Abstract
Netrin-4, a laminin-related secreted protein is an axon guidance cue recently shown essential outside of the nervous system, regulating mammary and lung morphogenesis as well as blood vascular development. Here, we show that Netrin-4, at physiologic doses, induces proliferation, migration, adhesion, tube formation and survival of human lymphatic endothelial cells in vitro comparable to well-characterized lymphangiogenic factors fibroblast growth factor-2 (FGF-2), hepatocyte growth factor (HGF), vascular endothelial growth factor-A (VEGF-A), and vascular endothelial growth factor-C (VEGF-C). Netrin-4 stimulates phosphorylation of intracellular signaling components Akt, Erk and S6, and their specific inhibition antagonizes Netrin-4–induced proliferation. Although Netrin receptors Unc5B and neogenin, are expressed by human lymphatic endothelial cells, suppression of either or both does not suppress Netrin-4–promoted in vitro effects. In vivo, Netrin-4 induces growth of lymphatic and blood vessels in the skin of transgenic mice and in breast tumors. Its overexpression in human and mouse mammary carcinoma cancer cells leads to enhanced metastasis. Finally, Netrin-4 stimulates in vitro and in vivo lymphatic permeability by activating small GTPases and Src family kinases/FAK, and down-regulating tight junction proteins. Together, these data provide evidence that Netrin-4 is a lymphangiogenic factor contributing to tumor dissemination and represents a potential target to inhibit metastasis formation.
Introduction
The lymphatic and blood vascular systems share many structural similarities, but with distinct functions. The lymphatic system is an open-ended network of endothelial cell-lined vessels working to maintain fluid homeostasis by unidirectionally transporting tissue fluid, extravasated plasma proteins, lipids, and cells from the interstitial space to the circulatory system via the thoracic duct. The lymphatic system has also been demonstrated to be a route for tumor metastasis.1
A vast number of lymphangiogenic factors, some previously identified as regulators of blood vascular endothelium,2 have been shown to induce physiologic and/or tumor lymphangiogenesis and tumor spreading.1 Netrins are laminin-like secreted proteins, initially identified as axonal guidance molecules.3 In mammals, the netrin family includes 5 ligands that act through 6 putative receptors, including deleted in colorectal cancer (DCC), neogenin, and the members of the Unc5 subfamily.3 Like Netrin-1, Netrin-4 promotes neurite outgrowth4 and regulates blood endothelial cell biology both positively and negatively.5-7 Nothing is known currently about the roles of Netrins in the lymphatic vasculature.
Here, we provide evidence that Netrin-4 functions as a pro-lymphangiogenic factor. We show that Netrin-4 induces proliferation, migration and survival of lymphatic endothelial cells through activation of p42/p44 MAPkinase, Akt/PI3kinase and mTor signaling pathways. We demonstrate that neogenin and Unc5b are expressed by lymphatic endothelial cells, yet their silencing does not suppress Netrin-4–induced biologic effects. Moreover, overexpression of Netrin-4 in mouse skin or in human breast tumors increases the density of lymphatics. Finally, we show that mice bearing Netrin-4–overexpressing tumors develop more metastases by an increased lymphatic permeability. Taken together, the data demonstrate that Netrin-4 functions as a pro-lymphangiogenic factor.
Methods
Refer to supplemental Methods for details (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Animals and in vivo experiments
Cell culture and in vitro assays
Lymphatic dermal human microvascular endothelial cells (HMVEC-dLys), dermal human microvascular endothelial cells (HMVEC-ds), and human umbilical vein endothelial cells (HUVECs) were obtained from Lonza and cultured in EBM-2 supplemented with the EGM-2MV kit according to manufacturer's instructions (Lonza) for 7 passages maximum. Human MCF7 breast cancer cells were a kind gift of Dr Alex Swarbrick (Garvan Institute, Australia) and were grown according to the ATCC recommendations. Mouse 66C14 mammary carcinoma line was provided by Dr Gary Sahagian (Tufts University). In vitro assays were performed as described7,10 and in supplemental Methods.
Statistical analysis
Data are shown as mean plus or minus SEM of 6 to 9 samples from 2 to 3 independent experiments. All statistical analysis was carried out using Statview (SAS Institute) and a standard Student 2-tailed t test or a Fisher exact test (for the determination of the lymph node metastasis score). A P value less than .05 was defined as statistically significant.
Results
Netrin-4 is a lymphatic endothelial mitogen and chemoattractant
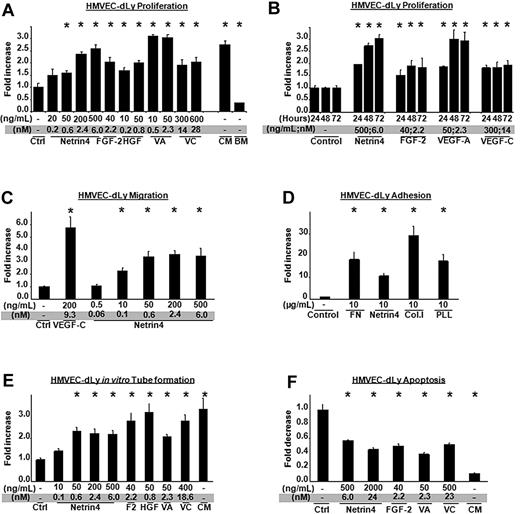
Netrin-4 regulates angiogenesis in vitro and in vivo.5,7 Because the blood and lymphatic systems share common structural and functional features, we asked if the lymphatic system also responded to Netrin-4. We initially studied Netrin-4 effects on the proliferation, migration, adhesion, tube formation, and survival of HMVEC-dLys. The mitogenic effects of Netrin-4 were compared with known lymphangiogenic factors FGF-2, HGF, VEGF-A (VA) and VEGF-C (VC) and complete media (CM) or basal media (BM), and quantified using the colorimetric Cell Counting kit-8 Dojindo. Results were expressed as fold increase compared with control. As shown in Figure 1A, Netrin-4 led to a dose-dependent increase in cell proliferation with a maximum of activity at 500 ng/mL (6nM) when HMVEC-dLys were treated for 72 hours and also induced a time-dependent cell proliferation (Figure 1A-B). Lymphatic endothelial cell proliferation was also induced by a higher concentration of Netrin-4 (10 μg/mL, supplemental Figure 1A). Lymphangiogenic activity of certain growth factors is suppressed by inhibiting VEGF-C/VEGFR-3 pathway.11 To rule out a similarly indirect role of Netrin-4, HMVEC-dLys were treated for 24 hours with 1 μg/mL of VEGFR-3 extracellular domain/human Fc construct in presence of Netrin-4, VEGF-C, HGF (supplemental Figure 1B) and their proliferation determined as described in the previous paragraph. As expected, VEGF-C–induced proliferation was blocked by the VEGFR-3/Fc construct while no effect was detected with Netrin-4 or HGF treatments.
Netrin-4 induces HMVEC-dLy proliferation, migration, tube formation and survival. (A) Mitogenic potential of different doses of Netrin-4 on lymphatic dermal human microvascular endothelial cells (HMVEC-dLys) compared with several known lymphangiogenic growth factors (FGF-2 or bFGF, HGF, VEGF-A (VA), VEGF-C (VC), and complete (CM) or basal cell (BM) culture media. Cell number was assessed using dojindo reagent 72 hours after treatment and expressed as fold increase versus control. (B) HMVEC-dLy proliferation under a single dose of Netrin-4 (500 ng/mL), FGF-2 (40 ng/mL), VEGF-A (50 ng/mL), or VEGF-C (300 ng/mL) assessed every 24 hours for 3 days. (C) Chemotactic effects of different doses of Netrin-4 and VEGF-C on HMVEC-dLys in a Boyden chamber assay. (D) HMVEC-dLy adhesion on various matrixes: Fibronectin (FN), Netrin-4, Collagen I (Col. I), and Poly-L-Lysin (PLL) at 10 μg/mL. (E) In vitro tube formation by HMVEC-dLys under different doses of Netrin-4, FGF-2 (bF), HGF, VEGF-A (VA), VEGF-C (VC), or complete media (CM). (F) Inhibition of serum deprivation–induced HMVEC-dLys apoptosis under different doses of Netrin-4, FGF-2, VEGF-A (VA), VEGF-C (VC), and complete media (CM). *P < .05.
Netrin-4 induces HMVEC-dLy proliferation, migration, tube formation and survival. (A) Mitogenic potential of different doses of Netrin-4 on lymphatic dermal human microvascular endothelial cells (HMVEC-dLys) compared with several known lymphangiogenic growth factors (FGF-2 or bFGF, HGF, VEGF-A (VA), VEGF-C (VC), and complete (CM) or basal cell (BM) culture media. Cell number was assessed using dojindo reagent 72 hours after treatment and expressed as fold increase versus control. (B) HMVEC-dLy proliferation under a single dose of Netrin-4 (500 ng/mL), FGF-2 (40 ng/mL), VEGF-A (50 ng/mL), or VEGF-C (300 ng/mL) assessed every 24 hours for 3 days. (C) Chemotactic effects of different doses of Netrin-4 and VEGF-C on HMVEC-dLys in a Boyden chamber assay. (D) HMVEC-dLy adhesion on various matrixes: Fibronectin (FN), Netrin-4, Collagen I (Col. I), and Poly-L-Lysin (PLL) at 10 μg/mL. (E) In vitro tube formation by HMVEC-dLys under different doses of Netrin-4, FGF-2 (bF), HGF, VEGF-A (VA), VEGF-C (VC), or complete media (CM). (F) Inhibition of serum deprivation–induced HMVEC-dLys apoptosis under different doses of Netrin-4, FGF-2, VEGF-A (VA), VEGF-C (VC), and complete media (CM). *P < .05.
Netrin-4 also exhibited a concentration-dependent chemotactic activity on HMVEC-dLys in a modified Boyden chamber (Figure 1C). Maximum migration was obtained at a dose of Netrin-4 as low as 50 ng/mL (0.6nM). As Netrin-4 shares homology to the N-terminal parts of the laminin short arms,4 we wondered if Netrin-4 could promote HMVEC-dLys adhesion. Cell culture wells were coated with bovine serum albumin (BSA), Fibronectin (FN), Collagen I (Col.I), Poly-L-Lysine (PLL), or Netrin-4. Cells were added for 30 minutes, washed, and the number of attached cells quantified using the colorimetric dojindo. As seen in Figure 1D, HMVEC-dLys adhered most to collagen I, followed by fibronectin and poly-L-Lysine. Netrin-4 also induced cell adhesion but at a lower level (Figure 1D).
To assess the capacity of Netrin-4 to induce in vitro tube formation, HMVEC-dLys were seeded on the top of growth factor-reduced Matrigel and treated with various doses of Netrin-4 for 12 hours. Formation of vascular tubes was assessed by counting lengths per photograph using ImageJ (National Institutes of Health) and represented as fold increase in tube length per surface area compared with control. Netrin-4 promoted a dose-dependent tube formation as VEGF-A (Figure 1E). Finally, Netrin-1 has been demonstrated to suppress endothelial cell death.12 To determine whether Netrin-4 could induce HMVEC-dLys survival, cells were incubated for 24 hours in serum-free media supplemented with different doses of Netrin-4. Cell death was quantified by the Cell Death Detection Elisa kit (Roche) and expressed as fold decrease in apoptosis versus control. Netrin-4 strongly reduced serum-deprivation–induced cell death (Figure 1F).
Taken together, these data showed that Netrin-4 directly induces lymphangiogenesis in vitro and at a level at least comparable to already-identified lymphangiogenic factors.
Netrin-4 activates intracellular signaling pathways in lymphatic endothelial cells
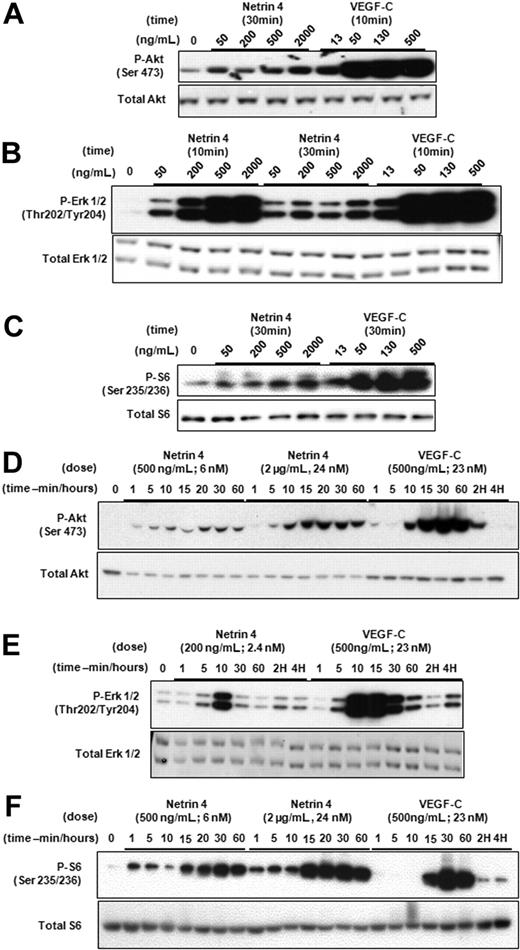
Stimulation of HMVEC-dLys in vitro by Netrin-4 suggested that this factor could activate intracellular signaling pathways as has been reported for VEGF-C, IGFs and PDGF-BB.1 We thus investigated phosphorylation of MAP Kinase Erk1 and 2, Akt and Ribosomal protein S6, a target of mTor. HMVEC-dLys were treated with Netrin-4 at variable doses (Figure 2A-C) or for variable times (Figure 2D-F). Both Netrin-4 and VEGF-C induced phosphorylation of Erk1/2, Akt, and S6 in concentration and time-dependent manners (Figure 2A-F). To rule out the possibility of a nonproteinaceous contamination of our Netrin-4 solution, we incubated Netrin-4 with native or heat-inactivated proteinase K, and alternatively, filtered the Netrin-4 solution through a 30-kDa exclusion column. Netrin-4 exposed to heat inactivated, but not native, proteinase K could activate Erk1/2 (supplemental Figure 2). Furthermore, Erk1/2 phosphorylation is only observed with the more than 30-kd fraction.
Netrin-4 activates intracellular signaling pathways of HMVEC-dLys. Determination of the phosphorylation of Akt (Ser 473; Serine 473, panels A and D, respectively), p42/p44 (Thr 202/Tyr 204; Threonin 202/Tyrosine 204, panels B and E, respectively), and Ribosomal protein S6 (Ser 235/236; Serine 235/236, panels C and F) by Western blotting after Netrin-4 treatment of HMVEC-dLy. Experiments were performed in triplicate.
Netrin-4 activates intracellular signaling pathways of HMVEC-dLys. Determination of the phosphorylation of Akt (Ser 473; Serine 473, panels A and D, respectively), p42/p44 (Thr 202/Tyr 204; Threonin 202/Tyrosine 204, panels B and E, respectively), and Ribosomal protein S6 (Ser 235/236; Serine 235/236, panels C and F) by Western blotting after Netrin-4 treatment of HMVEC-dLy. Experiments were performed in triplicate.
To determine whether these intracellular signaling pathways had functional relevance to Netrin-4's biologic activity, we treated HMVEC-dLys with Netrin-4 (500 ng/mL) in the absence or presence of U0126, LY294001, Akt inhibitor IV, and rapamycin, which are MEK, PI3K, Akt, and mTor inhibitors, respectively. Degrees of phosphorylation inhibition were then examined by Western blotting (supplemental Figure 3A,C,E,G), densitometry (supplemental Figure 3B,D,F,H), and inhibitors effect examined by a proliferation assay (supplemental Figures 3I-K, 4A-B). Although Netrin-4–stimulated Erk1/2 phosphorylation was completely inhibited by 10 μm U0126 (supplemental Figure 3A-B), this concentration only partially suppressed Netrin-4–induced cell proliferation (supplemental Figure 3I). Interestingly, both PI3K and Akt inhibitors strongly inhibited Netrin-4–promoted cell proliferation (supplemental Figure 3J and S4A) and Akt activation (supplemental Figure 3C-D and E-F). Although Rapamycin completely inhibited Netrin-4–induced phospho-S6 (supplemental Figure 3G-H), its effect on Netrin-4 proliferation was only partial (supplemental Figure 3K). Finally, simultaneous blockade of Erk, Akt and S6 pathways strongly inhibited Netrin-4–stimulated proliferation (supplemental Figure 4B). These data show that Netrin-4 induces phosphorylation of intracellular signaling pathways essential for Netrin-4–mediated lymphangiogenesis.
Netrin-4–mediated in vitro effects are not mediated by Unc5B or neogenin
We next investigated whether the effects of Netrin-4 in lymphatic endothelium were mediated by the canonical neuronal Netrin-1 receptors, DCC, or Unc5 family members.3 We first determined which Netrin-1 receptors were expressed in prox-1-positive HMVEC-dLys (supplemental Figure 5A). Quantitative RT-PCR detected only Unc5b and at a much lower level, neogenin mRNAs (supplemental Figure 5B). Inactivation of Netrin-1 receptors was conducted using a small interfering RNA (siRNA) strategy and examined by qRTPCR (supplemental Figure 5C,G,K). Control or Netrin-1 receptor siRNA transfected cells underwent migration (supplemental Figure 5D,H,L), proliferation (supplemental Figure 5E,I,M) or Erk1/2 activation (supplemental Figure 5F,J,N) assay as indicated previously. Unc5B mRNA level was reduced by 80% in the Unc5b versus control siRNA or untransfected conditions (supplemental Figure 5C), a knockdown score largely sufficient to detect its potential actvity as reported by Castets et al.12 Nevertheless, neither Netrin-4–induced migration (supplemental Figure 5D), proliferation (supplemental Figure 5E) nor Erk1/2 phosphorylation (supplemental Figure 5F) was reduced or abolished in the Unc5B siRNA transfected cells. Similar results were obtained using a second set of Unc5B siRNA (Dharmacon; data not shown). Complete depletion of neogenin receptor (supplemental Figure 5G) resulted in increased apoptosis as reported previously by Srinivasan et al13 (data not shown). However, Netrin-4–mediated effects were still preserved (supplemental Figure 5 H-J). Finally, the dual knockdown had also no effect (supplemental Figure 5K-N).
Adenosine receptor A2b has been reported as another potential Netrin-1 receptor and is expressed by HMVEC-dLys (supplemental Figure 6A). DPSPX or Enprofylline, both inhibitors of A2b, failed to suppress Netrin-4–stimulated cell proliferation (supplemental Figure 6B) or Erk1/2 activation (supplemental Figure 6C). These data provide evidence that none of the canonical Netrin-1 receptors mediate Netrin-4 function in lymphatic endothelium.
Netrin-4 is expressed in the lymphatic vasculature in mouse embryos and adults
Netrin-4 expression has been identified in basement membranes of kidney, ovaries, and blood vasculature.4 We asked whether Netrin-4 expression was also associated with mouse developmental and adult lymphangiogenesis. Netrin-4 was detected by immunostaining in the developing dorsal aorta (DA) and the anterior cardinal vein (ACV) in embryonic days (E) 10.5 mouse embryos (supplemental Figure 7A-C). Moreover, many of the prox1-positive lymphatic progenitor cells budding from the ACV also expressed Netrin-4 (supplemental Figure 7C). By E14.5, Netrin-4 is still detected in differentiated prox1-positive lymphatic endothelial cells lining the lymph sac (LV) and the blood vascular system; jugular vein (JV) and carotid artery (CA; supplemental Figure 7D-F). LYVE-1-positive lymphatic vessels costained with Netrin-4 in adult mouse intestinal villi and crypts, lymph nodes and skin (supplemental Figure 7G-I, J-L, and M-O, respectively). Interestingly, Netrin-4 was also detected in human breast tumor lymphatic and blood vessels (supplemental Figure 7P-R). Together, these data demonstrate that Netrin-4 is expressed in association with embryonic and adult lymphatic vessels and might play an important role in lymphatic development and maintenance of its integrity.
Netrin-4 overexpression in mouse skin induces in vivo lymphangiogenesis
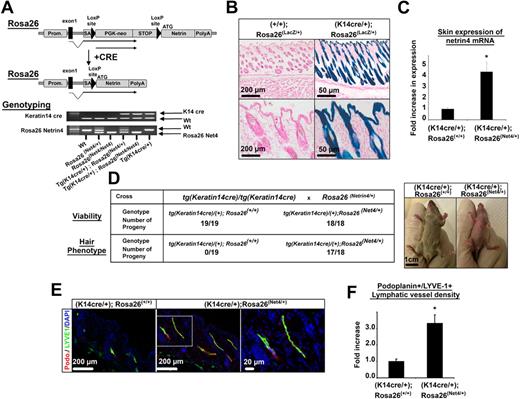
To define a biologic function of Netrin-4 in vivo, we generated a transgenic mouse in which Netrin-4 was ectopically expressed in skin under control of the keratin 14 promoter (Figure 3A). The allele, Rosa26 Netrin-4, contains the Netrin-4 cDNA inserted downstream of the murine Rosa26 promoter. Between the promoter and the cDNA is a strong transcriptional stop signal flanked by loxP sites. Expression of Cre recombinase excises the stop signal and allows expression of Netrin-4. Rosa26 Netrin-4/+ mice were bred with Keratin 14 Cre mice, with the expectation that progeny carrying both genes would express Netrin-4 in basal cells of stratified squamous epithelia and hair follicles (Figure 3B). Tg (K14 cre/+); Rosa26 Netrin-4/+ mice showed a 4-fold increase of Netrin 4 mRNA in skin compared with littermate controls (Figure 3C) leading to a protein expression level equal to 1 ng/μg protein of total skin lysate (supplemental Figure 8A). These mice were also smaller, redder and had poorly developed fur, but were fully viable (Figure 3D). Interestingly, a similar phenotype has been reported for mice overexpressing VEGF-C in skin. Immunostaining of skin sections of 3-week-old mice for the lymphatic specific markers, LYVE1 and podoplanin, or for blood vessel marker, PECAM-1, showed an increase in lymphatic and blood vessel density in Netrin-4–overexpressing versus control mice (Figure 3E and supplemental Figure 8B). Hair follicles, which strongly express K14 Cre, were surrounded by lymphatic or blood vessels in Netrin-4 but not control mice (Figure 3E and supplemental Figure 8B). Quantification of LYVE-1/Podoplanin or PECAM-1-positive structures confirmed that lymphatic and blood vessel density was significantly higher in Netrin-4 expressing mice than littermate controls (Figure 3F and supplemental Figure 8B). Macrophages have been shown to promote lymphangiogenesis and angiogenesis by secreting various growth factors.14 Immunostaining on skin sections using the macrophage marker F4/80, did not show any difference in macrophage density in Netrin-4–overexpressing versus control mice (supplemental Figure 8C).
Netrin-4 overexpression in mouse skin results in an increased lymphatic density. (A) Schematic of the Netrin-4 overexpression system and genotyping of the various mice. Netrin-4 cDNA was inserted into the constitutively active Rosa26 locus downstream of a LoxP-flanked transcriptional stop signal. In presence of a cre recombinase driven by the keratin14 promoter (indicated as Prom.), the stop signal is excised allowing expression of Netrin-4. (B) Expression of the LacZ gene reporter (Rosa26 LacZ/+) in mouse skin keratinocytes in presence of the Keratin14 cre recombinase (K14 cre/+). Scale bars: 200 and 50 μm. (C) Quantification of Netrin-4 mRNA by qRT-PCR in the skin of Netrin-4–overexpressing mice versus littermate controls. (D) Three-week-old skin-overexpressing Netrin-4 mice are viable but hairless, smaller, and redder than controls (scale bar: 1 cm). Pictures were taken on an Olympus IX71 microscope, at 100× and 400× magnification using a DP30BW Olympus camera and the MicroSuite Basic Edition Olympus software. (E) Skin sections of 3-week-old Netrin-4–overexpressing and littermates control mice costained with an anti–LYVE-1 and an antipodoplanin (indicated as podo.) antibody (dotted box represents the enlarged area shown; scale bars: 200 and 20 μm). (F) Quantification of the LYVE1+/podoplanin+ staining density using ImageJ and normalized to the control group. Pictures in panel E are representative of the quantification reported in panel F.
Netrin-4 overexpression in mouse skin results in an increased lymphatic density. (A) Schematic of the Netrin-4 overexpression system and genotyping of the various mice. Netrin-4 cDNA was inserted into the constitutively active Rosa26 locus downstream of a LoxP-flanked transcriptional stop signal. In presence of a cre recombinase driven by the keratin14 promoter (indicated as Prom.), the stop signal is excised allowing expression of Netrin-4. (B) Expression of the LacZ gene reporter (Rosa26 LacZ/+) in mouse skin keratinocytes in presence of the Keratin14 cre recombinase (K14 cre/+). Scale bars: 200 and 50 μm. (C) Quantification of Netrin-4 mRNA by qRT-PCR in the skin of Netrin-4–overexpressing mice versus littermate controls. (D) Three-week-old skin-overexpressing Netrin-4 mice are viable but hairless, smaller, and redder than controls (scale bar: 1 cm). Pictures were taken on an Olympus IX71 microscope, at 100× and 400× magnification using a DP30BW Olympus camera and the MicroSuite Basic Edition Olympus software. (E) Skin sections of 3-week-old Netrin-4–overexpressing and littermates control mice costained with an anti–LYVE-1 and an antipodoplanin (indicated as podo.) antibody (dotted box represents the enlarged area shown; scale bars: 200 and 20 μm). (F) Quantification of the LYVE1+/podoplanin+ staining density using ImageJ and normalized to the control group. Pictures in panel E are representative of the quantification reported in panel F.
Netrin-4 promotes tumor lymphangiogenesis and tumor metastasis
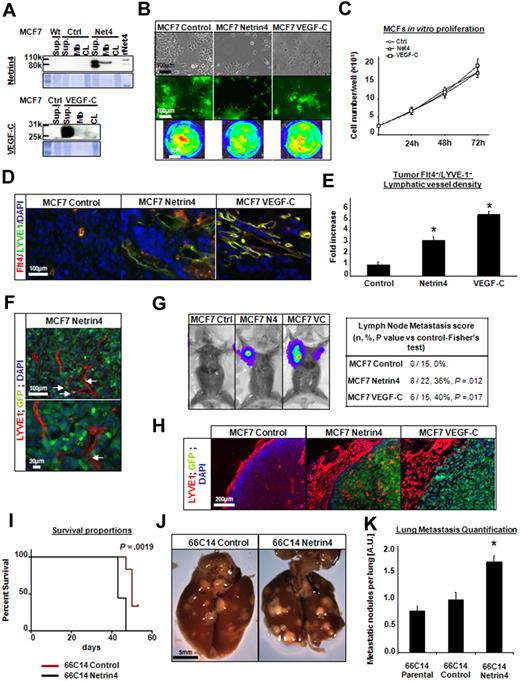
Metastasis is the principal cause of cancer mortality. In the past decade, converging data have illuminated the importance of tumor lymphatic vasculature in breast cancer dissemination. We investigated the capacity of Netrin-4 to promote tumor lymphangiogenesis and subsequent dissemination in 2 Netrin-4–overexpressing breast cancer models: a subcutaneous xenograft of poorly invasive human breast carcinoma MCF-7 cells; and an orthotopic transplant of metastatic murine mammary carcinoma 66c14 cells. Netrin-4 was only detected in MCF-7s stably transfected with a vector encoding mouse Netrin-4 and was secreted into the cell supernatant (SUP; like VEGF-C) or bound to the plasma membrane (Mb; Figure 4A). Interestingly, 2 bands were seen in the supernatant fraction compared with the membrane-bound fraction, suggesting partial cleavage of Netrin-4 during its release from the plasma membrane.15 MCF7 morphology and proliferation were not altered either by Netrin-4 or VEGF-C overexpression (Figure 4B-C). Green fluorescent protein (GFP) and firefly luciferase activity, provided by the expression vector or a second vector, were expressed at a similar level by all 3 lines (Figure 4B). To determine whether Netrin-4 could induce tumor lymphangiogenesis, MCF7 tumors were grown subcutaneously on the backs of NOD/SCID mice for 12 weeks, sectioned and stained for lymphatic or blood-specific markers, LYVE-1/VEGFR-3 or CD31, respectively. Netrin-4 tumors showed a higher density of intratumoral LYVE-1+/VEGFR-3+, CD31 stained lymphatic and blood vessels, which were only detected at tumor margins in the control group (Figure 4D and supplemental Figure 9A). More intratumoral lymphatic vessels were also found in VEGF-C tumors, where they formed dense continuous networks compared with the dilated and individualized vessels found in Netrin-4 groups. Quantification of LYVE-1+/VEGFR-3+ or CD31-positive structures confirmed that vessel density was significantly higher in Netrin-4 and VEGF-C expressing tumors than controls (Figure 4E and supplemental Figure 9A). F4/80 immunostaining of tumor sections revealed that density of tumor-associated F4/80+ macrophages was not changed in Netrin-4 versus control group (supplemental Figure 9B). Interestingly, GFP-positive tumor cells were noticed inside or tightly surrounding the enlarged dilated lymphatic vessels in Netrin-4 tumors (Figure 4F). This sign of tumor cell invasion was confirmed by intraperitoneal injection of luciferin substrate into tumor-bearing mice and subsequent bioluminescence detection. Indeed, axillary lymph nodes contained viable luciferase-expressing tumor metastases in 8 of 22 Netrin-4 tumor-bearing mice (36%) and 6 of 15 VEGF-C tumor bearing mice (40%). No lymphatic metastases were observed in the control tumor cohort (0 of 15, 0%; Figure 4G). Microscopic analysis of luciferase-positive lymph nodes confirmed the presence of GFP-positive metastases in mice bearing Netrin-4– or VEGF-C–expressing tumors, but none in control groups (Figure 4H). Moreover, dense layers of lymphatic and blood vessels surrounded cancer cell–invaded lymph nodes compared with controls (Figure 4H and supplemental Figure 9C).
Netrin-4–overexpressing MCF7 tumors have more lymphatic vessels and are more metastatic. (A) Human breast cancer MCF7 cells (WT) stably transduced with a Netrin-4 (Net4), VEGF-C or empty vector (control). Protein expression was determined in the cell supernatant (SUP), the fraction secreted and bound to the cytoplasmic membranes (Mb) or in the total cell lysate (CL) by Western blot using an anti–Netrin-4 or an anti–VEGF-C antibody. (rNet4; Recombinant Netrin-4). All control, Netrin-4, and VEGF-C MCF7 tumor cells also express at similar levels both GFP and luciferase (B) and proliferate in vitro at an identical rate (C; Ctrl; control empty vector, Net4; Netrin-4; scale bars in panel B: 100 μm and 1 cm). (D) Control, Netrin-4, and VEGF-C–overexpressing MCF7 cells injected subcutaneously into the midline of the back of NOS/SCID mice. Tumors were removed after 12 weeks, sectioned and costained with an anti–VEGFR-3/Flt-4 and anti–LYVE-1 antibody (scale bar: 100 μm). (E) VEGFR-3+/LYVE-1+ costaining density was quantified using ImageJ and represented as fold increase over the control-empty vector tumor condition. (F) GFP-positive tumor cells in the lumen of tumor lymphatic vessels stained with LYVE-1 antibody in the Netrin-4–overexpressing tumors (scale bars: 100 and 20 μm). (G) Luciferase-positive tumor cells metastasized into the auxillary lymph nodes of the Netrin-4 and VEGF-C tumor-bearing mice. (H) luciferase-positive lymph nodes contain GFP-positive tumor cells and are surrounded by enlarged LYVE-1 stained vessels in mice bearing Netrin-4 and VEGF-C–overexpressing tumors, but not in control condition (scale bar: 200 μm). 66C14 murine mammary carcinoma line metastasizing via the lymphatic system to lungs were stably transfected with a Netrin-4 encoding or empty-vector and injected into the exposed inguinal right mammary fat pad of Balb/C mice. Netrin-4–overexpressing 66C14 tumor bearing mice die faster than controls (I) and present more metastatic nodules per lung (J; scale bar: 5 mm). Pictures were taken on an Olympus IX71 microscope, at 100×, 200×, and 400× magnification using a DP30BW Olympus camera and the MicroSuite Basic Edition Olympus software. The number of metastatic nodules per lung was quantified by visual inspection (K). *P < .05.
Netrin-4–overexpressing MCF7 tumors have more lymphatic vessels and are more metastatic. (A) Human breast cancer MCF7 cells (WT) stably transduced with a Netrin-4 (Net4), VEGF-C or empty vector (control). Protein expression was determined in the cell supernatant (SUP), the fraction secreted and bound to the cytoplasmic membranes (Mb) or in the total cell lysate (CL) by Western blot using an anti–Netrin-4 or an anti–VEGF-C antibody. (rNet4; Recombinant Netrin-4). All control, Netrin-4, and VEGF-C MCF7 tumor cells also express at similar levels both GFP and luciferase (B) and proliferate in vitro at an identical rate (C; Ctrl; control empty vector, Net4; Netrin-4; scale bars in panel B: 100 μm and 1 cm). (D) Control, Netrin-4, and VEGF-C–overexpressing MCF7 cells injected subcutaneously into the midline of the back of NOS/SCID mice. Tumors were removed after 12 weeks, sectioned and costained with an anti–VEGFR-3/Flt-4 and anti–LYVE-1 antibody (scale bar: 100 μm). (E) VEGFR-3+/LYVE-1+ costaining density was quantified using ImageJ and represented as fold increase over the control-empty vector tumor condition. (F) GFP-positive tumor cells in the lumen of tumor lymphatic vessels stained with LYVE-1 antibody in the Netrin-4–overexpressing tumors (scale bars: 100 and 20 μm). (G) Luciferase-positive tumor cells metastasized into the auxillary lymph nodes of the Netrin-4 and VEGF-C tumor-bearing mice. (H) luciferase-positive lymph nodes contain GFP-positive tumor cells and are surrounded by enlarged LYVE-1 stained vessels in mice bearing Netrin-4 and VEGF-C–overexpressing tumors, but not in control condition (scale bar: 200 μm). 66C14 murine mammary carcinoma line metastasizing via the lymphatic system to lungs were stably transfected with a Netrin-4 encoding or empty-vector and injected into the exposed inguinal right mammary fat pad of Balb/C mice. Netrin-4–overexpressing 66C14 tumor bearing mice die faster than controls (I) and present more metastatic nodules per lung (J; scale bar: 5 mm). Pictures were taken on an Olympus IX71 microscope, at 100×, 200×, and 400× magnification using a DP30BW Olympus camera and the MicroSuite Basic Edition Olympus software. The number of metastatic nodules per lung was quantified by visual inspection (K). *P < .05.
To validate our finding that Netrin-4 promoted metastasis via tumor lymphatic vasculature in a second model; we used the mouse mammary cancer cell line, 66c14, known to metastasize via the lymphatic system.16 Netrin-4 overexpression did not modify in vitro cell morphology, adhesion or proliferation rates compared with empty vector–transfected or untransfected cells (data not shown). Neither was a significant difference observed for the growth rate between Netrin-4–overexpressing, control, and parental primary tumors (data not shown). However, Netrin-4 tumor-bearing mice died more quickly than control and parental cohorts (Figure 4I). Macroscopic analysis of lungs revealed a significant increase in the number of metastatic nodules per lung from Netrin-4–expressing tumors versus control tumors (Figure 4J), was confirmed by quantification of metastatic nodules per lung (Figure 4K). Together, these data demonstrate that Netrin-4 induces tumor lymphangiogenesis and increases metastasis formation via the lymphatic system.
Netrin-4 stimulates in vitro and in vivo lymphatic permeability
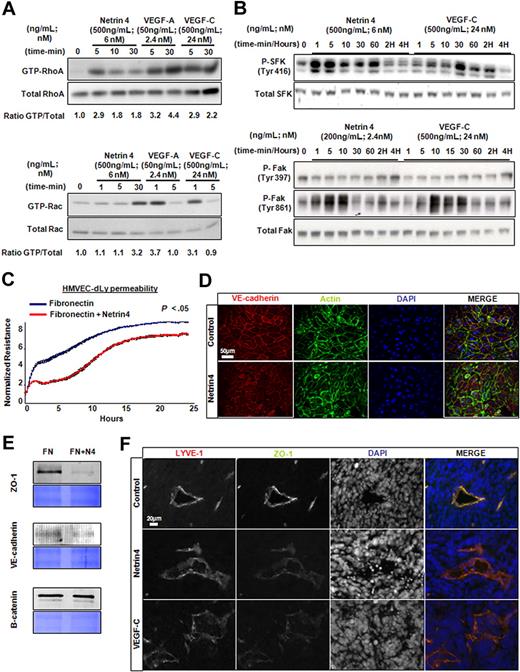
Lymphatic vessel structure and integrity as well as increased invasiveness or migration properties of cancer cells have been identified as avenues for tumor dissemination via the lymphatic vasculature.17 Specialized cell-cell junctions expressing VE-cadherin and tight junction-associated proteins such as Zona Occludens 1 (ZO-1) have been reported between lymphatic endothelial cells.18 Disruption of functional organization of such structures might lead to vascular fragility and increase in permeability. We therefore examined whether Netrin-4 could change lymphatic permeability. VEGF-A–induced endothelial cell permeability can be regulated by both small GTPases and protein kinases.19 Similarly, we found that Netrin-4 increased active RhoA (GTP-bound) and, to a lower extent, Rac1 in HMVEC-dLys, (Figure 5A). Phosphorylation of Src family kinase (SFK, on Tyrosine 416) and FAK (on Tyrosine 861 but not 397) was also induced by Netrin-4 stimulation of HMVEC-dLys in a time-dependent manner (Figure 5B). Netrin-4 also increased permeability, as measured by a decrease in transendothelial resistance, in a HMVEC-dLys monolayer in vitro (Figure 5C). VE-cadherin and actin staining of HMVEC-dLy revealed looser and more punctate cell junctions in Netrin-4 versus control (Figure 5D). Expression of the tight junction protein ZO-1 and VE-cadherin were down-regulated in the membrane fraction of HMVEC-dLys after stimulation with Netrin-4 compared with the control. However, beta-catenin or p120 catenin levels were unchanged (Figure 5E and data not shown). These results prompted us to examine whether expression of cell junction molecules were changed in lymphatic vessels in Netrin-4 versus control tumors. We found that ZO-1 immunostaining was fainter, thinner and more punctate in LYVE1-positive structures in both Netrin-4– and VEGF-C–overexpressing tumors compared with control tumors (Figure 5F). To further investigate Netrin-4 effect on lymphatic leakage, Tg (K14 cre/+); Rosa26 Netrin-4/+ mice (mice overexpressing Netrin-4 mice in the skin) and their littermate controls, Tg (K14 cre/+); Rosa26 +/+ were tested in a modified Miles assay.20 As shown in supplemental Figure 10, higher lymphatic-leakage was detected in Netrin-4–overexpressing versus control mice. These data provide evidence that Netrin-4 induces lymphatic permeability by disorganizing lymphatic cell-cell junctions.
Netrin-4 induces in vitro lymphatic permeability. (A) Induction of GTP-RhoA and Rac1 by Netrin-4, VEGF-A, and VEGF-C treatment of HMVEC-dLys. (B) Stimulation of the phosphorylation of Tyrosine 416 of Src kinase family (SFK, Tyr416) and the Tyrosin 861 but not the Tyrosine 391 of focal adhesion kinase (FAK; Tyr861 and Tyr391) by Netrin-4 (500 ng/mL) and VEGF-C (500 ng/mL). (C) Measurement of the electrical resistance of the cell monolayer over 24 hours using the ECIS system or (D) immunostained using an anti–VE-cadherin antibody to visualize cell junctions (scale bar: 50 μm) of HMVEC-dLys seeded either on Fibronectin or Fibronectin plus Netrin-4. (E) Membrane fraction proteins prepared from HMVEC-dLys seeded as previously mentioned in panels C and D analyzed for ZO-1, VE-cadherin, and beta-catenin expression (equivalent loading assessed by coomassie blue staining). (F) Control, Netrin-4, VEGF-C overexpressing MCF7 tumor sections stained for the cell junction protein ZO-1 or the lymphatic marker LYVE1 (scale bar: 20 μm). Data presented in panels A through E are from 1 experiment and representative of 2 independent experiments. Pictures were taken on an Olympus IX71 microscope, at 400× magnification using a DP30BW Olympus camera and the MicroSuite Basic Edition Olympus software.
Netrin-4 induces in vitro lymphatic permeability. (A) Induction of GTP-RhoA and Rac1 by Netrin-4, VEGF-A, and VEGF-C treatment of HMVEC-dLys. (B) Stimulation of the phosphorylation of Tyrosine 416 of Src kinase family (SFK, Tyr416) and the Tyrosin 861 but not the Tyrosine 391 of focal adhesion kinase (FAK; Tyr861 and Tyr391) by Netrin-4 (500 ng/mL) and VEGF-C (500 ng/mL). (C) Measurement of the electrical resistance of the cell monolayer over 24 hours using the ECIS system or (D) immunostained using an anti–VE-cadherin antibody to visualize cell junctions (scale bar: 50 μm) of HMVEC-dLys seeded either on Fibronectin or Fibronectin plus Netrin-4. (E) Membrane fraction proteins prepared from HMVEC-dLys seeded as previously mentioned in panels C and D analyzed for ZO-1, VE-cadherin, and beta-catenin expression (equivalent loading assessed by coomassie blue staining). (F) Control, Netrin-4, VEGF-C overexpressing MCF7 tumor sections stained for the cell junction protein ZO-1 or the lymphatic marker LYVE1 (scale bar: 20 μm). Data presented in panels A through E are from 1 experiment and representative of 2 independent experiments. Pictures were taken on an Olympus IX71 microscope, at 400× magnification using a DP30BW Olympus camera and the MicroSuite Basic Edition Olympus software.
Discussion
Although blood and lymphatic systems perform different functions, they share a variety of common features. Netrins, initially characterized as axon-guidance cues, have been recently described as regulators of blood vasculature.5-7,12,21 In the present study, we provide evidence that Netrin-4 acts in vitro and in vivo as a lymphangiogenic factor comparable in activity to FGF-2, HGF, VEGF-A and VEGF-C, but appears to act independent of the canonical Netrin-1 receptors, neogenin and Unc5B.
First, we show that that Netrin 4 induces proliferation, migration, tube formation and survival of human adult HMVEC-dLys in a time- and dose-dependent manner. This is consistent with a report showing that Netrin-4 stimulated in vitro cell functions of HMVECs at low concentrations, but was inhibitory at higher doses, and the demonstration that HUVECs and HUAECs in vitro showed a bi-phasic response to Netrin-1.6,7,12,21 Netrins, as axon guidance cues, were originally described as bifunctional factors, promoting attraction or repulsion, dependent on their concentration, cell expression or dimerization status of its receptors.3 A combination of receptor expression, receptor affinity, receptor dependence and ligand concentration might also be invoked to explain the multiple activities of Netrins in the endothelium.6,7,12,21
We observed that HMVEC-dLys express canonical neuronal Netrin-1 receptors Unc5B and neogenin mRNA but their inhibition failed to suppress Netrin-4–promoted in vitro effects. Interestingly, we noticed significant cell death in neogenin-knocked down cells compared with control cells, despite the extremely low amount of siRNA (in nanomolar range), while no significant change of morphology was observed with the Unc5B RNAi. A similar phenotype was noticed in neogenin knockout mice in comparison to littermate controls.13 These data might indicate that neogenin signals continuously to induce lymphatic cell survival in presence of its ligand, Netrin-1, which is also expressed by lymphatic cells (data not shown), according to the ligand/receptor dependence model.12 There is a single report that Neogenin and Unc5B receptors mediate Netrin-4 inhibitory effects in HUAECs pretreated with VEGF-A5 . The apparent discrepancy between our findings and data published by Lejmi et al might be explained by the different types and origins of the cells studied and/or by their different proliferation status after VEGF-A or FGF-2 stimulation. Interestingly, Nacht et al, who also showed a Netrin-4 inhibitory effect on HMVECs, failed to detect any binding of Netrin-4 to DCC, neogenin or Unc5B by both coimmunoprecipitation and Biacore surface Plasmon resonance based technology6 ; nor has Netrin-4 binding to canonical Netrin-1 receptors been seen in neurons.3 Together, these data indicate that the function of Netrins in endothelium is much more complicated than that of other endothelial factors such as VEGFs. This is likely to be compounded by the multiplicity of receptors and by ligand-receptor dependency effects. Further, the existence of unidentified netrin receptors is strongly probable; new noncanonical Netrin-1 receptors on nonendothelial cells have been reported in the past years.3,22 Also, because Netrins are laminin like proteins,15 the ECM could either regulate local concentrations of Netrins by serving as a growth factor reservoir, or could act as a coreceptor.22
We showed that Netrin-4 stimulated phosphorylation of protein kinase ERK1/2 and Akt (PKB) in a time- and concentration-dependent fashion. Although intracellular signaling pathways controlling lymphatic endothelial cell migration, proliferation, and survival have not been studied exhaustively, similar results were reported for the lymphangiogenic factors PDGF-BB, IGF-1 and 2, VEGF-A and VEGF-C1 . More interestingly, we have demonstrated that the ribosomal protein S6 kinase, target of the mTor complex, was activated by both Netrin-4 and VEGF-C, as previously shown for the lymphangiogenic factor FGF-2.23 In addition, the Netrin-4–induced proliferation of HMVEC-dLys was partially inhibited in vitro by specific kinase inhibitors and Rapamycin, a specific inhibitor of mTOR, which has also been shown to inhibit in vivo lymphangiogenesis and metastasis formation.24
The kinetics of Netrin-4 action, similar to VEGF-C or PDGF-BB, and the absence of effect using a VEGFR-3/Fc chimera argue strongly that this factor stimulates in vitro lymphangiogenesis directly, for example, not by changing the levels of expression of other endothelial growth factors.
We show that Netrin-4 is expressed by the first differentiated lymphatic endothelial cells and the adult lymphatic vasculature. Interestingly, Netrin-4 is also detected, at a higher extent in blood vessels particularly in the intestine. The relevance of this expression pattern is still unknown, and its resolution awaits the ability to mutate Netrin-4 specifically in each vascular bed using a blood or lymphatic specific cre recombinase and a Netrin-4 floxed allele. It also remains undetermined if Netrin-4 acts in vivo on the vasculature as a growth factor and/or in an autocrine manner. Indirect in vivo effects cannot be ruled out totally, as Netrin-1 has already been shown to act directly on endothelial, smooth muscle, inflammatory or even cancer cells.12,25-27 In addition, functional redundancy between Netrin-1 and Netrin-4 could also be envisioned as Netrin-1 expression has also detected in both mouse blood and lymphatic vasculature (data not shown).
We have demonstrated that Netrin-4 induces in vivo lymphangiogenesis in several different, standard lymphangiogenic models: overexpression in mouse skin; and 2 tumor models. Overexpression of Netrin-4 in the mouse skin phenocopied the reduction in fur development and increased lymphangiogenesis induced by overexpression of VEGF-C, although it did not lead to a hyperplasia of lymphatic vasculature induced by VEGF-C.28 The more robust phenotype with VEGF-C might be explained by the transgenesis strategy used by Jeltsch et al, leading to multiple insertions of the construct in the mouse genome and a subsequent higher expression.28
Netrin-4 overexpression also induced tumor lymphangiogenesis. Lymphatic vessels with wider lumens containing cancer cells were observed in Netrin-4 tumors and were correlated with increased tumor metastasis to axillary lymph nodes. A previous study in which subcutaneous MCF7 tumors overexpressed VEGF-C cells led to similar results,9 emphasizing the relevance of lymphatic vessels in metastatic disease. Several mechanisms have been shown to support metastatic dissemination including increased tumor blood and/or lymphatic density, or cancer cell migration and invasiveness.17 Stimulation of tumor lymphangiogenesis is thought to increase the surface area of tumor cells in contact with lymphatic endothelial cells and then to facilitate tumor dissemination because of their structures.29
Control of lymphatic permeability might also be a crucial element contributing to metastasis. Several reports showed that RhoA and Rac1, as well as focal adhesion kinase (FAK) and Src family kinases (SFK) are responsible of VEGF-A–mediated vascular permeability.19 Here we showed that Netrin-4 activates Rac1, RhoA and SFK, FAK phosphorylation and increased permeability of lymphatic cells similar to known lymphatic factors.30 Phosphorylation of FAK and SFK have been shown to correlate with enhanced cancer cell migration and invasion, while RhoA and Rac1 levels are up-regulated in numerous metastatic cancers.31-33 Vascular permeability in the lymphatic system is also regulated by cell-cell junctions composed of VE-cadherin and tight junction-associated proteins such as ZO-1.18 Expression of VEGF-A/VEGFR-2 has been shown to promote vascular permeability through the endocytosis of VE-cadherin.19 Disruption of tight junctions using a VE-cadherin inhibitory antibody increased lung metastasis.33 Similarly, expression of ZO-1 in endothelial tight junctions is down-regulated in metastatic breast and liver tumors.34,35 We demonstrated that both VE-cadherin and ZO-1 are reduced at the surface of HMVEC-dLy treated in vitro with Netrin-4, and vascular structures in Netrin-4– and VEGF-C–overexpressing breast tumors show a decrease in ZO-1 expression, leading to open gaps between lymphatic endothelial cells. Given our results, the contribution and mechanisms regulating such specialized lymphatic cell-cell junctions18 might be clearly investigated during tumor metastasis.
Our findings clearly show that Netrin-4 is a lymphangiogenic factor that induces metastasis via 2 probable mechanisms1 ; by increasing lymphatic density and consequently augmenting surface contact with cancer cells and2 by increasing lymphatic permeability and facilitating intravasation and/or extravasation of tumor cells.
Our data suggest that Netrin-4 may contribute to lymphatic metastasis, and support the strategy of targeting not only tumor-derived functions, but also targeting functions of the host that support the tumor. These data also warrant future studies to fully identify the functional receptors for Netrins in endothelium and therefore clarify their contribution in vascular biology and oncology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully thank Dr Charles L. Murtaugh for help with microscopy and Dr Lisa Urness for a critical reading of the manuscript. Podoplanin antibody (clone 8.1.1) was obtained from the Developmental Studies Hybridoma bank, University of Iowa. 66c14 cells were provided by Pr G. Gary Sahagian (Tufts University).
This work was supported by grants from the Fondation pour la Recherche Médicale (F.L.-L.), the American Heart Association (F.L.-L. and D.Y.L.), the Huntsman Cancer Institute Foundation (A.L.W.), the US Department of Defense Breast Cancer Research Program (A.L.W. and D.Y.L.), the US National Institutes of Health, the H.A. and Edna Benning Foundation, the Juvenile Diabetes Research Foundation, the Burroughs Wellcome Fund, and the Flight Attendants Medical Research Institute (D.Y.L.).
National Institutes of Health
Authorship
Contribution: F.L.-L., A.L.W., and K.R.T. contributed new reagents, F.L.-L. and A.L.W. performed experiments, and F.L.-L. and D.Y.L. designed the research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors are or were previously employed by the University of Utah, which has filed intellectual property surrounding the therapeutic uses of vascular guidance cues and with the intent to license this body of intellectual property for commercialization.
Correspondence: Dean Y. Li, University of Utah, Eccles Institute of Human Genetics, Bldg 533, Rm 4450, 15 North 2030 East, Salt Lake City, UT 84112; e-mail: dean.li@hmbg.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal