Abstract
Myelodysplastic syndromes (MDS) are a group of clonal disorders of the bone marrow characterized by peripheral cytopenias. Standard treatment in low- and intermediate-I–risk MDS is supportive therapy consisting of regular transfusions and growth factors, that is, erythropoietin (Epo) and granulocyte-colony-stimulating factor (G-CSF). Because flow cytometric analysis of MDS bone marrow samples can identify clinically relevant subgroups regarding transfusion dependency and disease progression, we addressed the question whether flow cytometry (FCM) was instrumental in predicting response. In 46 patients with low- and intermediate-I–risk MDS that were treated with Epo/G-CSF, low Epo level and low transfusion need were associated with response to Epo/G-CSF. Interestingly, aberrant phenotype of myeloblasts identified nonresponders among patients with the greatest response probability according to the predictive model of Hellström-Lindberg et al. Moreover, aberrant FCM of myeloblasts acted as a significant biomarker for treatment failure in multivariate analysis. A new predictive model based on the basis FCM combined with previously validated Epo levels is proposed defining 3 subgroups with 94%, 17%, and 11% response probability. In conclusion, FCM may add significantly to well-known predictive parameters in selecting MDS patients eligible for Epo/G-CSF treatment. This is of relevance regarding prevention of treatment failure.
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal disorders of the bone marrow characterized by peripheral cytopenia in one or more cell lineages. Standard treatment for patients with low- and intermediate-I–risk MDS is supportive therapy consisting of regular transfusions and growth factors, that is, erythropoietin (Epo) and human recombinant granulocyte-colony-stimulating factor (G-CSF). The administration of Epo has been shown to ameliorate hemoglobin (Hb) levels and reduce red blood cell transfusion requirements in patients experiencing anemia, with improvement in quality of life and overall survival (OS).1,2 The addition of G-CSF has been shown to induce responses in patients resistant to Epo alone.3-6 Approximately 40% to 50% of anemic MDS patients demonstrate erythroid response to the combined administration of Epo and G-CSF.2,7-11 Response to growth factor treatment can be predicted by a model created on the basis of pretreatment serum Epo levels (< 100, 100-500, and > 500 U/L) and transfusion need (< or ≥ 2 units per month).8 This validated scoring system distinguishes 3 patient groups: 1 with a high probability of erythroid responses (74%), 1 intermediate (23%), and 1 poor (7%).9
Recently, it was demonstrated that flow cytometric (FCM) analysis of MDS bone marrow samples adds significantly in the distinction of clinically relevant subgroups in MDS with respect to transfusion dependency and progression of disease.12-14 Aberrant expression of the lymphoid antigen CD7 on myeloid blasts, for instance, correlates with poor clinical outcome.13,15,16 Therefore, we addressed the question whether FCM analysis was instrumental in predicting response to a standardized Epo/G-CSF regimen.
Methods
Patient characteristics and routine diagnostics
Forty-six patients with low- and intermediate-I–risk MDS (median age, 69 years; range, 40-90 years) were enrolled in this study from 2004 until 2007 and followed up until December 2008. Diagnosis of MDS was made by 2 experienced hematologists (A.A.v.d.L. and G.J.O.) according to World Health Organization (WHO) 2001 classification.17 Bone marrow samples were evaluated for chromosomal anomalies according to International System for Cytogenetic Nomenclature guidelines.18 In those cases in which no metaphases could be analyzed, additional fluorescence in situ hybridization was performed according to recently published recommendations.19 Patients were monitored with respect to peripheral blood cell counts, blast counts in bone marrow, and the occurrence of any event related to MDS, for instance, transfusion requirements. Epo levels were assessed by radioimmunoassay (EPO-Trac). All samples were drawn after written informed consent in accordance with the Declaration of Helsinki; the study was approved by the Medical Ethics Committee of VU University Medical Center. Risk assessment was on the basis of international prognostic scoring system (IPSS) and the WHO classification-based prognostic score system (WPSS).20-22 Patient characteristics are summarized in Table 1.13
Characteristics of responders and nonresponders to Epo/G-CSF treatment
| . | Responders . | Nonresponders . | P* . |
|---|---|---|---|
| No. patients | 18 | 28 | |
| Median age, y (range) | 69 (47-87) | 68 (40-90) | .964 |
| Sex, M/F | 10/8 | 20/8 | |
| WHO 2001, n (%)† | .248 | ||
| RA(RS) | 8 (44) | 10 (36) | |
| RCMD(RS) | 10 (56) | 16 (57) | |
| RAEB-1 | 1 (3.5) | ||
| MDS-U | 1 (3.5) | ||
| IPSS, n (%)† | .183 | ||
| Low | 12 (67) | 13 (46) | |
| Intermediate-I | 6 (33) | 15 (54) | |
| Median WPSS (range) | < .001 | ||
| Very low | 5 (28) | 0 (0) | |
| Low | 11 (61) | 11 (39) | |
| Intermediate | 2 (11) | 11 (39) | |
| High | 5 (18) | ||
| Not classifiable | 1 (3.5) | ||
| Previous transfusion support, number of patients (%)† | 2 (12) | 18 (62) | .010 |
| Median blast percentage (range) | 1.9 (0.6-4.2) | 2.2 (0.5-9.7) | .257 |
| Median Hb, mmol/L (range) | 5.9 (4.8-6.3) | 5.1 (4.1-6.6) | .001 |
| Median time from diagnosis to study entry, mo (range) | 0 (0-58) | 0 (0-78) | .426 |
| Median serum Epo at study entry, U/L (range) | 76 (19-587) | 187 (33-6000) | .001 |
| Median ferritin at study entry, mg/L (range) | 576 (47-1992) | 786 (47-3543) | .168 |
| Median LDH at study entry, U/L (range) | 341 (192-569) | 350 (205-2599) | .276 |
| Aberrant flow cytometry, n (%)† | 2 (11%) | 21 (72%) | < .001 |
| . | Responders . | Nonresponders . | P* . |
|---|---|---|---|
| No. patients | 18 | 28 | |
| Median age, y (range) | 69 (47-87) | 68 (40-90) | .964 |
| Sex, M/F | 10/8 | 20/8 | |
| WHO 2001, n (%)† | .248 | ||
| RA(RS) | 8 (44) | 10 (36) | |
| RCMD(RS) | 10 (56) | 16 (57) | |
| RAEB-1 | 1 (3.5) | ||
| MDS-U | 1 (3.5) | ||
| IPSS, n (%)† | .183 | ||
| Low | 12 (67) | 13 (46) | |
| Intermediate-I | 6 (33) | 15 (54) | |
| Median WPSS (range) | < .001 | ||
| Very low | 5 (28) | 0 (0) | |
| Low | 11 (61) | 11 (39) | |
| Intermediate | 2 (11) | 11 (39) | |
| High | 5 (18) | ||
| Not classifiable | 1 (3.5) | ||
| Previous transfusion support, number of patients (%)† | 2 (12) | 18 (62) | .010 |
| Median blast percentage (range) | 1.9 (0.6-4.2) | 2.2 (0.5-9.7) | .257 |
| Median Hb, mmol/L (range) | 5.9 (4.8-6.3) | 5.1 (4.1-6.6) | .001 |
| Median time from diagnosis to study entry, mo (range) | 0 (0-58) | 0 (0-78) | .426 |
| Median serum Epo at study entry, U/L (range) | 76 (19-587) | 187 (33-6000) | .001 |
| Median ferritin at study entry, mg/L (range) | 576 (47-1992) | 786 (47-3543) | .168 |
| Median LDH at study entry, U/L (range) | 341 (192-569) | 350 (205-2599) | .276 |
| Aberrant flow cytometry, n (%)† | 2 (11%) | 21 (72%) | < .001 |
The data of some patients (25/46, ie, 9 RA[RS], 14 RCMD[RS], 1 RAEB-1, and 1 MDS-U) were used in a previous study.13
Epo indicates erythropoietin; F, female; G-CSF, granulocyte-colony-stimulating factor; Hb, hemoglobin; IPSS, international prognostic scoring system; LDH, lactate dehydrogenase; M, male; MDS, myelodysplastic syndromes; MDS-U, myelodysplastic syndrome, unclassified; RA, refractory anemia; RAEB-1 or RAEB-2, refractory anemia with excess blasts type 1 or 2; RCMD, refractory anemia; RS, ring sideroblasts; and WPSS, World Health Organization-based prognostic scoring system.
P values indicate comparison between responders and nonresponders.
Percentages in parentheses depict percentage of cases within responder or nonresponder group.
Treatment
All patients started with Epo if symptomatic at an Hb of less than 10 g/dL (6.2mM) independent of endogenous Epo level. Epo (NeoRecormon, Epoetin-beta; Roche) was started at a dose of 30 000 IU once weekly. In absence of an increase in Hb of at least 1 g/dL (0.62mM) within 6 weeks, Epo dose was escalated to 60 000 IU according to Hellström-Lindberg et al.4 If still no response was achieved within 12 weeks, G-CSF (Neupogen [Filgrastim]; Amgen BV) was added (300-480 μg dependent on weight, 3 times weekly). Dose reduction of G-CSF was performed if leukocyte counts increased greater than 30 × 109/L.
Response criteria
Erythroid response was evaluated according to IWG2006 response criteria.23 In short, erythroid response is defined by an increase of Hb level by more than 1.5 g/dL (0.93mM) in patients with baseline Hb less than 11 g/dL (6.8mM) or a relevant reduction of red blood cell transfusions of at least 4 per 8 weeks compared with number of transfusions in the previous 8 weeks. Only transfusions given for Hb of 9.0 g/dL or less (5.6mM) were taken into account. Transfusion dependency was evaluated and defined as requirement of 3 units of packed cells per month for a period of at least 4 months. Disease progression was defined as an increase in WHO subgroup to at least RAEB-1 and/or AML within 18 months after diagnosis of MDS. Both time to response and response duration were documented.
FCM analysis of bone marrow samples
Bone marrow samples drawn at diagnosis were analyzed by 4-color FCM as previously described.13 Analysis was performed on total nucleated bone marrow cells; ammonium chloride lysis of erythrocytes was performed before the staining procedure as proposed by the European LeukemiaNet Working Party.24 Monoclonal antibodies used in this study included fluorescein isothiocyanate–conjugated CD5 (clone DK23) and CD16 (DJ130c) from Dako; CD7 (M-T701), CD15 (MMA), CD34 (HPCA2), and HLA-DR (L243) from BD Biosciences; CD36 (CLB-IVC7) from Sanquin; phycoerythrin-conjugated CD7 (M-T701), CD11b (D12), CD13 (L138), CD19 (SJ25C1), CD33 (P67.6), CD56 (My31), CD117 (104D2), and CD123 (9F5) from BD Biosciences; peridinin-chlorophyll protein-conjugated CD45 (2D1) from BD Biosciences; allophycocyanin (APC)–conjugated CD11b (D12), CD13 (L138), CD14 (MoP9), CD33 (P67.6), CD34 (HPCA2), and HLA-DR (L243) from BD Biosciences; and CD117 (104D2) from Dako as described previously.13
Samples were analyzed with the use of a FACSCalibur (BD Biosciences); data were analyzed with CellQuest Software (BD Biosciences). Different cell compartments were identified by the use of CD45 expression and sideward light scatter (SSC).24 Our main focus was analysis of myeloid blasts; myeloid blasts were defined as CD45dimSSClow/int with expression of CD34 and/or a myeloid marker such as CD13 or CD117; at least 250 events within this compartment were acquired.13,24 Upon analysis CD34-APC–positive blast cells were back gated in the CD45/SSC plot. Back gating strategies were performed to exclude debris, nonviable cells, and doublets. This gate also was used to check myeloid commitment by the use of CD117 or CD13 and CD19. Marker expression in a defined subpopulation was determined compared with isotype controls or unstained cells; on the basis of currently used cutoffs in routine immunophenotyping diagnostics of leukemia, a cutoff of 20% was applied in the evaluation of aberrant marker expression.
Statistical analyses
Comparison between responders and nonresponders were statistically tested by the use of the Fisher exact test for categorical data and the Mann-Whitney U test for continuous data; univariate and multivariate regression analyses were performed to analyze value of markers predictive for response (SPSS 15.0 software).
Results
Response to growth factor therapy in IPSS and WPSS risk groups
Most patients were scheduled to Epo 60 000 IU/week (n = 44), and 42 patients received G-CSF in addition. G-CSF dose was temporarily reduced in 5 patients. Of all the patients in this study, 39% (18/46) responded to the standardized Epo/G-CSF regimen according to IWG2006 criteria, with a median time to response of 3 months and a median duration of 12 months (range, 3.5-51 months). Four patients became transfusion independent. Disease progression was observed in 9 patients; 2 patients in the responder (11%) and 7 in the nonresponder group (25%). Patients with a low-risk IPSS showed hematologic improvement in 48% of the cases (12/25; Table 1) and patients with intermediate-I–risk MDS in 29% (6/21). When WPSS was used to classify patients, all of the very low-risk patients (WPSS 0, n = 5) responded to treatment, whereas 50% response was observed in low-risk patients (WPSS 1, n = 22). Only 18% of the intermediate-risk patients (WPSS 2, n = 13) and none in high-risk group (WPSS 3, n = 5) were responsive to treatment. In 1 patient (classified by WHO morphology as MDS unclassified), WPSS risk group could not be determined.
Response to growth factor therapy in relation to FCM characteristics
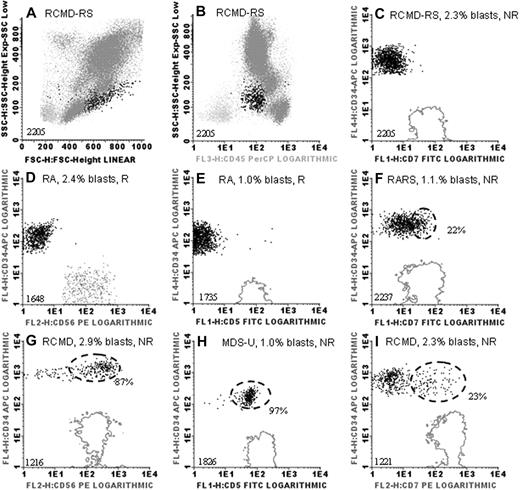
Median blast percentage in patients' bone marrow samples at diagnosis was 2.0% of total white blood cells (range, 0.5%-9.7%) as assessed by FCM; no differences in blast percentages were observed between responders and nonresponders (P = .472; Table 1). A dense cluster of at least 20% of the myeloid blast fraction (mainly consisting of CD34+ cells) that showed expression of, for example, lineage infidelity markers was considered as aberrant (Figure 1). Median percentage of aberrant blasts in these cases was 43% (range, 22%-97%; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Examples of immunophenotypic analysis of myeloid blasts in low- and intermediate-I–risk MDS. (A) Forward light scatter (FSC) and sideward light scatter (SSC) properties of a bone marrow sample of a myelodysplastic syndrome (MDS) patient are depicted (unique patient numbers are depicted in lower left corners). FSC (x-axis) and SSC (y-axis) reflect size and granularity, respectively. (B) CD45 staining (x-axis) versus SSC (y-axis) in this patient; CD45 is expressed on all white blood cells (highlighted in dark gray and black). CD34+ myeloid blasts, characterized by their diminished level of CD45 and low-to-intermediate SSC, are highlighted in black in all panels. (C-I) CD34 is depicted on the y-axes and lymphoid markers on the x-axes. Fluorescence 1 (FL1), FL2, FL3, and FL4 indicate fluorescein isothiocyanate–, phycoerythrin-, peridinin-chlorophyll protein–, and APC-conjugated antibodies, respectively. Solid gray lines indicate expression levels of lymphoid markers CD5, CD7, and CD56 in the reference lymphocyte population (x-axes in panels C-I). (D) The natural killer cell population was too small to generate a contour line; therefore, the reference population is depicted as gray dots. Marker expression was compared with unstained cells or appropriate isotype controls. Dashed black lines are depicted in case more than 20% of myeloid blasts expressed a lymphoid marker (aberrant flow cytometry); percentages of aberrant blasts are indicated as percentage of CD34+ cells. R indicates Epo responder; and NR, nonresponder. Graphs were generated with the use of Infinicyt software (Cytognos).
Examples of immunophenotypic analysis of myeloid blasts in low- and intermediate-I–risk MDS. (A) Forward light scatter (FSC) and sideward light scatter (SSC) properties of a bone marrow sample of a myelodysplastic syndrome (MDS) patient are depicted (unique patient numbers are depicted in lower left corners). FSC (x-axis) and SSC (y-axis) reflect size and granularity, respectively. (B) CD45 staining (x-axis) versus SSC (y-axis) in this patient; CD45 is expressed on all white blood cells (highlighted in dark gray and black). CD34+ myeloid blasts, characterized by their diminished level of CD45 and low-to-intermediate SSC, are highlighted in black in all panels. (C-I) CD34 is depicted on the y-axes and lymphoid markers on the x-axes. Fluorescence 1 (FL1), FL2, FL3, and FL4 indicate fluorescein isothiocyanate–, phycoerythrin-, peridinin-chlorophyll protein–, and APC-conjugated antibodies, respectively. Solid gray lines indicate expression levels of lymphoid markers CD5, CD7, and CD56 in the reference lymphocyte population (x-axes in panels C-I). (D) The natural killer cell population was too small to generate a contour line; therefore, the reference population is depicted as gray dots. Marker expression was compared with unstained cells or appropriate isotype controls. Dashed black lines are depicted in case more than 20% of myeloid blasts expressed a lymphoid marker (aberrant flow cytometry); percentages of aberrant blasts are indicated as percentage of CD34+ cells. R indicates Epo responder; and NR, nonresponder. Graphs were generated with the use of Infinicyt software (Cytognos).
Observed immunophenotypic aberrancies12 on myeloid blasts in this patient group were the expression of a lineage infidelity marker (CD5 [n = 1], CD7 [n = 15], or CD56 [n = 3], CD5 in combination with CD56 [n = 1]), loss of CD45 in addition to CD7 expression (n = 1), or loss of myeloid antigen CD33 (n = 1). Strikingly, aberrancies within the myeloid blast compartment were mainly found in nonresponding patients (21 [75%] of 28). Only 2 of 18 patients who responded to treatment (11%) showed an aberrant phenotype (CD7); however, response duration in these particular patients was just 3.5 and 6 months compared with a median of 12 months in all responders. Overall, 70% of patients (16/23) with normal myeloid blasts by FCM responded to Epo/G-CSF treatment, whereas 91% of the patients (21/23) with aberrant FCM were nonresponsive. Thus, aberrant immunophenotype of myeloid blasts is highly associated with treatment failure (P < .001).
Response to growth factor therapy in relation to endogenous Epo levels
The authors of several studies9-11 have validated the application of serum Epo levels at diagnosis to predict response to treatment. Epo/G-CSF treatment is recommended if Epo levels are less than 500 U/L and no or low transfusion need.25 In line with this, levels of endogenous Epo differed significantly between responders and nonresponders within our patient group (P = .001; Table 1). Only 44% of patients (17/39) that had Epo levels below the threshold of 500 U/L responded to treatment, whereas response at greater Epo levels was only 14% (1/7). In recently published studies, response rates were greatest in those patients who had serum Epo level less than 200 U/L.2,11 Other reports state that Epo levels less than 100 U/L are indicative of a greater probability of response to Epo/G-CSF.7,9,26 This cutoff level of 100 U/L appeared to be most discriminatory in our patient group: at an Epo level less than 100 U/L, 71% of patients (15/21) were responsive to Epo/G-CSF treatment, whereas 88% of the patients (22/25) with Epo levels greater than 100 U/L failed to respond. Thus, Epo levels greater than 100 U/L are highly associated with treatment failure (P < .001).
Additional markers in response prediction
Several other parameters are known to influence response to therapy, for instance, longer disease duration before Epo/G-CSF treatment affects outcome.10 Furthermore, patients requiring less than 2 units of red blood cell per month have a greater probability of response to Epo/G-CSF.9 Most patients in this study were newly diagnosed as MDS, and time to treatment was similar in the responder and nonresponder groups (P = .426; Table 1), although Hb was significantly lower in nonresponders compared with responders (P = .001; Table 1). As a result, transfusion dependency before treatment was more frequent among nonresponders compared with responders (P = .010). Transfusion dependency often is associated with the risk of iron overload, which adversely affects survival of the patients.21,27 Ferritin levels correlated significantly with transfusion need before growth factor treatment (P = .004; Table 1). Of note, only a few patients received additional iron chelation therapy.
When according to the current validated predictive model9 transfusion requirement was taken into account next to Epo levels, response rates were 53% in the group with a high-probability-to-respond group (17/32), 10% in the intermediate group (1/10), and 0% in the poor-probability-to-respond group (0/4). Interestingly, the presence of aberrant myeloid blasts might have identified 11 of 15 nonresponders among those patients who were supposed to have a good probability to respond.
Combination of validated response markers and FCM in response prediction
Because aberrant FCM was significantly associated with treatment failure, it might add to the well-known validated predictive response parameters to select those patients who are likely to respond to Epo/G-CSF. As illustrated in Table 2, 13 of 14 patients with low Epo levels and normal FCM responded to therapy. Of note, 6 patients were nonresponders despite their high response probability on the basis of their low Epo level (< 100 U/L); 5 of these patients might have been identified as nonresponders on the basis of the presence of aberrant myeloid blasts. Approximately one-third of patients responded that had either aberrant FCM and low Epo levels or normal FCM and Epo levels greater than 100 U/L, whereas none of the patients who had Epo levels greater than 100 U/L and aberrant FCM responded to treatment.
Epo levels and immunophenotype of myeloid blasts at start of Epo/G-CSF treatment
| . | Epo < 100 U/L . | Epo > 100 U/L . | ||
|---|---|---|---|---|
| nFCM . | aFCM . | nFCM . | aFCM . | |
| Responders | 13 | 2 | 3 | 0 |
| Nonresponders | 1 | 5 | 6 | 16 |
| Response rate | 94% | 29% | 33% | 0% |
| . | Epo < 100 U/L . | Epo > 100 U/L . | ||
|---|---|---|---|---|
| nFCM . | aFCM . | nFCM . | aFCM . | |
| Responders | 13 | 2 | 3 | 0 |
| Nonresponders | 1 | 5 | 6 | 16 |
| Response rate | 94% | 29% | 33% | 0% |
aFCM indicates aberrant immunophenotype of myeloid blasts; Epo, erythropoietin; G-CSF, granulocyte-colony-stimulating factor; and nFCM, normal immunophenotype of myeloid blasts.
FCM as biomarker in a new predictive model for response
Because aberrant FCM seems to be so strongly associated with treatment failure, a multivariate logistic regression analysis was performed to analyze whether aberrant FCM is an independent predictor of response. All variables with a P value less than .1 in univariate analysis (data not shown) were included in the multivariate analysis. Despite significance in the univariate analysis, Hb level and WPSS were not included in the multivariate analysis because Hb level is biased by pretreatment transfusions and WPSS is used to evaluate transfusion dependency. Transfusion dependency was included in the analysis. Epo levels were logarithmically transformed because of non-Gaussian distribution. In the final multivariate model aberrant FCM, Epo level and transfusion requirement before treatment were entered. In our cohort, only aberrant FCM and Epo levels were significant predictors of response to Epo/G-CSF treatment (Table 3). When Epo levels were grouped according to the model of Hellström-Lindberg et al,9 probability to respond was 37 times less in case of aberrant FCM and approximately 10 times less in case of greater Epo levels (P = .001, odds ratio 0.027 and P = .003, odds ratio 0.099, respectively).
Multivariate logistic regression analysis of prediction of response to Epo/G-CSF
| . | P . | Odds ratio . | 95% CI . |
|---|---|---|---|
| Aberrant flow cytometry | .001 | 0.035 | 0.005-0.274 |
| Serum Epo at study entry | .019 | 0.245 | 0.076-0.795 |
| Pretreatment RBC transfusions | .291 | 0.294 | 0.030-2.850 |
| . | P . | Odds ratio . | 95% CI . |
|---|---|---|---|
| Aberrant flow cytometry | .001 | 0.035 | 0.005-0.274 |
| Serum Epo at study entry | .019 | 0.245 | 0.076-0.795 |
| Pretreatment RBC transfusions | .291 | 0.294 | 0.030-2.850 |
CI indicates confidence interval; Epo, erythropoietin; G-CSF, granulocyte-colony-stimulating factor; and RBC, red blood cell.
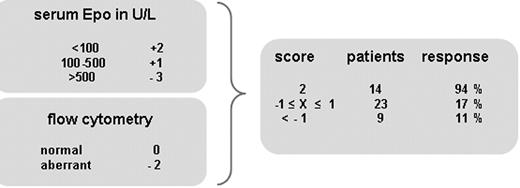
On the basis of our data, we propose a new predictive model (Figure 2); this model is solely created on the basis of previously validated Epo thresholds9 in combination with normal or aberrant FCM of myeloid blasts. Score for Epo levels was applied according to the model of Hellström-Lindberg et al, that is, less than 100 U/L: 2 points, 100 to 500 U/L: 1 point, and greater than 500 U/L: −3 points.9 Regression analysis of FCM against Epo at a cutoff of 100 U/L revealed approximately the same regression coefficients, P values, and odds ratios (data not shown); therefore, same weight of points was applied: in case of aberrant FCM, 2 points were subtracted; no points were added in case of normal FCM. Application of the proposed model distinguishes 3 subgroups with 94%, 17%, and 11% probability to respond to a standardized regimen of Epo/G-CSF. The first subgroup concerned patients with normal FCM and Epo levels less than 100 U/L (n = 14); the second subgroup had Epo levels less than 100 U/L in combination with aberrant FCM or Epo levels between 100 and 500 U/L with either normal or aberrant FCM (n = 20); the third subgroup had the greatest Epo levels, most of them with aberrant FCM (n = 4). Median response duration in the first subgroup was 14.5 months compared with 5 months in the other subgroups.
Endogenous Epo levels and flow cytometry of myeloid blasts as biomarkers in the prediction of response to Epo/G-CSF treatment in low-/intermediate-I–risk MDS. Points granted for Epo level in this model are exactly as in the validated model of Hellström-Lindberg et al.9 Normal and aberrant FCM score 0 and −2 points, respectively. Applying this new model defines 3 subgroups with 94%, 17%, and 11% probability to respond to growth factor treatment.
Endogenous Epo levels and flow cytometry of myeloid blasts as biomarkers in the prediction of response to Epo/G-CSF treatment in low-/intermediate-I–risk MDS. Points granted for Epo level in this model are exactly as in the validated model of Hellström-Lindberg et al.9 Normal and aberrant FCM score 0 and −2 points, respectively. Applying this new model defines 3 subgroups with 94%, 17%, and 11% probability to respond to growth factor treatment.
Discussion
FCM analysis of MDS bone marrow samples was shown to add significantly in the recognition of clinically relevant subgroups with respect to transfusion dependency and progressive disease.13 In this study, we showed that immunophenotypic analysis of myeloid blasts is also instrumental in predicting response to a standardized Epo/G-CSF regimen in low- and intermediate-I–risk MDS. In fact, aberrant immunophenotype of myeloid blasts was highly associated with treatment failure. The most common aberrancy was expression of lymphoid antigen CD7, a marker known to be associated with poor clinical outcome.13,15,16
Most responders had low Epo level and low transfusion requirement, hallmarks of the current validated predictive model.9 Interestingly, with the use of FCM we could identify nonresponders among those patients who should have a good probability to respond according to the latter model. Multivariate regression analysis identified aberrant FCM of myeloid blasts and increased Epo level as significant biomarkers for prediction of treatment failure. Therefore, a new predictive model on the basis of FCM and previously validated Epo levels was proposed (Figure 2). By applying this model, we were able to identify 3 subgroups with 94%, 17%, and 11% probability to respond. The first subgroup concerned 14 patients with normal FCM and Epo levels less than 100 U/L; the second subgroup of 23 patients had Epo levels less than 100 U/L in combination with aberrant FCM or Epo levels between 100 and 500 U/L with either normal or aberrant FCM; the third subgroup of 9 patients had the greatest Epo levels, most of them with aberrant FCM. Of note, a cutoff of 20% of aberrant marker expression, common in routine diagnostics, was used. Smaller percentages of aberrant blast cells, that is below 20%, might be of importance. It cannot be excluded that these represent a dysplastic or preleukemic clone, this is being evaluated by regularly monitoring bone marrow samples.
On the basis of recent studies, low- and intermediate-I–risk MDS patients who are responsive to treatment with Epo/G-CSF might benefit from an increased OS.2,10,11 Whether or not duration of response or time to response is predictive for this OS benefit is not yet elucidated. From our data we hypothesize that 94% of patients with a predictive score of 2 are highly likely to respond to Epo/G-CSF, thereby selecting patients who might benefit from a prolonged OS. However, patients with a very low probability to respond to treatment should not be selected for Epo/G-CSF treatment. Patients with an intermediate score might be selected for Epo/G-CSF for a maximum of 6 months. Our data demonstrate that median response duration in these patients was only 5.5 months (range, 3.5-6 months) compared with a median of 14.5 months (range, 6-51 months) in those patients with a high probability to respond. Our proposed model might have major financial impact because it can identify patients less likely to respond to an expensive Epo/G-CSF regimen better than the current predictive model.
In MDS, bone marrow residual normal and dysplastic hematopoiesis coexist. A preferential outgrowth of normal progenitors might correlate with response to treatment.28 Therefore, next to the value of FCM in predicting response to growth factor treatment, FCM might contribute to the management of low-/intermediate- I–risk MDS patients. Changes in immunophenotypic aberrancies over time might provide information on response to treatment or further progression of disease, especially when no other disease parameters, such as molecular and cytogenetic parameters, are available.29 Preliminary data indicate that immunophenotypic MDS-related abnormalities in bone marrow cells are no longer detectable or decrease in number in responding patients compared with pretreatment analysis.30
In conclusion, our data underscore observations that Epo/G-CSF is an effective first-line treatment regimen in a subgroup of patients with low- and intermediate-I–risk MDS with a low serum Epo level and low transfusion need. Notably, aberrant immunophenotype of myeloid blasts acted as a significant and cost-effective biomarker for response to Epo/G-CSF treatment in combination with Epo levels at validated thresholds. Importantly, FCM analysis of myeloid blasts in MDS bone marrow is a relatively easy technique for any laboratory with experience in the analysis of leukemia samples and monitoring minimal residual disease. Thus, FCM may add significantly to the well-known validated predictive parameters in the selection of patients likely to respond to Epo/G-CSF. In patients with MDS with an intermediate or poor response probability to Epo/G-CSF, one might consider alternative treatment strategies. Prospective studies are currently being conducted to validate the role of FCM in the diagnosis, prognostication, and disease monitoring of low-/intermediate-I–risk MDS during Epo/G-CSF and new emerging drugs such as lenalidomide and 5-azacitidine.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Claudia Cali, Linda van Dreunen, and Yvonne van der Veeken for technical assistance; Hans Berkhof (Department of Epidemiology and Biostatistics, VU University Medical Center, Amsterdam) for statistical assistance; and Drs van Maanen (Westfriesgasthuis, Hoorn), Timmers (Ziekenhuis Amstelveen), and van der Linden (Kennemer Gasthuis, Haarlem) for their contribution to patient accrual.
This study was supported (in part) by a Young Investigators grant (2007-2008) from the Myelodysplastic Syndrome Foundation Inc (A.A.v.d.L.) and an unrestricted educational grant of Roche BV.
Authorship
Contribution: T.M.W. wrote the manuscript, collected and analyzed data, and discussed results; C.A. collected and analyzed data and discussed results; M.E.D.C. provided patient material, collected and analyzed data, and discussed results; M.J.D.L.v.d.V. collected and analyzed clinical data and discussed results; C.E. handled sample logistics and provided patient material; G.J.O. analyzed clinical data and discussed results; and A.A.v.d.L. designed the study, provided patient material, collected and analyzed clinical data, and discussed results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arjan A. van de Loosdrecht, MD, PhD, VU University Medical Center, Department of Hematology, VU Institute of Cancer and Immunology (V-ICI), Cancer Center Amsterdam (CCA), De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: a.vandeloosdrecht@vumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal