In this issue of Blood, Kang and colleagues describe a molecular signaling network involving Notch, COUP-TFII, and Prox-1, along with other supporting molecules that regulates the specification and maintenance of lymphatic endothelial cells.
The functional importance of the lymphatic vessels is tremendous. They drain excessive fluid and serve as a major route of transportation of absorbed lipids, macromolecules, cell debris, immune cells, and even cancer cells from the interstitial space into regional draining lymph nodes and eventually to the systemic circulation.1 Thus, lymphatic vessels are crucial to maintain life. Growth of lymphatic vessels (lymphangiogenesis) is essential for normal embryonic development, normal postnatal physiologic function, as well as pathologic conditions such as inflammation or tumor dissemination.1 Lymphatic vessels are composed of a thin wall of monolayered lymphatic endothelial cells (LECs), which possess a very loose basement membrane and contain neither pericytes or smooth muscle cells. This unique architecture accounts for their specialized function and high efficiency in absorption and transportation. Lymphatic vessels gather to form the collecting lymphatic vessels, which have flap-like valves and are sparsely covered by a thin layer of smooth muscle cells that ensure a unidirectional delivery of lymph. In this regard, the collecting lymphatic vessels closely resemble venules both morphologically and functionally. In fact, the lymphatic vessels are derived from preformed veins around embryonic development days 9.5 to 10.5 in mice and weeks 6 to 7 in humans. A subset of endothelial cells (ECs) in the cardinal vein become committed to the LECs by a process orchestrated mainly by the master transcriptional factor Prox-1, secretory vascular endothelial growth factor (VEGF-C), and its cognate receptor, VEGF receptor-3 (VEGFR-3).1,2 This nascent lymphatic structure expands in a centrifugal pattern to establish the lymphatic network, which develops under the regulation of several key transcriptional factors, growth factors, and corresponding receptors.1
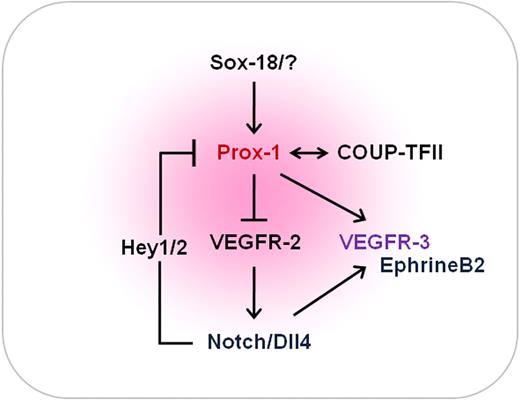
The molecular fate-determining network of lymphatic endothelial cells. Lymphatic endothelial cell specification is regulated by a dynamic molecular network of opposing and complementary forces. Notch, COUP-TFII, and Prox-1 are the 3 major players. Activation of Notch signaling suppresses the expression of Prox-1 and COUP-TFII through the action of Hey1/2 in lymphatic endothelial cells. Endogenous Prox-1 and COUP-TFII down-regulate VEGFR-2 and therefore ultimately suppress the expression of VEGF-A. In parallel, Notch signaling displays positive influence on VEGFR-3 and EphrinB2 signaling. SOX18 is also known to be capable of up-regulating Prox-1 in certain conditions. COUP-TF II indicates chicken ovalbumin upstream transcription factor II; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
The molecular fate-determining network of lymphatic endothelial cells. Lymphatic endothelial cell specification is regulated by a dynamic molecular network of opposing and complementary forces. Notch, COUP-TFII, and Prox-1 are the 3 major players. Activation of Notch signaling suppresses the expression of Prox-1 and COUP-TFII through the action of Hey1/2 in lymphatic endothelial cells. Endogenous Prox-1 and COUP-TFII down-regulate VEGFR-2 and therefore ultimately suppress the expression of VEGF-A. In parallel, Notch signaling displays positive influence on VEGFR-3 and EphrinB2 signaling. SOX18 is also known to be capable of up-regulating Prox-1 in certain conditions. COUP-TF II indicates chicken ovalbumin upstream transcription factor II; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
ECs establish a monolayered barrier at the innermost surface of blood and lymphatic vessel. They are morphologically and functionally flexible and display considerable plasticity. They can be roughly categorized into 3 groups: arterial, venous, and LECs, which all share common receptors, signaling pathways, and transcriptional factors, but individually adopt unique receptors, signaling pathways, and transcriptional factors, resulting in striking heterogeneity. Although these 3 different cell types are thought to be derived from common angioblasts, their fate decisions are complex and governed by several different as well as common regulators.
Among the many molecules that participate to regulate the differentiation of ECs, Notch, chicken ovalbumin upstream transcription factor-II (COUP-TFII), and Prox-1 deserve extra attention. Notch signaling pathway is known to preferentially give ECs an arterial identity. It induces the primary vascular plexus to encroach and form a complex but hierarchical network composed of the arteries, capillaries, and veins.3,4 COUP-TFII and yet unidentified factors are responsible for the differentiation of venous ECs. Prox-1 is the master transcriptional factor leading venous ECs to become LECs. Animal studies revealed that defects in any of these 3 key factors in ECs cause an ambiguity in their identity, dysfunctional vascular morphogenesis, and functional disturbance.1-4 To ensure proper EC specification, the expression of Notch receptors and ligands are exclusively confined to the arterial components, whereas COUP-TFII is mainly restricted to the venous compartment. Interestingly, however, lymphatic-fated ECs are found to express not only Prox-1, but also Notch and COUP-TFII. This unexpected finding raises a series of questions: What are the missions of these 3 musketeers and how do they organize their duties for lymphatic specification and maintenance during and after development? How do they coordinate themselves to regulate lymphangiogenesis during tumor growth or inflammation? Unlike arterial or venous ECs, why do LECs require all 3 players and how do they balance each other and eventually exhibit only one cell phenotype?
In this issue of Blood, Kang et al propose a novel theory of the molecular mechanism underlying the regulation by these 3 players in governing lymphatic specification and maintenance.5 Based on their extensive molecular and biochemical analyses using primary cultured human dermal LECs, the authors unraveled an exquisite feedback regulatory network controlling lymphatic specification and maintenance by the 3 endothelial fate regulators—Notch, COUP-TFII, and Prox-1. They show that activation of Notch signaling suppresses the expression of Prox-1 and COUP-TFII through the action of Hey1/2 in LECs. They also demonstrate that endogenous Prox-1 and COUP-TFII suppress VEGF-A signaling by down-regulation of its receptor VEGFR-2 and neurophilin-1 in LECs. In fact, VEGF-A acts as an upstream component of Notch signaling to induce arterial endothelial cell specification. Conversely, the inhibition of VEGF-A interferes with normal arterial de-velopment. In parallel, Notch signaling promotes VEGFR-3 and EphrinB2 signaling.6,7 Moreover, the homeobox transcriptional factor SOX18 can up-regulate Prox-1 in ECs during the initial stage of lymphatic specification, but not in LECs of the differentiated stages.8 The authors conclude that the 3 musketeers along with other supporting players regulate opposing, but complementary, mechanisms to establish a delicate feedback regulatory network that finely tunes the speci-fication and maintenance of LECs (see figure).
Although the study definitely advances our knowledge on endothelial cell fate decision, subsequent investigations will be required to extend their findings to the in vivo settings. Moreover, it brings up more questions such as how the current study is clinically applicable and how we can take advantage of their cross-regulations to treat vascular disease. No doubt, further studies using animal models of these 3 major players on growing and quiescent lymphatic vessels would be extremely useful to address the questions. We look forward to seeing that this study be followed by more basic and transitional research focusing on endothelial cell fate specification, which may lead to therapeutic opportunities in the field of vascular diseases such as vascular malformations.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal