Abstract
Chronic myelogenous leukemia (CML) is a clonal myeloproliferative disease (MPD) initiated by p210-BCR-ABL–mediated transformation of hematopoietic stem cells (HSCs). Inhibition of the ABL kinase alone is not sufficient to eradicate leukemic stem cells (LSCs). We have previously shown that the deficiency of Rac2 GTPase signaling, but not Rac1, in p210-BCR-ABL–transduced hematopoietic cells prolonged survival of mice with MPD. Here we demonstrate that absence of Rac2 GTPase prolongs survival of HSC-initiated, inducible Scl/p210-BCR-ABL (Scl/p210) binary transgenic mice, it induces apoptosis, and, unlike in normal HSC and progenitor (HSC/P), impairs LSC and progenitor (LSC/P) proliferation in vivo. As a result, Rac2 deficiency causes functional exhaustion of the LSC pool in vivo. This defect is not due to impaired interaction with the hematopoietic microenvironment as reflected by its unaltered adhesion, migration, and homing to recipient organs. In summary, Rac2 deficiency exhausts the LSC pool in vivo through impairment of oncogene-induced proliferation and survival signals.
Introduction
Chronic myelogenous leukemia (CML) is a hematopoietic stem cell (HSC) malignancy induced by p210-BCR-ABL, characterized by myeloproliferation in the bone marrow (BM), and egress of leukemic stem cells and progenitors (LSC/P).1-3 Expression of p210-BCR-ABL confers proliferative advantage to HSC, induces abnormal adhesion and migration of hematopoietic progenitors, and appears to be directly responsible for the development of a transformed phenotype.4,5 Although CML is considered a HSC-initiated disease, studies indicate that granulocyte-monocyte progenitors isolated from advanced-phase CML patients reacquire self-renewal properties and therefore may also serve as LSC.6 Inhibition of ABL-kinase alone is not sufficient to completely eliminate LSC/Ps, presumably due to drug refractoriness.7 However, mechanistic studies on downstream signals responsible for the BCR/ABL-mediated transformation phenotype are hindered by the lack of studies in HSC-initiated in vivo models.8
Rac GTPases play integral roles in HSC and progenitor (HSC/P) proliferation, survival, homing, and engraftment.9,10 Using a retroviral transduction model, we showed that the deficiency of the hematopoietic-specific Rac2 GTPase, but not Rac1 alone, prolonged survival of mice with myeloproliferative disease (MPD).11 However, rapid-progressing leukemia within a relatively shorter latency hindered analysis of CML-initiating and propagating stem cells during the disease development in vivo.
We have used a stem cell leukemia (Scl) promoter-driven, tetracycline-inducible (Scl/p210-BCR-ABL) binary transgenic mouse model. In this model, expression of p210-BCR-ABL is inducible and restricted to the HSC/P compartment, leading to a much longer latency of disease development and allowing the study of the HSC/P-intrinsic molecular changes during leukemogenesis in vivo.12,13 We show that the absence of hematopoietic-specific Rac2 GTPase significantly prolongs the survival of Scl/p210 mice. Mechanistically, prolonged survival was associated with reduced proliferation and increased apoptosis of CML-initiating and sustaining HSC/Ps in vivo. Scl/p210;Rac2−/− HSCs eventually failed to outcompete wild-type (WT) HSCs leading to the functional exhaustion of the LSC/P pool in vivo. These data confirm and further strengthen Rac GTPases as new molecular targets in BCR-ABL–transformed cells.
Methods
Generation of Scl/p210;Rac2−/− mice, genotyping, and b3a2 quantitation
The generation of Scl-tTA;TRE-p210-BCR-ABL (Scl/p210) and C57Bl/6 Rac2−/− mice have been previously described.12,14 Animal studies were approved by the Cincinnati Children's Hospital Medical Center Institutional Animal Care and Use Committee. FVB/N-backcrossed Scl/p210 mice (kindly provided by Dr Claudia Huettner, Blood Center of Southeastern Wisconsin, Milwaukee) were crossed with Rac2−/− mice to generate F2 Scl/p210;Rac2−/− mice. Rac-expressing, Scl/p210 littermates were used as the controls. Mice weaned from doxycycline for 3 months were used for all the assays. For competitive repopulation assays, B6.SJLPtprca Pep3b/BoyJ competitor cells and recipient mice were used.10 Mice were genotyped as described,10,12 and genomic DNA b3a215 and murine ApoB levels were quantified by quantitative polymerase chain reaction (qPCR).
Homing and competitive repopulation
For homing, Scl/p210 or Scl/p210;Rac2−/− splenocytes were intravenously transplanted into lethally-irradiated (11.75 Gy) recipient mice and homing frequency was calculated as previously described.10 For competitive repopulation experiments, we transplanted 1.5 × 106 splenocytes into lethally-irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ recipients along with 3 × 106 CD45.1+ BM cells, and engraftment after transplantation was measured by chimera assessment by flow cytometry and qPCR of peripheral blood.
Proliferation, survival, adhesion, and migration
Results and discussion
Stem cell leukemia (Scl) promoter-driven and tetracycline-inducible (tet-off: 12 weeks) expression of p210-BCR-ABL (b3a2) was observed in lin−c-kit+ (LK) and lin−c-kit+Sca1+ (LSK) cells;myeloproliferation was associated with increased proliferation of LSK cells in vivo and with activation of downstream signaling effectors CrkL, p38–mitogen-activated protein kinase and c-Jun N-terminal kinase (supplemental Figure 1A-C, available on the Blood Web site, see the Supplemental Materials link at the top of the online article). There was a 3-fold increase in the number of circulating and splenic HSCs, but not in the BM, in Scl/p210 mice compared with nontransgenic (Non-Tg) mice, suggesting egress of LSC/Ps (supplemental Figure 1D-E6-8 ). Scl/p210 mice also shared similar characteristics of HSC/P transformation as observed in human CML, including reduced adhesion to fibronectin, increased migration toward CXCL12 (Figure 1E-F) and increased expression of the adhesion molecule CD44 on HSC/Ps (supplemental Figure 1F11 ). Similar to a recent report,17 the MPD developed in the primary mice was transplantable to primary and secondary littermate recipients (supplemental Figure 2).
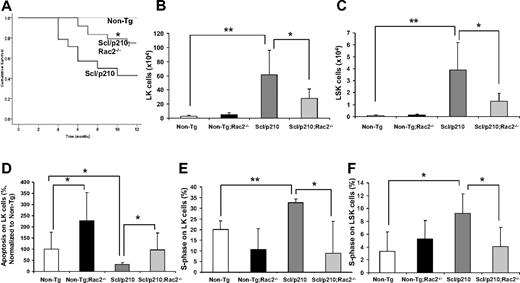
Loss of Rac2 GTPase increases survival of Scl/p210;Rac2−/− mice by impairing the LSC/P pool size in vivo. (A) Cumulative survival using the Kaplan-Meier log-rank P test performed among Non-Tg, Scl/p210, and Scl/p210;Rac2−/− mice (n = 14-22 mice per group). (B-C) Content of (B) Lin−Sca1−c-kit+ (LK) and of (C) Lin−Sca1+c-kit+ (LSK) cells in Non-Tg, Non-Tg;Rac2−/−, Scl/p210 and Scl/p210;Rac2−/− spleens. Data represent mean ± SD (n = 8 mice per group). (D) Apoptosis of Non-Tg, Non-Tg;Rac2−/−, Scl/p210 and Scl/p210;Rac2−/− hematopoietic progenitors in vivo. Data (normalized with respect to Non-Tg control; mean ± SEM) represent the average of 2 independent experiments including a minimum of 8 mice per group. (E-F) Proliferation of (E) LK and of (F) LSK cells in Non-Tg, Non-Tg;Rac2−/−, Scl/p210 and Scl/p210;Rac2−/− spleens in vivo. Proliferation was determined by measuring the uptake of BrdU in vivo. Data (mean ± SD) represent 1 of 3 independent experiments with identical results (n = 4 mice per group). *P < .05 and **P < .005 between the respective groups.
Loss of Rac2 GTPase increases survival of Scl/p210;Rac2−/− mice by impairing the LSC/P pool size in vivo. (A) Cumulative survival using the Kaplan-Meier log-rank P test performed among Non-Tg, Scl/p210, and Scl/p210;Rac2−/− mice (n = 14-22 mice per group). (B-C) Content of (B) Lin−Sca1−c-kit+ (LK) and of (C) Lin−Sca1+c-kit+ (LSK) cells in Non-Tg, Non-Tg;Rac2−/−, Scl/p210 and Scl/p210;Rac2−/− spleens. Data represent mean ± SD (n = 8 mice per group). (D) Apoptosis of Non-Tg, Non-Tg;Rac2−/−, Scl/p210 and Scl/p210;Rac2−/− hematopoietic progenitors in vivo. Data (normalized with respect to Non-Tg control; mean ± SEM) represent the average of 2 independent experiments including a minimum of 8 mice per group. (E-F) Proliferation of (E) LK and of (F) LSK cells in Non-Tg, Non-Tg;Rac2−/−, Scl/p210 and Scl/p210;Rac2−/− spleens in vivo. Proliferation was determined by measuring the uptake of BrdU in vivo. Data (mean ± SD) represent 1 of 3 independent experiments with identical results (n = 4 mice per group). *P < .05 and **P < .005 between the respective groups.
We have previously shown that the deficiency of Rac2 GTPase signaling, but not Rac1 alone, in p210-BCR-ABL–transduced hematopoietic cells prolonged survival of p210-BCR-ABL–initiated MPD.11 However, intrinsic problems associated with gene expression of a retroviral-driven oncogene and premature death of retrovirally transduced/transplanted mice precluded the analysis of the function of Rac2 in LSC/P in vivo. Leukemic development progresses slowly in FVB/N × C57Bl/6 Scl/p210 mice (50% mortality after 8 months; Figure 1A) allowing the analysis of LSC/P progressive disease. Rac2 deficiency alone significantly prolonged survival of Scl/p210 mice in vivo (Figure 1A). Prolonged survival of Scl/p210;Rac2−/− mice was associated with lower content of tumor-initiating LK and LSK cells in vivo (Figure 1B-C), despite similar level of expression of BCR-ABL in LK cells (average ΔCtScl/p210 = 7.00 vs average ΔCtScl/p210;Rac2−/− = 6.01; pull of 4 mice per group, where Ct is the cycle threshold). However, Rac2 deficiency did not alter the content of normal HSC/Ps in the Non-Tg mice (Figure 1B-C). In addition, Rac2-deficient Scl/p210 mice also had 2-fold reduced frequency of circulating LSK cells compared with Scl/p210 mice (0.014% ± 0.007% vs 0.036% ± 0.02%, respectively; P < .05, n = 10 mice per group).
Rac2 GTPase plays an important role in the survival, but not proliferation, of normal HSCs.10 We analyzed whether the prolonged survival of Scl/p210;Rac2−/− mice was due to defective LSC/P. As expected, Rac2−/−, Scl/p210, and normal LK cells show increased apoptosis in vivo (Figure 1D, supplemental Figure 10). However, the absence of Rac2 significantly reduced proliferation of Scl/p210 to levels similar to nonleukemic HSC/P, but did not modify the proliferation of normal LK and LSK cells in vivo (Figure 1E-F, supplemental Figure 9).
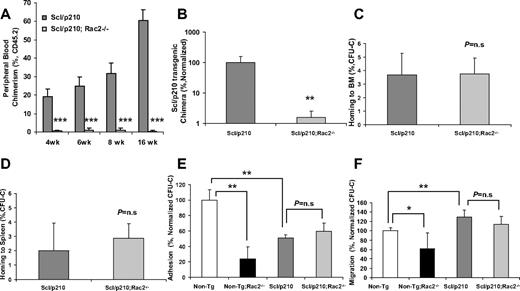
Scl/p210;Rac2−/− cells showed significantly reduced long-term HSC engraftment and unlike the Scl/p210 LSC, they were unable to compete with normal HSCs from primary to secondary recipient mice in a competitive repopulation assay (Figure 2A-B, supplemental Figures 3-5). Rac proteins have been involved in homing and interaction with the hematopoietic microenvironment. For that reason, we analyzed whether the increased survival of Scl/p210;Rac2−/− mice was related to defective homing of HSCs. Similar to normal HSC/P,10 Scl/p210 and Scl/p210;Rac2−/− hematopoietic progenitors home to BM and spleen similarly (Figure 2C-D). In addition, unlike in normal HSC/P,9 deletion of Rac2 did not affect the adhesion and migration-related transformation phenotype of Scl/p210 progenitors (Figure 2E-F) or modify the increased cell-surface expression of CD4418 observed in Scl/p210 HSC/Ps (supplemental Figure 1F). These data indicate that reduced proliferation and increased apoptosis of Rac2 deficiency induce removal of the leukemic pool faster than it is replenished by the expression of the oncogene BCR/ABL in the LSC/P compartment and limited the leukemia-initiation and propagation abilities of the stem cell pool resulting in defective engraftment in vivo.
Scl/p210;Rac2−/− LSCs fail to outcompete with wild-type HSCs during competitive repopulation in vivo. (A) Evolution of peripheral blood chimerism of CD45.2+ Scl/p210 or Scl/p210;Rac2−/− splenocytes, competitively transplanted with CD45.1+ WT BM cells, into lethally-irradiated CD45.1+ mice. Data represent mean ± SD (n = 8 mice per group). (B) Scl/p210-transgenic chimera in peripheral blood of WT recipient mice after 6 weeks of competitive transplantation as determined by genomic DNA b3a2 content of peripheral blood leukocytes. Data represent mean ± SD (n = 8 mice per group). (C-D) Colony-forming unit–cell (CFU-C) homing of Scl/p210 and Scl/p210;Rac2−/− splenic progenitors into lethally-irradiated recipients (C) BM and to (D) spleen in vivo (n = 3 mice per group); P = n.s; not significant). (E) Adhesion to recombinant fibronectin (CH-296) and (F) migration toward CXCL12 of Non-Tg, Non-Tg;Rac2−/−, Scl/p210 and Scl/p210;Rac2−/− hematopoietic progenitors in vitro. Data (mean ± SD) represent 1 of the 2 independent experiments performed each per triplicate of pools of 5 mice per group and experiment. *P < .05, **P = .01, and ***P < .005 between the respective groups.
Scl/p210;Rac2−/− LSCs fail to outcompete with wild-type HSCs during competitive repopulation in vivo. (A) Evolution of peripheral blood chimerism of CD45.2+ Scl/p210 or Scl/p210;Rac2−/− splenocytes, competitively transplanted with CD45.1+ WT BM cells, into lethally-irradiated CD45.1+ mice. Data represent mean ± SD (n = 8 mice per group). (B) Scl/p210-transgenic chimera in peripheral blood of WT recipient mice after 6 weeks of competitive transplantation as determined by genomic DNA b3a2 content of peripheral blood leukocytes. Data represent mean ± SD (n = 8 mice per group). (C-D) Colony-forming unit–cell (CFU-C) homing of Scl/p210 and Scl/p210;Rac2−/− splenic progenitors into lethally-irradiated recipients (C) BM and to (D) spleen in vivo (n = 3 mice per group); P = n.s; not significant). (E) Adhesion to recombinant fibronectin (CH-296) and (F) migration toward CXCL12 of Non-Tg, Non-Tg;Rac2−/−, Scl/p210 and Scl/p210;Rac2−/− hematopoietic progenitors in vitro. Data (mean ± SD) represent 1 of the 2 independent experiments performed each per triplicate of pools of 5 mice per group and experiment. *P < .05, **P = .01, and ***P < .005 between the respective groups.
In summary, Rac2 GTPase deficiency prolongs survival of Scl/p210 mice in a HSC-initiated model of disease through decreased proliferation and survival but without affecting homing, adhesion, and migration. The dramatic loss of engraftment of Scl/p210;Rac2−/− HSCs suggests Rac2 as a direct target of p210-BCR-ABL–dependent transformation in vivo and highlights the importance of the HSC/P-intrinsic signaling molecules that can functionally distinguish normal HSC/Ps from LSC/Ps.19 Studies showing a differential cell-intrinsic molecular program that can functionally distinguish a normal HSC from a LSC are limiting. Our results indicate that Rac2 deficiency leads to exhaustion of a phenotypically and functionally identified LSC/P pool in vivo and unveils an unexpected role of Rac2 in LSC/P regulation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Claudia Huettner for providing the Scl/p210-BCR-ABL mice, Rebecca Santho (Cincinnati Children's Hospital Medical Center) for technical assistance, and Margaret O'Leary (Hoxworth Blood Center) for editing the manuscript. We also thank Cincinnati Children's Hospital Medical Center Experimental Hematology & Cancer Biology Mouse and Flow Cytometry Cores for services.
A.S. is a recipient of The Institute of Cancer Research (United Kingdom)/Lady Tata Postdoctoral Fellowship in Leukaemia. This work was supported by the National Institutes of Health (NIH R01-HL087159, J.A.C.; R01 DK62757, D.A.W.), the Department of Defense (CM064050; J.A.C.), the Alex Lemonade Stand Foundation Award (J.A.C.), and The Leukemia & Lymphoma Society (D.A.W.).
National Institutes of Health
Authorship
Contribution: A.S., J.A., S.D., and J.A.C. performed experiments; D.A.W. participated in the research design and reviewed the manuscript; and A.S. and J.A.C. participated in the design and interpretation of data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jose A Cancelas, Division of Experimental Hematology & Cancer Biology, 3333 Burnet Ave, and Hoxworth Blood Center, University of Cincinnati, Cincinnati, OH 45229; e-mail: jose.cancelas@cchmc.org or jose.cancelas@uc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal