Abstract
The objective of this case-control study was to compare the efficacy and toxicity of lenalidomide plus dexamethasone (len/dex) versus thalidomide plus dexamethasone (thal/dex) as initial therapy for newly diagnosed myeloma. We retrospectively studied 411 newly diagnosed patients treated with len/dex (228) or thal/dex (183) at the Mayo Clinic. The differences were similar in a matched-pair analysis that adjusted for age, sex, transplantation status, and dexamethasone dose. The proportions of patients achieving at least a partial response to len/dex and thal/dex were 80.3% versus 61.2%, respectively (P < .001); very good partial response rates were 34.2% and 12.0%, respectively (P < .001). Patients receiving len/dex had longer time to progression (median, 27.4 vs 17.2 months; P = .019), progression-free survival (median, 26.7 vs 17.1 months; P = .036), and overall survival (median not reached vs 57.2 months; P = .018). A similar proportion of patients in the 2 groups experienced at least one grade 3 or 4 adverse event (57.5% vs 54.6%, P = .568). Main grade 3 or 4 toxicities of len/dex were hematologic, mainly neutropenia (14.6% vs 0.6%, P < .001); the most common toxicities in thal/dex were venous thromboembolism (15.3% vs 9.2%, P = .058) and peripheral neuropathy (10.4% vs 0.9%, P < .001). Len/dex appears well-tolerated and more effective than thal/dex. Randomized trials are needed to confirm these results.
Introduction
Multiple myeloma (MM) is a malignant plasma cell proliferative disorder that accounts for more than 11 000 deaths each year in the United States.1,2 For more than 40 years, melphalan and prednisone (MP) remained the standard of care for elderly patients. For more than a decade, a combination of vincristine, doxorubicin, and dexamethasone (VAD) was used as pretransplantation induction therapy for patients eligible for stem cell transplantation (SCT).2-4 The combination of thalidomide plus dexamethasone (thal/dex) has shown significant activity in newly diagnosed MM. Indeed, 2 randomized phase 3 trials compared thal/dex with high-dose dexamethasone alone and reported higher response rates and prolonged time to progression (TTP) in patients receiving thal/dex, even though this did not translate into overall survival (OS) improvement, albeit with relatively short follow-up.5,6 The main toxicities related to thalidomide therapy were deep vein thrombosis (DVT; 13%-19%) and peripheral neuropathy (3%-7%).5-7 A randomized study that compared MP with the thal/dex combination in patients not eligible for SCT found that thal/dex resulted in a higher proportion of very good partial response (VGPR) rate and partial response (PR), but TTP, progression-free survival (PFS), and OS were better for MP because of the toxicity of high-dose dexamethasone, especially in patients older than 75 years.7 A prospective randomized trial comparing thal/dex with standard VAD as pretransplantation induction regimen showed a higher response rate after induction in patients treated with thal/dex, although the benefit was not sustained 6 months after SCT because VGPR rates were almost identical after the high-dose melphalan.8
Lenalidomide (CC-5013), an analog of thalidomide, is more potent in preclinical assays than thalidomide9,10 and has fewer nonhematologic side effects compared with the parent drug.11,12 In newly diagnosed patients, lenalidomide plus high-dose dexamethasone was compared with high-dose dexamethasone alone in a double-blinded, placebo-controlled trial and was demonstrably superior to high-dose dexamethasone in terms of both response rates and 1-year PFS, but no differences in OS have been reported to date.13 Another recent phase 3 study compared the combination of lenalidomide plus low-dose dexamethasone with the lenalidomide plus high-dose dexamethasone regimen: major grade 3 or higher toxic effects, including thrombosis (25% vs 9%) and infections (16% vs 6%), were significantly higher in the lenalidomide plus high-dose dexamethasone group, and 1-year OS was significantly better with the association of lenalidomide plus low-dose dexamethasone. The differences were confirmed in both younger and elderly patients.14
No randomized trial of thal/dex versus lenalidomide plus dexamethasone (len/dex) has been reported so far, and unfortunately none is ongoing or planned. Thalidomide has significant nonhematologic toxicity, including a high risk of peripheral neuropathy. On the other hand, lenalidomide has more hematologic toxicity than thalidomide, and is not widely available in many countries. The goal of this case-control study was to compare the efficacy and the toxicity of thal/dex versus len/dex as primary therapy for newly diagnosed MM patients.
Methods
Patients and treatment schedule
After approval from the Mayo Clinic Institutional Review Board, data from 411 consecutive patients with newly diagnosed symptomatic MM seen at the Mayo Clinic and treated with thal/dex (183 patients) or len/dex (228 patients) were obtained by review of medical records and our existing database. We included all patients started on these agents, regardless of whether or not the treatment was administered as part of a trial to avoid bias in patient selection. Patients in the thal/dex group were treated from January 2000 through March 2008, whereas patients in the len/dex group were treated from March 2004 through December 2008. Thalidomide was given at a dose ranging from 100 mg/day to 400 mg/day continuously; lenalidomide dose was 25 mg/day, days 1 to 21 on a 28-day cycle. All patients received dexamethasone, either at high dose (40 mg orally on days 1-4, 9-12, and 17-20) or at low dose (40 mg orally on days 1, 8, 15, and 22); each cycle was repeated every 4 weeks. Patients were risk-stratified into 2 groups according to genetic abnormalities. The high-risk group was defined by the presence of at least one of the following abnormalities: deletion of p53 (locus 17p13), translocation t(4;14) or t(14;16) by fluorescent in situ hybridization, or loss of chromosome 13 or its long arm or hypodiploidy by metaphase cytogenetics.15 The standard-risk group included patients without any of these abnormalities. In addition to studying all patients, we also identified for accurate outcome comparison an equal number of pair mates among patients who received high-dose dexamethasone in the thal/dex and len/dex groups. Case matching was performed with respect to age, sex, and SCT status (patients treated with len/dex who received SCT were matched with patients treated with thal/dex who received SCT; patients treated with len/dex who did not receive SCT were matched with patients treated with thal/dex who did not received SCT).
Assessment of efficacy and safety
The response criteria used were standard International Myeloma Working Group Uniform Response Criteria.16 Briefly, a PR was defined as a 50% or higher decrease in the serum monoclonal protein (M-protein) levels from baseline and a greater than 90% reduction in 24-hour urine M-protein excretion or less than 200 mg/24 hours (if M-protein was immeasurable, a 50% or higher decrease in the difference between involved and uninvolved free light chain or a 50% or higher reduction in bone marrow plasma cells); for patients with soft-tissue plasmacytomas, a 50% size reduction was required. A VGPR required a 90% or greater reduction in serum M-protein and urinary M-protein less than 100 mg/24 hours or M-protein detectable by immunofixation but not on electrophoresis. A complete response (CR) was defined as negative serum and urine immunofixation, disappearance of any soft tissue plasmacytoma, and less than 5% plasma cells on bone marrow examination. Disease that did not satisfy the criteria for PR, VGPR, CR, or progressive disease was classified as stable disease. Disease progression required any of the following: 25% or greater increase in serum M-protein (absolute ≥ 0.5 g/dL) or urine M-protein (absolute ≥ 200 mg/dL) or, in case of immeasurable M-protein, in the difference between involved and uninvolved free light chain (absolute > 10 mg/dL) or 25% increase in bone marrow plasma cell percentage; development of new bone lesions, or plasmacytomas; and disease-related hypercalcemia. All responses needed to be confirmed in at least 2 consecutive assessments. TTP was calculated from the start of therapy until progression, relapse, or last known remission (death from causes other than progression were censored); PFS was calculated from the start of therapy until the date of progression, relapse, death from any cause, or known remission; OS was calculated from the start of therapy until the date of death or the date the patient was last known to be alive. All adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria (Version 3.0).17
Statistical analysis
The endpoint of this study was to compare the efficacy (response rate, PFS, TTP, and OS) and the toxicity profile (rate of grade 3 or 4 AEs) of these 2 regimens. Outcomes were analyzed on an intention-to-treat basis. The χ2 test or 2-sided Fisher exact test was used to compare differences in nominal variables, and the rank-sum test was used for continuous variables. Time-to-event analysis was performed using the Kaplan-Meier method.18 All comparisons were determined by the log-rank test and by the Cox proportional hazards model to estimate crude hazard ratios (HRs) and 95% confidence intervals (95% CIs). Analyses were performed using SAS software, Version 9.1. Times of observation were censored on May 5, 2009.
Results
Patient characteristics
Patient characteristics are listed in Table 1. Median age was similar in the 2 groups, although in the thal/dex group 68.3% of patients were younger than 65 years compared with 55.7% in the len/dex group (P = .009). A similar proportion of len/dex and thal/dex patients presented with International Staging System (ISS) stage I/II at diagnosis (77.4% vs 75.4%, P = .673). Cytogenetic data were available in only 33.3% of patients who received len/dex and 24.6% of patients treated with thal/dex. The proportions of evaluable patients presenting with high-risk cytogenetic in both groups were similar (32.9% vs 37.8%, respectively, in len/dex and thal/dex groups, P = .586). The majority of patients received thalidomide and lenalidomide not as part of a clinical trial. Only 64 of 183 (35.0%) patients and 96 of 228 (42.1%) were treated as part of a clinical trial in the thal/dex and len/dex groups, respectively. A high proportion of patients in the thal/dex group received high-dose dexamethasone compared with patients in the len/dex group (73.7% vs 31.6%, P < .001), reflecting changing in clinical practice. Patients were allowed to proceed to SCT if they wished and were deemed eligible for such therapy: 39.9% versus 47.5% of patients, respectively (P = .121), in the len/dex and thal/dex groups received SCT as front-line therapy within 9 months after initial diagnosis. Overall, 48.7% versus 60.1% of patients, respectively (P = .021), received transplantation at some point during their clinical course. A significantly higher proportion of patients treated with len/dex received salvage therapy (as second-line only) with bortezomib-based regimens (bortezomib-based regimens: 28.9% vs 15.1%, respectively, with len/dex and thal/dex, P = .014; lenalidomide plus bortezomib-based regimens: 4.1% vs 0%, respectively, with len/dex and thal/dex, P = .028; Table 2). Patient characteristics in the subgroup of matched patients receiving high-dose dexamethasone were similar to those of the whole population.
Patient characteristics
| Characteristic . | All patients . | High-dose dexamethasone patients . | ||||
|---|---|---|---|---|---|---|
| thal/dex (n = 183), n (%) . | len/dex (n = 228), n (%) . | P . | thal/dex (n = 72) . | len/dex (n = 72) . | P . | |
| Median age, y (range) | 60.2 (22.2-79.5) | 62.9 (29.0-92.9) | .061 | 63.3 (36.6-78.7) | 63.5 (29.0-78.4) | .952 |
| Less than 65 y | 125 (68.3) | 127 (55.7) | .009 | 40 (55.6) | 40 (55.6) | > .999 |
| Male sex | 109 (59.6) | 142 (62.3) | .574 | 44 (61.1) | 43 (59.7) | .865 |
| International Staging System | ||||||
| I/II* | 95 (75.4) | 151 (77.4) | .673 | 41 (71.9) | 53 (81.5) | .208 |
| III* | 31 (24.6) | 44 (22.6) | .673 | 16 (28.1) | 12 (18.5) | .208 |
| Missing | 57 (31.2) | 33 (14.5) | — | 15 (20.8) | 7 (9.7) | — |
| Type of M protein | ||||||
| IgG | 105 (57.4) | 136 (59.6) | .642 | 45 (62.5) | 40 (55.6) | .397 |
| IgA | 34 (18.6) | 43 (18.9) | .942 | 18 (25.0) | 17 (23.6) | .846 |
| No serum M protein | 2 (1.1) | 4 (1.8) | .696 | 0 (0) | 1 (1.4) | > .999 |
| Biclonal | 3 (1.6) | 5 (2.2) | .737 | 0 (0) | 2 (2.8) | .497 |
| Light-chain only | 19 (10.4) | 35 (15.4) | .138 | 5 (6.9) | 9 (12.5) | .400 |
| Missing | 20 (10.9) | 5 (2.2) | — | 4 (5.6) | 3 (4.2) | — |
| Cytogenetic | ||||||
| High-risk* | 17 (37.8) | 25 (32.9) | .586 | 4 (40.0) | 9 (33.3) | .715 |
| Data missing | 138 (75.4) | 152 (66.7) | — | 62 (86.1) | 45 (62.5) | — |
| Treatment | ||||||
| High-dose dexamethasone | 135 (73.7) | 72 (31.6) | < .001 | 72 (100) | 72 (100) | > .999 |
| Transplantation | 110 (60.1) | 111 (48.7) | .021 | 37 (51.4) | 37 (51.4) | > .999 |
| Characteristic . | All patients . | High-dose dexamethasone patients . | ||||
|---|---|---|---|---|---|---|
| thal/dex (n = 183), n (%) . | len/dex (n = 228), n (%) . | P . | thal/dex (n = 72) . | len/dex (n = 72) . | P . | |
| Median age, y (range) | 60.2 (22.2-79.5) | 62.9 (29.0-92.9) | .061 | 63.3 (36.6-78.7) | 63.5 (29.0-78.4) | .952 |
| Less than 65 y | 125 (68.3) | 127 (55.7) | .009 | 40 (55.6) | 40 (55.6) | > .999 |
| Male sex | 109 (59.6) | 142 (62.3) | .574 | 44 (61.1) | 43 (59.7) | .865 |
| International Staging System | ||||||
| I/II* | 95 (75.4) | 151 (77.4) | .673 | 41 (71.9) | 53 (81.5) | .208 |
| III* | 31 (24.6) | 44 (22.6) | .673 | 16 (28.1) | 12 (18.5) | .208 |
| Missing | 57 (31.2) | 33 (14.5) | — | 15 (20.8) | 7 (9.7) | — |
| Type of M protein | ||||||
| IgG | 105 (57.4) | 136 (59.6) | .642 | 45 (62.5) | 40 (55.6) | .397 |
| IgA | 34 (18.6) | 43 (18.9) | .942 | 18 (25.0) | 17 (23.6) | .846 |
| No serum M protein | 2 (1.1) | 4 (1.8) | .696 | 0 (0) | 1 (1.4) | > .999 |
| Biclonal | 3 (1.6) | 5 (2.2) | .737 | 0 (0) | 2 (2.8) | .497 |
| Light-chain only | 19 (10.4) | 35 (15.4) | .138 | 5 (6.9) | 9 (12.5) | .400 |
| Missing | 20 (10.9) | 5 (2.2) | — | 4 (5.6) | 3 (4.2) | — |
| Cytogenetic | ||||||
| High-risk* | 17 (37.8) | 25 (32.9) | .586 | 4 (40.0) | 9 (33.3) | .715 |
| Data missing | 138 (75.4) | 152 (66.7) | — | 62 (86.1) | 45 (62.5) | — |
| Treatment | ||||||
| High-dose dexamethasone | 135 (73.7) | 72 (31.6) | < .001 | 72 (100) | 72 (100) | > .999 |
| Transplantation | 110 (60.1) | 111 (48.7) | .021 | 37 (51.4) | 37 (51.4) | > .999 |
Percentages may not total 100 because of rounding.
— indicates not applicable.
Percentage calculated on number of patients for whom data were available.
Second-line salvage regimens
| Regimen . | thal/dex (n = 139), n (%) . | len/dex (n = 97), n (%) . | P . |
|---|---|---|---|
| Transplantation | 14 (10.1) | 18 (18.6) | .061 |
| Bortezomib-based regimen | 21 (15.1) | 28 (28.9) | .014 |
| Lenalidomide-based regimen | 34 (24.5) | 22 (22.7) | .752 |
| Lenalidomide-bortezomib based regimen | 0 (0) | 4 (4.1) | .028 |
| Thalidomide-based regimen | 23 (16.6) | 10 (10.3) | .174 |
| Others | 47 (33.8) | 15 (15.5) | .002 |
| Regimen . | thal/dex (n = 139), n (%) . | len/dex (n = 97), n (%) . | P . |
|---|---|---|---|
| Transplantation | 14 (10.1) | 18 (18.6) | .061 |
| Bortezomib-based regimen | 21 (15.1) | 28 (28.9) | .014 |
| Lenalidomide-based regimen | 34 (24.5) | 22 (22.7) | .752 |
| Lenalidomide-bortezomib based regimen | 0 (0) | 4 (4.1) | .028 |
| Thalidomide-based regimen | 23 (16.6) | 10 (10.3) | .174 |
| Others | 47 (33.8) | 15 (15.5) | .002 |
Response to therapy
Based on standard International Myeloma Working Group criteria, the response rate was significantly higher in len/dex patients compared with thal/dex patients (Table 3). On intention-to-treat analysis, considering all 411 patients, a significantly higher proportion of patients achieved at least a PR with len/dex compared with thal/dex (80.3% vs 61.2%, respectively, P < .001). A significant difference between the 2 groups was also found in terms of both VGPR or better (34.2% vs 12.0%, P < .001) and CR rate (13.6% vs 3.3%, P < .001), respectively. These differences remained significant when the analysis was restricted to 144 patients who received high-dose dexamethasone; len/dex (n = 72) patients obtained a significantly higher proportion of PR or better (90.3% vs 61.1%, P < .001), VGPR or better (50.0% vs 9.7%, P < .001), and CR (22.2% vs 2.8%, P < .001).
Best responses to treatment
| Response . | All patients . | High-dose dexamethasone patients . | ||||
|---|---|---|---|---|---|---|
| thal/dex (n = 183), n (%) . | len/dex (n = 228), n (%) . | P . | thal/dex (n = 72), n (%) . | len/dex (n = 72), n (%) . | P . | |
| CR or VGPR | 22 (12.0) | 78 (34.2) | < .001 | 7 (9.7) | 36 (50.0) | < .001 |
| PR or better | 112 (61.2) | 183 (80.3) | < .001 | 44 (61.1) | 65 (90.3) | < .001 |
| CR | 6 (3.3) | 31 (13.6) | < .001 | 2 (2.8) | 16 (22.2) | < .001 |
| VGPR | 16 (8.7) | 47 (20.6) | < .001 | 5 (6.9) | 20 (27.8) | .001 |
| PR | 90 (49.2) | 105 (46.1) | .528 | 37 (51.4) | 29 (40.3) | .181 |
| SD | 42 (22.9) | 26 (11.4) | .002 | 21 (29.2) | 3 (4.2) | < .001 |
| PD | 1 (0.6) | 5 (2.2) | .232 | 1 (1.4) | 1 (1.4) | > .999 |
| NA | 28 (15.3) | 14 (6.1) | — | 6 (8.3) | 3 (4.2) | — |
| Response . | All patients . | High-dose dexamethasone patients . | ||||
|---|---|---|---|---|---|---|
| thal/dex (n = 183), n (%) . | len/dex (n = 228), n (%) . | P . | thal/dex (n = 72), n (%) . | len/dex (n = 72), n (%) . | P . | |
| CR or VGPR | 22 (12.0) | 78 (34.2) | < .001 | 7 (9.7) | 36 (50.0) | < .001 |
| PR or better | 112 (61.2) | 183 (80.3) | < .001 | 44 (61.1) | 65 (90.3) | < .001 |
| CR | 6 (3.3) | 31 (13.6) | < .001 | 2 (2.8) | 16 (22.2) | < .001 |
| VGPR | 16 (8.7) | 47 (20.6) | < .001 | 5 (6.9) | 20 (27.8) | .001 |
| PR | 90 (49.2) | 105 (46.1) | .528 | 37 (51.4) | 29 (40.3) | .181 |
| SD | 42 (22.9) | 26 (11.4) | .002 | 21 (29.2) | 3 (4.2) | < .001 |
| PD | 1 (0.6) | 5 (2.2) | .232 | 1 (1.4) | 1 (1.4) | > .999 |
| NA | 28 (15.3) | 14 (6.1) | — | 6 (8.3) | 3 (4.2) | — |
Percentages may not total 100 because of rounding.
SD indicates stable disease; PD, progressive disease; NA, not available; and —, not applicable.
Survival
The median duration of follow-up for survivors from diagnosis was 20.9 months in the len/dex group and 46.5 months in the thal/dex group. Duration of therapy was significantly longer in the len/dex patients compared with the thal/dex patients: 36.7% vs 12.6% of patients who did not stop treatment to receive SCT were still receiving immunomodulatory drugs at 1 year (P < .001). A significantly higher proportion of patients in the len/dex group was still receiving therapy at the time of analysis compared with patients in the thal/dex group (18.4% vs 7.1%, respectively, P = .001). In the following analyses, patients who received SCT and patients who switched to another chemotherapy regimen before progression were censored at the date of transplantation/chemotherapy.
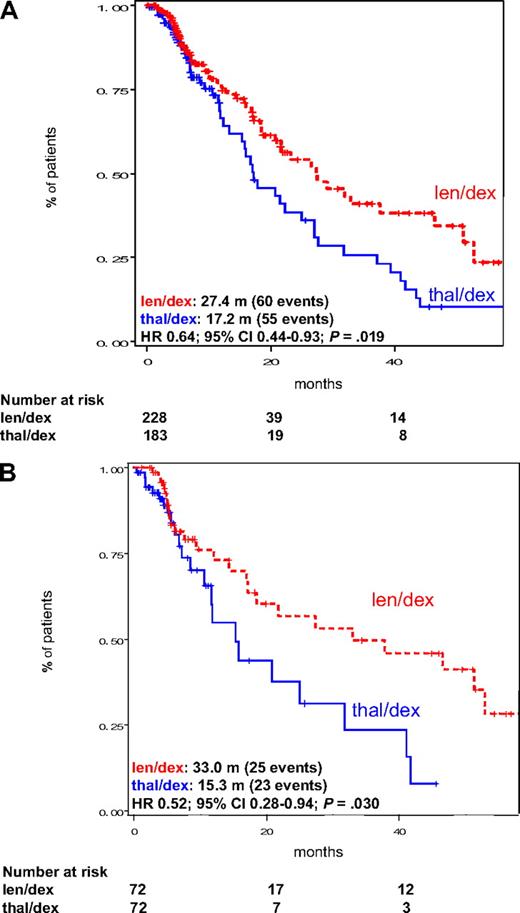
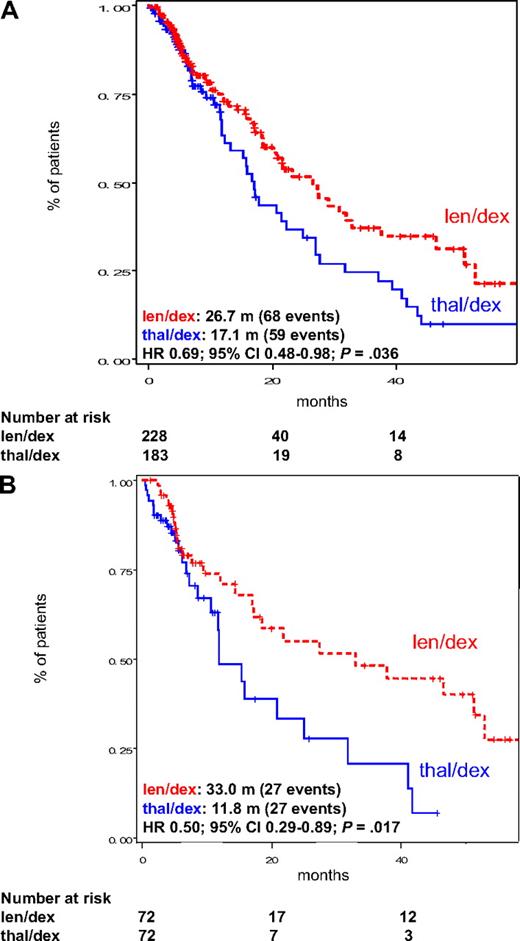
Among all patients, TTP was significantly better in the len/dex group (median, 27.4 months) than in patients receiving thal/dex (median, 17.2 months, HR = 0.64; 95% CI, 0.44-0.93; P = .019; Figure 1A). This was also confirmed in the subgroup of pair mates receiving high-dose dexamethasone (median TTP, 33.0 months vs 15.3 months, HR = 0.52; 95% CI, 0.28-0.94; P = .030; Figure 1B). Similarly, PFS was significantly higher in len/dex patients, both considering all patients (median, 26.7 months vs 17.1 months, HR = 0.69; 95% CI, 0.48-0.98; P = .036; Figure 2A) and pair mates receiving high-dose dexamethasone only (33.0 months vs 11.8 months, HR = 0.50; 95% CI, 0.29-0.89; P = .017; Figure 2B). These analyses were then repeated without censoring at the date of therapy patients who received SCT or switched to another chemotherapy regimen. Both TTP and PFS, for all patients and for high-dose dexamethasone pair mates only, remained significantly better with len/dex (survival curves are provided in the supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article).
TTP in the intention-to-treat population of patients treated with len/dex and thal/dex. (A) TTP in all patients, regardless of dexamethasone dose. (B) TTP in pair mates who received high-dose dexamethasone. Median TTP is provided in the figure. m indicates months.
TTP in the intention-to-treat population of patients treated with len/dex and thal/dex. (A) TTP in all patients, regardless of dexamethasone dose. (B) TTP in pair mates who received high-dose dexamethasone. Median TTP is provided in the figure. m indicates months.
PFS in the intention-to-treat population of patients treated with len/dex and thal/dex. (A) PFS in all patients, regardless of dexamethasone dose. (B) PFS in pair mates who received high-dose dexamethasone. Median PFS is provided in the figure. m indicates months.
PFS in the intention-to-treat population of patients treated with len/dex and thal/dex. (A) PFS in all patients, regardless of dexamethasone dose. (B) PFS in pair mates who received high-dose dexamethasone. Median PFS is provided in the figure. m indicates months.
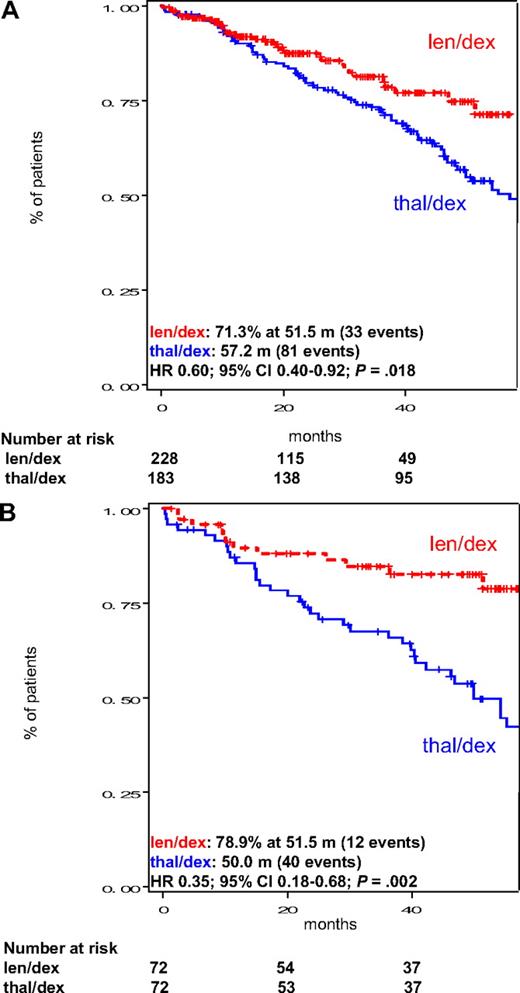
OS was significantly higher among len/dex patients, both considering all patients (median not reached vs 57.2 months, HR = 0.60; 95% CI, 0.40-0.92; P = .018; Figure 3A) and matched patients receiving high-dose dexamethasone only (median not reached vs 50.0 months, HR = 0.35; 95% CI, 0.18-0.68; P = .002; Figure 3B). Early deaths (during the first 4 months of therapy) were reported in 6 of 228 (2.6%) patients receiving lenalidomide and 4 of 183 (2.2%) patients treated with thalidomide in the 2 groups (P > .999).
OS in the intention-to-treat population of patients treated with len/dex and thal/dex. (A) OS in all patients, regardless of dexamethasone dose. (B) OS in pair mates who received high-dose dexamethasone. Median OS is provided in the figure. m indicates months.
OS in the intention-to-treat population of patients treated with len/dex and thal/dex. (A) OS in all patients, regardless of dexamethasone dose. (B) OS in pair mates who received high-dose dexamethasone. Median OS is provided in the figure. m indicates months.
Given the longer duration of therapy in patients treated with len/dex, probably in part related to a higher treatment discontinuation rate because of AEs in thal/dex group (“Toxicity and deaths”), survival analyses were repeated excluding patients who stopped treatment for toxicity in both groups: again, TTP, PFS, and OS were significantly longer for patients who received lenalidomide.
Subgroup analyses
We studied the effect of therapy by ISS stage. In patients who presented with ISS stage I or II at diagnosis, OS was better in patients treated with len/dex compared with thal/dex, both considering all patients (HR = 0.57; 95% CI, 0.32-1.00; P = .052) and high-dose pair mates only (HR = 0.20; 95% CI, 0.08-0.50; P < .001). In contrast, in patients with stage III ISS, no survival differences were found between patients receiving lenalidomide and patients treated with thalidomide, but this may be a function of small sample size.
There were no significant differences in OS of patients presenting with high-risk cytogenetics and treated with len/dex compared with patients treated with thal/dex (HR = 0.76; 95% CI, 0.23-2.51; P = .652), nor for patients with standard-risk cytogenetics (HR = 0.87; 95% CI, 0.31-2.43; P = .795), which again is probably the result of small sample size.
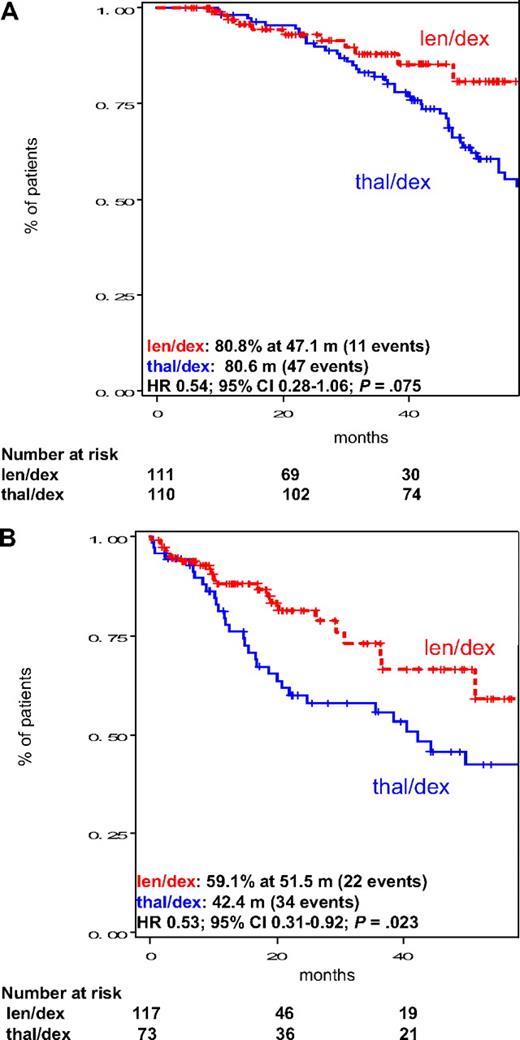
In the subgroup of patients who underwent SCT, there was a trend toward better OS in the len/dex group compared with thal/dex treated patients (median not reached vs 80.6 months, HR = 0.54; 95% CI, 0.28-1.06; P = .075; Figure 4A). In the subgroup of patients who did not receive SCT, OS was significantly longer with len/dex (median not reached vs 42.4 months, HR = 0.53; 95% CI, 0.31-0.92; P = .023; Figure 4B). In this subgroup of patients, response rate and TTP were significantly better with len/dex, too. Considering only patients who stopped treatment to pursue SCT (similar treatment duration in the 2 groups), OS was significantly longer in len/dex patients compared with thal/dex (median not reached vs 54.4 months, respectively, in len/dex and thal/dex, HR = 0.38; 95% CI, 0.15-0.96; P = .040).
Subgroup analysis of OS in the intention-to-treat population of patients treated with len/dex and thal/dex according to transplantation status. (A) OS in patients who received transplantation. (B) OS in patients in patients who did not receive transplantation. Median OS is provided in the figure. m indicates months.
Subgroup analysis of OS in the intention-to-treat population of patients treated with len/dex and thal/dex according to transplantation status. (A) OS in patients who received transplantation. (B) OS in patients in patients who did not receive transplantation. Median OS is provided in the figure. m indicates months.
To evaluate whether the availability of better rescue therapies introduced after 2004 influenced survival, we compared len/dex patients and thal/dex patients treated after March 2004: OS was significantly longer in the 228 patients treated with len/dex compared with 108 patients treated with thal/dex (median not reached for len/dex vs 54.4 months for thal/dex, HR = 0.60; 95% CI, 0.37-0.96; P = .033). In addition, subgroup analysis of OS of thal/dex patients only, treated before March 2004 and after March 2004, showed no differences between the 2 groups (HR = 1.07; 95% CI, 0.67-1.73; P = .767).
Toxicity and deaths
Major grade 3 or 4 toxicities observed with len/dex and thal/dex are listed in Table 4. A total of 131 (57.5%) patients receiving len/dex and 100 (54.6%) patients receiving thal/dex experienced at least one grade 3 or higher toxicity (P = .568). Toxicities were different in the 2 groups. The main toxicities of len/dex were hematologic, in particular neutropenia, reported in 14.0% patients in the len/dex group compared with 0.6% in the thal/dex group (P < .001).
Grade 3 or 4 adverse events
| . | All patients . | High-dose dexamethasone patients . | ||||
|---|---|---|---|---|---|---|
| thal/dex (n = 183), n (%) . | len/dex (n = 228), n (%) . | P . | thal/dex (n = 72), n (%) . | len/dex (n = 72), n (%) . | P . | |
| Treatment-related deaths | 1 (0.5) | 3 (1.3) | .632 | 1 (1.4) | 2 (2.8) | > .999 |
| At least one grade 3 or 4 adverse event | 100 (54.6) | 131 (57.5) | .568 | 44 (61.1) | 51 (70.8) | .218 |
| Hematologic | ||||||
| Anemia | 0 (0) | 10 (4.4) | .003 | 0 (0) | 3 (4.2) | .245 |
| Thrombocytopenia | 0 (0) | 11 (4.8) | .002 | 0 (0) | 3 (4.2) | .245 |
| Neutropenia | 1 (0.6) | 32 (14.0) | < .001 | 0 (0) | 10 (13.9) | .001 |
| Extrahematologic | ||||||
| Peripheral neuropathy | 19 (10.4) | 2 (0.9) | < .001 | 3 (4.2) | 0 (0) | .245 |
| Fatigue | 13 (7.1) | 23 (10.1) | .288 | 5 (6.9) | 10 (13.9) | .275 |
| Gastrointestinal toxicity | 12 (6.6) | 14 (6.1) | .863 | 5 (6.9) | 7 (9.7) | .764 |
| Constipation | 9 (4.9) | 0 (0) | .001 | 4 (5.6) | 0 (0) | .120 |
| Diarrhea | 0 (0) | 8 (3.5) | .01 | 0 (0) | 3 (4.2) | .245 |
| Venous thromboembolism | 28 (15.3) | 21 (9.2) | .058 | 14 (19.4) | 8 (11.1) | .165 |
| DVT | 21* (11.5) | 16 (7.0) | .117 | 9* (12.5) | 6 (8.3) | .413 |
| Pulmonary embolism | 7 (3.8) | 5 (2.2) | .385 | 5 (6.9) | 2 (2.8) | .441 |
| Dermatologic | 12 (6.6)† | 21 (9.7)‡ | .258 | 6 (8.3) | 5 (6.9)‡ | > .999 |
| Infections | 15 (8.2) | 30 (13.1) | .109 | 6 (8.3) | 10 (13.8) | .289 |
| Pneumonia | 5 (2.7) | 16 (7.0) | .070 | 1 (1.4) | 8 (11.1) | .033 |
| Sepsis | 2 (1.1) | 4 (1.8) | .696 | 1 (1.4) | 0 (0) | > .999 |
| Cardiovascular | 10 (5.5) | 10 (4.4) | .614 | 5 (6.9) | 5 (6.9) | > .999 |
| Transaminase increase | 0 (0) | 7 (3.1) | .019 | 0 (0) | 4 (5.6) | .120 |
| Myopathy | 4 (2.2) | 1 (0.4) | .177 | 2 (2.8) | 1 (1.4) | > .999 |
| . | All patients . | High-dose dexamethasone patients . | ||||
|---|---|---|---|---|---|---|
| thal/dex (n = 183), n (%) . | len/dex (n = 228), n (%) . | P . | thal/dex (n = 72), n (%) . | len/dex (n = 72), n (%) . | P . | |
| Treatment-related deaths | 1 (0.5) | 3 (1.3) | .632 | 1 (1.4) | 2 (2.8) | > .999 |
| At least one grade 3 or 4 adverse event | 100 (54.6) | 131 (57.5) | .568 | 44 (61.1) | 51 (70.8) | .218 |
| Hematologic | ||||||
| Anemia | 0 (0) | 10 (4.4) | .003 | 0 (0) | 3 (4.2) | .245 |
| Thrombocytopenia | 0 (0) | 11 (4.8) | .002 | 0 (0) | 3 (4.2) | .245 |
| Neutropenia | 1 (0.6) | 32 (14.0) | < .001 | 0 (0) | 10 (13.9) | .001 |
| Extrahematologic | ||||||
| Peripheral neuropathy | 19 (10.4) | 2 (0.9) | < .001 | 3 (4.2) | 0 (0) | .245 |
| Fatigue | 13 (7.1) | 23 (10.1) | .288 | 5 (6.9) | 10 (13.9) | .275 |
| Gastrointestinal toxicity | 12 (6.6) | 14 (6.1) | .863 | 5 (6.9) | 7 (9.7) | .764 |
| Constipation | 9 (4.9) | 0 (0) | .001 | 4 (5.6) | 0 (0) | .120 |
| Diarrhea | 0 (0) | 8 (3.5) | .01 | 0 (0) | 3 (4.2) | .245 |
| Venous thromboembolism | 28 (15.3) | 21 (9.2) | .058 | 14 (19.4) | 8 (11.1) | .165 |
| DVT | 21* (11.5) | 16 (7.0) | .117 | 9* (12.5) | 6 (8.3) | .413 |
| Pulmonary embolism | 7 (3.8) | 5 (2.2) | .385 | 5 (6.9) | 2 (2.8) | .441 |
| Dermatologic | 12 (6.6)† | 21 (9.7)‡ | .258 | 6 (8.3) | 5 (6.9)‡ | > .999 |
| Infections | 15 (8.2) | 30 (13.1) | .109 | 6 (8.3) | 10 (13.8) | .289 |
| Pneumonia | 5 (2.7) | 16 (7.0) | .070 | 1 (1.4) | 8 (11.1) | .033 |
| Sepsis | 2 (1.1) | 4 (1.8) | .696 | 1 (1.4) | 0 (0) | > .999 |
| Cardiovascular | 10 (5.5) | 10 (4.4) | .614 | 5 (6.9) | 5 (6.9) | > .999 |
| Transaminase increase | 0 (0) | 7 (3.1) | .019 | 0 (0) | 4 (5.6) | .120 |
| Myopathy | 4 (2.2) | 1 (0.4) | .177 | 2 (2.8) | 1 (1.4) | > .999 |
One plus hemorrhage.
One Steven-Johnson syndrome and 1 toxic epidermal necrolysis.
One Stevens-Johnson syndrome.
The most common toxicity in patients treated with thal/dex was peripheral neuropathy: 28 (15.3%) patients compared with 8 (3.5%) patients treated with len/dex (P < .001) developed a grade 2 to 4 peripheral neuropathy (grade 3 or 4, 10.4% vs 0.9%, P < .001). Thrombotic events were more frequent in the thal/dex group, but the difference was not significant (15.3% vs 9.2%, P = .058). Similar rates of infections (13.1% vs 8.2%, P = .109), fatigue (10.1% vs 7.1%, P = .288), dermatologic toxicity (9.7% vs 6.6%, P = .258), and cardiovascular events (4.4% vs 5.5%, P = .614) were reported in patients who received len/dex and thal/dex, respectively. The incidence of gastrointestinal events was not significantly different in the 2 groups (6.1% and 6.6%, respectively, in len/dex and thal/dex groups, P = .863), but toxicities were different: mainly diarrhea in patients who received lenalidomide and constipation in patients treated with thalidomide. When the analysis was restricted to pair mates who received high-dose dexamethasone, most of the data were confirmed. Incidence of peripheral neuropathy in the thal/dex group was lower if compared with the whole cohort of thal/dex patients, and the difference between thal/dex and len/dex patients was no more significant; however, even in this subgroup, patients who received thalidomide had a higher rate of neuropathy than patients treated with lenalidomide (grade 2-4, 8.3% vs 2.8%, respectively, P = .275; grade 3 or 4, 4.2% vs 0%, respectively, P = .245). Incidence of venous thromboembolism was slightly higher in both len/dex and thal/dex patients treated with high-dose dexamethasone compared with the all cohort of len/dex and thal/dex patients, as was the incidence of pneumonia in len/dex high-dose dexamethasone patients. Twenty-two (9.7%) patients treated with len/dex discontinued treatment for AEs (main reason was dermatologic toxicity in 27.3%) compared with 33 (18.0%) patients receiving thal/dex (P < .013; main reason for thalidomide discontinuation was peripheral neuropathy in 30.3%). There was 1 toxic death in the thal/dex group (resulting from pulmonary embolism) and 3 in the len/dex patients (1 sepsis, 1 pneumonia, and 1 gastrointestinal perforation).
Discussion
In newly diagnosed MM patients, 2 randomized studies have shown that the thal/dex regimen is better than high-dose dexamethasone alone: in the first study, the response rate was significantly higher with thal/dex than with dexamethasone alone (63% vs 41%, P < .002)5 ; the second study showed that TTP was also improved in the thal/dex group (P < .001).6 No differences in survival were reported, but the trials were not powered to detect survival differences and follow-up was relatively short. The increased response rates and TTP with thal/dex needed to be balanced against the increased toxicity. In the thal/dex group compared with the dexamethasone group, the rates of any grade 3 or 4 toxic effects in the first 4 months were significantly higher (45% vs 21%, P = .001) as were DVT (17% vs 3%) and grade 3 or 4 peripheral neuropathy (7% vs 4%).5
A prospective randomized study confirmed the efficacy of the thal/dex regimen also in comparison with the standard VAD regimen. The frequency of at least a VGPR was 25% after thal/dex induction compared with 7% after VAD (P < .003). However, the benefit was not sustained at 6 months after SCT (VGPR rates, 44% vs 42%, P = .87). DVT was higher in the thal/dex group (23% vs 8%, P = .004).8 In elderly patients not eligible for SCT, thal/dex resulted in a higher proportion of VGPR rate (26% vs 13%, P = .006) and PR rate (68% vs 50%, P = .002) than did standard MP, but OS was inferior with thal/dex resulting from the increase in toxicity seen with thal/dex, particularly in patients older than 75 years.7
Although thal/dex has emerged as an efficacious oral induction regimen for myeloma, more effective and safer regimens are needed. Recent studies show that lenalidomide, an analog of thalidomide, is also highly active and, with a different toxicity profile, may potentially be safer than the parent drug. A combination trial with lenalidomide plus dexamethasone showed improved activity more than historic controls with lower toxicity in a phase 2 clinical trial.19,20 Large phase 3 trials are ongoing to investigate the role of lenalidomide plus dexamethasone in newly diagnosed MM. Preliminary results show that lenalidomide plus high-dose dexamethasone resulted in a higher CR rate (22.1% vs 3.8%) and 1-year PFS (77% vs 55%, P = .002) than did high-dose dexamethasone alone.13 Preliminary results also show that the combination of lenalidomide plus low-dose dexamethasone has further benefit in terms of 2-year OS (87% vs 75%, P < .001) and AEs compared with lenalidomide plus high-dose dexamethasone.14
Although thal/dex and len/dex are 2 of the most common regimens used in the treatment of newly diagnosed myeloma, no formal comparison has yet been done between them. In an attempt to address this issue, we have analyzed a series of newly diagnosed MM patients treated with either thal/dex or len/dex at the Mayo Clinic. The response rates and survival with thal/dex and len/dex are comparable with that reported in the literature.5-8,13,14 In our cohort, the 2 groups were comparable for baseline characteristics (age, sex, and ISS stage).
We clearly demonstrated that len/dex is superior to thal/dex. On intention-to-treat analysis, PR rates were significantly higher with len/dex, as was the depth of response, with higher CR and VGPR rates noted with len/dex. Our findings were corroborated among the subgroup of matched pair mates who received high-dose dexamethasone alone because it is known that the dose of dexamethasone may affect outcome.14 Similarly, we adjusted for the effect of transplantation by comparing the 2 regimens in patients who received transplantation as well as in the subset of patients who did not receive transplantation. Again, the findings were sustained.
The AEs reported were consistent with the established toxicity profile for both lenalidomide and thalidomide. The rate of grade 3 or 4 AEs was similar in both groups, but the toxicity profile was different and consistent with what is reported in the literature.6-8,13,14 Venous thromboembolism has been confirmed as one of the most frequent grade 3 or 4 AEs related to thalidomide and grade 3 or 4 peripheral neuropathy as the main reason for thalidomide discontinuation. Thromboembolic events occurred at a similar rate of patients in both groups, as did skin rash. Despite a higher incidence of neutropenia in patients receiving len/dex, the rate of grade 3 or 4 infections was not significantly higher. The use of high-dose dexamethasone has been associated with an increase in the toxicity.14 In the subgroup analysis of patients receiving high-dose dexamethasone, the rate of grade 3 or 4 AEs is higher compared with the whole cohort of 411 patients.
The better tolerability of lenalidomide is of course one of the advantages of lenalidomide treatment compared with thalidomide treatment, which translated in significantly longer treatment duration. Probably, longer treatment is one of the reasons for better survival in len/dex patients, but because duration of therapy is affected by tolerability as well as true superior efficacy of one regimen more than the other, it is not possible to compare patients with equivalent treatment duration. We think that longer treatment resulting from better tolerance is not the only reason for better survival in len/dex patients because, when we restricted the survival analysis to patients who stopped treatment to pursue transplantation (with no significant differences in treatment duration), OS for lenalidomide-treated patients was still significantly longer.
There are some limitations to the study. Because this was not a randomized trial, the patient populations were not treated contemporaneously, the choice of postinduction therapy was not standardized, and len/dex patients may have had better salvage options. This could in part explain the absence of survival differences in patients with high-risk cytogenetic abnormalities. Although salvage therapies will affect OS, they will have no impact on response rate or TTP, which are assessed before salvage therapies are used. To evaluate whether difference salvage options could have affected survival, we compared patients treated after March 2004, and we confirmed that OS was significantly better in patients who received len/dex. After March 2004, when both drugs were available, more patients received len/dex compared with thal/dex: this represents the feeling among physicians, based on relapsed myeloma trials where lenalidomide appeared to be more potent with less neurotoxicity than thalidomide. The impact of dexamethasone dose on outcome has been demonstrated in the phase 3 E4A03 trial, when used in combination with lenalidomide. Patients in this analysis received a mix of the high- and low-dose dexamethasone regimens. This limitation was overcome by the matched-pair analysis, in which patients treated with thalidomide or lenalidomide combined with high-dose dexamethasone were compared. The matched analysis confirmed the superiority of len/dex over thal/dex observed in the overall cohort. In addition, patients in the thal/dex group received different thalidomide doses: in this retrospective study, it is hard to evaluate the impact of different doses and dose modifications. However, a randomized trial more correctly addressed this issue and found comparable 1-year OS between patients treated with thalidomide 100 mg and thalidomide 400 mg.21 Another limitation is that, because not all patients were treated as part of a clinical trial, the rates of toxicity may be underestimated. Despite these limitations, this is the first study to compare the efficacy and safety of thalidomide versus lenalidomide, and is of importance because a true randomized comparison of these 2 regimens probably won't occur.
In conclusion, results of this case-control analysis suggest the superiority of len/dex compared with thal/dex in terms of response rates, survival, and toxicity. Moreover, len/dex treatment, although more active, was not associated with increased toxicity. In particular, len/dex has a lower incidence of peripheral neuropathy, the main reason for thalidomide discontinuation. Ideally, randomized prospective phase 3 studies are necessary to confirm these results. Comparison of len/dex with other active regimens, such as bortezomib/dexamethasone and bortezomib/thalidomide/dexamethasone, are also needed to determine the optimum initial therapy for MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Cancer Institute, National Institutes of Health (grants CA62242 and CA107476) and the Eastern Cooperative Oncology Group (S.V.R.).
National Institutes of Health
Authorship
Contribution: F.G. and S.V.R. analyzed data and wrote the manuscript; S.R.H., M.Q.L., F.B., M.A.G., S.K., A.D., J.R.M., P.L.B., D.D., C.B.R., J.A.L., S.J.R., S.R.Z., T.E.W., R.F., R.A.K., P.R.G., A.K.S., and S.V.R. provided study patients; and S.K., A.D., M.A.G., V.R., P.R.G., C.B.R., R.A.K., and A.K.S. provided critical review and edits to the manuscript.
Conflict-of-interest disclosure: M.A.G. has received honoraria from Celgene, Millennium, Genzyme, and Amgen. S.K. has received research support from Celgene, Novartis, Millennium, Bayer, and Genzyme. M.Q.L., A.D., T.E.W., and P.R.G. have received research funding/grants from Celgene. P.L.B. has received research funding from Merck and has been a member of the advisory board for Celgene, Genentech, and Amgen. R.F. has provided consultancy for Celgene, Medtronic, Genzyme, Amgen, Bristol-Myers Squibb, and Otsuka American Pharmaceutical. The remaining authors declare no competing financial interests.
Correspondence: S. Vincent Rajkumar, Department of Internal Medicine, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: rajks@mayo.edu.