In this issue of Blood, Burns and colleagues report that the lymphocytes of a Wiskott-Aldrich syndrome patient with an activating WASP mutation display abnormal L-selectin–dependent adhesion and flow behavior in conjunction with altered cell topography and actin polymerization.
A distinguishing feature of hematopoietic cells is their ability to live free yet be exquisitely competent to make cell-to-cell and cell-to-substratum adhesion contacts when conditions and timing are right. Such properly functioning adhesion contacts are required for cell localization, migration, homing, and also activation, proliferation, cytotoxicity, and phagocytosis. Because all of these processes are affected, Wiskott-Aldrich syndrome (WAS), which was once of interest to only a few clinical immunologists and platelet specialists, has gained broad significance.
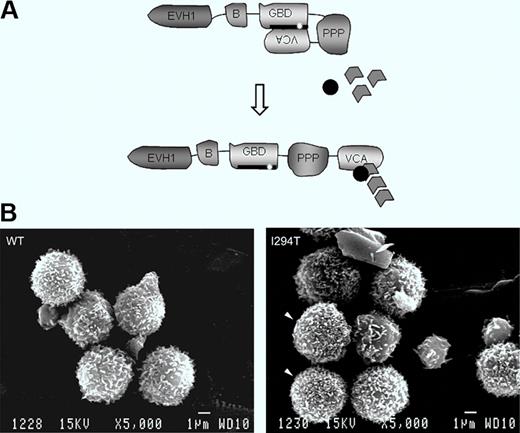
Schematic showing WASP autoinhibition in normal resting cells and conformational activation. The patient's mutation is indicated. Bottom: Lymphocytes of a normal healthy donor and WASPI294T lymphocytes of the patient. See the complete figure by Burns et al beginning on page 5355.
Schematic showing WASP autoinhibition in normal resting cells and conformational activation. The patient's mutation is indicated. Bottom: Lymphocytes of a normal healthy donor and WASPI294T lymphocytes of the patient. See the complete figure by Burns et al beginning on page 5355.
WAS is an inherited combined disease involving bleeding caused by thrombocytopenia and immune deficiency of varying severity.1,2 The spectrum of cell lineages now known to be affected includes not only T and B lymphocytes and platelets, as initially appreciated, but also natural killer (NK) and NKT cells, macrophages and dendritic cells, and to a lesser extent, neutrophils. The affected gene product WAS protein (WASP) is exclusive to hematopoietic cells. Its function is to integrate cell activation signals and, in cooperation with actin-related proteins (Arps), to induce cytoskeletal remodeling. In resting cells, WASP is found in a closed autoinhibited conformation by virtue of binding of the GTPase binding domain (GBD) to the C-terminal verprolin homology, cofilin homology, acidic region (VCA domains).3 On cell activation, the GTP-bound form of the small Rho GTPase cdc42 binds to the WASP-GBD and this, along with other changes, disrupts the autoinhibited structure. Activated WASP with its exposed VCA domain can then interact with Arp2/3 and actin monomers to initiate actin polymerization (figure, see schematic).
In a rare form of the disease, patients inherit one of several mutations in the GBD that disrupt the autoinhibited structure and result in enhanced actin-polymerizing activity.4-7 These mutations cause a distinct clinical phenotype, called X-linked neutropenia (XLN), involving severe neutropenia and myelocytopenia due to arrest of myelopoiesis in association with abnormalities of actin cytoskeletal structure. In this issue of Blood, Burns et al focus on the lymphocytes of a patient with one of these activating mutations, WASPI294T.8 They report that particularly high densities of microvillus projections were more frequent on patient lymphocytes compared with normal lymphocytes when the two were compared using scanning electron microscopy, and that microvilli were also more dense and were dysmorphic in a cell line model of WASPI294T. The cell line model also showed increased cellular content of F-actin. When examined under flow conditions, primary patient lymphocytes and the WASPI294T expressing model lymphoid cells displayed increased average rolling velocity on the L-selectin ligand sialyl Lewis-X (sLex) and showed marked fluctuations in rolling velocity (jerky rolling) compared with counterpart cells expressing normal WASP. The increased average velocity and jerky rolling behavior of lymphoid cells expressing constitutively activated WASP suggest defects in contact between L-selectin and its ligand. The authors were able to eliminate a number of possible abnormalities that could cause this phenomenon, including L-selectin surface density, its localization to microvilli, and activation-induced ectodomain shedding, and also expression and activation state of the cytoskeletal anchorage proteins ezrin-radixin-moesin because none of these differed between WASPI294T and WASPWT lymphoid cells. Finally, the authors used time-lapse interference reflectance microscopy to monitor persistence of lymphoid cell–substratum adhesion contacts. This was done under static conditions because of the extremely transient nature of adhesion contacts under flow conditions. They found that WASPI294T lymphoid cells had a higher percentage of relatively long-lived adhesions, that is, they had decreased turnover (decreased dynamics) of contact adhesions, compared with WASPWT cells. This was true when the cells were plated on sLex, but contact adhesion dynamics were similar for WASPI294T and WASPWT cells when plated on the nonspecific adhesion substrate poly-L-lysine.
The presented data are the first extensive study of lymphocyte defects in XLN; they substantially extend the earlier finding that B lymphocytes with this mutation showed increased migration toward CXCL13.9 Relating these defects to the clinical impact of WASP-activating GBD mutations on lymphocytes is not straightforward because of the rarity of these patients, the variability of their clinical status, and the coexistence of severe neutropenia. Nonetheless, patients with XLN are thought to have attenuated lymphocyte dysfunction. Within the larger framework of WASP function, abnormalities of microvillus structures are not novel. Indeed, the reciprocal finding, decreased density along with structural abnormalities of microvillus projections on lymphocytes10 and lymphoid cell lines11 of patients with classical WAS, was arguably the first structural defect directly linked to the WAS disease gene, which at the time was still unidentified.11 The increase of microvilli on lymphocytes bearing the WASP autoactivating mutation8 verifies that WASP plays a role in lymphocyte microvillus formation, even in the resting state, and renders the current findings directly relevant to classical WAS and to WASP function in normal lymphocytes. The novelty and importance of the study lie in the connection of autoactivated lymphocyte WASP to increased cellular F-actin, dysmorphic nature and increased density of microvillus structures, altered dynamics of adhesion contact turnover, and defective rolling behavior on L-selectin ligand. For better understanding, the question of whether or not these connections are direct will need to be addressed, and the apparent role of WASP in regulating lymphocyte adhesion contact dynamics also deserves further exploration. Finally, it will be important to learn whether WASP's role in regulating adhesion contact dynamics is limited to lymphocytes and L-selectin ligand, or is a more general property of cell-substratum and cell-cell adhesion contacts of hematopoietic cells. The diversity of cell lineages and cell functions affected in WAS strongly suggests a broader involvement.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal