Abstract
Determining how normal and leukemic stem cells behave in vivo, in a dynamic and noninvasive way, remains a major challenge. Most optical tracking technologies rely on the use of fluorescent or bioluminescent reporter genes, which need to be stably expressed in the cells of interest. Because gene transfer in primary leukemia samples represents a major risk to impair their capability to engraft in a xenogenic context, we evaluated the possibility to use gene transfer–free labeling technologies. The lipophilic dye 3,3,3′,3′ tetramethylindotricarbocyanine iodide (DiR) was selected among 4 near-infrared (NIR) staining technologies. Unfortunately we report here a massive transfer of the dye occurring toward the neighbor cells both in vivo and in vitro. We further demonstrate that all lipophilic dyes tested in this study (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine perchlorate [DiI], DiD, DiR, and PKH26) can give rise to microenvironmental contamination, including when used in suboptimal concentration, after extensive washing procedures and in the absence of phagocytosis or marked cell death. This was observed from all cell types tested. Eventually, we show that this microenvironmental contamination is mediated by both direct cell-cell contacts and diffusible microparticles. We conclude that tracking of labeled cells using non–genetically encoded markers should always be accompanied by drastic cross validation using multimodality approaches.
Introduction
Despite recent animated discussions,1 xenotransplantation of human cells in immunodeficient animals remains the only available technique to analyze the “stemness” of human cancer-initiating cells.2-5 Despite the wide use of this model, homing and dynamic behavior of injected cells depending on time remain largely unknown both for normal and malignant cells. Whole-body imaging (WBI) and intravital microscopy (IVM) provide powerful tools to longitudinally monitor the behavior of cancers in vivo6,7 and to track and analyze normal and cancer-initiating cells in their microenvironment or niche.8,9 Near-infrared optical imaging (NIR) represents a very attractive technology in this context by offering a relatively easy and cheap access to multimodality and multiscale in vivo imaging.10,11
In vivo cell tracking using optical imaging technologies largely relies on the use of ectopic expression of reporter genes such as Luciferase (for bioluminescence) or on fluorescent proteins. To obtain such expression, cells have to be engineered in vitro so that the reporter gene can be integrated in their genome for stable expression over time. Because in vitro manipulation of primary leukemia samples represents a real risk to impede the functionality of the cells, gene transfer–free approaches would represent a major improvement in this context.12 Various strategies have been reported to fluorescently label cells in vitro for subsequent tracking in vivo.13-16 In the field of immunohematology, the succinimidyl ester of carboxyfluorescein diacetate (5(6)-CFDA SE), or the closely related but distinct carboxyfluorescein succinimidyl ester (CFSE) as well as PKH26 are the gold standard techniques for cell tracking. Gene transfer–free labeling of cells relies mainly on 3 technologies. Amine-reactive probes (such as CFSE) diffuse passively into cells where they will react with cytosolic amine-containing residues to form dye-protein adducts that are retained in cells. We have tested here a far-red derivative of this technology is the DDAO-SE. Lipophilic dyes (such as PKH26) are based on membrane labeling by stable incorporation of long-chain carbocyanine dye containing long aliphatic tails into lipid regions of the cell membrane.17 A wide variety of these molecules have been developed in terms of spectral properties, including in the far-red and NIR windows, which make them attractive candidates for WBI and confocal IVM (DiD or 3,3,3′,3′ tetramethylindotricarbocyanine iodide [DiR]). Nanoparticles are the third type of technology usable for cell labeling. Fluorescent quantum dots are small nanocrystals (1-10 nm) made of cadmium, an inorganic semiconductor material, possessing several unique optical properties well suited for in vivo imaging. The dots are very photo stable, remain resistant for long periods of time in vivo, and are available in a large variety of colors, including in the NIR region (quantum dot 705 nm). In addition, their excitation spectrum makes them ideal tools for 2-photon excitation.18 Eventually, we also evaluated a fourth family of strategies consisting of using an antibody labeling kit (IRDye 800CW) to covalently stain the extracellular domain of membrane proteins. As previous studies have nicely shown that carbocyanine lipophilic dyes are very effective tools for WBI and/or IVM tracking of normal and malignant bone marrow (BM)–derived cells without affecting their functionality,19-23 we aimed at using similar strategies to track primary human acute myeloid leukemia (AML) and hematopoietic stem cells (HSCs).
Methods
Human and mouse cells
Cord blood cells were obtained under research ethics committee approval and informed consent at the Royal London Hospital in accordance with the Declaration of Helsinki. Mononuclear cells were obtained by density centrifugation.
The human HL-60 cell line was provided by the cell culture service at the Institute and cultivated in RPMI supplemented with 10% fetal calf serum (FCS), l-glutamine, and penicillin/streptomycin. Transduction of HL-60 cells was performed with an HIV-1–based self-inactivating (SIN) lentiviral vector (pHR′SINcPPT-SEW-SffV-IRES-eGFP lentivirus), which carries the enhanced green fluorescent protein (eGFP) reporter gene under the control of the spleen focus-forming virus LTR (a kind gift from Prof A. Thrasher, Institute of Child Health, London, United Kingdom) as previously described.24
The human osteosarcoma cell line Cal-72 (purchased from German Collection of Microorganisms and Cell Cultures [DSMZ], http://www.dsmz.de) and maintained in Dulbecco modified Eagle medium with 10% FCS + 2mM l-glutamine + 1× insulin-transferrin-sodium selenite. Human umbilical vein endothelial cells (HUVECs; Clonetics) were cultured in endothelial growth medium 2 (Clonetics), and GP293T cells (kind gift from Prof Eric So, Institute of Cancer Research, London, United Kingdom) were maintained in Dulbecco modified Eagle medium supplemented with 10% FCS and 1% antibiotics.
Mouse hematopoietic stem and progenitor cell staining and fluorescence-activated cell sorting
Bone marrow from either ROSA26-eGFP (Biological Resource Unit at Clare Hall Institute, London, United Kingdom) or β-actin–Luciferase (Xenogen-Caliper) mice was obtained by crushing the bones with a mortar and a pestle in PBS supplemented with 2% FCS, and cells were harvested after Ficoll gradient centrifugation and stained using anti–Sca-1–phycoerythrin (PE)–cyanin 7, CD117–allophycocyanin (APC), and lineage (Lin)–biotinylated (subsequently labeled with PE-conjugated streptavidin)–specific monoclonal antibodies (see antibody list in supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) following the manufacturer's instructions. Dead cells (DAPI+ [4,6 diamidino-2-phenylindole–positive]) and doublets (displaying side scatter width) were excluded. EGFP+ mature cells (Lin+), early progenitors (Lin− Sca− Kit+; LK), and stem-enriched cells (Lin− Sca+ Kit+) were sorted after exclusion of dead cells (DAPI+) and doublets using a MoFlow cytometer (Beckman Coulter) equipped with a 70-μm nozzle.
Vital fluorescent dyes for cell tracking
CellTrace CFSE Cell Proliferation Kit (CFSE), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; DiIC18(3); DiI), CellTrace Far Red DDAO-SE (DDAO), Q-tracker 705 Cell Labeling Kits (Q-tracker 705), Vybrant DiD cell-labeling solution (DiD), and the long-chain lipophylic 1,1′-dioctadecyl-3,3,3′,3′ tetramethylindotricarbocyanine iodide (DiR; DiIC18(7)) were purchased from Molecular Probes; PKH26 Red Fluorescent Cell Linker Kit (PKH) was purchased from Sigma-Aldrich; and IRDye 800CW protein labeling kit was purchased from Li-Cor Biosciences; all products were used following the manufacturer's instructions.
Adoptive transfer of human HL-60 cells in immunodeficient mice
All animal experiments were performed in compliance with Home Office and Cancer Research UK (CRUK) guidelines. Nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice were originally obtained from Dr Leonard Schultz (The Jackson Laboratory) and bred at Charles River Laboratories. They were kept in microisolators and fed with sterile food and acidified water. Mice aged 8 to 12 weeks were irradiated at 375 cGy (137Cs source) 24 hours before intravenous injection of 5 to 10 × 106 HL-60 cells.
Recovery of the bone marrow of mice that underwent transplantation for flow cytometric analysis
Animals were killed and bones (femur, tibia, helium) and spleen were collected. The bone marrow was harvested either by flushing long bones25 or by crushing the bone as described in “Mouse hematopoietic stem and progenitor cell staining heading.” The cells were filtrated on a 70-μm cell strainer, and leukocytes were recovered after red cells underwent ammonium chloride lysis.
In vitro analysis of biocompatibility
Competitive proliferation assay using HL60 cells.
Untransduced HL60 cells (HL60-eGFP−) were stained with different NIR dyes at different doses. After repeated washing and incubations, cells were mixed together with 30% of unstained HL60-eGFP+ cells and cultured in standard conditions. At different times points, cells were harvested and analyzed by flow cytometry. The principle of this “competitive proliferation assay” is based on the respective proliferation kinetics of NIR-stained (eGFP−) and NIR-unstained (eGFP+) HL60 cells. In the absence of any toxic or cytostatic effect associated with the dye, the relative proportion of both subpopulations should remain constant. In the case of toxicity related to NIR treatment, the amount of cells initially stained with a NIR dye should decrease.
Optimization of staining procedures on selected murine progenitors.
Lineage (lin)–negative cells from β-actin–Luciferase mice bone marrow cells were obtained using the mouse hematopoietic progenitor cell enrichment kit (StemCell Technologies) following the manufacturer's instructions. After fluorescent staining of the cells, they were plated in serum-free medium supplemented with optimized cytokine cocktail24 either in 24-well plates for flow cytometric (FC) analysis of viability and fluorescence intensity on day 1 or in triplicates in a black 96-well plate for bioluminescence imaging on day 7.
In vitro bioluminescence imaging
Just before imaging, 100 μL of luciferin 2× (300μg/mL; Xenogen-Caliper) was added to the 100 μL of cell suspension in each well. After 5-minute incubation, bioluminescence activity was acquired in a prewarmed (37°C) IVIS Spectrum (Xenogen-Caliper) using a 3-second exposure, binning 16 over a total period of 15 minutes. Data were processed using the Living Image 3.1 software (Xenogen-Caliper) after drawing regions of interests of identical size (duplications of the region of interest) around individual wells. Bioluminescence imaging activity was quantified as a flux (photon per second). Mean and standard values were calculated for each condition and expressed as a percentage of the respective control.
In vitro near-infrared scanning
Near-infrared scanning was performed using the Odyssey infrared imaging system (Ly-Cor Biosciences). DDAO-SE and Q-tracker 705 nm were imaged using the 700-nm channel; DiR and IRDye 800CW fluorescence, using the 800-nm channel.
In vivo near-infrared scanning
Near-infrared scanning was performed using the Odyssey infrared imaging system (Ly-Cor Biosciences) as described for in vitro scanning. At 12 hours to 3 weeks after injection of the leukemic cells, animals were killed by CO2 exposure, and shaved by first using a clipper and then hair remover cream. Matched controls (age- and sex-matched animals) were imaged on the same scan to provide reliable and objective representation of the background and/or autofluorescence signals. Postacquisition image processing was realized using the Odyssey MousePOD Module (Version 2.1) software. To determine what a specific signal on a test animal was, the same anatomic location was analyzed on a proper negative control animal. Image displays were set up so that fluorescence did not come from the control region of interest.
Cocultures of hematopoietic and stromal cells
After different staining and washing procedures (detailed in each experiment), HL60 or eGFP-HL60 cells were plated on pre-established confluent layers of primary human umbilical vein endothelial cells (HUVECs) or a fibroblastic cell line (GP293T). At the end of the culture, nonadherent and adherent cells were harvested by trypsynization. All the cells from the same well were pooled together for subsequent staining with anti–human CD45 (hCD45) and anti-hCD33 and flow cytometric analysis.
Similarly mouse mature progenitors (Lin− Sca− Kit−; L3−), early progenitors (Lin− Sca− Kit+; LK), and a population enriched in functionally defined stem cells (Lin− Sca+ Kit+; LSK) were sorted from β-actin–Luciferase transgenic animals (no phenotype described to date26 ). Cells were stained using published procedures27,28 that were further optimized for this study: 2μM DiI for 10 minutes in pure PBS at 37°C, followed by 2 washing steps with PBS, 2% FCS and subsequent overnight incubation in StemSpan (StemCell Technologies) serum-free medium supplemented with previously optimized cytokine cocktail.24 Cells were then washed and plated on pre-established confluent monolayers of HUVECs or GP293T cells in collagen-coated CellBIND (Corning) 96-well plates optimized for high-quality imaging. After overnight incubation, 1.5 μL of anti–murine CD45 (mCD45)–APC was added in each well, and the plate was incubated for 30 minutes at 37°C before confocal imaging and subsequent harvesting of the cells for flow cytometry (similar procedures as for HL60 cocultures). A second plate was analyzed 48 hours after initiation of the coculture.
Flow cytometric analysis
Depending on the need, mice and/or human cells were stained using fluorescently labeled antibodies25 (see antibody list in supplemental Methods). Washed cells were suspended in PBS containing DAPI (4,6 diamidino-2-phenylindole) and analyzed using a BD Life Science Research (LSRII) flow cytometer.
Absolute quantification of dye transfer.
After in vivo or in vitro incubation of stained cells, flow cytometry was used to determine the absolute amount of fluorescence associated with stained versus neighbor cells. The total amount of fluorescence was calculated by multiplying the number of positive events by the mean fluorescence intensity of the whole population. The same operation was applied for stained cells (HL60 or purified hematopoietic stem and/or progenitor cells [HSPCs], depending on the experiment) and for neighbor cells (recipient or stromal cells, depending on the experiment). The respective contribution of each population was then expressed as a percentage of total fluorescence.
Confocal imaging
Cells in suspension.
Cells in suspension were imaged using an upright Zeiss LSM 510 equipped with a pulsed Ti-Sapphire laser tuned at 870 nm and a 633-nm laser to excite the quantum dots and the eGFP and the NIR dyes, respectively. Fluorescence was collected using band-pass filters at 500 to 550 nm and 650 to 710 nm.
HSPCs/stroma cocultures.
At the end of the coculture, anti–mCD45-APC antibodies were added to each well and placed in the 37°C environmental chamber of an inverted LSM510 Zeiss confocal equipped with a Plan-Apochromat 20×/0.75 numeric aperture Zeiss lens. DiI and APC were imaged separately (multitrack configuration) by successive excitations at 543 nm and 633 nm and collection of fluorescence using 560-nm and 650-nm long-pass filters, respectively. A 10-μm stack was acquired (2-μm step) using a zoom 3 magnification. Resulting images were processed using Imaris 6.3.0 software (Bitplane) for brightness adjustment and 3-dimensional reconstruction. Individual and merged channels are displayed for the cocultures of LSK and LK on endothelial cells.
Statistical analysis
Averages, standard deviation, and Student t tests were calculated using Microsoft Excel 2004 for Mac (Version 11.5.5). P values less than .05 were considered as significant.
Results
We first characterized 4 different dyes based on 4 distinct families of vital staining procedures (DDAO-SE, DiR, IRDye 800CW, and Q-tracker705) to obtain near-infrared (NIR) labeling of human acute myeloid leukemia (AML) cell line HL60. The DIR dye was our best candidate in terms of brightness, stability, lack of toxicity, and compatibility with multimodality imaging (supplemental Figure 1).
In vivo tracking of DiR-stained human leukemia cells by NIR-WBI
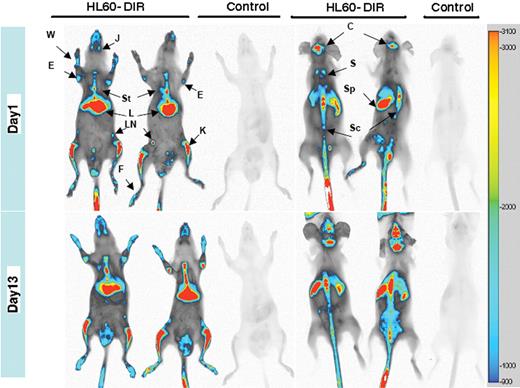
DiR-stained HL-60 cells injected intravenously in irradiated NOD/SCID mice gave rise to a strong signal nicely associated with bones 24 hours after transplantation (Figure 1 and supplemental Figure 2) with no apparent decay over a 2-week period of time (Figure 1 bottom panels). This was quite surprising, knowing that HL-60 cells proliferate rapidly in vivo.
DiR allows sensitive NIR whole-body imaging tracking of human leukemic cells and analysis of their homing in immunocompromised recipients. HL60 cells (107) stained with 20μM DiR (HL60-DiR) were injected in 4 animals, whereas 2 controls were injected with unlabeled cells. At 24 hours after injection, 1 control and 2 animals injected with HL60-DiR were killed and prepared for whole-body imaging. Frontal acquisition (top left panel) and dorsal acquisition (top right panel) are displayed. The same operations have been realized 13 days after the injection (lower panels). The intensity settings for the acquisition and the processing of the images are identical for the 4 images. Fluorescence intensity scale is displayed on the right side of the images. J indicates jaws; St, sternum; E, elbow; L, liver; W, wrist; LN, lymph node; K, knee; F, foot; C, calvaria; S, scapula; Sc, spinal column; and Sp, Spleen.
DiR allows sensitive NIR whole-body imaging tracking of human leukemic cells and analysis of their homing in immunocompromised recipients. HL60 cells (107) stained with 20μM DiR (HL60-DiR) were injected in 4 animals, whereas 2 controls were injected with unlabeled cells. At 24 hours after injection, 1 control and 2 animals injected with HL60-DiR were killed and prepared for whole-body imaging. Frontal acquisition (top left panel) and dorsal acquisition (top right panel) are displayed. The same operations have been realized 13 days after the injection (lower panels). The intensity settings for the acquisition and the processing of the images are identical for the 4 images. Fluorescence intensity scale is displayed on the right side of the images. J indicates jaws; St, sternum; E, elbow; L, liver; W, wrist; LN, lymph node; K, knee; F, foot; C, calvaria; S, scapula; Sc, spinal column; and Sp, Spleen.
Assessing the specificity of the signal
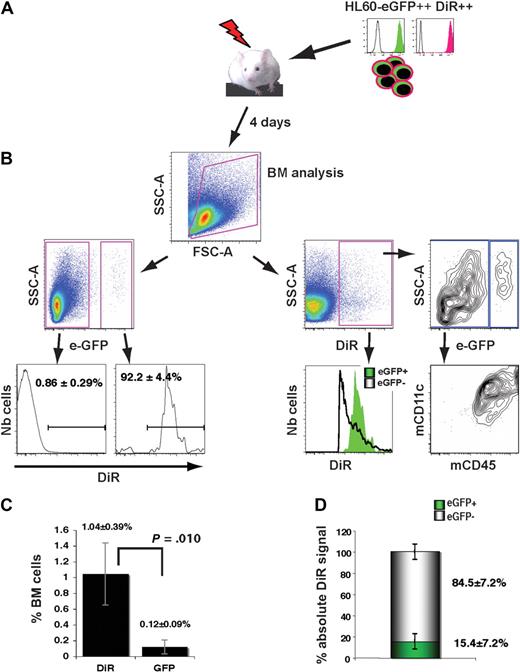
To address the specificity of the signal, transduced HL60-eGFP–bright sorted cells were stained using 5μM DiR for 30 minutes in complete medium following published procedures,19 incubated overnight in culture and intravenously injected in sublethally irradiated NOD/SCID mice (Figure 2A). Fluorescence intensity and cell viability (> 90%) were controlled before the adoptive transfer (Figure 2A). As expected, 4 days after injection, virtually all eGFP+ human cells displayed a bright NIR fluorescence and the presence of DiR+ cells within mouse cells (eGFP−) was quite low (0.86% ± 0.29% of total BM; Figure 2B left panel). However, analyzing the same datasets by first gating on the DiR+ population provides a very different view. Surprisingly, eGFP+ cells represented only 11.5% (± 2.5%) of DiR+ cells (Figure 2B right panel). The DiR+ eGFP− cells were of murine origin (mCD45+) and coexpressed the dendritic marker CD11c, suggesting a phagocytic process for the dye transfer. Within the total BM, the frequency of DiR+ cells was 8 times higher than GFP+ cells (1.04% ± 0.39% versus 0.12% ± 0.09%, respectively; P = .010; Figure 2C). Quantification of absolute DiR signal in mouse and human cells further revealed that only 15.4% (± 7.2%) of the DiR signal was specific, that is, coming from human cells (Figure 2D).
In vivo transfer of DiR dye from human leukemic cells toward mouse cells. (A) Sublethally irradiated mice were intravenously injected with 20 × 106 HL60-eGFP–bright cells stained with 5μM DIR and preincubated overnight at 37°C before the infusion. BM cells were analyzed by FC 4 days afterward. (B left panels) Frequency and analysis of DiR+ cells within human (eGFP+) and mouse (eGFP−) cells. (Right panels) Detailed phenotype of live cells positive for DiR. (C) FC quantification of human cell frequencies within the BM of recipients based on the detection of DiR+ or eGFP+ events (n = 4). (D) Determination of the absolute contribution of mouse and human cells within the total DiR signal: for each animal (n = 4), the absolute contribution of human (eGFP+) DiR+ cells was calculated by multiplying the mean fluorescence intensity by the number of events. The same operation was done for mouse cells (eGFP−) and for the total population of DiR+ cells. Results are expressed as a percentage of total DiR absolute signals.
In vivo transfer of DiR dye from human leukemic cells toward mouse cells. (A) Sublethally irradiated mice were intravenously injected with 20 × 106 HL60-eGFP–bright cells stained with 5μM DIR and preincubated overnight at 37°C before the infusion. BM cells were analyzed by FC 4 days afterward. (B left panels) Frequency and analysis of DiR+ cells within human (eGFP+) and mouse (eGFP−) cells. (Right panels) Detailed phenotype of live cells positive for DiR. (C) FC quantification of human cell frequencies within the BM of recipients based on the detection of DiR+ or eGFP+ events (n = 4). (D) Determination of the absolute contribution of mouse and human cells within the total DiR signal: for each animal (n = 4), the absolute contribution of human (eGFP+) DiR+ cells was calculated by multiplying the mean fluorescence intensity by the number of events. The same operation was done for mouse cells (eGFP−) and for the total population of DiR+ cells. Results are expressed as a percentage of total DiR absolute signals.
Optimization of the staining procedure to reduce/abrogate the microenvironmental contamination
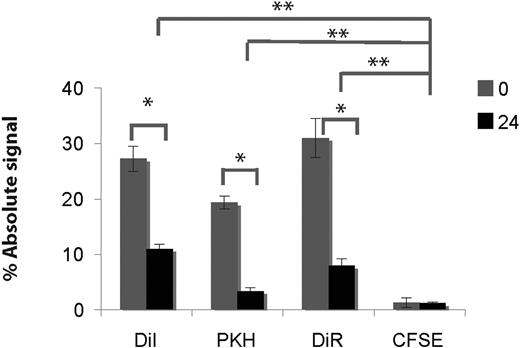
Although the viability of injected cells was good, the phenotype of DiR+ murine cells suggested the dye transfer to be mediated by a phagocytosis process, corroborating and extending recent reports.29,30 We thus tested, using the HL60 cell line, 3 different lipophilic dyes (DiI, DiR, and PKH) and an amine-reactive dye (CFSE) at very low doses (2 and 0.5μM31 ) to determine whether we can decrease this transfer. The stained cells were coincubated on a fibroblast layer unable to support hematopoiesis (GP293T cells) either after 2 washings or after overnight incubation in liquid culture. Despite suboptimal staining efficiency with the low dye concentration used (supplemental Figure 3), a substantial amount of fluorescence in stromal cells was still detected with the 3 lipophilic dyes (but not with the amine-reactive dye; Figure 3 and supplemental Figure 3). This transfer was significantly reduced but not abrogated by culturing stained HL60 cells overnight before starting the incubation with fibroblastic cells (Figure 3).
Microenvironment contamination occurs from all lipophylic dyes tested. HL60 cells were stained in PBS without serum with CFSE (0.5μM),31 DiI, DiR, or PKH26 (2μM) for 10 minutes and washed twice in PBS, 2% FCS. Stained cells were either directly plated on a confluent layer of fibroblasts (GP293T; condition 0 hours) or incubated for 24 hours in standard culture conditions before being plated on fibroblasts (condition 24 hours). After 24 hours of coculture at 37°C, cells were recovered by trypsinization, stained for hCD45 + hCD33 (both PE in the case of DiR, APC for all other dyes), and analyzed by flow cytometry. The absolute amount of fluorescence associated with the fibroblasts is displayed as a percentage of the total fluorescence for each dye (average and standard deviation obtained from 4 wells; data are representative of 2 independent experiments). Comparing 0 hours versus 24 hours for each dye, the differences are statistically significant (*P < .004 for all dyes except CFSE where P = .7). After 24-hour coculture, all dye transfers using lipophilic dyes are higher than with CFSE (**P < .001).
Microenvironment contamination occurs from all lipophylic dyes tested. HL60 cells were stained in PBS without serum with CFSE (0.5μM),31 DiI, DiR, or PKH26 (2μM) for 10 minutes and washed twice in PBS, 2% FCS. Stained cells were either directly plated on a confluent layer of fibroblasts (GP293T; condition 0 hours) or incubated for 24 hours in standard culture conditions before being plated on fibroblasts (condition 24 hours). After 24 hours of coculture at 37°C, cells were recovered by trypsinization, stained for hCD45 + hCD33 (both PE in the case of DiR, APC for all other dyes), and analyzed by flow cytometry. The absolute amount of fluorescence associated with the fibroblasts is displayed as a percentage of the total fluorescence for each dye (average and standard deviation obtained from 4 wells; data are representative of 2 independent experiments). Comparing 0 hours versus 24 hours for each dye, the differences are statistically significant (*P < .004 for all dyes except CFSE where P = .7). After 24-hour coculture, all dye transfers using lipophilic dyes are higher than with CFSE (**P < .001).
As our ultimate goal was to work with primary AML cells and knowing, from our experiments and others,12 that these cells are very fragile and that leukemia-initiating cells (LICs) are difficult to maintain in vitro, we thus wondered whether alternative procedures could be used to lower the dye transfer. First, we tested the possibility of performing the labeling incubation in serum-free medium to keep the cells in a suitable environment, but this abrogated the initial staining as one might have predicted (supplemental Figure 5A). After labeling the cells with DiI, DiD, or DiR, we assessed the beneficial impact of 2, 3, or 4 washing steps, or even fluorescence-activated cell sorting (FACS) of the stained cells before starting the coculture (supplemental Figure 5B-C). None of these procedures was effective. In our hands, reduction in dye concentration plus overnight incubation was the best strategy to reduce the transfer and was used in subsequent experiments.
Can we reduce the microenvironmental contamination using primary stem and progenitor cells?
At that stage, we had only evidence of the dye transfer using a cell line. During the course of this work, very impressive studies have demonstrated that lipophilic dyes are very useful tools for tracking of different primary cells in the bone marrow of live animals by intravital microscopy.19,23,27,32,33 We thus wondered whether using primary cells the transfer could be decreased. In preliminary experiments, we observed that human cord blood cells stained with DiR could induce transfer in coculture with osteoblastic cells. The transfer induced by stained osteoblasts was also demonstrated.
The biocompatibility of the staining procedure was further optimized on selected murine progenitor cells (supplemental Figure 6). During this step, we noticed that cell viability was slightly affected by the 10-minute incubation at 37°C in PBS alone (used for DiI and DiR staining) and even more using buffer C (used for PKH staining; supplemental Figure 6A representative of 2 independent experiments) even in the absence of any dye addition. Based on quantification of viable cell numbers by in vitro bioluminescence, we found that DiI at low concentration gave the best results in terms of biocompatibility (supplemental Figure 6C). The relatively low staining efficiency further encouraged us to use this condition (supplemental Figure 6B).
To determine whether primary hematopoietic stem and/or progenitor cells (HSPCs) could be affected by this dye transfer in a short-period of time, stem cell–enriched early progenitors (Lin− Sca-1+ Kit+ [LSK]) were purified, stained with 2μM DiI for 10 minutes, washed, incubated overnight in serum-free medium supplemented with cytokines,24 and washed again before being cultivated on a confluent layer of unstained endothelial cells for 18 hours. Live confocal imaging of the cells demonstrated clear transfer in this very stringent condition (Figure 4B), and absolute quantification revealed that more than 30% of DiI fluorescence was associated with endothelial cells (Figure 4C). This result demonstrates that, even in the absence of marked cell death (Figure 4A), transfer occurs not only with primary cells but also with HSPCs. In an independent experiment, similar results were obtained when LSK cells stained with DiR were cultivated on a complex stroma composed of human osteoblasts, endothelial cells, and murine stromal cells for 12 hours (data not shown). Although the intensity of the transfer is affected by the duration of the incubation, overall substantial amounts of dye transfer occurred during this period of time (supplemental Figure 4). Furthermore, we wondered whether we could used this strategy for “tattooing” the stromal cells directly interacting with HSCs in vivo (stem cell niche), we performed the same experiment in parallel with more engaged progenitors devoid of stem cell activity (Lin− Sca-1− Kit+ [LK] and Lin− Sca-1− Kit− [L3−[). Because endothelial cells might display the capability of supporting both stem cells and more committed progenitors, the experiment was conducted in parallel on hematopoietic nonsupportive fibroblastic cells. As expected, hematopoietic cell viability was slightly reduced after coincubation with fibroblasts compared with endothelial cells (Figure 4A), illustrating different functionalities of the 2 stromal cell types used. A transfer could be detected and quantified in all conditions tested (Figure 4B-C), demonstrating that this phenomenon is not a specific feature of stem cells, and that a stem cell niche–type interaction is not necessary for this to occur.
Microenvironment contamination occurs from sorted HSPCs but does not reflect a stem cell niche–type interaction with stromal cells. Different populations of HSPCs (Lin− Sca-1+ Kit+ [LSK], Lin− Sca-1− Kit+ [LK], Lin− Sca-1− Kit− [L3−]) that were FACS purified (purity > 90% for all fractions) were stained with 2μM DiI, washed, and incubated overnight in serum-free medium complemented with cytokines. After washing, cells were plated on confluent layers of endothelial cells (HUVECs) or fibroblastic cells (GP293). After the coculture, dye transfer was analyzed by confocal imaging (B) and FC (A,C). (A) Cells were recovered by trypsinization and stained with anti–mCD45-APC. After washing, cells were suspended in DAPI containing PBS, 2% FCS for FC analysis. After elimination of debris and doublets, viability (DAPI−) was determined on HSPCs (CD45+). (B) After overnight coculture (18 hours), hematopoietic cells were stained in situ by an anti–mCD45-APC antibody. A 10-μmstack was acquired for each region of interest (2-μm step) using a zoom 3 magnification. Displayed are 3-dimensional reconstructions for individual and merged channels for the cocultures of LSK and LK on endothelial cells. Scale bar represents 20 μm. (C) Absolute quantification of DiI distribution after 22 hours of coculture: using the same strategy as in Figure 2D, absolute quantification of DiI fluorescence was measured for HSPCs (CD45+) and stromal cells (CD45−) and expressed as percentage of total DiI fluorescence in the different conditions.
Microenvironment contamination occurs from sorted HSPCs but does not reflect a stem cell niche–type interaction with stromal cells. Different populations of HSPCs (Lin− Sca-1+ Kit+ [LSK], Lin− Sca-1− Kit+ [LK], Lin− Sca-1− Kit− [L3−]) that were FACS purified (purity > 90% for all fractions) were stained with 2μM DiI, washed, and incubated overnight in serum-free medium complemented with cytokines. After washing, cells were plated on confluent layers of endothelial cells (HUVECs) or fibroblastic cells (GP293). After the coculture, dye transfer was analyzed by confocal imaging (B) and FC (A,C). (A) Cells were recovered by trypsinization and stained with anti–mCD45-APC. After washing, cells were suspended in DAPI containing PBS, 2% FCS for FC analysis. After elimination of debris and doublets, viability (DAPI−) was determined on HSPCs (CD45+). (B) After overnight coculture (18 hours), hematopoietic cells were stained in situ by an anti–mCD45-APC antibody. A 10-μmstack was acquired for each region of interest (2-μm step) using a zoom 3 magnification. Displayed are 3-dimensional reconstructions for individual and merged channels for the cocultures of LSK and LK on endothelial cells. Scale bar represents 20 μm. (C) Absolute quantification of DiI distribution after 22 hours of coculture: using the same strategy as in Figure 2D, absolute quantification of DiI fluorescence was measured for HSPCs (CD45+) and stromal cells (CD45−) and expressed as percentage of total DiI fluorescence in the different conditions.
Mechanisms of transfer of the lipophilic dyes
In preliminary experiments, where DiR-stained cord blood mononuclear cells (MNCs) were incubated with osteoblasts, the 2 cell populations being separated by a microporous membrane, we observed that the transfer on osteoblasts was only partly reduced, suggesting that diffusible elements could be involved in the process and that a direct contact between donor and acceptor populations was not absolutely required (data not shown).
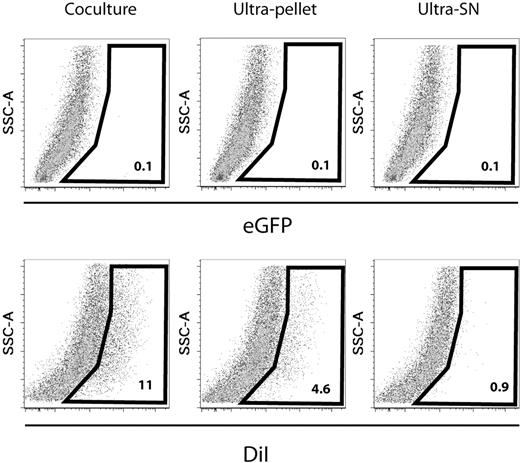
Because the transfer occurred similarly with different subpopulations of murine HSPCs, human cord blood MNCs, and HL60 cell line, we chose to further dissect the mechanism of transfer with HL60 cells considered as a homogeneous population (Figure 5). HL60 cells stained with DiI were cultivated overnight. Cells were recovered after centrifugation, and the upper part of the supernatant was further fractionated by ultracentrifugation to produce the ultrapellet and the ultrasupernatant. Similar quantities of cells, and equivalents of ultrapellet and ultrasupernatant, were dispatched on confluent stromal monolayers and incubated at 37°C. We observed that both direct cell-cell contact and the ultrapellet gave rise to transfer. Of interest, no transfer was obtained from the ultrasupernatant fraction. Similar data were obtained using DiR in 2 independent experiments, whereas no transfer could be detected when eGFP-bright HL60 cells were used. This confirmed our preliminary observations on cord blood and osteoblastic cells and further demonstrated that transfer can occur from diffusible elements that can be recovered by ultracentrifugation, which implies that it is not mediated by the release of free dye molecules in the environment.
Microenvironment contamination is mediated by direct cell-cell interactions and diffusible particles but not free dye molecules. HL60 cells were stained using 4μM DiI for 10 minutes in PBS without FCS at 37°C, washed, and plated in standard liquid culture conditions. As a control, eGFP-bright cells were treated in identical conditions without staining. Before and after culture, cell viability was higher than 90% (not shown). After 24-hour culture, cells were centrifuged (300g for 7 minutes at 4°C). The upper third part of the supernatant was harvested for ultracentrifugation (compare below); the remaining one was discarded. Cells were washed and 100 000 cells from each condition were plated on a confluent layer of HUVECs in a well of a 24-well plate. The supernatant from the first centrifugation step was further ultracentrifuged (61 600g for 2 hours 30 minutes at 4°C). The resulting supernatant (ultra-SN) and pellet (ultrapellet) were harvested separately. We determined the amount of ultra-SN and ultrapellet that would be equivalent to the number of cells plated on HUVECs and added this amount into separate confluent HUVEC wells. After 24 hours of culture, hematopoietic and stromal cells were recovered and analyzed by FACS after staining with anti–human CD45-APC and anti–human CD33-APC monoclonal antibody. DiI contamination of stromal cells (CD45−) after direct coculture or after exposure to similar amounts of ultrapellet or ultra-SN is displayed. No eGFP signal was found associated with stromal cells, whatever the condition (upper line). These data are representative of 2 experiments, which also included DiR.
Microenvironment contamination is mediated by direct cell-cell interactions and diffusible particles but not free dye molecules. HL60 cells were stained using 4μM DiI for 10 minutes in PBS without FCS at 37°C, washed, and plated in standard liquid culture conditions. As a control, eGFP-bright cells were treated in identical conditions without staining. Before and after culture, cell viability was higher than 90% (not shown). After 24-hour culture, cells were centrifuged (300g for 7 minutes at 4°C). The upper third part of the supernatant was harvested for ultracentrifugation (compare below); the remaining one was discarded. Cells were washed and 100 000 cells from each condition were plated on a confluent layer of HUVECs in a well of a 24-well plate. The supernatant from the first centrifugation step was further ultracentrifuged (61 600g for 2 hours 30 minutes at 4°C). The resulting supernatant (ultra-SN) and pellet (ultrapellet) were harvested separately. We determined the amount of ultra-SN and ultrapellet that would be equivalent to the number of cells plated on HUVECs and added this amount into separate confluent HUVEC wells. After 24 hours of culture, hematopoietic and stromal cells were recovered and analyzed by FACS after staining with anti–human CD45-APC and anti–human CD33-APC monoclonal antibody. DiI contamination of stromal cells (CD45−) after direct coculture or after exposure to similar amounts of ultrapellet or ultra-SN is displayed. No eGFP signal was found associated with stromal cells, whatever the condition (upper line). These data are representative of 2 experiments, which also included DiR.
Discussion
In this study, we have conducted a comprehensive analysis concerning a variety of vital dyes based on 4 distinct technologies including amine-reactive dyes (CFSE [green] or DDAO-SE [far red]), lipophilic dyes (DiI [red], PKH26 [red], DiD [far red] and DiR[NIR]), nanocrystals (quantum dots 705 [NIR]), and a kit for labeling antibodies (IRDye 800CW [NIR]). At the end of a thorough assessment, we were not able to find a single dye that would fulfill all of our initial criteria of multimodal imaging combining WBI, IVM, and flow cytometry and also provide good evidence of reliability. CFSE displayed very good biocompatibility and staining efficiency, while not displaying microenvironmental contamination, which make this strategy very suitable to track cells in different settings. Although the green fluorescence emitted by CFSE is not suitable for sensitive whole-body imaging,10 our data confirm it is a very reliable tool for microscopy (including 2-photon microscopy) and flow cytometric analyses. The infrared version DDAO-SE is compatible with all imaging techniques tested but was toxic for the cells in our study. Although quantum dots did not display major toxicity on HL60 cells, they provided very heterogeneous staining, which is not suitable for IVM imaging. Although the main limiting factor observed for IRDye 800CW was linked to its suboptimal excitation by 633-nm lasers used for IVM and FACS, we did not try to use an antibody labeling kit more compatible with our imaging systems because it does stain membrane proteins. Indeed, the transfer of membrane antigen was reported and might lead to microenvironmental contamination (MEC) in a similar manner as what we describe for lipophilic dyes.34,37,38
To our knowledge, this study is the first to demonstrate that lipophilic dyes (DiI, PKH-26, DiD, and DiR) used to stain human leukemic cells, cord blood MNCs, but also different sorted populations of murine HSPCs, can rapidly induce (within 15-24 hours) MEC in the absence of phagocytosis (Figures 3–4 and supplemental Figures 3-4). Fluorescent MEC could not be detected using amine-reactive CFSE or eGFP-encoded reporter vector, excluding a general mechanism involving all types of fluorochromes and demonstrating that, although endothelial cells are capable of phagocytosis, this mechanism plays only a marginal role in this model (Figure 3 and supplemental Figures 3-4). We observed, as previously described, important release of unbound CFSE within the first hours after staining31 (data not shown). Of interest, this phenomenon did not lead to significant transfer, at least in vitro. Conversely, no such fluorescence intensity drop was observed for lipophilic dyes. Considering the nature of lipophilic dyes, which become fluorescent in a lipidic environment, and knowing that exchanges of microdomains of cell membrane do occur, we believe it is an effective mechanism for lipophilic dye transfer. Direct contacts between donor and acceptor populations are not necessary for the transfer to occur, reinforcing this hypothesis (Figure 5). Altogether, our data suggest that lipophilic dyes induced MEC occurring through exchanges of membrane microdomains via cell-cell interaction, a mechanism described in the immune system as trogocytosis,34 and at distance (without cell-cell contacts) probably involving microvesicle and/or exosome35,36 transfer. Of interest, recent reports demonstrated that within an in vitro reconstructed model of human osteoblastic stem cell niche, exchanges of membranes could be a new way of reciprocal communication between HSCs and their niche.37 This suggests that potentially any type of membrane-based labeling strategy could possibly give rise to MEC, a hypothesis that will need to be assessed in future studies.
In recent developments of in vivo imaging, DiI, DiD, or DiR lipophilic dyes have been widely used for cell tracking.19-23,27,32,33 This strategy is currently recognized as a validated—not to mention, state of the art—technique for in vivo tracking of individual hematopoietic stem cells.28,39 Although careful validation of biocompatibility was performed,27 our work is the first to demonstrate the importance of optimizing the staining procedure for each cell type and, more importantly, to verify whether the fluorescent signal actually remains associated with the cells of interest in vivo and in vitro. Although the duration of incubation before imaging is a key factor to limit dye transfer, our in vitro data showed that it can be massive during short overnight incubations, thus pleading for the systematic implementation of rigorous controls to document and quantify the occurrence of MEC. Careful reexamination of the published data using lipophilic dye–based cell tracking allowed us to identify at least 3 situations where MEC could be involved: long-term tracking, analysis of cell division kinetics, and phagocytosis. Although this does not impede on the general message of this article, it is likely that MEC did occur 70 days after the transplantation of HSPCs stained with 10μM DiR for 30 minutes, even though it was performed in complete medium, which reduces the staining efficiency (data not shown).19 More recently, we noticed small discrepancies among 3 studies concerning the kinetics of cell divisions in the first hours after injection in irradiated recipients. Indeed, 2 of these studies report that only a minority of eGFP or CFSE-labeled HSPCs actually divide within the first 2 days after injection in conditioned recipients.40,41 Surprisingly, a study using DiD and DiI (5μM and 7.5μM, respectively, for 10 minutes at 37°C in PBS without serum) to track highly purified stem cells in vivo reports that from day 1 onward, the majority of clusters are constituted of 2 or more cells, while nicely demonstrating in parallel that HSCs always seeded a given BM place individually. At the present time, it is difficult to determine whether this discrepancy among the studies is related mainly to different kinetics of divisions due to functional differences between the calvarium versus long bones niches and/or whether an apparent multiplication of cells could arise from MEC phenomenon.27 Another very impressive tracking of human HSPCs mostly realized in nonirradiated SCID mice looked at the impact of leukemia development on the localization of normal human HSPCs. Considering that SCID mice maintain a relatively high degree of immune competence (especially in the phagocytic compartment), and based on recent confirmations concerning the critical necessity of preconditioning for engraftment to occur27,40,42 and personal observations (F.L., manuscript in preparation), it would not be surprising if a relatively high level of leakage (through phagocytosis and/or MEC) occurred from human cells stained with 12.5μM DiR for 30 minutes at 37°C. This highlights the need for further validation of some of these observations (specially those obtained at later time points), which might concern endogenous phagocytes rather than human HSPCs.32
During the course of this study, we have established that both optimization of the labeling procedures and thorough control of dye transfer are critically needed when establishing tracking experiments involving lipophilic dyes, both in vitro and in vivo. A variety of parameters have to be considered: the concentration of the dye (and its vehicle), duration, temperature, and quality of the medium used during the staining step (compare supplemental Figures 1, 4, 5, and 6). Obviously, the washing procedure is also critical. The viability of the stained cells, fluorescence intensity, stability, and homogeneity of the staining over time should also be evaluated. It is also important in the initial phases of development to analyze whether the functionality of the cells is affected by the staining procedure. To accurately assess dye transfer, it is important to start the flow cytometric analysis by gating first the whole population of fluorescent cells (positive for the dye), without prediscrimination of the population of interest (compare results in Figure 2). The intensity of the transfer can be precisely quantified. To determine the contribution of MEC in any model, it would be suitable to systematically measure the amount of fluorescence associated with the cells of interest versus other cell types (compare Figures 2D, 3, 4C, and supplemental Figures 4, 5C). However, such quantification requires obtaining a clearly identifiable population by flow cytometry, a condition that might not be fulfilled when small numbers of highly purified stem cells are injected systemically.27,28,33
Thus, the main conclusion here is not only to inform the community about the risk of dye contamination and MEC, which might have led to misinterpretation of some results, but also to propose a simple, reliable, and quantitative approach for systematically assessing the specificity of any staining procedure that might be useful in the development of improved cell-tracking models, a critical step for the quality management of cell therapy imaging.29,30,39,43-47
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Shai Senderovich and Christopher Ridler, and the Animal Care Facility, the Flow Cytometry, the Equipment Park, and the Light Microscopy core facilities at London Research Institute for valuable technical help. We acknowledge Dr Erik Sahai for providing pivotal technical help.
This work was funded by CRUK and by a European grant (contract no. 037632; D.B.).
Authorship
Contribution: F.L. led the conception and design of the experiments, collection, analysis, and interpretation of the data, and wrote the paper; E.G. provided material for the study, and helped in conception and design of experiments; and D.B. helped in conception and design, data analysis, interpretation, and redaction of paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dominique Bonnet, Hematopoietic Stem Cell Laboratory, CRUK, London Research Institute, 44 Lincoln's Inn Fields, London, WC2A 3PX; United Kingdom; e-mail: d.bonnet@cancer.org.uk.

![Figure 4. Microenvironment contamination occurs from sorted HSPCs but does not reflect a stem cell niche–type interaction with stromal cells. Different populations of HSPCs (Lin− Sca-1+ Kit+ [LSK], Lin− Sca-1− Kit+ [LK], Lin− Sca-1− Kit− [L3−]) that were FACS purified (purity > 90% for all fractions) were stained with 2μM DiI, washed, and incubated overnight in serum-free medium complemented with cytokines. After washing, cells were plated on confluent layers of endothelial cells (HUVECs) or fibroblastic cells (GP293). After the coculture, dye transfer was analyzed by confocal imaging (B) and FC (A,C). (A) Cells were recovered by trypsinization and stained with anti–mCD45-APC. After washing, cells were suspended in DAPI containing PBS, 2% FCS for FC analysis. After elimination of debris and doublets, viability (DAPI−) was determined on HSPCs (CD45+). (B) After overnight coculture (18 hours), hematopoietic cells were stained in situ by an anti–mCD45-APC antibody. A 10-μmstack was acquired for each region of interest (2-μm step) using a zoom 3 magnification. Displayed are 3-dimensional reconstructions for individual and merged channels for the cocultures of LSK and LK on endothelial cells. Scale bar represents 20 μm. (C) Absolute quantification of DiI distribution after 22 hours of coculture: using the same strategy as in Figure 2D, absolute quantification of DiI fluorescence was measured for HSPCs (CD45+) and stromal cells (CD45−) and expressed as percentage of total DiI fluorescence in the different conditions.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/26/10.1182_blood-2009-05-224030/4/m_zh89991052340004.jpeg?Expires=1766040027&Signature=mHwS56Uvl8506L~PhHcL6K6qWrwW13OYtBLVCWCcsQlv-J8QL91KDhOzECTEUjswnkXKE0uG1xaUrI6VQi4BfJi56Q2tk-U8~HTcgzbbn8quSgqmpWEpArU3s-6148PqCcEqw1chQAgSIUZe3D3fxw6usbOwwnJ2WOtcRb7fFt29MnMLUtc6udme5aSHM3P2N05-DcowoHGYGZaeXgjIVd1sb73YaoKbepamcmVDs24zsLmRCGSE3UUNCyiLRX3MSsNMfu2hNUfGPst4ItIJJRsYC-vI8LtTZTqlT6qF5LDRReNVr7xoAzUFEGmN2M52FSnd7K2pod42FvfEwh2U4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)