Abstract
The nature of viral vectors is suggested to be a significant contributor to undesirable immune responses subsequent to gene transfer. Such viral vectors, recognized as danger signals by the host immune system, activate dendritic cells (DCs), causing unwanted antivector and/or transgene product immunity. We recently reported efficient induction of immune tolerance to coagulation factor IX (FIX) by direct intramuscular injection of adeno-associated virus (AAV)–FIX. AAV vectors are nonpathogenic and elicit minimal inflammatory response. We hypothesized that the nonpathogenic nature of AAV plays a critical role in induction of tolerance after AAV gene transfer. We observed inefficient recruitment and activation of DCs subsequent to intramuscular injection of AAV. To further validate our hypothesis, we examined immune responses to FIX after intramuscular injection of AAV with simultaneous activation of DCs. We were able to achieve phenotypic and functional activation of DCs after administration of lipopolysaccharide and anti-CD40 antibody. However, we observed efficient induction of FIX tolerance irrespective of DC activation in mice with different genetic and major histocompatibility complex backgrounds. Furthermore, activation of DCs did not exaggerate the immune response induced after intramuscular injection of AAV serotype 2 vector. Our results demonstrate that induction of FIX tolerance after AAV gene transfer is independent of DC activation status.
Introduction
Gene therapy is emerging as a valuable alternative treatment for human diseases. However, adverse immune responses subsequent to gene transfer, such as severe cytotoxic T lymphocyte response and formation of inhibitory antibodies against transgene products,1-4 need to be addressed for successful application of gene therapy in human patients. The primary step would be to determine critical factors that may decide or control the ultimate immunologic outcomes in gene transfer. This will provide a fundamental insight into the mechanism accounting for the immune responses subsequent to gene transfer, leading to a better understanding and then final resolution of the adverse immune responses.
A variety of inherent factors, in conjunction with gene transfer, can be encountered and recognized as a class of danger signals by the host immune system.2,5 Such danger signals elicit the innate immunity of the host, thus stimulating and causing the maturation and activation of quiescent antigen-presenting cells (APCs).2,5,6 The activated APCs in turn present the processed antigen with appropriate major histocompatibility complex (MHC) molecules to antigen-specific T cells, to initiate the relevant immune response.2,5 Dendritic cells (DCs) are a major type of professional APCs, which act as the central decision maker of the immune system.6-8 Activation of DCs by the danger signal is a critical step in deciding the ultimate immunologic outcome.5,6,8,9 Quiescent (immature or mature) DCs are considered tolerogenic and capable of inducing T-cell deletion, anergy, or regulatory T cells. Whether a T cell is tolerized or activated to become an effector cell depends on the activation status of the APCs. The mature and activated DCs initiate priming of the antigen-specific CD4+ helper T cells, leading to immune responses to relevant targets such as the delivery vector.6,9,10 Not all transgene products are immunogenic, and therefore not recognized as danger by the host immune system. However, the antiviral vector immunity can instigate collateral adverse immune responses against the transgene product.2 If the transgene product itself is immunogenic, the antiviral vector immunity can worsen the intensity of the undesirable immune responses against the transgene product.
A critical factor in relation to gene transfer that can be identified as a danger signal by the host organisms is the nature of the viral gene delivery vector. Many gene transfer vectors are recombinant derivatives of viruses, such as adenovirus and retrovirus, the majority of which are pathogenic and immunotoxic.1,2 Although the final recombinant viral vectors that are used in gene delivery are devoid of potential pathogenic viral component, vector-related immunotoxic incidents have been observed in many gene transfer studies.1 Among all viral vectors tested in gene therapy studies, adeno-associated virus (AAV) is the only virus that is not associated with any known human disease. The nonpathogenic nature of AAV does not present itself as a danger signal to the host. It therefore causes only a minimum level of vector-related toxicity and immune responses in AAV-based gene transfers.2,11,12 This makes AAV an attractive gene transfer vector compared with other gene transfer vectors derived from pathogenic viruses.
Coagulation factor IX (FIX) gene transfer for hemophilia B treatment is a good model for gene therapy studies. Multiple strategies have been used for FIX gene transfer, including the use of viral vectors. AAV has been extensively tested for hemophilia B gene therapy. Direct intramuscular injection of AAV has been shown to be a convenient, safe, and potentially effective approach for FIX gene transfer.13-15 Intramuscular injection of AAV does not cause severe cellular immune response such as cytotoxic T lymphocyte response in rodent, canine, or human patients. Such severe immune responses were, however, substantial after gene transfers using recombinant adenoviral vector.16 Formation of inhibitory anti-FIX antibodies, which is the major complication in FIX replacement to treat hemophilia B, has also been observed in preclinical studies of intramuscular AAV gene transfer.13,17-19
Efficient induction of immune tolerance to FIX is critical for the success of hemophilia treatment. Many factors have been proposed to contribute to induction of immune tolerance or immunity to FIX after muscular AAV gene transfer.13,14,17-19 It is, however, inconclusive and still an ongoing debate as to the immunologic consequence and the significance of these factors. It needs further investigation.
We recently reported efficient induction of immune tolerance to FIX by direct intramuscular injection of AAV serotype 1 vector (AAV1) in mice with diverse genetic and immunologic backgrounds.13-15 AAV has proven to be much less immunogenic in contrast to other pathogenic viral vectors such as recombinant adenoviral vectors.11,12 It is conceivable that lower incidence of immunity against transgene product in AAV gene transfer may be ascribed to the nonpathogenicity of AAV and the consequent inefficiency of activated DCs to stimulate an immune response. It was also reported that excess local immunity at the injection site could increase anti-FIX immunity in the context of intramuscular AAV gene transfer.20 We therefore hypothesized that the nonpathogenic property of the AAV vector is one of the major factors that facilitates induction of immune tolerance to FIX after intramuscular AAV1-FIX gene transfer.
In the current study, we first examined the status of DCs in the context of intramuscular AAV gene transfer. We found that the DCs remained in a steady state after intramuscular injection of either AAV1 or AAV serotype 2 vector (AAV2). We then investigated immune responses to FIX after intramuscular injection of AAV1 concomitant with full activation of DCs by lipopolysaccharide (LPS) and anti-CD40 antibody. Our data demonstrated that despite activation of DCs, no change in the ultimate outcome of immune tolerance induced by intramuscular injection of AAV1 vectors was observed. We accordingly concluded that induction of FIX tolerance by intramuscular AAV gene transfer is independent of the activation status of DCs.
Methods
AAV vector production, animal care, and procedures
The AAV–human FIX (hFIX) vectors were made using a 3-plasmid transfection scheme, as previously described.13,15 C57BL/6, BALB/c, and C3H mice were purchased from The Jackson Laboratory. All the mice were maintained in pathogen-free animal facilities at Mount Sinai School of Medicine, and treated in accordance with the guidelines of the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine, which approved this study. AAV injections, animal procedures, and plasma sample collection were conducted, as previously described.13,15 For in vivo DC activation, 8- to 10-week-old mice were injected with 100 μg/mouse anti-CD40 antibody (rat anti–mouse CD40, immunoglobulin G [IgG] 2a, clone FGK45; Axxora) in the footpad and 100 μg/mouse LPS (Sigma-Aldrich) via intraperitoneal injection or anti-CD40 antibody alone on day 0. The draining lymph nodes and spleens were collected 6, 24, 72, and 120 hours after AAV and anti-CD40 antibody with or without LPS injection for characterization of DCs. Two units of recombinant hFIX (rhFIX; Genetics Institute) emulsified in 100 μL of complete Freund's adjuvant (CFA; Pierce Biotechnology) was injected subcutaneously for boosting the immune response to hFIX or for verification of hFIX immune tolerance.
Detection of hFIX antigen and anti-hFIX antibodies
Phenotypic characterization of DCs
Single-cell suspensions were prepared by mechanical disintegration of the isolated draining lymph nodes and spleen, followed by red blood cell lysis (BD Pharmingen). The cells were suspended in 100 μL of fluorescence-activated cell sorter (FACS) staining buffer (5 mL of phosphate-buffered saline [PBS]; 100 μL each of normal mouse, rabbit, and human serum; 333 μL of 30% bovine serum albumin; 5 mL of Hanks balanced salt solution complete with bovine serum albumin; and 100mM EDTA [ethylenediaminetetraacetic acid]), and then stained with the following fluorochrome-conjugated antibodies CD11c phycoerythrin (PE; 1:200), CD80 allophycocyanin (1:200), CD86 allophycocyanin (1:200; BD Pharmingen), and MHCII biotin (eBiosciences). The cells were incubated with the antibody mixture for 30 minutes on ice and washed twice by the addition of 1 mL of FACS buffer. Cells that received biotin-conjugated antibodies were incubated an additional 30 minutes with avidin allophycocyanin Cy7 (BD Pharmingen). The cells were then washed twice and stored in 1× formalin buffer before analysis. The cells were read on a LSRII flow cytometer equipped with FACSDiva software Version 5.0.2 (both BD Biosciences). The data were analyzed using FlowJo analysis software Version 8.4.6 (TreeStar).
Cytokine profile
Splenocytes were suspended in complete RPMI 1640 and cultured for 4 hours at 37°C in a CO2 incubator. GolgiSTOP (4 μL/6 mL culture; BD Pharmingen) was added and incubated for an additional 4 hours at 37°C. The cells were collected and stained for surface antigen CD11c PE (1:200; BD Pharmingen). The cells were then fixed and permeabilized per manufacturer's recommendation (BD Cytofix/Cytoperm Plus Fixation/Permeabilization kit; BD Pharmingen). The cells were subsequently stained for intracellular cytokines interleukin (IL)-12 allophycocyanin, IL-6 PE, and IL-10 fluorescein isothiocyanate (BD Pharmingen). The cells were evaluated on BD LSRII flow cytometer equipped with the BD FACSDiva software and analyzed using FlowJo analysis software.
Statistical analysis
The data were analyzed using GraphPad Prism Version 3.0cx (GraphPad). Statistical differences between the various experimental groups were evaluated by 2-tailed, unpaired t test. A P value less than .05 was considered statistically significant.
Results
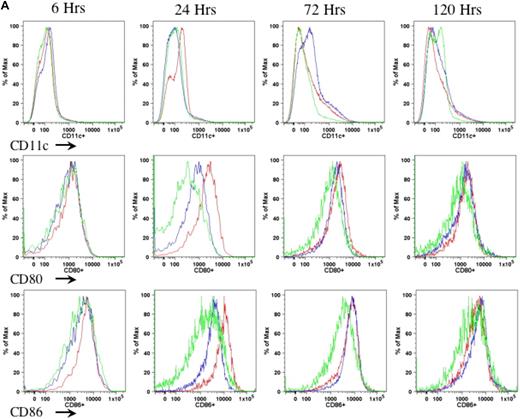
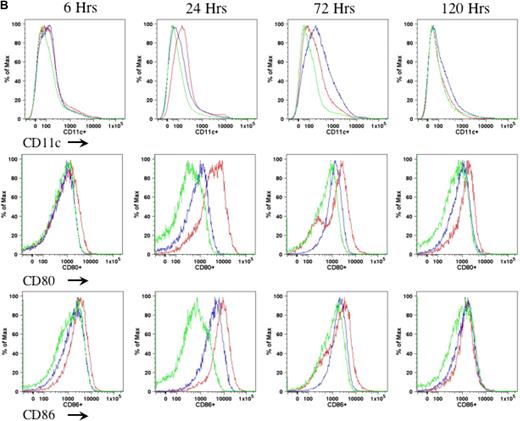
In the current study, we proposed to examine the role of DCs in AAV1-induced immune tolerance to FIX. For a sensitive as well as dependable evaluation of DC motivation, recruitment, and activation after intramuscular injection of AAV, we performed preliminary experiments to determine the appropriate duration, route, and mode of DC activation. We compared different agents that are known and defined for DC activation, such as LPS and anti-CD40 antibody administered either subcutaneously (footpad) or intraperitoneally.21-23 We examined the number of CD11c+ cells as well as expression of MHCII and costimulatory molecules (CD80 and CD86) on CD11c+ cells in draining lymph nodes (Figure 1A) and spleen (Figure 1B) of the mice at 6, 24, 72, and 120 hours after administration of anti-CD40 antibody alone or LPS plus anti-CD40 antibody or PBS. We observed that the combination of LPS and anti-CD40 antibody was optimal for the activation of DCs (Figure 1A-B). We also observed that DC activation peaked at 24 hours upon administration of the DC stimulator via the subcutaneous (footpad) route (Figure 1A-B). We thus decided to evaluate the status of DCs at 24 hours after intramuscular injection of AAV and/or administration of anti-CD40 antibody together with LPS.
Kinetics of DC activation upon administration of LPS and anti-CD40 antibody. Eight- to 10-week-old C57BL/6 mice (n = 4 per cohorts) received LPS (100 μg/mouse) plus anti-CD40 antibody (100 μg/mouse; red line), or anti-CD40 antibody alone (blue line), or PBS (green line). Cells from draining lymph nodes (A) and splenocytes (B) were collected at 6, 24, 72, and 120 hours after injection and stained for cell surface antigens of CD11c (top panel), CD80 (middle panel), and CD86 (bottom panel). The cells were first gated on the CD11c+ population of live cells. Expression of CD80 and CD86 was determined from the gated CD11c population. A representative of 2 independent experiments for each time point is shown.
Kinetics of DC activation upon administration of LPS and anti-CD40 antibody. Eight- to 10-week-old C57BL/6 mice (n = 4 per cohorts) received LPS (100 μg/mouse) plus anti-CD40 antibody (100 μg/mouse; red line), or anti-CD40 antibody alone (blue line), or PBS (green line). Cells from draining lymph nodes (A) and splenocytes (B) were collected at 6, 24, 72, and 120 hours after injection and stained for cell surface antigens of CD11c (top panel), CD80 (middle panel), and CD86 (bottom panel). The cells were first gated on the CD11c+ population of live cells. Expression of CD80 and CD86 was determined from the gated CD11c population. A representative of 2 independent experiments for each time point is shown.
Direct intramuscular injection of AAV vectors does not motivate or activate DCs
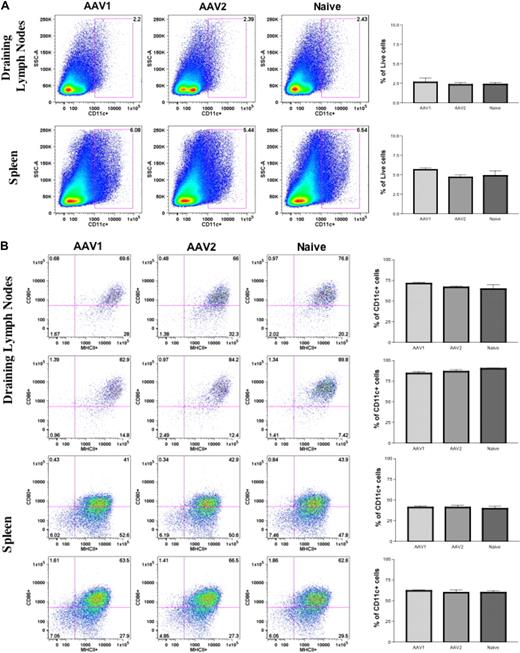
We first examined the numbers and activation status of DCs subsequent to intramuscular injection of AAV vectors. Cohorts of 8- to 10-week-old C57BL/6 mice (n = 4 per cohort) received intramuscular injection of 1011 vector genomes (vg) of AAV1-hFIX or 6 × 1010 vg of AAV2-hFIX vectors, as described. Twenty-four hours after AAV injection, cells from the draining lymph nodes and spleen of the mice were harvested for detection of cell surface markers by flow cytometry. The size of draining lymph nodes and spleen in the AAV1-injected and AAV2-injected mice was similar to those in the naive, untreated mice. We also observed no change in the number of CD11c+ cells in the draining lymph nodes and spleen of AAV1- or AAV2-injected mice compared with naive, untreated mice (Figure 2A). Baseline levels of cells expressing CD11c remained approximately 2% in draining lymph nodes and approximately 5% to 6% in the spleen in both experimental and untreated groups. We observed no statistically significant difference in the expression of costimulatory molecules on the CD11c+ cells in both the draining lymph nodes and spleen of AAV1- and AAV2-injected mice compared with naive untreated mice (Figure 2B). In summary, we did not observe significant up-regulation in the numbers or activation of DCs after intramuscular injection of AAV.
Direct intramuscular injection of AAV vectors does not activate DCs. Eight- to 10-week-old C57BL/6 mice (n = 4 per cohorts) received intramuscular injection of 1011 vg of AAV1-hFIX or 6 × 1010 vg of AAV2-hFIX. Control mice of similar age were administered PBS. After 24 hours, cells from the draining lymph nodes and spleen were collected and stained for the appropriate antibodies, and flow cytometry analyses were performed, as described. (A) No change in DC numbers in draining lymph nodes and spleen after intramuscular injection of AAV. The dot plots show the expression of CD11c on cells. Numbers indicate the percentage of CD11c+ cells among the total live cells in either the draining lymph nodes or spleen. (B) No activation of DCs in draining lymph nodes and spleen after intramuscular injection of AAV. The dot plots show the expression of either MHCII and CD80 or MHCII and CD86 on cells gated with CD11c. Numbers indicate the percentage of MHCII+CD80+ or MHCII+CD86+ among the CD11c+ population. Data shown are mean ± SEM. A representative of 2 independent experiments is shown.
Direct intramuscular injection of AAV vectors does not activate DCs. Eight- to 10-week-old C57BL/6 mice (n = 4 per cohorts) received intramuscular injection of 1011 vg of AAV1-hFIX or 6 × 1010 vg of AAV2-hFIX. Control mice of similar age were administered PBS. After 24 hours, cells from the draining lymph nodes and spleen were collected and stained for the appropriate antibodies, and flow cytometry analyses were performed, as described. (A) No change in DC numbers in draining lymph nodes and spleen after intramuscular injection of AAV. The dot plots show the expression of CD11c on cells. Numbers indicate the percentage of CD11c+ cells among the total live cells in either the draining lymph nodes or spleen. (B) No activation of DCs in draining lymph nodes and spleen after intramuscular injection of AAV. The dot plots show the expression of either MHCII and CD80 or MHCII and CD86 on cells gated with CD11c. Numbers indicate the percentage of MHCII+CD80+ or MHCII+CD86+ among the CD11c+ population. Data shown are mean ± SEM. A representative of 2 independent experiments is shown.
LPS and anti-CD40 antibody can efficiently recruit DCs and induce complete activation of DCs
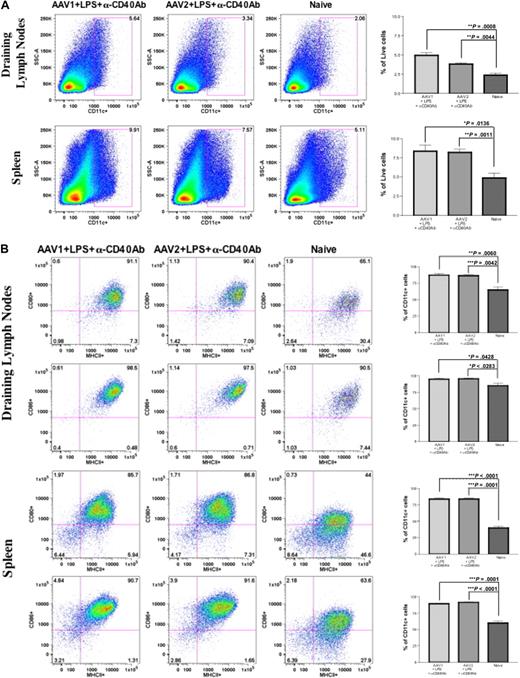
To further validate our preliminary observation and hypothesis, we proposed to investigate the effect of DC activation on immune responses to FIX after intramuscular injection of AAV1. Intramuscular injection of AAV alone does not affect the number and activation status of DCs. Therefore, we injected mice with LPS and anti-CD40 antibody to activate DCs, as described. Anti-CD40 antibody has been proven to be a potent activator of cells such as DCs, B cells, and epithelial cells, causing strong inflammation.22,23 Cohorts of 8- to 10-week-old C57BL/6 mice received intramuscular injection of 1011 vg of AAV1-hFIX and 6 × 1010 vg of AAV2-hFIX with or without administration of LPS and anti-CD40 antibody (n = 4 for each cohort). Twenty-four hours after intramuscular injection of AAV1/AAV2 and/or administration of LPS and anti-CD40 antibody, cells from the draining lymph nodes and spleen of the mice were harvested for detection of cell surface markers by flow cytometry. The size of draining lymph nodes and spleen in the mice that received LPS and anti-CD40 antibody was considerably larger compared with naive, untreated mice, or the mice that received only intramuscular injection of AAV. We observed a significant increase in the number of CD11c+ cells in both the draining lymph nodes as well as spleen of mice that received LPS and anti-CD40 antibody compared with naive and untreated mice (Figure 3A). Significant expression of MHCII and costimulatory molecules (CD80 and CD86) was also detected in the CD11c+ cells in both the draining lymph nodes and spleen of the mice that received LPS and anti-CD40 antibody, compared with naive and untreated mice (Figure 3B). These results demonstrated significantly increased numbers and complete phenotypic activation of DCs in experimental mice after a single administration of LPS and anti-CD40 antibody.
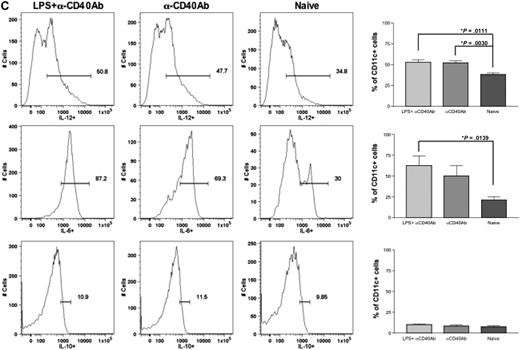
Anti-CD40 antibody and LPS recruit and activate DCs in the draining lymph nodes and spleen. Eight- to 10-week-old C57BL/6 mice (n = 4 per cohorts) received intramuscular injection of 1011 vg of AAV1-hFIX plus 100 μg of LPS plus 100 μg of anti-CD40 antibody (in the footpad) or 6 × 1010 vg of AAV2-hFIX plus 100 μg of LPS plus 100 μg of anti-CD40 antibody (in the footpad) on the same day. Control mice received PBS. Cells from the draining lymph nodes and spleen were collected after 24 hours and stained with the appropriate antibodies, and flow cytometry analysis was performed, as described. (A) Increase in DC numbers in draining lymph nodes and spleen after administration of LPS and anti-CD40 antibody in comparison with naive mice. The dot plots show the expression of CD11c on cells. Numbers indicate the percentage of CD11c+ cells among the total live cells in either the draining lymph nodes or the spleen. (B) Activation of DCs in draining lymph nodes and spleen after administration of LPS and anti-CD40 antibody. The dot plots show the expression of either MHCII and CD80 or MHCII and CD86 on cells gated with CD11c. Numbers indicate the percentage of MHCII+CD80+ or MHCII+CD86+ among the CD11c+ population. Data shown are mean ± SEM. A representative of 2 independent experiments is shown. (C) Cytokine profile of splenocytes 24 hours after administration of LPS and anti-CD40 antibody. The histograms show the expression of IL-12 (top panel), IL-6 (middle panel), and IL-10 (bottom panel) on cells gated with CD11c. Numbers indicate the percentage of CD11c+ cells that are expressing the particular cytokine. Data shown are mean ± SEM, n = 4 for each cohort. A representative of 2 independent experiments is shown.
Anti-CD40 antibody and LPS recruit and activate DCs in the draining lymph nodes and spleen. Eight- to 10-week-old C57BL/6 mice (n = 4 per cohorts) received intramuscular injection of 1011 vg of AAV1-hFIX plus 100 μg of LPS plus 100 μg of anti-CD40 antibody (in the footpad) or 6 × 1010 vg of AAV2-hFIX plus 100 μg of LPS plus 100 μg of anti-CD40 antibody (in the footpad) on the same day. Control mice received PBS. Cells from the draining lymph nodes and spleen were collected after 24 hours and stained with the appropriate antibodies, and flow cytometry analysis was performed, as described. (A) Increase in DC numbers in draining lymph nodes and spleen after administration of LPS and anti-CD40 antibody in comparison with naive mice. The dot plots show the expression of CD11c on cells. Numbers indicate the percentage of CD11c+ cells among the total live cells in either the draining lymph nodes or the spleen. (B) Activation of DCs in draining lymph nodes and spleen after administration of LPS and anti-CD40 antibody. The dot plots show the expression of either MHCII and CD80 or MHCII and CD86 on cells gated with CD11c. Numbers indicate the percentage of MHCII+CD80+ or MHCII+CD86+ among the CD11c+ population. Data shown are mean ± SEM. A representative of 2 independent experiments is shown. (C) Cytokine profile of splenocytes 24 hours after administration of LPS and anti-CD40 antibody. The histograms show the expression of IL-12 (top panel), IL-6 (middle panel), and IL-10 (bottom panel) on cells gated with CD11c. Numbers indicate the percentage of CD11c+ cells that are expressing the particular cytokine. Data shown are mean ± SEM, n = 4 for each cohort. A representative of 2 independent experiments is shown.
Upon activation, DCs express proinflammatory cytokines. We thereby examined cytokine expression of the activated DCs to further validate the complete activation of DCs after administration of LPS and anti-CD40 antibody. We analyzed the expression of cytokines in these cells by flow cytometry. We observed that there was a significant increase in the number of CD11c+ cells expressing proinflammatory cytokines IL-6 and IL-12 after administration of either LPS and anti-CD40 antibody (P = .011 for IL-12; P = .014 for IL-6) or anti-CD40 antibody alone (P = .003 for IL-12) in comparison with CD11c+ cells from naive and untreated mice (Figure 3C). However, there was no difference in the expression of IL-10 among the experimental and naive groups. This further validated complete activation of these DCs by LPS and anti-CD40 antibody.
Activation of DCs does not abrogate the FIX tolerance induced by intramuscular injection of AAV1
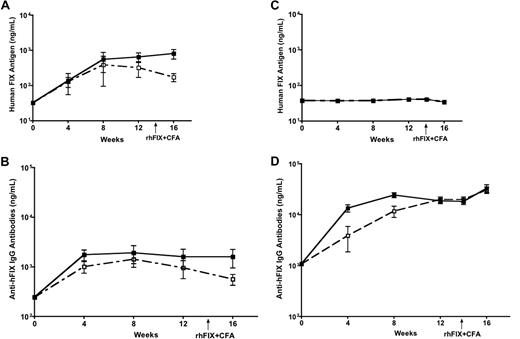
In steady state without inflammatory mediators, DCs are known to capture, process, and present antigen to T cells leading to immune tolerance. Upon inflammatory stimulation, DCs are activated to initiate and promote immunity. We thereby deduced that induction of inflammation and DC activation at the same time as AAV injection would have the potential to change the immunologic outcome after intramuscular injection of AAV. We then evaluated the effect of DC activation on FIX tolerance induced by intramuscular injection of AAV1 vector. After intramuscular injection of AAV1 with or without coadministration of LPS and anti-CD40 antibody, we examined levels of hFIX antigen and anti-hFIX antibody in the circulation of the experimental mice. Low levels of anti-hFIX antibody and equivalent levels of circulating hFIX antigen were detected in the mice regardless of the activation of DCs by coadministration of LPS and anti-CD40 antibody (Figure 4A-B; n = 5 for each cohort). The levels of anti-hFIX antibody in mice that received LPS and anti-CD40 antibody are comparable with those that did not receive LPS and anti-CD40 antibody. We are aware of the detection of low levels of anti-hFIX antibody in mice after intramuscular injection of AAV1, which are slightly higher than background anti-hFIX antibody in naive isogenic mice. Such low levels of anti-hFIX antibodies do not exhibit inhibitory activity.15 We also observed that activation of DCs does not exaggerate the immune response induced by intramuscular injection of AAV2. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 6 × 1010 vg of AAV2-hFIX with or without anti-CD40 antibody (n = 5 for each cohort). We observed barely detectable levels of hFIX antigen levels in both experimental groups. Very high levels of anti-hFIX antibodies were observed, which were further increased on immunogenic challenge (Figure 4C-D).
Activation of DCs does not abrogate FIX tolerance induced by intramuscular injection of AAV1 in C57BL/6 mice. Plasma was collected every 4 weeks after AAV and LPS plus anti-CD40 antibody injection, and measured for hFIX antigen and anti-hFIX IgG antibodies by ELISA. Data shown are mean ± SEM, n = 5, for each cohort. (A) hFIX antigen. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 1011 vg of AAV1-hFIX alone (■) or with LPS and anti-CD40 antibody (□). (B) Anti-hFIX IgG antibodies. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 1011 vg of AAV1-hFIX alone (■) or with LPS and anti-CD40 antibody (□). (C) hFIX antigen. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 6 × 1010 vg of AAV2-hFIX alone (■) or with anti-CD40 antibody (□). (D) Anti-hFIX IgG antibodies. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 6 × 1010 vg of AAV2-hFIX alone (■) or with anti-CD40 antibody (□).
Activation of DCs does not abrogate FIX tolerance induced by intramuscular injection of AAV1 in C57BL/6 mice. Plasma was collected every 4 weeks after AAV and LPS plus anti-CD40 antibody injection, and measured for hFIX antigen and anti-hFIX IgG antibodies by ELISA. Data shown are mean ± SEM, n = 5, for each cohort. (A) hFIX antigen. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 1011 vg of AAV1-hFIX alone (■) or with LPS and anti-CD40 antibody (□). (B) Anti-hFIX IgG antibodies. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 1011 vg of AAV1-hFIX alone (■) or with LPS and anti-CD40 antibody (□). (C) hFIX antigen. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 6 × 1010 vg of AAV2-hFIX alone (■) or with anti-CD40 antibody (□). (D) Anti-hFIX IgG antibodies. Eight- to 10-week-old C57BL/6 mice received intramuscular injection of 6 × 1010 vg of AAV2-hFIX alone (■) or with anti-CD40 antibody (□).
Furthermore, no change of hFIX antigen levels and anti-FIX antibody titers in the AAV1- and DC activator-injected mice after immunogenic challenge of rhFIX emulsified in CFA, demonstrating definite immune tolerance to FIX in these mice (Figure 4A-B). In contrast, injection of rhFIX/CFA in AAV2-injected mice, irrespective of whether they received anti-CD40 antibody or not, increased the titers of anti-FIX antibodies (Figure 4C-D). These results clearly demonstrate that activation of DCs cannot affect the immunologic outcome of FIX tolerance induced by intramuscular injection of AAV1.
We also examined expression of FIX antigen at early time points after intramuscular injection of AAV1-FIX. We detected hFIX antigen in AAV1-injected mice as early as 24 hours after intramuscular injection of AAV1-FIX, which became significant by day 5 (mean = 165.3 ± SEM = 55.27 ng/mL, n = 5). Detection of immediate expression of FIX antigen at early time points post-AAV injection demonstrates exposure of the host immune system to the FIX antigen when DCs are activated by the inflammatory stimulation of LPS and anti-CD40 antibody.
DC activation-independent FIX tolerance induced by intramuscular injection of AAV1 is irrespective of genetic and MHC backgrounds of mice
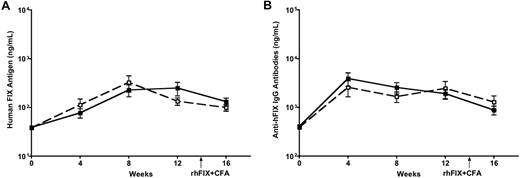
It was reported that mice with C57BL/6 are more tolerogenic than other strains of mice, such as BALB/c and C3H.24,25 We then moved on to test whether activation of DCs could reverse the immune outcome in mice with different genetic and MHC backgrounds by using BALB/c (H2d) and C3H (H2k) mice. Eight- to 10-week-old BALB/c and C3H mice received intramuscular injection of 3 × 1011 vg of AAV1-hFIX (a dose required to induce FIX tolerance)13 alone or with LPS and anti-CD40 antibody (n = 5 for each cohort). We examined levels of hFIX antigen and anti-hFIX antibodies in the circulation of the experimental mice. A peak in circulating FIX antigen levels was observed at 8 weeks, followed by a stable expression of FIX until week 16 in both BALB/c (Figure 5A) and C3H mice (data not shown). A slight peak in anti-FIX antibodies was observed initially at week 4, followed by very low levels until week 16 in both BALB/c (Figure 5B) and C3H mice (data not shown). The levels of both circulating hFIX antigen and anti-hFIX antibodies were comparable irrespective of whether the DCs were activated with LPS and anti-CD40 antibody or not. DC activation failed to reverse the tolerance observed after intramuscular injection of AAV1 (Figure 5 depicts BALB/c results; C3H mice data not shown). This indicates that activation of DCs does not change the immunologic outcome in mice with diverse genetic and immunologic backgrounds.
Activation of DCs does not abrogate FIX tolerance induced by intramuscular injection of AAV1 in BALB/c mice. Eight- to 10-week-old BALB/c mice received intramuscular injection of 3 × 1011 vg of AAV1-hFIX alone (■) or with LPS and anti-CD40 antibody (□; n = 5 for each cohort). Plasma was collected every 4 weeks after AAV and LPS plus anti-CD40 antibody injection, and measured for hFIX antigen (A) and anti-hFIX IgG antibodies (B) by ELISA. Data shown are mean ± SEM.
Activation of DCs does not abrogate FIX tolerance induced by intramuscular injection of AAV1 in BALB/c mice. Eight- to 10-week-old BALB/c mice received intramuscular injection of 3 × 1011 vg of AAV1-hFIX alone (■) or with LPS and anti-CD40 antibody (□; n = 5 for each cohort). Plasma was collected every 4 weeks after AAV and LPS plus anti-CD40 antibody injection, and measured for hFIX antigen (A) and anti-hFIX IgG antibodies (B) by ELISA. Data shown are mean ± SEM.
Discussion
Adverse immune responses after gene transfer are considered one of the major causes of the minimal success in gene therapy clinical trials to date.2-4 It is of great importance to elucidate the effect and the extent of gene transfer vector on adverse immunity for successful gene therapy. The nature of the gene transfer vectors is suggested as a foundation accounting for undesirable immune responses subsequent to gene transfer.1,2 Vectors, the majority of which are derived from viruses, are encountered and recognized as danger signals by the host immune system. Such viral vectors cause a certain extent of inflammation by stimulating and initiating activation of APCs, leading to unwanted antivector and/or transgene product immunity.1,2 Very little is known about the exact nature of APCs that are implicated in the initiation of anti-hFIX immune responses subsequent to intramuscular injection of AAV.
DCs are the most potent and the major type of professional APCs with a unique T-cell stimulatory aptitude.6,7 DCs are the essential link between the innate and the adaptive immune responses.7 The activation status of DCs has been proven to play a major role in determining the immunologic outcome. Immature or mature DCs in a steady state have been shown to lead to T-cell tolerance.6,9,10 The activation of the DCs and their function in immune regulation, in turn, are dictated by the innate immune responses to different pathogens and the consequent danger signals.6,7 Such innate immune responses and the relevant danger signal trigger migration, maturation, differentiation, and activation of the DCs by significantly up-regulating expression of MHC and costimulatory molecules, which are critical in initiating/promoting T-cell priming and proliferation for effective immunity.6 Activation of DCs not only precedes adaptive immune responses, it also determines and controls the final outcomes of the immune responses.6
The nonpathogenic AAV vectors elicit negligible inflammation and thus have minimal effect on DC activation.11,12 It is conceivable that inefficiency in activation of DCs would favor lower immunity against vector as well as the transgene product. It was reported that there was insignificant immunity subsequent to AAV gene transfer.26 We hypothesized that inefficiency of DC activation subsequent to AAV gene transfer is a factor that promotes FIX tolerance.
The objective of this study was to investigate the effect of activation status of the major APC on humoral immune responses to FIX (formation of inhibitory anti-FIX antibodies or FIX tolerance) after intramuscular AAV gene transfer for hemophilia B treatment. The results of our studies first revealed absence of, or inefficient recruitment and activation of DCs subsequent to intramuscular AAV gene transfer. This is consistent with the nonpathogenic nature of the AAV vector, thus in support of our hypothesis.
If the absence of DC activation were essential for induction of FIX tolerance in the context of intramuscular injection of AAV, activation of the DCs would abrogate the FIX tolerance and elicit anti-FIX immunity. We, however, observed efficient induction of immune tolerance to FIX subsequent to intramuscular injection of AAV1 regardless of full phenotypic and functional activation of DCs. Such an observation is contrary to our hypothesis and is also inconsistent with the danger signal theory.
Accordingly, complete activation of DCs locally and systemically failed to initiate an anti-FIX immunity or obliterate the immune tolerance to FIX induced by intramuscular AAV1 gene transfer. This indicates the likelihood of the limited role that the effect of inflammation, innate immunity, and the subsequent activation of DCs play in determining the immune responses to transgene product in the context of intramuscular gene transfer. It also suggests that the nonpathogenic nature of AAV vectors and the consequent minimal inflammation of AAV gene transfer may not contribute as much to induction of immune tolerance to the transgene product, as postulated. In support of our results, immune tolerance to FIX was also observed in FIX gene transfer using adenoviral and lentiviral vectors targeting the liver.16,27 However, significant inflammation and DC activation were observed subsequent to gene transfer of adenoviral and lentiviral vectors.2,16,27 This also seemed to be inconsistent with the danger signal theory of immunology.2,5,6 A body of recent experimental evidence strongly suggests that maturation of DCs can also render them to be tolerogenic.6,9,10 We would like to point out that the LPS- and anti-CD40 antibody-stimulated DCs in this study are not only fully matured, but also completely activated. Administration of anti-CD40 antibody also causes a substantial level of general inflammation in addition to complete activation of many types of APCs, such as DCs, B cells, and even endothelial cells.22,23 To our knowledge, there is no report or proposal suggesting tolerance induction under the condition of complete activation of DCs.
Unique characteristics of gene transfer may make immune responses to trangene product in the context of gene transfer distinctive from the immunology of classical protein replacement. As for the immune responses to FIX after intramuscular injection of AAV vector, multiple factors, including property of the gene transfer vectors, vector dose, targeting organ/tissue, efficiency, and amount of the transgene product expressed, persistent expression of the transgene product, etc, may play a certain part in determining the ultimate immunologic outcome. High levels of FIX antigen were reported as critical in deciding the immune responses to FIX after muscular AAV gene transfer.13-15,18 It is, however, elusive as to whether there is a single decisive factor, or a synergy of several factors that command the eventual immunologic outcome in the context of AAV gene transfer. Our results in the current study clearly demonstrated that activation of DCs alone could not reverse induction of FIX tolerance in the context of intramuscular injection of AAV vectors. Ongoing efforts in our laboratory are investigating the interaction among multiple factors, such as AAV dose, antigen (FIX) level, DC differentiation status, etc, in deciding the ultimate immunologic outcome to FIX in the context of intramuscular AAV gene transfer.
It was also reported that the inefficiency of local inflammatory activation by AAV at injection sites could be overcome by an increase of adjuvant-like component(s) in the AAV vector preparation upon elevated dose of AAV vectors.20 It was proposed that the intensified local inflammation at the AAV injection site consequently led to higher risk of formation of anti-FIX antibodies.20 Inefficient expression of adequate levels of FIX antigen rather than the local inflammation caused by high-dose AAV seemed to play a major role in the higher risk of anti-FIX antibody formation in that report.13,15,18
In summary, we demonstrated that activation of DCs could not abrogate the FIX immune tolerance induced by intramuscular injection of AAV1 vectors. Our results provided critical insight into not only the immune responses to the transgene product and the relevant mechanisms after gene transfer, but also the universal principles and mechanisms governing general immune responses. Better understanding of the mechanisms involved in the immune responses to FIX after gene transfer will facilitate development of a successful gene therapy approach for hemophilia treatment and FIX tolerance induction.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health Grant R01-HL076699 (H.C.). H.C. is a National Heart, Lung, and Blood Institute/National Hemophilia Foundation researcher.
National Institutes of Health
Authorship
Contribution: H.C. designed the study, analyzed the data, and wrote the paper; A.S.B., M.K., and D.K. performed the experiments; and A.S.B. summarized the data and participated in data analysis and paper writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hengjun Chao, Division of Hematology/Oncology, Box 1079, Mount Sinai School of Medicine, One Gustave L. Levy Pl, New York, NY 10029; e-mail: hengjun.chao@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal