In this issue of Blood, Hertlein and colleagues present compelling in vitro and in vivo evidence that the novel HSP90 inhibitor 17-DMAG is a potent and selective killer of CLL cells that mediates its effects through the targeted inhibition of NF-κB.1 Their findings describe a promising new therapeutic approach for the treatment of this disease.
Heat shock protein 90 (HSP90) is a molecular chaperone that interacts with and stabilizes client proteins. It is commonly overexpressed in a range of human cancers and is thought to contribute to tumor cell growth and survival by stabilizing proteins involved in cell-cycle regulation and antiapoptotic signaling.2 In this way, we might consider HSP90 to be a “partner in crime” in chronic lymphocytic leukemia (CLL) cells by conferring a survival and/or proliferative advantage to the tumor cell. In this issue of Blood, Hertlein et al demonstrate that the novel HSP90 inhibitor 17-DMAG, a more soluble derivative of geldanamycin (17-AAG), is preferentially cytotoxic to primary CLL cells compared with normal lymphocytes. Importantly, they also provide some fascinating insights into the molecular mechanisms that underpin the cytotoxic effects of this agent and elucidate that HSP90 inhibitors can target distinct client proteins.
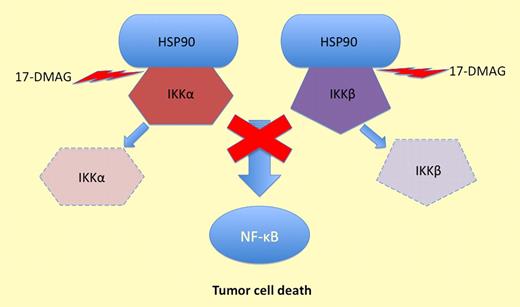
17-DMAG inhibits HSP90 and prevents it protecting IKKα and IKKβ. This stops NF-κB activation and induces tumor cell death.
17-DMAG inhibits HSP90 and prevents it protecting IKKα and IKKβ. This stops NF-κB activation and induces tumor cell death.
NF-κB is often constitutively activated in CLL3,4 but the reasons for this are not yet fully elucidated. In this article, the authors show that 17-DMAG markedly inhibited the I-kappa-B kinase (IKK) complex in CLL cells. This complex represents the cytoplasmic activation machinery for NF-κB. IKK inhibition was associated with a marked decrease in nuclear NF-κB and significant reductions in the transcription and protein expression of the antiapoptotic genes MCL1 and BCL2. Overexpression of these proteins has been associated with inferior clinical outcome in CLL, so direct or indirect targeting of these proteins represents a promising therapeutic approach.5,6 Unlike 17-AAG, 17-DMAG inhibited the IKKα and IKKβ subunits of the IKK complex, making it theoretically capable of blocking both the classical and alternative NF-κB activation pathways (see figure). Evidence for this dual inhibitory effect was presented in which CLL cells were cultured in the presence of CpG oligonucleotides (classical activation pathway) and CD40 ligand (alternative activation pathway). Under both sets of conditions, 17-DMAG was able to inhibit nuclear NF-κB and suppress the expression of MCL1. These experiments may be important as a proof of principle, as there is growing evidence that CLL cells receive additional survival signals in the tissue microenvironment that may prevent complete clearance of tumor cells after treatment and thereby promote the persistence of minimal residual disease. In this context, it will be interesting to extend these studies to include an assessment of the apoptotic responses induced by 17-DMAG under these same conditions.
Encouragingly, in vivo assessment of 17-DMAG in a TCL1-SCID transplant mouse model significantly reduced the tumor burden and prolonged survival of treated mice. However, SCID mice do not form lymph nodes so it remains to be seen whether 17-DMAG is capable of eradicating tumor cells from these microenvironments. With this in mind, clinical studies of HSP90 inhibitors suggest that they affect the stability of client proteins, but because of redundancy within signaling pathways, inhibiting HSP90 alone may not be sufficient.7,8 Moreover, it may be that HSP90 inhibitors will be most useful when coupled with conventional cytotoxic chemotherapy or other targeted therapies like monoclonal antibodies. In these combinations, HSP90 inhibitors may sensitize the tumor cells by suppressing antiapoptotic genes like MCL1 and BCL2. In so doing, they may increase the efficacy of existing chemotherapeutic agents and induce synergy.
Recent advances in treatment options for CLL have yielded higher response rates and improved progression-free and overall survival. However, CLL still remains an incurable malignancy in the majority of cases. This paper demonstrates that HSP90 inhibitors may be an important addition to the arsenal of targeted therapies used in the management of this disease. Additional studies of HSP90 inhibitors, including detailed profiling of the specific client proteins that they target, are clearly warranted in CLL and other hematologic malignancies.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal