Abstract
Human red cell differentiation requires the action of erythropoietin on committed progenitor cells. In iron deficiency, committed erythroid progenitors lose responsiveness to erythropoietin, resulting in hypoplastic anemia. To address the basis for iron regulation of erythropoiesis, we established primary hematopoietic cultures with transferrin saturation levels that restricted erythropoiesis but permitted granulopoiesis and megakaryopoiesis. Experiments in this system identified as a critical regulatory element the aconitases, multifunctional iron-sulfur cluster proteins that metabolize citrate to isocitrate. Iron restriction suppressed mitochondrial and cytosolic aconitase activity in erythroid but not granulocytic or megakaryocytic progenitors. An active site aconitase inhibitor, fluorocitrate, blocked erythroid differentiation in a manner similar to iron deprivation. Exogenous isocitrate abrogated the erythroid iron restriction response in vitro and reversed anemia progression in iron-deprived mice. The mechanism for aconitase regulation of erythropoiesis most probably involves both production of metabolic intermediates and modulation of erythropoietin signaling. One relevant signaling pathway appeared to involve protein kinase Cα/β, or possibly protein kinase Cδ, whose activities were regulated by iron, isocitrate, and erythropoietin.
Introduction
Red cell production results from erythropoietin (Epo)–driven survival, proliferation, and maturation of committed bone marrow progenitor cells. This process critically depends on cellular uptake of adequate bioavailable iron, provided in the form of diferric transferrin. Compromise in iron uptake or intracellular trafficking results in iron-restricted erythropoiesis, characterized by diminished marrow responsiveness to Epo.1 Epo acts during an early phase of erythroid development, between late (erythroid burst-forming unit) and early pronormoblast stages, before the initiation of hemoglobin synthesis.1-3 Therefore, iron restriction serves as a checkpoint to restrain Epo-driven progenitor expansion in the face of limited iron stores. It has been documented in zebrafish, in which defects in intracellular iron utilization block early erythroid differentiation,4 and in mammals, in which dietary iron deficiency impairs the transition from erythroid colony-forming unit to pronormoblast.5 In the clinical setting, iron-restricted erythropoiesis underlies many of the anemias that are refractory to Epo treatment (eg, anemias of chronic renal disease and inflammation).1
The lineage- and stage-selective nature of the erythroid iron restriction response has suggested involvement of a specialized signaling pathway distinct from the iron depletion response that occurs in a wide variety of cell types treated with chelators. Supporting this notion, chelator-induced iron depletion in MCF-7 cells causes cell-cycle arrest in G1 phase followed by apoptosis,6 neither of which is seen in iron-restricted erythropoiesis in vivo.2,6,7 Thus, to identify mechanisms applicable to the erythroid iron restriction response, an in vitro model was created in which primary human hematopoietic progenitors underwent culture in medium with transferrin saturation levels that allowed normal granulopoiesis and megakaryopoiesis but precluded normal erythropoiesis. In this system, early erythroid progenitors showed impaired growth and maturation in response to Epo but no increase in apoptosis.
Intracellular iron response pathways use several kinase cascades, hypoxic response factors, Smad proteins, Notch signaling, and iron-response proteins 1 and 2 (IRP1 and IRP2).8-16 In the best-characterized iron regulatory system, IRP1 functions as cytosolic aconitase (ACO1) under high-iron conditions and converts to an RNA-binding factor under low-iron conditions. This latter function, involving engagement of cis-acting iron response elements in specific mRNAs (eg, ferritin and transferrin receptor 1), has been widely accepted to mediate many features of the mammalian iron deprivation response.15
The current studies examined signaling alterations associated with the erythroid iron deprivation response, based on the hypothesis that a discrete pathway may connect iron availability with Epo responsiveness. From screening known iron response pathways, we identified aconitase enzymatic activity as correlating most closely with the lineage-specific iron restriction response. A functional role for these enzymes was supported by multiple experimental approaches, including enzymatic inhibition and provision of exogenous metabolic product. In the latter experiments, isocitrate strikingly reversed the erythroid iron restriction response in vitro as well as in vivo, raising the possibility for a novel therapeutic approach to Epo-resistant anemias.
Possible mechanisms for erythroid regulation by aconitase and isocitrate include production of adenosine triphosphate (ATP), reduced form of nicotinamide adenine dinucleotide (NADH), nicotinamide adenine dinucleotide phosphate (NADPH), and succinyl coenzyme A (CoA), the last of which serves as a precursor for heme biosynthesis. However, multiple independent experimental approaches supported a novel signaling function. In particular, the iron-aconitase-isocitrate pathway played a critical role in Epo-mediated down-regulation of protein kinase Cα/β (PKCα/β) activation and also affected PKCδ activation status. Accordingly, hyperactivation of PKC induced by iron deprivation contributed to multiple aspects of the erythroid iron restriction response. A model for iron regulation of erythropoiesis is presented that integrates these findings with the recent report of physical and functional interaction between PKC and aconitase.17
Methods
Cell cultures and treatments
Purified human CD34+ progenitors derived from granulocyte-colony stimulating factor–mobilized peripheral blood cells of healthy donors were provided by the Hematopoietic Cell Processing Core of the Fred Hutchinson Cancer Research Center as previously described.18 The cells were grown at 37°C with 5% CO2 in serum-free medium consisting of Iscove modified Dulbecco medium with β-mercaptoethanol, lot-tested bovine serum albumin, and the indicated cytokines (PeproTech). To establish cultures with defined transferrin saturations, the medium was supplemented with defined ratios of the insulin/transferrin/selenium A and B preparations from StemCell Technologies. After thawing, the CD34+ progenitors first underwent approximately 48 hours of prestimulation in Iscove modified Dulbecco medium with β-mercaptoethanol, BIT 9500 supplement (StemCell Technologies), and cytokines consisting of 100 ng/mL stem cell factor (SCF), 100 ng/mL FMS-like tyrosine kinase 3 ligand, 100 ng/mL thrombopoietin, and 50 ng/mL interleukin-3. After prestimulation, the cells were washed and resuspended in unilineage differentiation medium at 1.5 × 105 cells/mL. Erythroid medium contained 4.5 U/mL Epo and 25 ng/mL SCF; granulocytic medium contained 10 ng/mL granulocyte colony-stimulating factor, 10 ng/mL interleukin-3, and 25 ng/mL SCF; and the megakaryocytic medium contained 40 ng/mL thrombopoietin, 100 ng/mL SDF1α, and 25 ng/mL SCF with cells grown on fibronectin-coated plates. Cell numbers and viabilities were determined manually with the use of a hemacytometer and trypan blue staining. Barium fluorocitrate (Sigma-Aldrich) was converted to sodium fluorocitrate with the use of the approach of Paulsen et al.19 L+- and D−-isocitrate trisodium salts were obtained by saponification of the corresponding enantiopure isocitric acid lactones (3 equivalents NaOH, 80°C, 4 hours). The L+- and D−-isocitric acid lactones were synthesized from dimethyl L- and D-malate, respectively, by the method of Schmitz et al.20 The cell-permeable α-ketoglutarate derivative, trifluoromethyl benzyl-α-ketoglutarate (TaKG), was synthesized as previously described.21 Racemic sodium isocitrate, sodium citrate, sodium α-ketoglutarate, and chloramphenicol were all purchased from Sigma-Aldrich. Small-molecule inhibitors, including bisindolylmaleimide I hydrochloride and Gö6983 were purchased from EMD/Calbiochem. Knockdown of ACO2 expression in K562 and CD34+ cells used the LMP retroviral vector (Open Biosystems) containing short hairpin RNA (shRNA) template subcloned from the pSM2c construct V2HS_231437 (Open Biosystems). Transduction followed by analysis of green fluorescent protein–positive (GFP+) cells was performed as previously described.22
Flow cytometry
Flow cytometric analysis of erythroid differentiation by costaining for glycophorin A (GPA) and CD41 was performed as previously described.18,22 Staining of cells with annexin V–phycoerythrin and 7-amino-actinomycin D used the Annexin 5-phycoerythrin Apoptosis Detection Kit I (BD PharMingen). For cell-cycle analysis, cells underwent fixation with ethanol followed by staining with propidium iodide in the presence of RNAase. Analyses were performed on a FACSCalibur instrument (BD Biosciences) with the use of FlowJo software Version 8.6.3 (TreeStar Inc).
Aconitase, IRP, and metabolic assays
Assays for aconitase activites of ACO1/ACO2, iron-responsive element (IRE)–binding activities of IRP1/IRP2, and IRP2 protein levels were performed as previously described.23 For detailed information on aconitase assays, as well as on measurements of cellular ATP, adenosine monophosphate (AMP), isocitrate, citrate, NADH, oxidized form of NADPH (NADP), and NADPH, see supplemental Experimental Procedures (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
In vivo models
The animal experiments were approved by the University of Virginia Animal Care and Use Committee. The mice consisted of C57BL/6 purchased from The Jackson Laboratory. In initial experiments to assay for toxicity, iron replete, 6-week-old female mice received sodium DL-isocitrate by daily intraperitoneal injection at 200 mg/kg/d for 5 days. To induce iron deficiency anemia, 3-week-old male weanlings were placed on the Iron Deficient Diet from Harlan Teklad (product no. TD 30 396), which contains 2 to 5 ppm of iron. For measurements of complete blood counts (CBCs), retro-orbital blood samples were analyzed on the Hemavet 850 FS automated CBC analyzer (Drew Scientific). Starting at 1 month of the diet, CBCs were regularly monitored. Treatments, initiated at onset of anemia (approximately day 40 of diet), consisted of daily intraperitoneal isocitrate or α-ketoglutarate (200 mg/kg) or equivalent volume of saline for 6 to 7 consecutive days.
Statistical analysis
Pairwise comparisons of values in treatment groups used a 2-tailed Student t test. P values less than or equal to .05 were considered significant.
Immunoblot
Whole-cell lysates underwent sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting as previously described.18 Antibodies included mouse anti–β-actin, and mouse antitubulin (Sigma-Aldrich); rabbit anti-ACO2 (generously provided by Dr Luke Szweda, Oklahoma Medical Research Foundation); rabbit antiferritin heavy chain and anti–5-aminolevulinate synthase e (ALAS-e; Santa Cruz Biotechnology); and rabbit anti–globin (Accurate Chemical & Scientific). Rabbit polyclonal antibodies to phosphorylated and total PKC isozymes were purchased from Cell Signaling Technology, with the exceptions of antibodies to phospho-PKCϵ and phospho-PKCη (Millipore).
Results
An in vitro model of iron-restricted erythropoiesis
An important aspect of iron-restricted erythropoiesis in vivo consists of lineage selectivity, manifesting as an isolated anemia with normal numbers of white cells and normal to elevated platelet counts.24 To recapitulate this phenomenon in vitro, we identified a critical threshold of transferrin saturation that allowed human CD34+ hematopoietic progenitors to undergo granulopoiesis and megakaryopoiesis but not erythropoiesis, with all cultures initiated at a density of 1.5 × 105 cells/mL. In particular, various transferrin saturations from 100% to 25% had no effect on any lineage, whereas a transferrin saturation of 15% caused an erythroid lineage-specific growth arrest and gradual decline in viability (Figure 1A). By contrast, 1% transferrin saturation decreased growth and viability in all 3 lineages (Figure 1A). Thus, transferrin saturation levels at 15% elicited an erythroid iron-restriction response and at 1% produced a non–lineage-specific response.
A primary human model system for iron restriction of erythropoiesis. (A) Effect of transferrin saturation (Sat) on growth and viability of hematopoietic lineages. CD34+ cells underwent unilineage culture in serum-free media. Results show mean ± SEM for 3 to 5 independent experiments. (B) Inhibition of early erythroid differentiation by iron restriction. Erythroid cultures with 100% or 15% transferrin saturation underwent flow cytometry (fluorescence-activated cell sorting [FACS]) with gating on viable cells. CD34/CD36 expression was analyzed on day 4. Indicated are percentages of cells in various quadrants. (C) Minimal apoptosis associated with erythroid iron restriction. Cells from panel B were analyzed by FACS for 7-amino-actinomycin D (7-AAD) and annexin V staining with gating on all cells. Whole cell lysates from day 5 cultures underwent immunoblotting (IB) for caspase 3 (bottom; arrow indicates cleavage product). (D) Minimal effect of erythroid iron restriction on cell-cycle parameters. Day 5 cultures stained with propidium iodide underwent FACS. Indicated are percentages of cells in various fractions, including subdiploid (Sub).
A primary human model system for iron restriction of erythropoiesis. (A) Effect of transferrin saturation (Sat) on growth and viability of hematopoietic lineages. CD34+ cells underwent unilineage culture in serum-free media. Results show mean ± SEM for 3 to 5 independent experiments. (B) Inhibition of early erythroid differentiation by iron restriction. Erythroid cultures with 100% or 15% transferrin saturation underwent flow cytometry (fluorescence-activated cell sorting [FACS]) with gating on viable cells. CD34/CD36 expression was analyzed on day 4. Indicated are percentages of cells in various quadrants. (C) Minimal apoptosis associated with erythroid iron restriction. Cells from panel B were analyzed by FACS for 7-amino-actinomycin D (7-AAD) and annexin V staining with gating on all cells. Whole cell lysates from day 5 cultures underwent immunoblotting (IB) for caspase 3 (bottom; arrow indicates cleavage product). (D) Minimal effect of erythroid iron restriction on cell-cycle parameters. Day 5 cultures stained with propidium iodide underwent FACS. Indicated are percentages of cells in various fractions, including subdiploid (Sub).
In a rodent model, iron-restricted erythropoiesis has been characterized by a developmental block at the transition from erythroid colony-forming unit to early erythroblast.5 Erythroid maturation of human progenitors can be monitored by flow cytometric quantitation of GPA, a lineage-specific surface marker whose expression increases as a direct function of cellular differentiation; in addition, the earliest detectable events of human erythroid lineage commitment are characterized by simultaneous down-regulation of CD34 and up-regulation of CD36.25 In vitro iron restriction in our system disrupted early erythroid differentiation, inhibiting the up-regulation of GPA and CD36, as well as the down-regulation of CD34 (Figure 1B). Previous studies comparing erythroid versus megakaryocytic output of primary human CD34+ cell cultures have used combined staining for GPA and CD41.26 By this approach, the erythroid blockade observed with 15% transferrin saturation was associated with increased proportions of CD41+ GPA− cells, most probably representing megakaryocytic precursors, a conclusion further supported by their structure on Wright-stained cytospins (see Figure 3C). The iron-restricted erythroid cultures also showed a slight increase in cell death. Importantly, this cell death appeared to be nonapoptotic, with no increase in annexin V staining among 7-amino-actinomycin D–negative cells and with no increase in caspase 3 cleavage (Figure 1C). Cell-cycle analysis showed no evidence for G1 or G2/M arrest as the basis for growth inhibition in Figure 1A, nor was a significant subdiploid (Sub) population seen (Figure 1D). Additional multiparametric flow cytometric studies confirmed the absence of annexin V staining and cell-cycle arrest within the GPA+ erythroid fraction (supplemental Figure 1). Furthermore, treatment of cells with a caspase inhibitor failed to alleviate the block in erythropoiesis caused by iron restriction (supplemental Figure 1).
Characterization of relevant pathways: a novel role for aconitase
To identify mediators of the erythroid iron-restriction response shown in Figure 1, candidate iron-regulated pathways were analyzed by 3 approaches: determination of signaling perturbations, pharmacologic pathway activation or blockade, or retroviral/genetic blockade. These approaches provided no evidence for involvement of hypoxia-inducible factor, Notch, c-Jun N-terminal kinase, p38, extracellular signal-regulated kinase, Janus kinase 2, caspase, heat shock protein 70, ferritin heavy chain, or bone morphogenetic protein–Smad (for details, see supplemental Tables 1-2).
Although aconitase inactivation occurs in vitro during cellular iron depletion,23 iron-deficient animals have shown little evidence for declines in specific activity in nonhematopoietic tissues. Specifically, Meyron-Holtz et al27 showed identical cytosolic (ACO1) and mitochondrial (ACO2) aconitase activites in liver extracts from iron-replete and iron-deficient mice, despite a clear increase in IRP2 activity in the iron-deficient samples. Along similar lines, comparison by Chen et al28 of iron-replete and -deficient rat livers showed no change in ACO1 activity and a 25% decrease in ACO2 activity that was attributable to a decrease in ACO2 protein levels. We examined the effect of iron restriction on aconitase activities in primary hematopoietic progenitors in a gel-based assay that resolves the mitochondrial and cytosolic isoforms. In these experiments, iron restriction reproducibly caused 60% decreases in both mitochondrial and cytosolic aconitase activities in erythroid samples (P < .05) but no significant changes in megakaryocytic or granulocytic enzyme activities (Figure 2A-B). The protein levels of mitochondrial aconitase remained unaffected by erythroid iron restriction (Figure 2A), despite the pronounced decrease in enzymatic activity. Cytosolic aconitase protein was below the limit of immunoblot detection in these cells. Consistent with the decrease in aconitase activities, iron-restricted erythroblasts showed a 3-fold decrease in intracellular isocitrate levels (P = .02) (supplemental Figure 2A). In 2 of 2 experiments, increased intracellular citrate occurred with iron restriction, but this finding was not statistically significant because of the variable magnitude of the change (supplemental Figure 2B). Concomitant activation of ATP-citrate-lyase (ACL), which promotes metabolic shunting of citrate to acetyl-CoA, may have prevented major citrate buildup during iron deprivation (supplemental Figure 2C). We also examined the activities of isocitrate dehydrogenase (IDH) and succinate dehydrogenase, enzymes which act downstream of aconitase. With the use of gel-based enzymography, 2 NADP-dependent and no NAD-dependent IDH activities were identified in erythroid cells, neither of which diminished with iron restriction; in fact, the upper species/activity appeared slightly increased with iron restriction (supplemental Figure 2E). succinate dehydrogenase, which contains an iron-sulfur cluster, showed no change in activity due to iron restriction (supplemental Figure 2F).
Implication of aconitase activity in iron regulation of erythropoiesis. (A) Erythroid-specific aconitase inactivation during iron restriction. Enzymography (Acon Activity) was performed on extracts from day 4 cultures in erythroid (Ery), megakaryocytic (Mk), or granulocytic (Gran) media with 100% or 15% transferrin saturation; controls consisted of K562 cells expressing shRNAs targeting ACO2 or ACO1. Expression of mitochondrial aconitase protein in samples (bottom panel, IB: ACO2). (B) Summary of 3 independent experiments as in panel A. The y-axis is the ratio of scanned signals obtained with 15% transferrin saturation to those obtained with 100% transferrin saturation, expressed as mean percentage ± SEM. **P < .001 for Ery ACO1 changes and 0.03 for Ery ACO2 changes. Mk and Gran signals showed no significant changes. (C) Direct aconitase inhibition impairs erythroid differentiation in a manner similar to iron restriction. Erythroid cultures with 100% transferrin saturation were supplemented with fluorocitrate (FC) and underwent FACS assessment of differentiation (top panels) and viability (forward [FSC] and side scatter [SSC] in bottom panels) on day 5. Where indicated, 10mM citrate was also included in culture. (D) Summary of 3 independent experiments as in panel C, using 200μM fluorocitrate.
Implication of aconitase activity in iron regulation of erythropoiesis. (A) Erythroid-specific aconitase inactivation during iron restriction. Enzymography (Acon Activity) was performed on extracts from day 4 cultures in erythroid (Ery), megakaryocytic (Mk), or granulocytic (Gran) media with 100% or 15% transferrin saturation; controls consisted of K562 cells expressing shRNAs targeting ACO2 or ACO1. Expression of mitochondrial aconitase protein in samples (bottom panel, IB: ACO2). (B) Summary of 3 independent experiments as in panel A. The y-axis is the ratio of scanned signals obtained with 15% transferrin saturation to those obtained with 100% transferrin saturation, expressed as mean percentage ± SEM. **P < .001 for Ery ACO1 changes and 0.03 for Ery ACO2 changes. Mk and Gran signals showed no significant changes. (C) Direct aconitase inhibition impairs erythroid differentiation in a manner similar to iron restriction. Erythroid cultures with 100% transferrin saturation were supplemented with fluorocitrate (FC) and underwent FACS assessment of differentiation (top panels) and viability (forward [FSC] and side scatter [SSC] in bottom panels) on day 5. Where indicated, 10mM citrate was also included in culture. (D) Summary of 3 independent experiments as in panel C, using 200μM fluorocitrate.
To determine whether loss of aconitase activity could contribute to impairment in erythropoiesis, erythroid cultures with 100% transferrin saturation underwent treatment with the active site inhibitor fluorocitrate. In vivo and in vitro pharmacologic studies, as well as x-ray crystallography, have all established aconitase enzymes as the main target of fluorocitrate action.29 The mechanism of inhibition occurs through conversion of fluorocitrate to 4-hydroxy-trans-aconitate, which then stably occupies the active site; however, at high levels fluorocitrate may also block citrate transport.30 Fluorocitrate treatment reproducibly blocked erythroid maturation in a dosage-dependent manner and was reversed by inclusion of excess citrate (Figure 2C-D). Although the highest dose of fluorocitrate (200μM) also diminished cellular viability by approximately 23%, this effect was less than the differentiation block of approximately 50%. Concerns about nonspecific toxicity due to residual barium in our fluorocitrate preparation also prompted us to test the effects of fluoroacetate, provided as a highly pure, ready-to-use sodium salt. Fluoroacetate, which undergoes conversion in cells to fluorocitrate, potently blocked erythroid differentiation and caused no decrease in cell viability (supplemental Figure 3A). Therefore, the effects of aconitase inhibition on erythroid differentiation are not attributable to nonspecific toxicity.
To determine whether ACO1 contributed to erythroid maturation, we compared the responses of wild-type and knockout mice31 to phenylhydrazine-induced hemolytic anemia, with no differences observed. Therefore, the role of ACO2 was assessed in erythroid progenitors in high-iron cultures with the use of shRNA targeting. ACO2 knockdown that used the retroviral construct from Figure 2A caused a reproducible albeit modest block in GPA expression (supplemental Figure 3B-C). Attempts at combined shRNA knockdown of both enzymes, followed by standard culture in normal erythroid medium with adequate iron and no exogenous isocitrate, yielded too few transfectants for meaningful analysis.
Isocitrate reversal of the erythroid iron-restriction response
In an attempt to bypass aconitase defects associated with iron deprivation, erythroid cultures were supplemented with exogenous DL-isocitrate at dosages similar to those routinely used for short-chain fatty acid induction of fetal hemoglobin in erythroid progenitors.32 Strikingly, DL-isocitrate reversed all of the phenotypic features of iron-restricted erythropoiesis, including abnormalities in the GPA/CD41 ratio, cellular structure, hemoglobinization, and globin expression (Figure 3A-E). The 5-mM dose reproducibly restored erythroid GPA expression to 84% of the levels seen with 100% transferrin saturation (Figure 3B). Maximal rescue of erythropoiesis, with essentially complete restoration of all parameters, was observed with 20mM isocitrate. Importantly, DL-isocitrate did not reverse the decline in ferritin levels associated with iron restriction (Figure 3E), suggesting that it does not affect cellular iron stores. However, ferritin levels may also be regulated at the transcriptional level. Therefore, direct assays of IRP activity were conducted as well. Analysis of IRP activity showed that 15% transferrin saturation activated both IRP1 and IRP2 (Figure 3F-G). Interestingly, DL-isocitrate reversed the activation of IRP1 by erythroid iron restriction but had minimal effects on activity or expression of IRP2, the more reliable sensor of intracellular iron.31 Trace iron contamination of the isocitrate was also ruled out by mass spectrometry and atomic emission spectroscopy. Furthermore, in erythroid cultures with limiting amounts of Epo, isocitrate failed to reverse the effects of iron restriction, confirming that its action occurs independently of iron delivery to cells (see Figure 5A-B). To rule out potential artifacts caused by isocitrate chelation of extracellular calcium, cells were treated with the extracellular calcium chelator EGTA (ethylene glycol tetraacetic acid), which showed no effects at doses ranging from 5μM to 1.5mM (not shown). As an additional specificity control, cells underwent treatment with citrate. Unlike isocitrate, citrate at 20mM exacerbated the effects of iron restriction (supplemental Figure 4).
Isocitrate abrogation of the erythroid iron-restriction checkpoint. (A) Isocitrate (IC) reverses defects in differentiation and viability. Five-day erythroid cultures with 15% transferrin saturation included trisodium isocitrate (IC) at 5mM. FACS analysis of GPA and CD41 expression with gating on viable fraction. (B) Summary of 3 independent experiments as in panel A. (C) Isocitrate reverses structural changes associated with iron restriction. Light microscopy of Wright-stained cytospins from cultures in panel A (400× magnification). Images were acquired with the use of an Olympus BX51 microscope equipped with an Olympus DP70 digital camera. The objective lens consisted of Uplan Fl 40×/0.75 NA. Image acquisition and processing used Adobe Photoshop, CS3/10.0 and CS2/9.0, respectively. (D) Isocitrate enhances hemoglobinization. Erythroid cultures with 100% or 15% transferrin saturation were supplemented with 20mM isocitrate. Photograph of day 5 cell pellets. (E) Isocitrate augments globin chain expression. Whole cell lysates from panel D underwent immunoblotting. (F) IRE-binding activity of IRP1 and IRP2: influences of transferrin saturation and isocitrate. RNA gel-shift assays were performed on extracts from day 4 CD34+ cultures in erythroid medium with 100% or 15% transferrin saturation and 20mM isocitrate. (G) IRP2 stabilization by iron deprivation: minimal effect of isocitrate. Cellular extracts from panel F underwent immunoblotting. FSC indicates forward scatter; and SSC, side scatter.
Isocitrate abrogation of the erythroid iron-restriction checkpoint. (A) Isocitrate (IC) reverses defects in differentiation and viability. Five-day erythroid cultures with 15% transferrin saturation included trisodium isocitrate (IC) at 5mM. FACS analysis of GPA and CD41 expression with gating on viable fraction. (B) Summary of 3 independent experiments as in panel A. (C) Isocitrate reverses structural changes associated with iron restriction. Light microscopy of Wright-stained cytospins from cultures in panel A (400× magnification). Images were acquired with the use of an Olympus BX51 microscope equipped with an Olympus DP70 digital camera. The objective lens consisted of Uplan Fl 40×/0.75 NA. Image acquisition and processing used Adobe Photoshop, CS3/10.0 and CS2/9.0, respectively. (D) Isocitrate enhances hemoglobinization. Erythroid cultures with 100% or 15% transferrin saturation were supplemented with 20mM isocitrate. Photograph of day 5 cell pellets. (E) Isocitrate augments globin chain expression. Whole cell lysates from panel D underwent immunoblotting. (F) IRE-binding activity of IRP1 and IRP2: influences of transferrin saturation and isocitrate. RNA gel-shift assays were performed on extracts from day 4 CD34+ cultures in erythroid medium with 100% or 15% transferrin saturation and 20mM isocitrate. (G) IRP2 stabilization by iron deprivation: minimal effect of isocitrate. Cellular extracts from panel F underwent immunoblotting. FSC indicates forward scatter; and SSC, side scatter.
Regulation of erythropoiesis by isocitrate: metabolic and nonmetabolic pathways
A possible mechanism for isocitrate regulation of erythropoiesis consists of its metabolic processing through the Krebs cycle to yield ATP, NAD(P)H, and succinyl CoA. Succinyl CoA provides a critical building block for heme synthesis through conjugation with glycine by ALAS-2, an erythroid enzyme deficient in patients with X-linked sideroblastic anemia.33 Evidence against such a mechanism was provided by analysis of cellular ATP, NADH, and NADPH levels, none of which showed significant change as a function of iron restriction or isocitrate rescue (Figure 4A). AMP kinase activation, which reflects cellular energy balance, was not enhanced by iron restriction; in addition, intracellular AMP levels were not increased (supplemental Figure 5A-B). Furthermore, exogenous α-ketoglutarate, provided as the bioactive cell-permeable ester TaKG,21 failed to reverse the erythroid iron-restriction response (supplemental Figure 5C). TaKG, like exogenous hemin, induced erythroid differentiation in K562 cells, consistent with its uptake and metabolism to heme (supplemental Figure 5D). As additional evidence suggesting a nonmetabolic mechanism of action, isocitrate, but not hemin, relieved an erythroid-differentiation blockade caused by chloramphenicol, an inhibitor of mitochondrial biogenesis (supplemental Figure 5E-F). Finally, enantiopure preparations of D- and L-isocitrate were compared for rescue of iron-restricted erythropoiesis. Only D-isocitrate can be metabolized by isocitrate dehydrogenase.34 Surprisingly, L-isocitrate restored cellular viability with an efficiency equivalent to that of D-isocitrate (Figure 4B). L-isocitrate also rescued erythroid differentiation (Figure 4C), albeit not as efficiently as D-isocitrate. In aggregate, these results suggest that isocitrate regulation of erythropoiesis may occur, at least in part, through a nonmetabolic mechanism.
Participation of a nonmetabolic isocitrate signaling pathway. (A) Cellular ATP, NADH, and NADPH levels in day 5 erythroid cultures. Shown are means ± SEMs for 3 independent experiments. (B) Viability effects of L- versus D-isocitrate in erythroid iron restriction. Erythroid cultures received 1mM, 5mM, and 10mM isocitrate (IC) enantiomers, with FACS analysis on day 5. Graph summarizes 3 independent experiments with the use of 10mM isocitrate. **P = .012 for D-IC + 15% versus 15% and 0.017 for L-IC + 15% versus 15%. No significant difference was seen for D-IC + 15% versus L-IC + 15%. (C) Effects of L- versus D-isocitrate on erythroid differentiation. FACS analysis of GPA and CD41 expression in cultures from panel B, with gating on viable fraction. **P = .04 for D-IC + 15% versus 15%; *P = .05 for L-IC + 15% versus 15%. In addition, P = .05 for D-IC + 15% versus L-IC + 15%.
Participation of a nonmetabolic isocitrate signaling pathway. (A) Cellular ATP, NADH, and NADPH levels in day 5 erythroid cultures. Shown are means ± SEMs for 3 independent experiments. (B) Viability effects of L- versus D-isocitrate in erythroid iron restriction. Erythroid cultures received 1mM, 5mM, and 10mM isocitrate (IC) enantiomers, with FACS analysis on day 5. Graph summarizes 3 independent experiments with the use of 10mM isocitrate. **P = .012 for D-IC + 15% versus 15% and 0.017 for L-IC + 15% versus 15%. No significant difference was seen for D-IC + 15% versus L-IC + 15%. (C) Effects of L- versus D-isocitrate on erythroid differentiation. FACS analysis of GPA and CD41 expression in cultures from panel B, with gating on viable fraction. **P = .04 for D-IC + 15% versus 15%; *P = .05 for L-IC + 15% versus 15%. In addition, P = .05 for D-IC + 15% versus L-IC + 15%.
Coupling of iron and isocitrate to Epo modulation of PKC
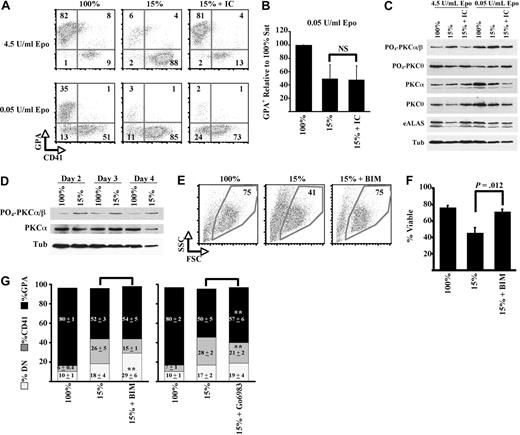
To determine the involvement of Epo signaling in isocitrate regulation of erythropoiesis, rescue experiments as in Figures 3 and 4 were conducted with a variety of Epo doses. Notably, a concentration of 0.05 U/mL Epo still permitted partial erythroid differentiation under iron-replete conditions but completely precluded isocitrate rescue of differentiation under iron restriction (Figure 5A-B). In the course of screening Epo-associated signal transduction pathways, an association was identified between responsiveness to isocitrate and the status of PKCα/β activation (T638 phosphorylation). In particular, in cultures with high levels of Epo (4.5 U/mL), iron restriction induced PKCα/β hyperactivation, which was reversed by inclusion of isocitrate (immunoblot in Figure 5C; scanning densitometry in supplemental Figure 6). By contrast, cells cultured with low levels of Epo (0.05 U/mL) showed PKCα/β hyperactivation regardless of iron or isocitrate levels. These findings were highly reproducible in 3 independent experiments and were specific for PKCα/β. Most other PKC isozymes such as θ (Figure 5C), ϵ (supplemental Figure 8A), η (not shown), and λ (not shown) showed no changes in activation as a function of iron, isocitrate, or Epo levels. Two notable exceptions consisted of PKCδ, which was consistently hyperactivated by iron restriction but unaffected by Epo deprivation, and PKCμ, whose activation diminished with Epo deprivation but not with iron restriction (supplemental Figure 8A). These assays cannot distinguish between activation of the highly homologous PKCα and PKCβisoforms, but only the expression of the former protein could be ascertained by immunoblot.
Crosstalk of iron and isocitrate with Epo signaling: PKCα/β as common target. (A-B) Epo levels influence isocitrate rescue. FACS analysis of 5 day cultures with indicated doses of Epo plus 25 ng/mL SCF; 100% or 15% transferrin, and 20mM isocitrate (IC) were included as indicated. Graph summarizes results of 3 independent experiments. (C) Iron restriction and Epo deprivation both induce PKCα/β hyperactivation. Epo levels influence the capacity of isocitrate to reverse the hyperactivation. Whole cell lysates from 4 day erythroid cultures underwent immunoblotting. (D) Kinetics of PKCα/β hyperactivation associated with erythroid iron restriction. (E-F) Complete reversal of viability defects by pan-PKC inhibitor. Erythroid cultures with 100% or 15% transferrin were treated with 0.5μM BIM, with FACS analysis on day 5. Graph represents 3 independent experiments. (G) Partial reversal of differentiation defects with selective PKC inhibitor. Cultures as in panel E were treated with either 0.5μM BIM or with 0.5μM Gö6983. Graphs summarize 3 independent experiments. DN indicates GPA− CD41− double-negative cells. **P = .02 for DN percentage in BIM + 15% versus 15%. **P = .01 for GPA percentage in Gö6983 + 15% versus 15%.
Crosstalk of iron and isocitrate with Epo signaling: PKCα/β as common target. (A-B) Epo levels influence isocitrate rescue. FACS analysis of 5 day cultures with indicated doses of Epo plus 25 ng/mL SCF; 100% or 15% transferrin, and 20mM isocitrate (IC) were included as indicated. Graph summarizes results of 3 independent experiments. (C) Iron restriction and Epo deprivation both induce PKCα/β hyperactivation. Epo levels influence the capacity of isocitrate to reverse the hyperactivation. Whole cell lysates from 4 day erythroid cultures underwent immunoblotting. (D) Kinetics of PKCα/β hyperactivation associated with erythroid iron restriction. (E-F) Complete reversal of viability defects by pan-PKC inhibitor. Erythroid cultures with 100% or 15% transferrin were treated with 0.5μM BIM, with FACS analysis on day 5. Graph represents 3 independent experiments. (G) Partial reversal of differentiation defects with selective PKC inhibitor. Cultures as in panel E were treated with either 0.5μM BIM or with 0.5μM Gö6983. Graphs summarize 3 independent experiments. DN indicates GPA− CD41− double-negative cells. **P = .02 for DN percentage in BIM + 15% versus 15%. **P = .01 for GPA percentage in Gö6983 + 15% versus 15%.
Time course analysis determined whether the kinetics of PKCα/β hyperactivation were consistent with a causal role in the erythroid iron-restriction response. Notably, PKCα/β hyperactivation occurred 2 days after initiating iron restriction and persisted through day 4 (immunoblot in Figure 5D; scanning densitometry in supplemental Figure 7). The phenotypic response was first manifested between days 3 and 4 (Figure 1A-C). Therefore, PKCα/β hyperactivation preceded the growth, viability, and differentiation effects of iron restriction. To further examine the role of PKCα/β hyperactivation, iron-restricted cells underwent treatment with 0.5μM bisindolylmaleimide I (BIM), a regimen that inhibits PKC but is not isozyme selective.35 Remarkably, BIM treatment completely reversed the decline in cellular viability associated with iron restriction (Figure 5E-F). With regard to differentiation, BIM diminished the proportion of megakaryocytic cells but did not alleviate the block in erythroid differentiation. To address concerns that BIM might also inhibit PKC isozymes that support erythroid differentiation, for example, PKCϵ,36,37 similar experiments were conducted with a more selective inhibitor, Gö6983, which targets classical isozymes as does its congener Gö6976 but also spares PKCμ.38 Gö6983 (0.5μM) significantly restored erythroid differentiation (P = .01) and diminished megakaryocytic differentiation (P = .003) in the iron-restricted cultures (Figure 5G). These results show an Epo-signaling requirement for isocitrate rescue of erythroid differentiation under conditions of iron restriction and specifically implicate signals involved in the down-modulation of PKCα/β and δ activation. Failure of this down-modulation may contribute, at least in part, to the phenotypic features of the erythroid iron-deprivation response.
To more specifically examine the contribution of PKCα to the erythroid iron-deprivation response, lentiviral shRNA constructs were used to knock down expression in primary erythroid progenitors. As shown in supplemental Figure 8B, 1 of 3 constructs used, sh 692, effectively knocked down erythroid PKCα expression. The knockdown of PKCα did not have a major effect on GPA expression during iron deprivation (supplemental Figure 8C). These results suggest functional redundancy in which PKCβ and PKCδ may also contribute to the iron-deprivation response.
Influence of isocitrate on erythropoiesis in vivo
In normal adult C57BL/6 mice, intraperitoneal injection of a trisodium DL-isocitrate solution at 200 g/kg/d for 5 consecutive days produced no evidence of toxicity on the basis of signs/symptoms and necropsy studies. Therefore, this regimen was tested in a murine model of dietary iron deficiency anemia. In this model, male C57BL/6 weanlings placed on a low-iron diet reproducibly developed a progressive anemia that began approximately between days 30 and 45 of the diet. In 2 independent experiments, isocitrate treatment completely reversed the progression of anemia, as assessed by red blood cell number and hematocrit (Figure 6). Examination of full hematologic indexes (supplemental Table 3) indicated that the stimulatory effects of isocitrate were essentially confined to these parameters. The lack of increase in mean corpuscular volume and the decline in mean corpuscular hemoglobin concentration may have resulted from isocitrate driving red cell production in the face of ever-decreasing iron stores. To address the possibility that isocitrate might be acting as a nonspecific energy source, an additional experiment compared equivalent doses of isocitrate and α-ketoglutarate in mice with iron deficiency anemia. In contrast to results obtained with isocitrate, progression of anemia was unaffected by α-ketoglutarate administration (Figure 6). Administration of exogenous citrate to mice has not been performed because of the potential for its conversion to isocitrate by nonerythroid marrow cells.
Influence of isocitrate on erythropoiesis in vivo. Isocitrate (IC) inhibits anemia progression during iron deprivation. Peripheral red blood cell (RBC) number and hematocrit (HCT) in mice on iron-deficient diets. Treatment with isocitrate (dashed lines) versus either normal saline (NS) or α-ketoglutarate (αKG; solid lines) was initiated on days indicated by arrows. Each point represents mean ± SEM for 6 mice/group. **Experiment 1 day 54, isocitrate versus saline, P values are .007 and .001 for RBC number and HCT. ##Experiment 2 day 45, isocitrate versus saline, P values are .015 and .018 for RBC number and HCT. †Comparison of isocitrate with α-ketoglutarate, day 56, P values are .044 and .046 for RBC number and HCT.
Influence of isocitrate on erythropoiesis in vivo. Isocitrate (IC) inhibits anemia progression during iron deprivation. Peripheral red blood cell (RBC) number and hematocrit (HCT) in mice on iron-deficient diets. Treatment with isocitrate (dashed lines) versus either normal saline (NS) or α-ketoglutarate (αKG; solid lines) was initiated on days indicated by arrows. Each point represents mean ± SEM for 6 mice/group. **Experiment 1 day 54, isocitrate versus saline, P values are .007 and .001 for RBC number and HCT. ##Experiment 2 day 45, isocitrate versus saline, P values are .015 and .018 for RBC number and HCT. †Comparison of isocitrate with α-ketoglutarate, day 56, P values are .044 and .046 for RBC number and HCT.
Discussion
Iron regulation of erythroid differentiation provides a checkpoint to prevent inappropriate iron utilization when body stores are limited. In the absence of such a checkpoint, erythropoiesis could potentially divert iron from vital functions in other tissues.15 To function in a protective manner, this checkpoint must act at stages before Epo-mediated expansion of the progenitor pool and subsequent hemoglobinization. In addition, it must act in a rheostatic manner to calibrate erythropoietic output to iron availability.
Comparison of iron deficiency anemia with other human disorders of hemoglobin synthesis highlights some distinctive features of the erythroid iron-restriction pathway. Primary disorders of globin and heme synthesis, for example, thalassemias and ALAS-2 deficiency, display erythroid hyperplasia in the bone marrow.33,39 By contrast, marrows from patients with iron deficiency anemia lack erythroid hyperplasia,40 despite markedly increased (∼ 4-fold) serum Epo levels.41 Furthermore, defective conversion of protoporphyrin IX to heme by ferrochelatase occurs in both iron deficiency anemia and erythropoietic protoporphyria, but only a minority of patients with erythropoietic protoporphyria develop anemia.42 These clinical distinctions imply that iron restriction of erythropoiesis in humans does not result simply from defects in globin or heme production.
The clinical relevance of this regulatory pathway derives from its role in anemias associated with inflammation, renal disease, malignancy, and myelodysplasia, in which defects in iron uptake or processing render erythroid progenitors resistant to Epo stimulation.1,43 In these anemias, treatment of patients with intravenous iron may partially restore Epo responsiveness, but this measure cannot be applied over the long term because of risks of iron overloading. Of additional clinical importance, this regulatory pathway has been exploited in the therapy of polycythemia vera, in which purposeful induction of iron deficiency by phlebotomy remains the treatment of choice in restraining overactive erythropoiesis.44
In the current studies, the human progenitor model captured several key features of in vivo iron-restricted erythropoiesis, that is, lineage specificity, inhibition of early differentiation, and growth suppression, while lacking features of the more generic cellular iron-depletion response, that is, the G1 arrest and apoptosis seen with iron chelation. The growth inhibition occurred in the absence of phase-specific arrest and may result from generalized slowing of the entire cell division cycle, as has recently been described in erythroid progenitors treated with desferrioxamine.45 Interestingly, the threshold of transferrin saturation required to achieve this effect, 15%, correlates with the anemic threshold seen in patients with iron deficiency.46 Normal transferrin concentrations in human serum (2-3 mg/mL) greatly exceed those in our cell culture system (0.05 mg/mL), but differences in bioavailability, matrix compositions, target cell densities, and competing tissue demands complicate in vivo versus in vitro comparisons of absolute levels. However, using percentage saturation as a point of comparison may conceivably correct for many of these differences.
Several distinct approaches have implicated aconitase activity as a control element within this pathway. First, the aconitase enzymes underwent inactivation in an erythroid-selective manner in response to 15% transferrin saturation. The protein levels of ACO2 were not significantly affected by iron restriction, indicating a posttranslational mechanism rather than IRE-mediated translational control.16,47 Why ACO2 protein levels did not decrease with iron restriction is not clear, but one possibility is that its IRE may require a greater degree of cellular iron depletion than was induced by our iron restriction. Second, fluorocitrate inhibition of aconitase activity elicited a response similar to that of iron restriction, that is, inhibition of erythroid growth and differentiation. Attempts to determine which of the 2 isoenzymes regulates erythropoiesis did not yield definitive results. ACO1 knockout had no effect on steady-state or stress erythropoiesis in mice, and ACO2 knockdown caused only partial inhibition of GPA up-regulation in human CD34+ cells. One possibility is that the 2 isoenzymes may function semiredundantly, ACO2 perhaps contributing more than ACO1. However, another probable possibility, discussed in Figure 7, is that the simple loss of enzymatic function may not fully explain the role of aconitase in the erythroid response to iron restriction. The mechanism for aconitase inactivation during iron restriction may result from destabilization of the iron-sulfur clusters within the active sites, a notion supported by the activation of IRP1 RNA binding activity associated with erythroid iron restriction (Figure 3F).
A potential confounding factor is that iron deprivation alters the cellular composition of the cultures, and this alteration could lead in a secondary manner to diminished aconitase activity. Several lines of evidence argue against this possibility. First, the aconitase assays were conducted on day 4 of culture, a point at which the megakaryocytic composition differs only by 20% in iron-replete versus iron-restricted cultures (Figure 1B). Second, erythroid aconitase activity does not increase as a function of cellular maturation (see supplemental Figure 2D), ruling out a role for the maturation block caused by iron restriction. Third, ample precedent exists for direct inactivation of cellular aconitase by iron deprivation.
Additional evidence implicating aconitase in the erythroid response to iron restriction is the striking ability of isocitrate to abrogate this response in vitro and in vivo. The mechanism underlying this phenomenon most probably involves both nonmetabolic and metabolic pathways. The participation of a nonmetabolic pathway is suggested by the ability of the metabolically inert L-isomer to completely rescue viability and partially rescue differentiation. The ability of D-isocitrate to contribute to heme biosynthesis may account for its greater potency in restoring erythroid differentiation. However, in addition to direct metabolic conversion, isocitrate could also contribute to heme synthesis in an indirect manner by restoring the expression of ALAS-2 (eALAS) in iron-deprived erythroid cells (see Figure 5C). That isocitrate metabolism alone is insufficient is suggested by the lack of rescue by α-ketoglutarate in vitro and in vivo. The prevention of IRP1 activation by isocitrate in Figure 3F is consistent with an ability to enter cells, bind aconitase, and stabilize active site iron-sulfur clusters, in keeping with described substrate protective effects.48
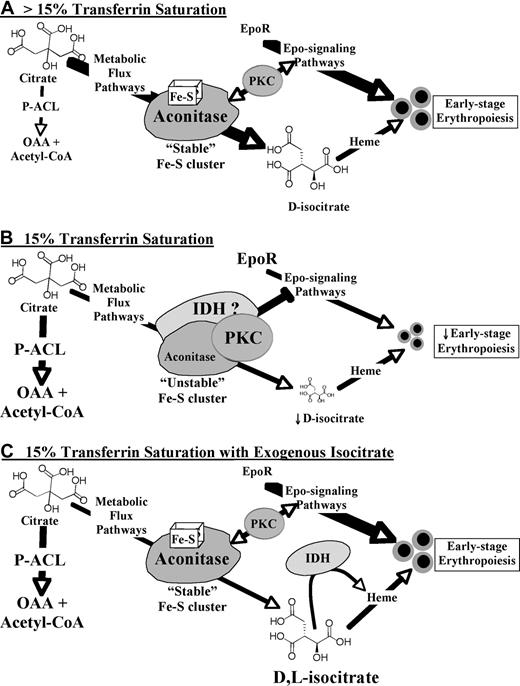
Figure 7 provides a model in which aconitase/isocitrate integrates signals from Epo, iron levels, and potentially other stimuli. With their active-site iron-sulfur clusters, aconitases may function as sensors for iron levels. Their production of isocitrate contributes to erythropoiesis in part through the metabolic generation of heme (Metabolic Flux Pathway). During iron restriction (Figure 7B), destabilization of the iron-sulfur cluster (see Figure 3F) is predicted to induce conformational changes and enhanced assembly of signaling complexes that include PKC. Along these lines, several recent studies have provided evidence for aconitase-kinase interactions, including ACO2 binding to conventional PKC.17,49,50 Provision of exogenous isocitrate (Figure 7C) has 3 predicted consequences that are nonmutually exclusive. The first, bypassing of the metabolic blockade, may permit heme biosynthesis but does not suffice to reverse the iron-restriction response, as discussed earlier. The second, stabilization of the iron-sulfur cluster (see Figure 3F), is predicted to restore aconitase to its “high iron” conformation and thereby dissociate the PKC-associated signaling complexes whose hyperactivation impairs Epo responsiveness. The third, binding to IDH, is probably relevant because L-isocitrate, although incapable of undergoing metabolic conversion, still retains the capacity for IDH binding.34 IDH has also been identified in complexes with signal-transducing proteins49 and represents an excellent candidate for participating, along with aconitase and PKC, in an iron-restriction signalosome, a possibility currently under investigation.
Model for erythropoietic regulation by an iron-aconitase-isocitrate pathway. In the absence of iron restriction (A; > 15% Transferrin Saturation) aconitase enzymes possess intact iron-sulfur clusters and function mainly in the conversion of citrate to isocitrate by the Metabolic Flux Pathway. In the presence of iron restriction (B; 15% Transferrin Saturation), destabilization of the aconitase iron-sulfur clusters induces assembly of a repressive signalosome which may act in part through PKC hyperactivation. In addition, diminished metabolic flux may compromise heme production and lead to shunting of citrate by activated ATP-citrate-lyase (P-ACL) to oxaloacetate (OAA) and acetyl-CoA. Isocitrate rescue (C; 15% Transferrin Saturation with Exogenous Isocitrate) may prevent assembly of a repressive signalosome by stabilization of aconitase iron-sulfur clusters and by binding to isocitrate dehydrogenase (IDH) enzymes. In addition, exogenous D- but not L-isocitrate may support heme biosynthesis. However, exogenous isocitrate does not prevent shunting of citrate by activated ATP-citrate-lyase.
Model for erythropoietic regulation by an iron-aconitase-isocitrate pathway. In the absence of iron restriction (A; > 15% Transferrin Saturation) aconitase enzymes possess intact iron-sulfur clusters and function mainly in the conversion of citrate to isocitrate by the Metabolic Flux Pathway. In the presence of iron restriction (B; 15% Transferrin Saturation), destabilization of the aconitase iron-sulfur clusters induces assembly of a repressive signalosome which may act in part through PKC hyperactivation. In addition, diminished metabolic flux may compromise heme production and lead to shunting of citrate by activated ATP-citrate-lyase (P-ACL) to oxaloacetate (OAA) and acetyl-CoA. Isocitrate rescue (C; 15% Transferrin Saturation with Exogenous Isocitrate) may prevent assembly of a repressive signalosome by stabilization of aconitase iron-sulfur clusters and by binding to isocitrate dehydrogenase (IDH) enzymes. In addition, exogenous D- but not L-isocitrate may support heme biosynthesis. However, exogenous isocitrate does not prevent shunting of citrate by activated ATP-citrate-lyase.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Luke Szweda (Oklahoma Medical Research Foundation) for providing antibodies, Eyal Gottlieb and Dan Tennant (Beatson Institute for Cancer Research) for providing the compound TaKG, and Klaus Ley and the University of Virginia Cardiovascular Research Center for the use of the Hemavet analyzer.
This work was supported by a Ruth L. Kirschstein National Research Service Award, by the National Institutes of Health (grant F32 HL0860046), and by a University of Virginia K12 Clinical Translational Career Development Award (G.C.B.); by the University of Virginia Cancer Center through the James and Rebecca Craig Cancer Research Scholars Award and the NCI Cancer Center Support (grant P30 CA44579), by the Biomedical Innovation Fund of the Ivy Foundation (J.J.C.); by the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01 DK079924; A.N.G.); and by the Roche Foundation for Anemia Research.
National Institutes of Health
Authorship
Contribution: G.C.B. designed the research, performed experiments, and wrote the manuscript; L.L.D., A.-L.T. and S.L.G. designed the research, performed experiments, and analyzed data; W.-H.T. and T.A.R. designed, performed, and interpreted studies of IRP1 and IRP2; B.D. and J.M.M. designed, performed, and interpreted NMR metabolomic studies; J.J.C. synthesized and analyzed vital new reagents (enantiopure isocitrates); and A.N.G. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adam N. Goldfarb, University of Virginia School of Medicine, PO Box 800904, Charlottesville, VA 22908; e-mail: ang3x@virginia.edu.

![Figure 1. A primary human model system for iron restriction of erythropoiesis. (A) Effect of transferrin saturation (Sat) on growth and viability of hematopoietic lineages. CD34+ cells underwent unilineage culture in serum-free media. Results show mean ± SEM for 3 to 5 independent experiments. (B) Inhibition of early erythroid differentiation by iron restriction. Erythroid cultures with 100% or 15% transferrin saturation underwent flow cytometry (fluorescence-activated cell sorting [FACS]) with gating on viable cells. CD34/CD36 expression was analyzed on day 4. Indicated are percentages of cells in various quadrants. (C) Minimal apoptosis associated with erythroid iron restriction. Cells from panel B were analyzed by FACS for 7-amino-actinomycin D (7-AAD) and annexin V staining with gating on all cells. Whole cell lysates from day 5 cultures underwent immunoblotting (IB) for caspase 3 (bottom; arrow indicates cleavage product). (D) Minimal effect of erythroid iron restriction on cell-cycle parameters. Day 5 cultures stained with propidium iodide underwent FACS. Indicated are percentages of cells in various fractions, including subdiploid (Sub).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/1/10.1182_blood-2009-10-251496/4/m_zh89991054600001.jpeg?Expires=1765915726&Signature=NMdCQAn-9OLk3vzll3-04wqIovKlU8sYakMV5qY6KDfkKHT2O4Ax5ulxmPR2Zbo13aAZzYbNerAYie38EolOePMF3WYuG-E3wuh1Tk-aCaFYX31j39HStEmtST0a2nZu4Q3nWB-f8Mn7UwSNavotGmQmSWVLG-z33dVJXK2nuqx4hebO8K1UGLo8zM~LvnwfWg3uLlMN95dIeZyZaVCnf4ccI~hN5ddgOd31cwBI4sYCbOImU-ncrCBmN49OVxgwbKxmT3o-Z5CL3ESoLXJeW2EP3UO1ms0z2ji76N4fn8FA3Mc-X8lZx8txiehncM0vMdRgUxC2LFP~z1wl0323kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Implication of aconitase activity in iron regulation of erythropoiesis. (A) Erythroid-specific aconitase inactivation during iron restriction. Enzymography (Acon Activity) was performed on extracts from day 4 cultures in erythroid (Ery), megakaryocytic (Mk), or granulocytic (Gran) media with 100% or 15% transferrin saturation; controls consisted of K562 cells expressing shRNAs targeting ACO2 or ACO1. Expression of mitochondrial aconitase protein in samples (bottom panel, IB: ACO2). (B) Summary of 3 independent experiments as in panel A. The y-axis is the ratio of scanned signals obtained with 15% transferrin saturation to those obtained with 100% transferrin saturation, expressed as mean percentage ± SEM. **P < .001 for Ery ACO1 changes and 0.03 for Ery ACO2 changes. Mk and Gran signals showed no significant changes. (C) Direct aconitase inhibition impairs erythroid differentiation in a manner similar to iron restriction. Erythroid cultures with 100% transferrin saturation were supplemented with fluorocitrate (FC) and underwent FACS assessment of differentiation (top panels) and viability (forward [FSC] and side scatter [SSC] in bottom panels) on day 5. Where indicated, 10mM citrate was also included in culture. (D) Summary of 3 independent experiments as in panel C, using 200μM fluorocitrate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/1/10.1182_blood-2009-10-251496/4/m_zh89991054600002.jpeg?Expires=1765915726&Signature=Yq0ljl2Zfab5NBz0dJb4igDs1e4Uqxj8urUsHUbD~mdyS7SdMhnhYgewcIRvuJy~YFEdI8ZM4laHeRPhEEFsZ93fjITH2SB9bFUYA8xDClhitZ8b~dmUvjp2fcvk9MvR1Z4DCNkKzkB8uc-4bvkdCJBCavvv-0G838NS8N7vP6KViLYIOC1hyZhYjdMerjr8w1kxiAyktDOAtOS5izP61V0ZP9HRUeqS8LqFe8aXX7t1Qa-etI5zyFmFSLovdG~owEHGN99~9m8UeEBgA2OFmlvdEx8aUoIADozPrU41PXiQrqONkIpGGvFy~awjHoobKsQ6qHeU~R1ZwtmZmYu9kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)