3C). Conversely, dnNb TG mice showed a marked decrease in absolute numbers of these 2 subpopulations (Figure 3D). The same results were obtained for 2 independent lines of either dnNb or Numb TG mice (supplemental Figure 2; unpublished observations, February 2008). Because DP thymocytes do not divide, differences in DP cellularity are originated at the DN stage; therefore, we analyzed by flow cytometry DN subpopulations in transgenic mice. We observed a block at the DN3 stage in dnNb TG mice, compared with WT mice, with a 2.5-fold increase in the percentage of DN3 thymocytes (Figure 3E). This block was absent in Numb TG and was reverted by Numb overexpression in dnNb × Numb TG mice (Figure 3E). The fact that sole expression of Numb is able to revert the dnNb TG phenotype indicated that the truncated dnNb protein is inhibiting exclusively Numb. Interestingly, we observed a gradation in thymus cell numbers as functional levels of Numb increased, starting with dnNb TG mice to reach a maximum in Numb TG mice (Figure 3F).
Impaired proliferation in the thymus of dnNb TG mice
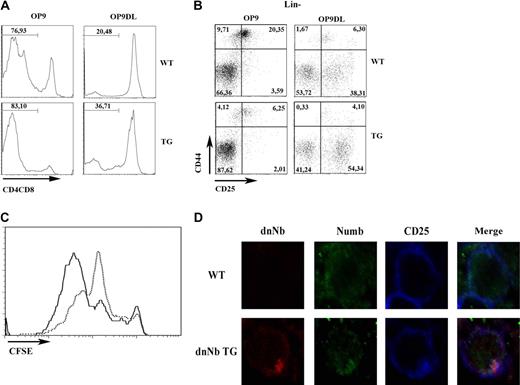
The results described in the previous subsection suggest that inhibiting Numb interferes seriously with thymic development. Because both proliferation and pre-TCR signaling start during the DN3 stage, we wanted to study the effect of Numb inhibition on these processes. To study thymocyte proliferation, we used the OP9 system,34 consisting on 2 cell lines, OP9 and OP9-DL1, the later expressing a Notch ligand that enhances Notch signaling and differentiation into the DP stage. We labeled day 15 fetal WT or dnNb TG thymocytes with CFSE (5,6-carboxyfluorescein diacetate succinimidyl ester) and coincubated them with OP-9 or OP9-DL1 cells for 6 days. Although few thymocytes expressing CD4 and/or CD8 developed on OP9 cells after 6 days of incubation, the number increased considerably in both WT and dnNb TG thymocytes incubated with OP9-DL1 cells (Figure 4A). As expected, very few DN3 and DN4 cells developed on OP9 cells, but these numbers were higher when incubated on OP9-DL1 cells, and the observed DN3 block in dnNb TG thymocytes was still observed (Figure 4B). Analysis of CFSE binding on the recovered thymocytes showed that dnNb thymocytes had proliferated to a lesser extent than WT thymocytes (Fig 4C). In addition, cell cycle analysis was performed at all DN stages, comparing dnNb TG and WT mice. Although no difference was found at the DN1 and DN2 stages, signs of S-phase arrest were observed at the DN3 stage, and less proliferating cells at the DN4 stage, in dnNb TG mice compared with WT mice (supplemental Figure 3A). This correlated with augmented DN3 absolute cell numbers in dnNb TG mice (supplemental Figure 3B). Thus, Numb inhibition results in decreased proliferative potential of DN thymocytes.
Proliferation block in DN thymocytes of dnNb TG mice. (A) Maturation of WT and dnNb TG fetal (15 days) thymocytes to the DP stage after 6 days of coculture with OP9 or OP9-Δ-like cells. The DP stage was monitored by expression of CD4 and CD8. (B) Differentiation of WT and dnNb TG fetal DN thymocytes after 6 days of coculture with OP9 or OP9-Δ-like. Lin− thymocytes were stained with CD44 and CD25. Quadrants represent DN1, DN2, DN3, and DN4 subpopulations (clockwise from top left to bottom left). (C) CFSE expression in fetal thymocytes of WT (solid line) or dnNb TG (broken line) mice cultured for 6 days over OP9-Δ-like cells. (D) Confocal images of fetal thymocytes of WT (top) and dnNb TG (bottom) mice stained with Numb (green), dnNb (anti-Myc; red) and CD25 (blue). Data are representative of at least 3 independent experiments.
Proliferation block in DN thymocytes of dnNb TG mice. (A) Maturation of WT and dnNb TG fetal (15 days) thymocytes to the DP stage after 6 days of coculture with OP9 or OP9-Δ-like cells. The DP stage was monitored by expression of CD4 and CD8. (B) Differentiation of WT and dnNb TG fetal DN thymocytes after 6 days of coculture with OP9 or OP9-Δ-like. Lin− thymocytes were stained with CD44 and CD25. Quadrants represent DN1, DN2, DN3, and DN4 subpopulations (clockwise from top left to bottom left). (C) CFSE expression in fetal thymocytes of WT (solid line) or dnNb TG (broken line) mice cultured for 6 days over OP9-Δ-like cells. (D) Confocal images of fetal thymocytes of WT (top) and dnNb TG (bottom) mice stained with Numb (green), dnNb (anti-Myc; red) and CD25 (blue). Data are representative of at least 3 independent experiments.
We suspected that dnNb inhibited Numb by competing for endogenous Numb binding sites. Obviously, dnNb would not be able to exert normal Numb function at those sites because of lacking the C-terminus. Therefore, we analyzed Numb and dnNb localization on CD25+ fetal thymocytes by confocal microscopy. The Numb antibody used in our studies is raised against the Numb C-terminus, which is absent from dnNb protein; therefore, it only recognizes endogenous Numb, although dnNb can be detected with the use of an anti-Myc antibody. We observed that, as we hypothesized, dnNb is localized at the cell membrane and polarizes together with endogenous Numb in CD25+ thymocytes (Figure 4D). This is consistent with competence between Numb and dnNb for binding sites: probably, dnNb partially blocks Numb access to its physiologic sites.
Increased Notch signaling and abnormal pre-TCR expression in dnNb TG DN thymocytes
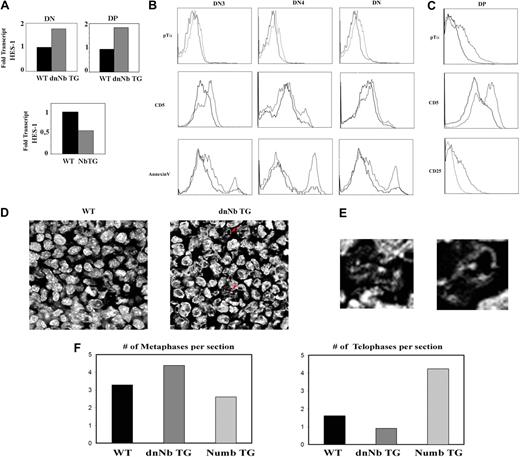
We hypothesized that Numb inhibition results in increased Notch signaling, evidenced by augmented Hes-1 mRNA levels in thymocytes, and the opposite would happen in Numb TG thymocytes. This was, indeed, the result obtained by analyzing Hes-1 expression in WT, dnNb, and Numb TG thymocytes by RT-PCR (Figure 5A). Taking into account the observed parallelism in asymmetric protein segregation between Notch and the pre-TCR, and the fact that Numb colocalizes with the pre-TCR, the question arose of whether Numb inhibition would enhance pre-TCR signaling, in a similar way to its described effect on Notch.
Increased surface pre-TCR levels, apoptosis, and premature differentiation in dnNb TG thymocytes. (A) Hes-1 gene expression levels, measured by RT-PCR, in WT (black) and dnNb TG (gray) thymocytes (top), or WT (black) and Numb TG (gray) T cells (bottom). (B) Pre-TCR (top), CD5 (middle), and Annexin V (bottom) flow cytometric measurement of DN3, DN4, or total DN WT or dnNb TG thymocytes; WT (solid line) and dnNb TG (broken line). (C) Pre-TCR (top), CD5 (middle), and CD25 (bottom) flow cytometric measurement in WT or dnNb TG DP thymocytes; WT (solid line) and dnNb TG (broken line). (D) Confocal images of fetal thymic frozen sections treated with TOPRO-3 to detect DNA. Apoptotic nuclei are marked with red arrows. (E) High magnification images of the nuclei marked with red arrows in panel D. (F) Average number of metaphases (left) and telophases (right) per fetal thymic section of WT (black), dnNb TG (dark gray), or Numb TG (light gray) mice. All data are representative of at least 3 independent experiments.
Increased surface pre-TCR levels, apoptosis, and premature differentiation in dnNb TG thymocytes. (A) Hes-1 gene expression levels, measured by RT-PCR, in WT (black) and dnNb TG (gray) thymocytes (top), or WT (black) and Numb TG (gray) T cells (bottom). (B) Pre-TCR (top), CD5 (middle), and Annexin V (bottom) flow cytometric measurement of DN3, DN4, or total DN WT or dnNb TG thymocytes; WT (solid line) and dnNb TG (broken line). (C) Pre-TCR (top), CD5 (middle), and CD25 (bottom) flow cytometric measurement in WT or dnNb TG DP thymocytes; WT (solid line) and dnNb TG (broken line). (D) Confocal images of fetal thymic frozen sections treated with TOPRO-3 to detect DNA. Apoptotic nuclei are marked with red arrows. (E) High magnification images of the nuclei marked with red arrows in panel D. (F) Average number of metaphases (left) and telophases (right) per fetal thymic section of WT (black), dnNb TG (dark gray), or Numb TG (light gray) mice. All data are representative of at least 3 independent experiments.
We looked first at pre-TCR surface levels by flow cytometry with the use of an antibody against the pTα chain and observed that dnNb TG DN thymocytes expressed higher pTα surface levels than WT DN thymocytes (Fig 5B). Increased pre-TCR levels on the surface of developing thymocytes would result in enhanced pre-TCR signaling; therefore, we analyzed CD5 expression on DN thymocytes of WT and dnNb TG mice. CD5 expression was increased at both DN3 and DN4 stages in dnNb TG, compared with WT thymocytes (Figure 5B). We could not detect CD5 expression on DN3 E cells,35 whereas CD5 expression was increased in DN3 L cells of dnNb TG compared with WT thymocytes (data not shown). In this aspect, it is interesting to note that CD4hiCD8int thymocytes of dnNb TG mice do also express increased CD5 levels (N.M.-B., G. Bretons, M. Canelles, manuscript submitted, December 2009). Conversely, DN thymocytes of Numb TG mice expressed lower CD5 levels, compared with the WT (data not shown).
If DN thymocytes of dnNb TG mice express higher surface pre-TCR levels and present increased CD5 expression as a consequence of enhanced pre-TCR signals, this may result in either death or premature differentiation.36 To assess whether a higher fraction of DN thymocytes undergoes cell death in dnNb TG compared with WT mice we performed Annexin-V staining and observed more cell death in DN thymocytes of dnNb TG mice (Figure 5B). However, the thymus of dnNb TG mice contained DP and SP thymocytes, indicating that not all the DN thymocytes die; we, therefore, wanted to investigate how excessive pre-TCR levels affect DN thymocytes that end up developing into SP cells. In mice deficient for Numb and Numblike, neural progenitors differentiate prematurely before expanding.8 We hypothesized that DN thymocytes receiving enhanced pre-TCR signaling could bypass the proliferation burst associated with the DN-DP transition, differentiating prematurely into DP thymocytes, which would contribute to the observed decreased thymus size. DN thymocytes must down-regulate both CD25 and pre-TCR levels previous to transitioning to the DP stage; therefore, an indicator of premature differentiation would be high surface CD25 and/or pre-TCR expression at the DP stage. We observed increased surface levels of both in DP thymocytes of dnNb mice, compared with WT mice (Figure 5C). In addition, CD5 levels were also increased in DP thymocytes of dnNb TG mice (Figure 5C). With the use of TOPRO, we observed an increase of apoptotic nuclei, with the characteristic DNA fragmentation, in dnNb TG compared with WT fetal thymi (Figure 5D-E). Together, these results indicate that excessive pre-TCR signaling induces cell death in a fraction of DN thymocytes and premature DN3-DP transition in another subset of DN thymocytes in dnNb TG mice.
Such changes in proliferation may affect the number of thymocytes undergoing each cell cycle stage; therefore, we counted the number of metaphases and telophases on fetal thymic sections of WT, dnNb TG, or Numb TG mice, stained with TOPRO. We found more metaphases and less telophases in dnNb TG than in WT fetal thymi; conversely, there were less metaphases and more telophases in Numb TG than in WT fetal thymi (Figure 5F). This may indicate that thymocytes progress faster through the cell cycle in Numb TG mice than in either WT or dnNb mice, whereas the opposite effect is caused by Numb inhibition in dnNb TG mice.
Defect in c-Cbl endosomal localization in dnNb TG DN thymocytes
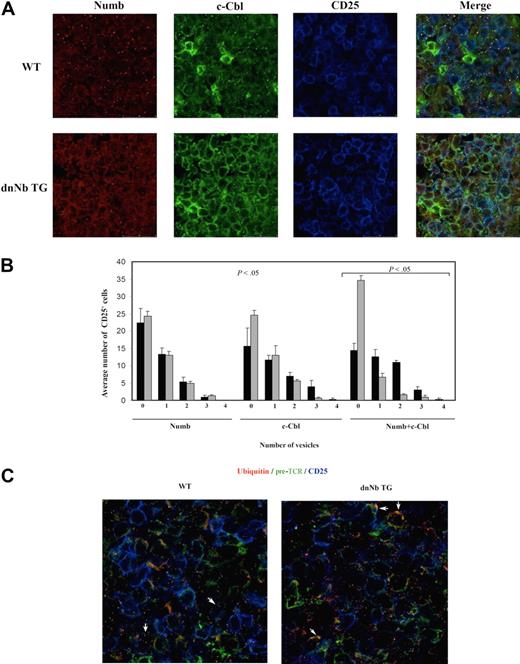
We hypothesized that dnNb protein must be interacting (perhaps even more efficiently than endogenous Numb) with proteins implicated in pre-TCR signaling. If this happened, endogenous Numb would be excluded from sites where it functions during normal development. It is interesting that c-Cbl, an essential player in pre-TCR signaling and early thymocyte development,37 coimmunoprecipitates with Numb.30 Moreover, there is a remarkable parallelism between the phenotypes of c-Cbl/Cbl-b double knockout and dnNb TG mice, both of which show reduced thymus size and abnormal pre-TCR surface expression on both DN and DP thymocytes. To assess whether dnNb expression affected c-Cbl function, we stained 15-day WT and dnNb TG fetal thymic sections with antibodies against Numb and c-Cbl. C-Cbl was expressed at high levels in both WT and dnNb TG thymi. However, although c-Cbl was localized in vesicles in fetal WT thymocytes, it was mostly on the membranes of dnNb TG fetal thymocytes (Figure 6A). Numb expression pattern was largely conserved in dnNb TG mice (Figure 6A). When both proteins were observed on the same image (Figure 6A Merge), there was a loss of “yellow dots” (c-Cbl+ Numb+ vesicles) in dnNb TG thymi, compared with WT. Quantification of vesicles containing Numb, c-Cbl, or both in CD25+ fetal thymocytes showed that, although the average number of Numb-containing vesicles per cell did not change in dnNb TG mice with respect to WT mice, there was an important decrease in the average number of both c-Cbl+ and DP Numb+/c-Cbl+ vesicles per cell in dnNb TG mice, compared with WT mice (Figure 6B).
Less endosomal Numb and more ubiquitylated membrane pre-TCR in fetal dnNb TG thymocytes. (A) Confocal images of fetal thymic frozen sections stained with antibodies against Numb (red), c-Cbl (green), and CD25 (blue). (B) Average number of CD25+ cells containing from zero to 4 c-Cbl+, Numb+, and c-Cbl+Numb+ vesicles. Four independent images for each line (WT and dnNb TG) were processed. For colocalization, analysis masks for c-Cbl (green) and for Numb (red) were made with Photoshop 7.0. Numbers of vesicles per CD25+ cell in each mask were counted. The same analysis was performed in masks containing Numb and c-Cbl together (yellow). An average of 34 and 45 CD25+ cells per image were counted for WT (black bars) and dnNb TG (gray bars), respectively. (C) Confocal images of fetal thymic frozen sections of WT (left) and dnNb TG (right) mice, stained with antibodies against ubiquitin (red), pre-TCR (green), and CD25 (blue). White arrows point to cells with intracellular ubiquitin+/pre-TCR+ vesicles (left) or membrane ubiquitin+/pre-TCR+ accumulations (right). All data are representative of at least 3 independent experiments.
Less endosomal Numb and more ubiquitylated membrane pre-TCR in fetal dnNb TG thymocytes. (A) Confocal images of fetal thymic frozen sections stained with antibodies against Numb (red), c-Cbl (green), and CD25 (blue). (B) Average number of CD25+ cells containing from zero to 4 c-Cbl+, Numb+, and c-Cbl+Numb+ vesicles. Four independent images for each line (WT and dnNb TG) were processed. For colocalization, analysis masks for c-Cbl (green) and for Numb (red) were made with Photoshop 7.0. Numbers of vesicles per CD25+ cell in each mask were counted. The same analysis was performed in masks containing Numb and c-Cbl together (yellow). An average of 34 and 45 CD25+ cells per image were counted for WT (black bars) and dnNb TG (gray bars), respectively. (C) Confocal images of fetal thymic frozen sections of WT (left) and dnNb TG (right) mice, stained with antibodies against ubiquitin (red), pre-TCR (green), and CD25 (blue). White arrows point to cells with intracellular ubiquitin+/pre-TCR+ vesicles (left) or membrane ubiquitin+/pre-TCR+ accumulations (right). All data are representative of at least 3 independent experiments.
To investigate whether the observed vesicles corresponded to endosomes, we stained fetal thymic sections with an antibody against ubiquitin, together with either Numb or c-Cbl. We found that both Numb- and c-Cbl–containing vesicles also contained ubiquitin (supplemental Figure 4A-B). The fact that c-Cbl access to endosomes is blocked in dnNb TG mice suggests that dnNb must be able to access sites normally occupied by endogenous Numb, displacing it from those sites. Numb is probably a mediator of c-Cbl access to endosomes; therefore, expressing a truncated Numb protein blocks it, interfering with pre-TCR degradation. This would result in more pre-TCR bound to ubiquitin on the surface of dnNb TG CD25+ thymocytes compared with WT thymocytes. We confirmed this by visualizing pre-TCR and ubiquitin on fetal thymic sections (Figure 6C). We also analyzed TCRβ expression on CD25+ fetal thymocytes and observed high TCRβ expression on the membrane of dnNb TG DN thymocytes (supplemental Figure 4C). This is in agreement with excessive pre-TCR signaling in dnNb TG thymocytes.
Symmetric Numb segregation in Numb TG mice
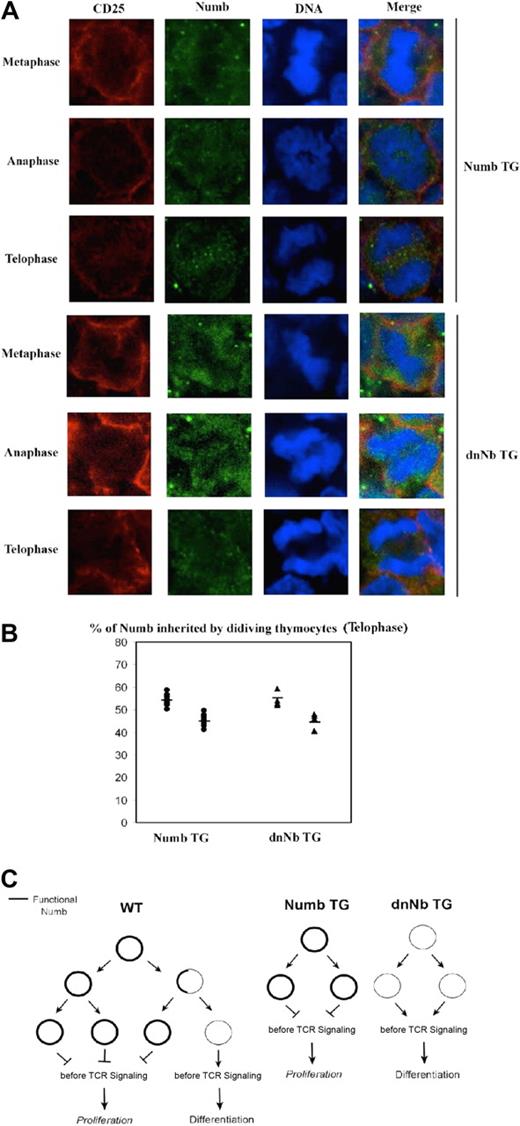
It is known that little Numb is necessary to guarantee normal asymmetric division38 ; however, it is not known how excessive Numb levels affect this process. Visualization of Numb expression in cycling Numb TG CD25+ DN thymocytes on fetal sections showed homogeneous, high Numb expression on both daughter cells at all stages of cell division (Figure 7A top). Interestingly, similar results were obtained by analyzing dnNb TG mice (Figure 7A bottom). Numb signal was quantified for both TG lines, observing that both Numb TG and dnNb TG thymocytes segregated Numb in a symmetric fashion, similar to Rag−/− thymocytes (Figure 7B; Table 2). Accordingly, average standard deviation of DC#1 and DC#2 is significantly lower than that of Numb in the WT (5.11 in Numb TG and 6.22 in dnNb TG vs 10.31 in WT). The fact that dnNb TG mice show a more symmetric Numb segregation in thymocytes than WT mice is irrelevant in terms of asymmetric division, given that endogenous Numb function is blocked by dominant-negative Numb. It rather adds to the notion that Numb degradation is inhibited by dominant-negative Numb. These data show that, during asymmetric division in the thymus, it is not the quantity of Numb inherited by each cell, but its functionality as an internalization adaptor, that determines the different daughter cell fates.
Symmetric Numb segregation in Numb and dnNb TG DN thymocytes. (A) Confocal images of mitotic Numb TG (top) or dnNb TG (bottom) fetal thymocytes at metaphase, anaphase, and telophase stages stained with antibodies against CD25 (red), Numb (green), and treated with TOPRO-3 iodide (blue). The images are representative of at least 3 independent experiments. (B) Percentage of Numb inherited by each daughter cell at the telophase stage in Numb TG (●) and dnNb TG (▴) mice. A total of 10 (Numb TG mice) and 6 (dnNb TG mice) z-stacks were analyzed. Average standard deviation for DC#1 and DC#2 = 5.11 (Numb TG), 6.22 (dnNb TG). Horizontal lines represent average values. (C) A model of Numb influence on thymocyte decisions.
Symmetric Numb segregation in Numb and dnNb TG DN thymocytes. (A) Confocal images of mitotic Numb TG (top) or dnNb TG (bottom) fetal thymocytes at metaphase, anaphase, and telophase stages stained with antibodies against CD25 (red), Numb (green), and treated with TOPRO-3 iodide (blue). The images are representative of at least 3 independent experiments. (B) Percentage of Numb inherited by each daughter cell at the telophase stage in Numb TG (●) and dnNb TG (▴) mice. A total of 10 (Numb TG mice) and 6 (dnNb TG mice) z-stacks were analyzed. Average standard deviation for DC#1 and DC#2 = 5.11 (Numb TG), 6.22 (dnNb TG). Horizontal lines represent average values. (C) A model of Numb influence on thymocyte decisions.
Percentages of Numb inherited by each daughter cell at telophase
| . | DC#1 . | DC#2 . | DC#1/DC#2 . |
|---|---|---|---|
| Nb TG | |||
| 58.77 | 41.23 | 1.42 | |
| 56.90 | 43.00 | 1.32 | |
| 56.00 | 44.00 | 1.27 | |
| 56.00 | 44.00 | 1.27 | |
| 54.52 | 45.48 | 1.19 | |
| 53.32 | 46.68 | 1.14 | |
| 53.00 | 47.00 | 1.12 | |
| 52.40 | 47.60 | 1.10 | |
| 52.20 | 47.80 | 1.09 | |
| 50.34 | 49.66 | 1.01 | |
| dnNb TG | |||
| 59.50 | 40.50 | 1.46 | |
| 59.32 | 40.68 | 1.45 | |
| 54.00 | 46.00 | 1.21 | |
| 52.91 | 47.09 | 1.12 | |
| 52.72 | 47.28 | 1.11 | |
| 52.00 | 48.00 | 1.08 |
| . | DC#1 . | DC#2 . | DC#1/DC#2 . |
|---|---|---|---|
| Nb TG | |||
| 58.77 | 41.23 | 1.42 | |
| 56.90 | 43.00 | 1.32 | |
| 56.00 | 44.00 | 1.27 | |
| 56.00 | 44.00 | 1.27 | |
| 54.52 | 45.48 | 1.19 | |
| 53.32 | 46.68 | 1.14 | |
| 53.00 | 47.00 | 1.12 | |
| 52.40 | 47.60 | 1.10 | |
| 52.20 | 47.80 | 1.09 | |
| 50.34 | 49.66 | 1.01 | |
| dnNb TG | |||
| 59.50 | 40.50 | 1.46 | |
| 59.32 | 40.68 | 1.45 | |
| 54.00 | 46.00 | 1.21 | |
| 52.91 | 47.09 | 1.12 | |
| 52.72 | 47.28 | 1.11 | |
| 52.00 | 48.00 | 1.08 |
DC indicates daughter cell.
Discussion
Although asymmetric division is associated with precursor differentiation in an increasing number of developing organs, the question of whether this also applies to thymocyte development has remained elusive. We have explored this by visualizing dividing thymocytes on fetal thymic frozen sections. Our finding that Numb asymmetrically segregates in a subset of dividing fetal thymocytes is in good agreement with the observed pattern of Numb segregation during mouse cortical neurogenesis.7,39 However, both Numb and the pre-TCR polarize early during the cell cycle, indicating a possible role for Numb in pre-TCR signaling. Localization of pre-TCR on lipid rafts40 and its polarization associated with signaling41 had been observed before. Therefore, despite its capacity to signal in a ligand-independent fashion, pre-TCR is probably able to signal more efficiently when the TCR signaling machinery is polarized. Moreover, Numb seems to be associated with this machinery in DN thymocytes. Membrane pre-TCR localization through the cell cycle indicated that, although pre-TCR synthesis is stopped early,26 its expression and signaling must continue through the cell cycle. On the basis of our data showing that Numb, but not pre-TCR, is asymmetrically segregated, it can be speculated that, when the division is asymmetric, the cell inheriting both Numb and pre-TCR continues proliferating, whereas the cell inheriting pre-TCR but not Numb differentiates further. This is a simple model, based on data from other systems in which asymmetric division has been studied in detail.4-6 In this regard, our observation that, in fetal thymus, just 30% of the precursors divide asymmetrically seems logical, because at this developmental stage proliferation must be promoted at the expense of differentiation to ensure correct numbers of thymocytes in the adult organ. Possibly, at later stages (eg, adult thymus) differentiation is promoted at the expense of proliferation, by changing the symmetric/asymmetric division rate. It is important to note that Numb may play a role in pre-TCR signaling at prophase and metaphase (when it is always polarized), and a different role as cell fate determinant at telophase (when it is only asymmetrically segregated in a fraction of dividing thymocytes).
In this context, the mild block at the DN3 stage observed in mice in which Numb and Numblike had been conditionally deleted (see Figure 4C in27 ) and the down-modulation of Notch target genes in thymocytes of mice overexpressing p61 Numb29 both support the idea that Numb may modulate important signaling events at early stages of development. The mentioned knockout study must be interpreted with caution, in the light of data showing that as little as 5% of normal levels of Numb or Numblike is enough to guarantee normal asymmetric division and development in mammalians.38 It is possible that, in the work by Wilson et al,27 remnants of Numb and Numblike in precursors were enough to allow thymocyte development, resulting in the observed mild defect. We have used a dominant-negative approach to avoid these and other problems related to knockout models.42 A dominant-negative protein binds to the same ligand than the endogenous protein but is functionally defective. In dnNb TG mice, the dnNb protein should bind to endogenous Numb PTB ligands, but proteins that are normally associated to the Numb C-terminus would be excluded from the complexes. In the case of c-Cbl, it is associated to endogenous Numb in endosomes of WT but not dnNb TG DN thymocytes. Therefore, dnNb does probably bind to Numb ligands within the TCR complex but is unable to bind to c-Cbl and help it translocate into the endosomes because of lacking the C-terminus.11,13 The similarity between c-Cbl/Cbl-b knockout37 and dnNb TG thymic phenotypes does also favor this notion. Thus, our data indicate a role for Numb in facilitating c-Cbl access to endosomes. Obviously, this affects pre-TCR degradation and signaling, because c-Cbl is directly involved in this function.37 It is important to note that protein degradation plays an essential role during development43 and asymmetric division44,45 ; therefore, a link between Numb and pre-TCR degradation seems a reasonable mechanism to regulate thymic development.
Another indication of the mechanism by which dnNb blocks Numb function comes from visualization of endogenous Numb in dnNb TG DN thymocytes. The fact that endogenous Numb is not polarized in premitotic thymocytes of dnNb TG mice indicates that dnNb associates with pre-TCR complexes on the membrane. However, without the C-terminus dnNb does not allow pre-TCR translocation into the endosomes. This results in pre-TCR accumulation on the cell surface and endogenous Numb cytoplasmic distribution in dnNb TG thymocytes. We have excluded the possibility that dominant-negative Numb may inhibit other PTB-containing proteins than Numb by showing that Numb overexpression reverts the dominant-negative phenotype. In addition, the observed opposite effects on thymus size by either inhibiting or overexpressing Numb and the lack of asymmetric Numb segregation in Numb TG thymocytes indicate that Numb and asymmetric division play a key role in regulating thymus size.
Together, the data support a model for thymocyte asymmetric division in which Numb presence in daughter cells mitigates pre-TCR signaling and allows further proliferation. However, dividing thymocytes that do not inherit Numb receive more pre-TCR signals and differentiate (Fig 7C). It seems, thus, clear that Numb directs thymocyte decisions between differentiation and division. This is in agreement with the proposed general role of asymmetric division as a way to diversify cell progeny.46 Future studies in the thymus and other organs will, hopefully, clarify what cues above Numb dictate at a certain developmental stage the rate of precursor differentiation versus proliferation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Weimin Zhong (Yale University) for discussion and support; Drs M. L. Toribio (CBMSO, CSIC, Spain), B. J. Fowlkes (NIAID, National Institutes of Health), and S. Hilfiker (IPBLN, CSIC, Spain) for discussion; B. J. Fowlkes for Numb and dnNb transgenic mice; Juan Carlos Zúñiga-Pflücker for OP9 and OP9-DL1 cells; Francisco Ferrer and Maria Isabel Gutierrez for expert technical mouse work; and Jose Luis Luque for expert technical help with microscopy.
The work was supported by the Spanish Science and Education Ministry (grants BFU2004-01771 and BFU2007-67476), a Special Intramural CSIC (Spanish Science Council) grant, by the Department of Science and Innovation of the Regional Government of Andalucia, Spain (Excellence Grant P06-CTS-02 112) and FEDER funds from the European Union. R.A. and M. Caraballo were supported by a CSIC scholarship.
National Institutes of Health
Authorship
Contribution: R.A., N.M.-B., and M. Caraballo planned, designed, and performed experiments; and M. Canelles planned, designed, performed experiments, and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matilde Canelles, Instituto de Parasitología y Biomedicina López-Neyra, CSIC, Parque Tecnológico Ciencias de la Salud, 18100 Granada, Spain; e-mail: mcanelles@ipb.csic.es.
References
Author notes
R.C. and N.M.-B. contributed equally to the study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal