Abstract
Factor XIII (FXIII) is a plasma transglutaminase that cross-links fibrin monomers, α2-plasmin inhibitor, and so forth. Congenital FXIII deficiency causes lifelong bleeding symptoms. To understand the molecular pathology of FXIII deficiency in vivo, its knockout mice have been functionally analyzed. Because prolonged bleeding times, a sign of defective/abnormal primary hemostasis, were commonly observed in 2 separate lines of FXIII A subunit (FXIII-A) knockout mice, a possible role or roles of FXIII in platelet-related function was investigated in the present study. Although platelet aggregation induced by adenosine diphosphate or collagen was normal, clot retraction (CR) was lost in the platelet-rich plasma (PRP) of FXIII-A knockout mice. In contrast, there was no CR impairment in the PRP of tissue transglutaminase-knockout mice compared with that of wild-type mice. Furthermore, a transglutaminase inhibitor, cystamine, halted CR in the PRP of wild-type mice. These results indicate that the enzymatic activity of FXIII is necessary for CR, at least in mice.
Introduction
Coagulation factor XIII (FXIII) is a pro-enzyme of plasma transglutaminase (TGase) consisting of 2 enzymatic A subunits (FXIII-A) and 2 noncatalytic B subunits, and plays a critical role in the generation of a stable hemostatic plug.1-3 FXIII catalyzes intermolecular cross-linking reactions between fibrin monomers, α2-plasmin inhibitor, fibronectin, etc. These reactions increase the mechanical strength of the fibrin clot and its resistance to proteolytic degradation, and enhance the assembly of the extracellular matrix.
Congenital FXIII deficiency is a rare autosomal recessive disorder, the cases of most of which are caused by defects in the FXIII-A gene.3 A lifelong bleeding tendency, abnormal wound healing, and recurrent spontaneous miscarriage are common symptoms of FXIII deficiency.1,4
FXIII-A exists extracellularly in plasma as well as intracellularly as a cytosolic protein in megakaryocytes/platelets and monocytes/macrophages, although the function(s) of intracellular FXIII-A remain(s) unknown.5,6 In particular, platelets cause clot retraction (CR) by retracting extended filopodia along fibrin strands.7 There have been conflicting reports about the effects of FXIII deficiency on CR; investigators reported that the absence of FXIII abolished,7-9 did not affect,10,11 or rather enhanced12 CR in patients with congenital FXIII deficiency. However, platelet aggregation induced by various agents is uniformly normal in patients with congenital FXIII deficiency.8,9,13,14
To understand the precise molecular pathology of FXIII deficiency in vivo, FXIII-A knockout (KO) mice have been analyzed. FXIII-A KO mice demonstrated a severe bleeding tendency.15 We also reported that FXIII-A KO mice developed severe uterine bleeding, resulting in spontaneous miscarriage in females, and male-specific intrathoracic hemorrhage as well as excessive cardiac fibrosis.16,17
Methods
Animal
Wild-type C57BL/6J mice were obtained from Japan SLC Inc. Gene-targeted mice of FXIII-A were generated as previously reported,18 and tissue TGase (tTG) KO mice were generously provided by Prof Melino of Rome Tor Vergata University.19 These KO mice were maintained on a C57BL/6 genetic background for more than 10 generations. The animals were given free access to a standard food and water supply under specific pathogen–free conditions throughout the period of this study. Experimental procedures were approved by the Animal Care and Use Committee of Yamagata University and were carried out in accordance with the guidelines of this committee and Japanese governmental law.
CR
Blood was collected from the jugular vein of mice and anticoagulated with 3.8% sodium citrate in a ratio of 1 part anticoagulant to 9 parts blood, and platelet-rich plasma (PRP) was obtained by centrifugation at 800g for 5 minutes. These samples were then activated with 1 U/mL thrombin (Sigma-Aldrich) and 5mM CaCl2 under gentle shaking, and the reaction mixtures were left unstirred at 37°C in siliconized test tubes. The extent of CR was assessed 10 and 60 minutes later by measuring the weight of each sample's fluid not incorporated into the clot, and was expressed as a percentage by calculating the ratio between clot weight and that of the whole reaction mixture (weight ratio).
Because the quantity of blood collectable from mice is limited, the time course of CR was monitored by taking photographic images of clots during time intervals, and measurement of clot areas was performed using ImageJ (Wayne Rasband, National Institutes of Health). The CR was expressed as a percentage by calculating the ratio between a specific clot area and that of the whole reaction mixture (area ratio) at indicated time intervals. The ratio at 0 minutes was set to 100%. Cystamine (Wako Pure Chemical) was added to PRP immediately before thrombin. Results are presented as mean plus or minus SD of 3 independent experiments, and were analyzed using an unpaired Student t test.
Results and discussion
Impaired CR in FXIII-A KO mice
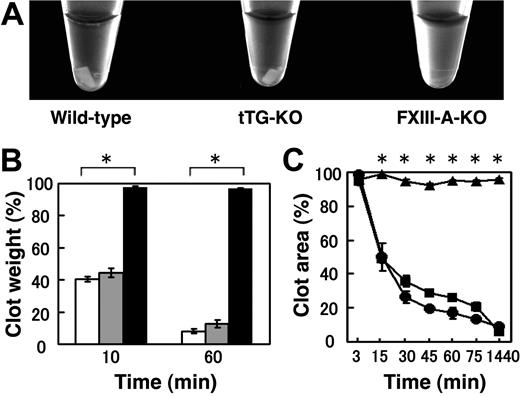
A possible role (or roles) for FXIII-A in the process of CR was investigated using PRP obtained from FXIII-A KO mice. Wild-type PRP showed significant retraction at 1 hour after thrombin treatment, whereas FXIII-deficient PRP showed no indication of retraction even after treatment of 1 hour at 37°C (Figure 1A). FXIII-A KO mice exhibited a significantly reduced rate of CR (a weight ratio in Figure 1B; an area ratio in Figure 1C), suggesting that FXIII-A is essential for the CR reaction.
Impaired CR in FXIII-A-KO mice but normal CR in tTG KO mice. (A) Photograph of CR in wild-type mice (left), tTG KO mice (middle), FXIII-A KO mice (right). PRP was incubated with 1 U/mL thrombin, 5mM CaCl2 at 37°C. The photograph was taken after 1 hour. (B) Quantitative analysis of CR by a weight ratio method. The extent of CR was assessed at 10 and 60 minutes by measuring the clot weight, for wild-type (open), tTG KO (shaded), or FXIII-A KO (filled) mice. Data are mean ± SD of triplicates. (C) Time-dependent CR measured by an area ratio method. The extent of CR was assessed at the indicated times by measuring the clot size, for wild-type (●), tTG KO (■), or FXIII-A KO (▴) mice. Data are mean ± SD of triplicates. *Statistically significant differences (P < .001) were observed at 10 and 60 minutes in panel B and at 15, 30, 45, 60, 75, and 1440 minutes in panel C between FXIII-A-KO versus wild-type mice.
Impaired CR in FXIII-A-KO mice but normal CR in tTG KO mice. (A) Photograph of CR in wild-type mice (left), tTG KO mice (middle), FXIII-A KO mice (right). PRP was incubated with 1 U/mL thrombin, 5mM CaCl2 at 37°C. The photograph was taken after 1 hour. (B) Quantitative analysis of CR by a weight ratio method. The extent of CR was assessed at 10 and 60 minutes by measuring the clot weight, for wild-type (open), tTG KO (shaded), or FXIII-A KO (filled) mice. Data are mean ± SD of triplicates. (C) Time-dependent CR measured by an area ratio method. The extent of CR was assessed at the indicated times by measuring the clot size, for wild-type (●), tTG KO (■), or FXIII-A KO (▴) mice. Data are mean ± SD of triplicates. *Statistically significant differences (P < .001) were observed at 10 and 60 minutes in panel B and at 15, 30, 45, 60, 75, and 1440 minutes in panel C between FXIII-A-KO versus wild-type mice.
A lack of FXIII-A but not tTG abolished the CR
Because platelets contain both FXIII-A and tTG,20 both of which are also present on the surface of activated platelets,21 we next examined whether the absence of tTG affected CR using tTG KO mice.19 CR, however, was normal in tTG-deficient PRP, as shown by the weight ratio in Figure 1B. Clots of wild-type and tTG-deficient PRPs were equally retracted by 1 hour at 37°C (average percentage of clot weight 8.2% and 12.7%, respectively). In addition, Figure 1C (the area ratio) clearly shows the time-dependent nature of CR in the wild-type and tTG KO mice. CR was completely halted in FXIII-A KO mice.
Enzymatic activity of FXIII-A required for CR
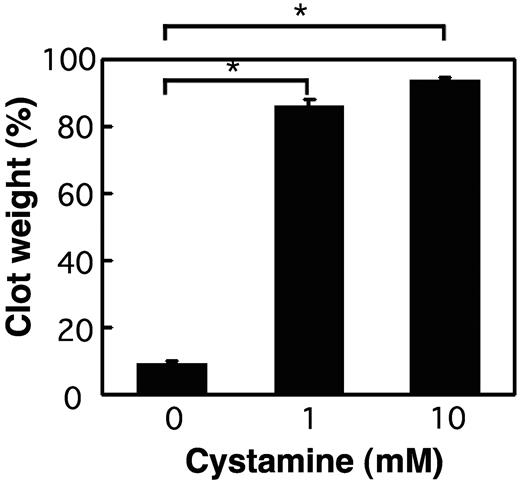
We also investigated the requirement of enzymatic activity of FXIII-A for CR. As shown in Figure 2, a potent inhibitor of tTG and FXIII-A,22 cystamine, completely inhibited CR in wild-type PRP. CR of wild-type PRP was also totally halted by the treatment with batroxobin, which converts fibrinogen to fibrin without activating FXIII, and by adding ethylenediaminetetraacetic acid in place of Ca2+ ions essential for TGase activity (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article). These findings indicate that the TGase activity of FXIII-A is a prerequisite to CR.
Impaired CR by a potent TGase inhibitor cystamine. PRP of wild-type mice was incubated with 1 U/mL thrombin, 5mM CaCl2 at 37°C for 1 hour in the absence (left) or the presence of 1mM (middle) or 10mM (right) cystamine. The extent of CR was assessed at 60 minutes by measuring the clot weight. Data are mean ± SD of triplicates. *Statistically significant difference (P < .001) was observed between the absence versus the presence of cystamine.
Impaired CR by a potent TGase inhibitor cystamine. PRP of wild-type mice was incubated with 1 U/mL thrombin, 5mM CaCl2 at 37°C for 1 hour in the absence (left) or the presence of 1mM (middle) or 10mM (right) cystamine. The extent of CR was assessed at 60 minutes by measuring the clot weight. Data are mean ± SD of triplicates. *Statistically significant difference (P < .001) was observed between the absence versus the presence of cystamine.
The indispensable role of the extracellular/plasma FXIII-A in the development of Ca2+-dependent CR was previously demonstrated in humans7 ; that is, when normal washed platelets were resuspended in FXIII-deficient plasma, the tension developed in the thrombin-generated clot was totally diminished compared with the control containing washed platelets resuspended in normal plasma. When purified FXIII was added to the FXIII-deficient plasma, the tension was restored to normal.7 However, replacement of extrinsic FXIIIs only partially restored impaired CR in the PRP of FXIII-A KO mice in our system (supplemental Figure 2), suggesting that both extracellular/plasma and intracellular/platelet FXIIIs are required for CR, at least in mice.
FXIII has been reported to cross-link fibrin, glycoprotein IIb/IIIa, actin, and myosin,23 which are involved in CR. Both actin and myosin are located in the platelet cytosol and are cross-linked by Ca2+-dependent TGase activity.23 The platelet FXIII-A can be activated by calpain, an endogenous intracellular protease.24 Thus, it is possible that intracellular/platelet FXIII-A may function for CR via the cross-linking of cytosolic actin and myosin. Recently, myosin IIA has been reported to mediate fibrin-independent platelet contraction.25 Moreover, extracellular/plasma fibrin cross-linked by FXIII-A must be indispensable for CR because afibrinogenemia (ie, fibrinogen deficiency in plasma) also causes impaired CR.9
The in vivo significance of CR for primary hemostasis is less understood: impaired CR may be, to some extent, responsible for the bleeding symptoms in patients with congenital FXIII deficiency.1,4 The precise molecular mechanism of FXIII-mediated CR remains to be explored in the future.
Presented in part at the 31st Meeting of the Japanese Society of Thrombosis and Hemostasis, Osaka, Japan, November 21, 2008.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof G. Melino of Rome Tor Vergata University for providing tTG KO mice and Ms L. Boba for assisting in preparation of the manuscript.
This work was supported in part by a Global Center of Excellence project to Yamagata University and Grant-in-Aid for Scientific Research.
Authorship
Contribution: K.K. performed research, analyzed data, and wrote the paper; M.S., M.K., and T.M. performed research; N.Y. performed research and analyzed data; and A.I. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akitada Ichinose, Department of Molecular Patho-Biochemistry and Patho-Biology, Yamagata University School of Medicine, Iida-Nishi 2–2-2, Yamagata 990-9585, Japan; e-mail: aichinos@med.id.yamagata-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal