Abstract
This study evaluated the efficacy and safety of single-agent bortezomib in indolent B-cell lymphoma that had relapsed from or was refractory to rituximab. Sixty patients enrolled: 59 were treated with bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 for up to eight 21-day cycles; responders could receive 4 additional cycles; maintenance was optional. Fifty-three evaluable patients completed more than 2 cycles. The median age was 70 years, 53% female, Ann Arbor stage III-IIIE (28%) and IV (65%); 43 patients (72%) had more than 2 prior regimens; and 6 patients went on to maintenance. Overall responses are as follows: 1 complete response (1.9%), 3 unconfirmed complete response (5.7%), 3 partial response (5.7%), 34 stable disease (64.2%), and 12 progressive disease (22.6%). Median time to response = 2.2 months (range, 1.2-5.3 months); duration of response = 7.9 months (2.8-21.3 months); 1-year survival was 73% and 2-year survival was 58%; median survival = 27.7 months (range, 1.4-30.9 months); median progression-free survival = 5.1 months (range, 0.2-27.7 months), median time to progression = 5.1 months (range, 0.2-27.7 months), and median event-free survival = 1.8 months (range, 0.2-27.7 months). Treatment-related grade 3 or 4 adverse events included: thrombocytopenia (20%), fatigue (10%), neutropenia (8.5%), and neuropathy and diarrhea (6.8% each). This study demonstrates that bortezomib has modest activity against marginal zone and follicular lymphoma; it has the potential for combination with other agents in low-grade lymphomas. Maintenance therapy should be explored further.
Introduction
Indolent B-cell lymphomas tend to be slow-growing but are incurable. The 5-year survival is 60% to 70%, but as many as one-third of these lymphomas will transform to a higher-grade form of lymphoma, usually diffuse large B cell. Our goal was to evaluate an agent with a novel mechanism of action that might result in a high durable complete remission rate.
Bortezomib (VELCADE, Millennium) is a small-molecule proteasome inhibitor, developed as an agent to treat human malignancies. The antineoplastic effect of bortezomib likely involves several distinct mechanisms, including inhibition of cell growth and survival pathways, induction of apoptosis, and inhibition of expression of genes that control cellular adhesion, migration, and angiogenesis. Thus, the mechanisms by which bortezomib elicits its antitumor activity may vary among tumor types, and the extent to which each affected pathway is critical to the inhibition of tumor growth could also differ. Bortezomib has a novel pattern of cytotoxicity in National Cancer Institute in vitro and in vivo assays.1 In addition, bortezomib has shown cytotoxic activity in a variety of xenograft tumor models, both as a single agent and in combination with chemotherapy and radiation.2-10 Notably, bortezomib induces apoptosis in cells that overexpress bcl-2, a genetic trait that confers unregulated growth and resistance to conventional chemotherapies.11
Currently, bortezomib is approved for the first- and second-line treatment of multiple myeloma. It is also approved for the treatment of mantle cell lymphoma in patients who have had at least one prior therapy.12 Bortezomib is thought to be efficacious in multiple myeloma via its inhibition of nuclear factor-κB activation, its attenuation of interleukin-6–mediated cell growth, a direct apoptotic effect, and possibly antiangiogenic and other effects.13
The mechanism of antitumor activity in non-Hodgkin lymphoma is not known; however, bortezomib inhibits the growth of various human lymphoma cell lines. In a phase 1 study, a single subject with previously treated refractory follicular lymphoma (FL) who received bortezomib 1.38 mg/m2 per dose had a partial response (PR; 50% shrinkage of mediastinal and intra-abdominal adenopathy) that was sustained for more than 12 months off therapy.14 O'Connor et al15 reported good responses in FL (1 complete response [CR], 1 unconfirmed complete response [CRu], and 5 PRs in 9 assessable patients [overall response rate 78%]); further, PRs were observed in the 2 subjects with marginal zone lymphoma treated with bortezomib monotherapy.16
Studies using bortezomib as monotherapy and in combination with other chemotherapy agents in relapsed/refractory indolent lymphoma are ongoing.17-20
This study was designed to evaluate the efficacy and safety of bortezomib as monotherapy, at the manufacturer's recommended dose of 1.3 mg/m2, in patients with indolent B-cell lymphoma who have relapsed after or who are refractory to rituximab therapy.
Methods
Study design
This was a prospective, open-label, phase 2, multicenter trial to determine disease response to bortezomib in patients with indolent lymphoma who had been previously treated and had progressed on prior therapy. In addition to response, the study evaluated time to progression (TTP), time to best response, duration of response, 1- and 2-year survival, progression-free survival (PFS), and toxicity. Responses were evaluated according to the International Working Group criteria21 ; additional analyses were done based on the Follicular Lymphoma International Prognostic Index.22
The protocol was approved by US Oncology's central institutional review board with jurisdiction over specific sites that registered patients on study. All patients were required to sign an informed consent form before being enrolled into the study in accordance with the Declaration of Helsinki. Accrual for this study began September 6, 2005; thus, it is not registered in any clinical trial registry.
Patients
Patients at least 18 years of age were registered to the study if they met all of the following key inclusion criteria: histologically proven indolent lymphoma, including small lymphocytic lymphoma (SLL), follicular center cell grades 1 and 2, or marginal zone (extranodal, nodal, and splenic) based on the World Health Organization 1997 classification; prior therapy with rituximab (Biogen Idec and Genentech USA) was required. The patient must have relapsed anytime after rituximab therapy or be refractory to rituximab (rituximab failure, or refractory to rituximab, was defined as failure to obtain any degree of response or relapse/progression within 6 months of completing rituximab therapy); prior high-dose chemotherapy was permitted with no limit on the number of prior regimens; measurable disease more than 1.5 cm in 2 perpendicular dimensions, which had not been previously irradiated (previously irradiated lesions that had progressed since prior irradiation could be used as measurable disease); Eastern Cooperative Oncology Group performance status 0 or 1; normal renal and hepatic function: platelets more than 50 × 109/L without transfusion support within 7 days before the assessment, hemoglobin more than or equal to 7.5 g/dL without transfusion support within 7 days before the assessment, and absolute neutrophil count more than or equal to 0.75 × 109/L without the use of growth factors; and not pregnant or breastfeeding.
Patients were excluded if they had any of the following: cutaneous lymphoma or any lymphoma not meeting the specific inclusion criteria; lymphocyte count more than or equal to 5000; currently on any investigational agent, or had received prior bortezomib for current or any disease; had received any of the following before day 1 cycle 1: antineoplastic drugs within 21 days, rituximab, alemtuzumab (Campath), or any other unconjugated therapeutic antibody within 4 weeks, nitrosoureas within 6 weeks, radioimmunoconjugates, or toxin immunoconjugates, such as ibritumomab tiuxetan (Zevalin) or tositumomab (Bexxar) within 10 weeks, or radiation therapy within 3 weeks; major surgery within 2 weeks before day 1 cycle 1, renal impairment (creatinine clearance < 20 mL/min); central nervous system involvement; peripheral neuropathy or neuropathic pain (National Cancer Institute Common Terminology Criteria for Adverse Events) more than or equal to grade 2; any serious uncontrolled intercurrent medical or psychiatric illness, known to be HIV+; myocardial infarction within 6 months of enrollment, or had New York Hospital Association23 class III or IV heart failure, uncontrolled angina, severe uncontrolled ventricular arrhythmias, or electrocardiographic evidence of acute ischemia or active conduction system abnormalities.
Treatment
Patients received 1.3 mg/m2 bortezomib administered as intravenous bolus over 3 to 5 seconds on days 1, 4, 8, and 11 of each 21-day cycle, up to a maximum of 8 cycles. Patients were treated until CR or other response as follows: CR, these patients received an additional 4 cycles of therapy once a CR was achieved, for a total maximum of 12 cycles. For any response less than CR, after completion of 8 21-day cycles, patients may have received maintenance doses (day 1, 4, 8, and 11 every 42 days) for up to 2 years or until progression. Patients on maintenance therapy were assessed every 6 weeks. If patients progressed, they were taken off treatment and were to be followed every 2 months for additional therapy, date of relapse, survival, and toxicities (for 30 days after last dose).
All patients received allopurinol 300 mg/day for days 1 to 30; acyclovir or famciclovir was given for herpes zoster prophylaxis at the discretion of the physician.
Assessments and response and toxicity criteria
At baseline, patients had the following assessments: medical history, complete physical examination, Eastern Cooperative Oncology Group performance status, clinical and radiologic assessment of disease, assessment of other lesions, bone marrow to confirm staging and bcl-2 status, complete blood count, complete metabolic profile, creatinine clearance, and lactic dehydrogenase. These were repeated within 7 days after the last dose. Patients were seen every 2 months thereafter; total study participation was limited to 2 years.
Responses were based on the International Working Group criteria for non-Hodgkin lymphoma.21 Radiologic assessment of tumors (chest computed tomograph, pelvic/abdominal computed tomograph) and evaluation of patient response were done between days 15 and 22 of each even-numbered cycle. Patients had to have completed 2 full cycles of therapy to be evaluable for response.
Adverse events were recorded throughout the trial and for up to 30 days after the last dose of any study drug. Toxicities and adverse events were graded using the Common Terminology Criteria for Adverse Events, Version 3.0.24
Statistical analysis
The primary objective of this study was to determine the response rate (CR, CRu, and PR) produced by bortezomib in patients with relapsed or refractory indolent lymphoma. The secondary objectives were to determine time to response, duration of response, TTP, response in patients who relapse within 6 months or later than 6 months of completing rituximab therapy, survival at 1 year and 2 years, PFS, event-free survival, and toxicity. The analysis between the sensitive and refractory patients was noncomparative; no significance testing was done.
A sample size of 60 indolent lymphoma patients was needed to determine that an increase in response rate, from 8.5% to 20% (95% confidence interval, 10%-32%), would deem bortezomib as “effective” using a 1-sample binomial exact method (STPlan Software), with an alpha of 0.05 and a power of 80% 1-sided test. A stopping rule was used; with ongoing review, at least 2 responses in the first 20 patients were required to continue.
Descriptive statistics were used to characterize the patient population. Response rate was determined based on the evaluable population, and a 2-sided 95% confidence interval of response rates was calculated. Among tumor responders, the duration of response was measured from the first date for CR, CRu, or PR (whichever status was recorded first) to the date of progression or death or the date of last contact. Survival (defined as the time from the date of start of treatment to the date of death or date of last contact), PFS (defined as time from the date of start of treatment to the date of progression or death to any cause), TTP (defined as time from the date of start of treatment to the date of progression or death to progression), and event-free survival (defined as time from the date of start of treatment to the date of treatment failure or death from any cause) were estimated using the method of Kaplan-Meier25 with point probabilities at every 3-month time point. If the patient had not failed, progressed, or died, the patient was censored on the date of last follow-up or the date of start of new treatment (if the new treatment was given before the progression date). The toxicity profile of bortezomib was evaluated in the safety population, defined as all patients who received at least one dose of study drug. SAS software (version 9.1) was used to perform analyses and R software (Version 2.6.0) was applied to prepare figures of survival analyses.
Results
Patient characteristics
Patient characteristics at baseline are summarized in Table 1. Sixty patients were enrolled on the protocol and 59 were treated; 53 patients completed more than 2 cycles of treatment and were evaluable for response. Patients were heavily pretreated; 43 patients (72%) had received at least 2 prior regimens; 50% of patients had received 3 or more prior treatment regimens (overall mean was 3.1 prior regimens; range, 1-12). Besides rituximab, prior treatments included chemotherapy (single-agent and combinations), radioimmunotherapy, and steroids.
Patient characteristics at baseline
| Characteristic . | Value . |
|---|---|
| No. of subjects enrolled | 60 |
| Median age, y (range) | 70.1 (40.4-87.2) |
| Sex, n (%) | |
| Male | 28 (46.7) |
| Female | 32 (53.3) |
| Race, n (%) | |
| White | 53 (88.3) |
| Black | 4 (6.7) |
| Hispanic | 2 (3.3) |
| Other | 1 (1.7) |
| Eastern Cooperative Oncology Group performance status* | |
| 0 | 33 (55.0) |
| 1 | 27 (45.0) |
| Laboratory values | |
| Normal hemoglobin | 35 (58.3) |
| Normal LDH | 44 (73.3) |
| Stage | |
| IE-IIE | 4 (6.7) |
| III | 15 (25.0) |
| IIIE | 2 (3.3) |
| IV | 39 (65.0) |
| Histology | |
| Follicular center cell, grade 1 | 23 (38.3) |
| Follicular center cell, grade 2 | 17 (28.3) |
| Small lymphocytic | 12 (20.0) |
| Marginal zone, extranodal | 5 (8.3) |
| Marginal zone, nodal | 2 (3.3) |
| Unspecified | 1 (1.7) |
| Prior therapy† | |
| Chemotherapy | 60 (100) |
| Hormone/biologic therapy | 1 (1.7) |
| Radiotherapy | 13 (21.7) |
| Sites of metastasis‡ | |
| Soft tissue/lymph node(s) | 59 (98.3) |
| Viscera | 21 (35.0) |
| Bone | 16 (26.7) |
| Characteristic . | Value . |
|---|---|
| No. of subjects enrolled | 60 |
| Median age, y (range) | 70.1 (40.4-87.2) |
| Sex, n (%) | |
| Male | 28 (46.7) |
| Female | 32 (53.3) |
| Race, n (%) | |
| White | 53 (88.3) |
| Black | 4 (6.7) |
| Hispanic | 2 (3.3) |
| Other | 1 (1.7) |
| Eastern Cooperative Oncology Group performance status* | |
| 0 | 33 (55.0) |
| 1 | 27 (45.0) |
| Laboratory values | |
| Normal hemoglobin | 35 (58.3) |
| Normal LDH | 44 (73.3) |
| Stage | |
| IE-IIE | 4 (6.7) |
| III | 15 (25.0) |
| IIIE | 2 (3.3) |
| IV | 39 (65.0) |
| Histology | |
| Follicular center cell, grade 1 | 23 (38.3) |
| Follicular center cell, grade 2 | 17 (28.3) |
| Small lymphocytic | 12 (20.0) |
| Marginal zone, extranodal | 5 (8.3) |
| Marginal zone, nodal | 2 (3.3) |
| Unspecified | 1 (1.7) |
| Prior therapy† | |
| Chemotherapy | 60 (100) |
| Hormone/biologic therapy | 1 (1.7) |
| Radiotherapy | 13 (21.7) |
| Sites of metastasis‡ | |
| Soft tissue/lymph node(s) | 59 (98.3) |
| Viscera | 21 (35.0) |
| Bone | 16 (26.7) |
Assessed before first treatment.
Subjects may have had more than 1 type of prior therapy.
Patients may have had more that 1 site of metastasis.
Treatment outcomes
The overall response rate (CR, CRu, and PR) was 13.3%; however, an additional 64% of patients had disease stabilization. Twelve patients had durable stable disease (SD) lasting 6 months or longer. Overall, one CR was achieved at cycle 6; the 3 CRu were achieved at a median of 4 cycles (range, 2-8). All other best responses of PR, SD, and progressive disease (PD) were determined at a median of 2 cycles (ranges, PR [2-2], SD [2-6], PD [1-2]). Median cycles until best response for rituximab refractory patients were 2 for all response categories (for CR and CRU range was not applicable; for SD and PD, range was 2-4 and 2-2, respectively); for rituximab-sensitive patients, best responses were achieved at cycle 6 for CR (range, not applicable) and CRu (range, 4-8) and cycle 2 for PR (range, not applicable), SD (range, 2-6), and PD (range, 1-2).
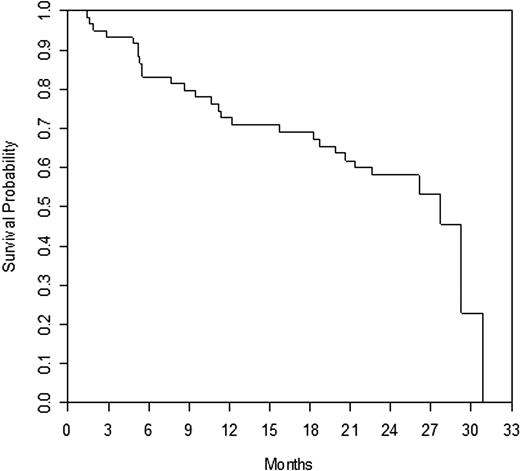
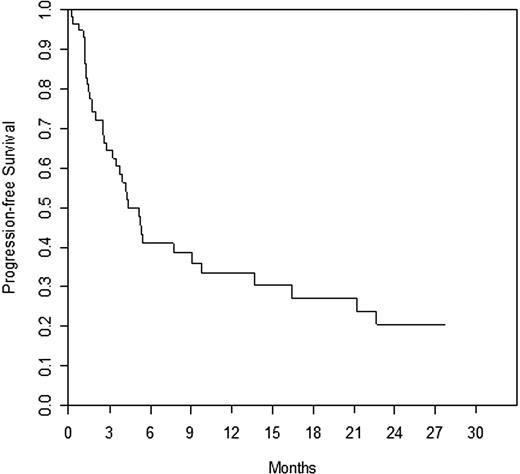
Efficacy is summarized in Table 2; time to response and duration of response are also presented. Responses by prior rituximab response status are presented in Table 3, and responses by histology subtype are summarized in Table 4. The response rates seen in the refractory and relapsed subgroups were similar. When broken down by histology, the majority of patients had FL (n = 36). The response rate was 17% in patients with follicular disease. There were only 6 patients with marginal zone lymphoma; however, there was one CRu in this small group of patients. There were no responses (CR or PR) in small lymphocytic disease, and all stabilization of disease was limited to 6 months or less. Table 5 summarizes patient status and reasons for treatment discontinuation. Survival at 1 year was 73% and at 2 years, 58%. Median survival = 27.7 months (range, 1.4-30.9 months); median PFS = 5.1 months (range, 0.2-27.7 months), median TTP = 5.1 months (range, 0.2-27.7 months), and median event-free survival = 1.8 months (range, 0.2-27.7 months). Overall survival and PFS are detailed in Figures 1 and 2, respectively.
Efficacy (n = 53 evaluable subjects)
| . | n (%) . | 95% CI . |
|---|---|---|
| Best response (all patients)* | ||
| CR | 1 (1.9) | 0.0-10.1 |
| CRu | 3 (5.7) | 1.2-15.7 |
| PR | 3 (5.7) | 1.2-15.7 |
| SD† | 34 (64.2) | 49.8-76.9 |
| PD | 12 (22.6) | 12.3-36.2 |
| . | n (%) . | 95% CI . |
|---|---|---|
| Best response (all patients)* | ||
| CR | 1 (1.9) | 0.0-10.1 |
| CRu | 3 (5.7) | 1.2-15.7 |
| PR | 3 (5.7) | 1.2-15.7 |
| SD† | 34 (64.2) | 49.8-76.9 |
| PD | 12 (22.6) | 12.3-36.2 |
Of 60 patients registered, 1 patient was not treated because of ECOG PS outside inclusion criterion on day 1 of cycle 1; 6 remaining patients were not evaluable because of intercurrent illness or injury (n = 1 lower extremity edema and n = 1 pathologic fracture of the femur) and n = 1 each disease progression, administration of prohibited concomitant medication, thrombocytopenia, and investigator decision that local radiotherapy would be a preferred treatment option.
Median time to response (n = 7) was 1.4 months (range, 1.2-5.3 months). Median duration of response was 7.9 months (range, 2.8-22.4 months).
Includes 11 with SD > 6 months.
Responses by rituximab status (refractory vs sensitive)
| . | n (%) . | 95% CI . |
|---|---|---|
| Best response (rituximab-refractory, n = 30) | ||
| CRu | 1 (3.3) | 0.1-17.2 |
| PR | 2 (6.7) | 0.8-22.1 |
| SD* | 19 (63.3) | 43.9-80.1 |
| PD | 8 (26.7) | 12.3-45.9 |
| Best response (rituximab-sensitive, n = 23) | ||
| CR | 1 (4.3) | 0.1-21.9 |
| CRu | 2 (8.7) | 1.1-28.0 |
| PR | 1 (4.3) | 0.1-21.9 |
| SD† | 15 (65.2) | 42.7-83.6 |
| PD | 4 (17.4) | 5.0-38.8 |
| . | n (%) . | 95% CI . |
|---|---|---|
| Best response (rituximab-refractory, n = 30) | ||
| CRu | 1 (3.3) | 0.1-17.2 |
| PR | 2 (6.7) | 0.8-22.1 |
| SD* | 19 (63.3) | 43.9-80.1 |
| PD | 8 (26.7) | 12.3-45.9 |
| Best response (rituximab-sensitive, n = 23) | ||
| CR | 1 (4.3) | 0.1-21.9 |
| CRu | 2 (8.7) | 1.1-28.0 |
| PR | 1 (4.3) | 0.1-21.9 |
| SD† | 15 (65.2) | 42.7-83.6 |
| PD | 4 (17.4) | 5.0-38.8 |
Refractory patients relapsed within 6 months of rituximab; sensitive patients relapsed more than 6 months after rituximab therapy.
Includes 6 with SD > 6 months.
Includes 5 with SD > 6 months.
Responses by histology subtype (n = 53 evaluable subjects)
| Characteristic . | n (%) . |
|---|---|
| Best response (by histology) | |
| Follicular (n = 36) | |
| CR | 1 (2.8) |
| CRu | 2 (5.6) |
| PR | 3 (8.3) |
| SD | 22 (41.5) |
| SD < 6 mo | 13 |
| SD > 6 mo | 9 |
| PD | 8 (22.2) |
| Marginal zone (n = 6) | |
| CRu | 1 (16.7) |
| SD | 2 (33.3) |
| SD < 6 mo | 0 |
| SD > 6 mo | 2 |
| PD | 3 (50.0) |
| Small lymphocytic (n = 10) | |
| SD | 9 (90.0) |
| SD < 6 mo | 9 |
| SD > 6 mo | 0 |
| PD | 1 (10.0) |
| Unknown/unspecified (n = 1) | |
| SD | 1 (100.0) |
| SD < 6 mo | 1 |
| Characteristic . | n (%) . |
|---|---|
| Best response (by histology) | |
| Follicular (n = 36) | |
| CR | 1 (2.8) |
| CRu | 2 (5.6) |
| PR | 3 (8.3) |
| SD | 22 (41.5) |
| SD < 6 mo | 13 |
| SD > 6 mo | 9 |
| PD | 8 (22.2) |
| Marginal zone (n = 6) | |
| CRu | 1 (16.7) |
| SD | 2 (33.3) |
| SD < 6 mo | 0 |
| SD > 6 mo | 2 |
| PD | 3 (50.0) |
| Small lymphocytic (n = 10) | |
| SD | 9 (90.0) |
| SD < 6 mo | 9 |
| SD > 6 mo | 0 |
| PD | 1 (10.0) |
| Unknown/unspecified (n = 1) | |
| SD | 1 (100.0) |
| SD < 6 mo | 1 |
Patient status
| Characteristic . | Value . |
|---|---|
| Total no. of subjects | 60 |
| Status, no. (%) | |
| Alive | 32 (53.3) |
| Dead | 28 (46.7) |
| Cause of death, no. (%) | |
| PD | 22 (36.7) |
| Lymphocytic leukemia | 2 (3.4) |
| Myelodysplastic syndrome | 1 (1.7) |
| Pneumonia | 1 (1.7) |
| Pneumonitis | 1 (1.7) |
| Unknown (no autopsy) | 1 (1.7) |
| Reason for discontinuation, no. (%) | |
| Progressive disease/recurrence | 25 (41.7) |
| Toxicity | 20 (33.3) |
| Investigator decision | 7 (11.7) |
| Patient request | 4 (6.7) |
| Normal completion | 2 (3.3) |
| Other* | 2 (3.3) |
| Median total of cycles received (range) | 4 (1-21) |
| Characteristic . | Value . |
|---|---|
| Total no. of subjects | 60 |
| Status, no. (%) | |
| Alive | 32 (53.3) |
| Dead | 28 (46.7) |
| Cause of death, no. (%) | |
| PD | 22 (36.7) |
| Lymphocytic leukemia | 2 (3.4) |
| Myelodysplastic syndrome | 1 (1.7) |
| Pneumonia | 1 (1.7) |
| Pneumonitis | 1 (1.7) |
| Unknown (no autopsy) | 1 (1.7) |
| Reason for discontinuation, no. (%) | |
| Progressive disease/recurrence | 25 (41.7) |
| Toxicity | 20 (33.3) |
| Investigator decision | 7 (11.7) |
| Patient request | 4 (6.7) |
| Normal completion | 2 (3.3) |
| Other* | 2 (3.3) |
| Median total of cycles received (range) | 4 (1-21) |
“Other” represents patients discontinued because of error in calculation of PD or protocol deviation (n = 1 each).
Six patients went on to receive maintenance therapy; 3 of these patients' best response was stabilization of disease, one patient achieved a PR after 9 cycles of therapy (total), responding after 1.2 months and held the response for 7.9 months before progressing. Two patients had CRu; time to response was 2.9 and 5.3 months and responses held for 29.3 and 6.4 months, respectively.
Drug delivery
The median number of cycles completed was 4 (range, 1-21). Six patients (10%) less than CR (including CRu) after 8 cycles went on to receive maintenance therapy. The mean relative dose intensity of bortezomib was 85.9% (median dose intensity, 89.0%); 33.9% of patients had a dose delay, 3.4% had a dose reduction, and 28.8% had a delay and reduction. Main reasons for delay were thrombocytopenia or fatigue; doses were reduced mainly due to thrombocytopenia and discontinued mainly because of fatigue.
Toxicity
Bortezomib was generally tolerated in this study, although the reason given for treatment discontinuation was toxicity in 33.3% of patients. Most of the toxicities were grades 1 to 3 with limited grade 4 events being reported. Grades 3 and 4 events were reported, whereas grade 1 and 2 events were treated symptomatically until resolution. The most frequent severe (grade 3) and very severe (grade 4) events were thrombocytopenia, fatigue, neutropenia, neuropathy, and diarrhea. Grade 1 to 4 treatment-related toxicities occurring in more than 2 patients are summarized in Table 6.
Grades 1-4 treatment-related toxicities occurring in more than 2 patients
| Adverse event . | Grade 1, n . | Grade 2, n . | Grade 3, n . | Grade 4, n . | Grades 3 + 4, n (%) . |
|---|---|---|---|---|---|
| Hematologic | |||||
| Thrombocytopenia | 3 | 5 | 8 | 4 | 12 (20.3) |
| Neutropenia | 2 | 8 | 5 | 0 | 5 (8.5) |
| Leukopenia | 3 | 1 | 2 | 0 | 2 (3.4) |
| Anemia | 4 | 3 | 0 | 0 | 0 |
| Nonhematologic | |||||
| Fatigue | 2 | 4 | 6 | 0 | 6 (10.2) |
| Neuropathy | 3 | 8 | 4 | 0 | 4 (6.8) |
| Diarrhea | 2 | 2 | 4 | 0 | 4 (6.8) |
| Constipation | 4 | 0 | 3 | 0 | 3 (5.1) |
| Weakness (generalized) | 1 | 1 | 3 | 0 | 3 (5.1) |
| Nausea | 6 | 2 | 2 | 0 | 2 (3.4) |
| Dehydration | 0 | 1 | 1 | 1 | 2 (3.4) |
| Hypokalemia | 0 | 0 | 1 | 1 | 2 (3.4) |
| Hypotension | 0 | 0 | 2 | 0 | 2 (3.4) |
| Adverse event . | Grade 1, n . | Grade 2, n . | Grade 3, n . | Grade 4, n . | Grades 3 + 4, n (%) . |
|---|---|---|---|---|---|
| Hematologic | |||||
| Thrombocytopenia | 3 | 5 | 8 | 4 | 12 (20.3) |
| Neutropenia | 2 | 8 | 5 | 0 | 5 (8.5) |
| Leukopenia | 3 | 1 | 2 | 0 | 2 (3.4) |
| Anemia | 4 | 3 | 0 | 0 | 0 |
| Nonhematologic | |||||
| Fatigue | 2 | 4 | 6 | 0 | 6 (10.2) |
| Neuropathy | 3 | 8 | 4 | 0 | 4 (6.8) |
| Diarrhea | 2 | 2 | 4 | 0 | 4 (6.8) |
| Constipation | 4 | 0 | 3 | 0 | 3 (5.1) |
| Weakness (generalized) | 1 | 1 | 3 | 0 | 3 (5.1) |
| Nausea | 6 | 2 | 2 | 0 | 2 (3.4) |
| Dehydration | 0 | 1 | 1 | 1 | 2 (3.4) |
| Hypokalemia | 0 | 0 | 1 | 1 | 2 (3.4) |
| Hypotension | 0 | 0 | 2 | 0 | 2 (3.4) |
Antivirals were given prophylactically (at the discretion of the physician); 23 patients received acyclovir or famciclovir, 3 patients received both, and 3 patients were treated solely with topical antivirals. Only 1 patient developed a herpes zoster infection while on treatment; this patient had received no antiviral prophylaxis. Serious infections were limited to sepsis (grade 3, n = 1) and pneumonia (grade 3, n = 2). Several patients had more minor infections (n = 3; 1 each respiratory, urinary tract, and sinus) or fever (n = 4; associated with pneumonia, viral illness, urinary tract infection, and sinus infections).
Discussion
This is the largest study to date of single-agent bortezomib in the treatment of indolent lymphomas, exploring the safety and efficacy of bortezomib in patients who were either refractory to rituximab (relapsing within 6 months of completing rituximab therapy) or had relapsed more than 6 months after rituximab. Responses (CR or PR) were comparable between the 2 groups.
The treatment of indolent lymphoma continues to present challenges; subtypes, such as FL, are typically more responsive than other subtypes15,26 ; however, Goy et al27 noted that other subtypes responded as well.
To date, only 2 full-length publications address single-agent bortezomib as a treatment for indolent lymphoma15,27 ; both studies were comparably small, with 24 and 50 assessable patients, respectively. Their results were comparable with our findings in patients with SLL. O'Connor et al noted that patients with SLL were slow to respond or failed to respond15 ; however, Goy et al27 reported a CR in 1 of 4 SLL patients. Follicular lymphoma patients fared better, although the sample size was limited. Goy et al27 noted that 1 of 5 FL patients achieved a CR in their study, and O'Connor et al15 noted that 6 of 10 FL patients achieved a CR (1), CRu (1), or PR (4), for a response rate of 60%.
This study is the first to report TTP as an objective in patients with relapsed or refractory indolent lymphoma; thus, there are no data for comparison of 12-, 24-, and 30-month PFS using single-agent bortezomib, either as induction or maintenance. Furthermore, the median duration of response was 7.9 months, with a median of 1.4 months elapsing before attainment of best response. This is a durable response, and it has been suggested that second and third responses may be achieved with additional courses of bortezomib; however, additional courses of bortezomib increase the likelihood of cumulative toxicities (ie, neuropathy and thrombocytopenia). The toxicity attributed to bortezomib (ie, thrombocytopenia, neuropathy, and fatigue) may limit its utility in combination given the fact that a third of patients discontinued study treatment because of adverse events.
This current study demonstrates that bortezomib has modest activity as a single agent with the potential to be combined with other agents for indolent lymphoma with the hope of improving the response rate and prolonging PFS; stabilization of disease was achieved in nearly two-thirds of patients, suggesting that a rather long treatment does not provide a higher rate of complete or partial responses. The current study population was heavily pretreated; 72% of patients had received 2 or more prior regiments for the treatment of their disease, which may explain (in part) the lower than anticipated response rates. The ongoing Lym 2001 study of rituximab with or without bortezomib may be of interest, although no results are currently available (Ali Nourbaksh, Millennium Pharmaceuticals, oral communication, December 2008). Any synergy that exists between these agents may be revealed in the final data.
Presented as a poster at the 50th annual meeting of the American Society of Hematology, December 6-9, 2008, San Francisco, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who shared their experiences with US Oncology Research Inc physicians listed in the Appendix, the site coordinators in the field (especially Beth Parker in Yakima, WA), project manager Julie Boston, data reviewer Tracy Locke, and biostatistician Jessica Donato, who assured the accuracy and integrity of the data.
This work was supported in part by a research grant from Millennium Pharmaceuticals Inc.
Authorship
Contribution: N.D.B. and R.T. conceived and designed the study, provided administrative support, provided study materials or patients, and analyzed and interpreted the data; K.K., T.B., R.R., D.B., E.W.C., P.Y.D., R.L., P.J.S., and S.J.V. provided study materials or patients; J.B. provided administrative support and collected and assembled the data; K.A.B. provided administrative support and analyzed and interpreted the data; Y.W. collected and assembled the data and analyzed and interpreted the data; L.A. conceived and designed the study, collected and assembled the data, and analyzed and interpreted the data; and all authors participated in writing the manuscript and had final approval of the manuscript.
Conflict-of-interest disclosure: R.T. has participated on a Millennium advisory board. R.L. has consulted for Amgen, GlaxoSmith-Kline, and Novartis in the past 2 years. The remaining authors declare no competing financial interests.
Correspondence: Nicholas Di Bella, Rocky Mountain Cancer Centers–Aurora, 1700 S Potomac, Aurora, CO 80012; e-mail: nick.dibella@usoncology.com.
Appendix
The following oncologists from the US Oncology Research network also participated in this study: Carlos A. Alemany, Orlando, FL; Mammo Amare, Dallas, TX; Stephen B. Beck, Birmingham, AL; James H. Bordelon, Fort Worth, TX; Matthew Brouns, Vancouver, WA; Kathy L. Christman, Greenville, SC; John M. Davis, Kansas City, MO; John R. Eckardt, Washington, MO; Carlos Encarnacion, Waco, TX; Thomas P. Flynn, Minneapolis, MN; Susan E. Myers Freeman, Aurora, CO; Edward R. George, Norfolk, VA; Robert H. Gersh, Spokane, WA; William Larry Gluck, Greenville, SC; Andrew R. Greenspan, Indianapolis, IN; Beth A. Hellerstedt, Austin, TX; Craig W. S. Howe, St Paul, MN; Maria Jorgensen, Yakima, WA; Pankaj Khandelwal, Odessa, TX; Kathryn S. Kolibaba, Vancouver, WA; Alan D. Kritz, Raleigh, NC; Scott Kruger, Hampton, VA; Gary L. Lee (deceased), Eugene, OR; Keith W. Logie, Fishers, IN; Regan M. Look, Portland, OR; Scott A. McKenney, Beaumont, TX; Mark A. O'Rourke, Greenville, SC; Alvin L. Otsuka, Thornton, CO; Michael Park, Lewisville, TX; John C. Paschold, Newport News, VA; Mark W. Redrow, Fort Worth, TX; Paul D. Richards, Salem, VA; John F. Schwerkoske, St Paul, MN; Kenneth M. Simon, Winter Park, FL; Guru Sonpavde, Webster, TX; Robert L. Talley, Lee's Summit, MO; Anthony Van Ho, Portland, OR; Charles H. Weissman, Latham, NY; and Lalan S. Wilfong, Dallas, TX.