Abstract
Allogeneic stem cell transplantation is the most potent form of effective adoptive immunotherapy. The graft-versus-leukemia (GVL) effect mediated by the allogeneic graft, however, is typically coexpressed with graft-versus-host disease (GVHD), which is the major complication of allogeneic stem cell transplantation. In this study, we used genetic and antibody-based strategies to examine the effect that blockade of interleukin 23 (IL-23) signaling had on GVH and GVL reactivity in murine transplantation recipients. These studies demonstrate that the selective protection of the colon that occurs as a consequence of inhibition of IL-23 signaling reduces GVHD without loss of the GVL effect. The separation of GVH and GVL reactivity was noted in both acute and chronic hematologic malignancy models, indicating that this approach was not restricted by the kinetic profile of the underlying leukemia. Furthermore, a potent GVL response could be mounted in the colon under conditions where tumor cells migrated to this site, indicating that this organ did not serve as a sanctuary site for subsequent systemic relapse in GVHD-protected animals. These studies demonstrate that blockade of IL-23 signaling is an effective strategy for separating GVH and GVL responses and identify IL-23 as a therapeutic target for the regulation of alloresponses in humans.
Introduction
The curative potential of allogeneic stem cell transplantation (SCT) derives, in part, from an antileukemia (graft-versus-leukemia [GVL]) effect that is conferred by donor T cells and other immune effectors within the allogeneic graft.1,2 Unfortunately, the GVL effect is typically coexpressed with graft-versus-host disease (GVHD), which is the major complication associated with allogeneic SCT.3-5 Thus, patients who are potentially cured of their disease may die of GVHD-related complications or require protracted immune-suppressive therapy that impairs their quality of life6,7 and renders them susceptible to opportunistic infections.8 One of the longstanding but elusive goals in the field, therefore, has been the development of viable strategies for the separation of GVL and GVH responses so that GVHD-associated mortality and morbidity do not negate the benefit derived from disease eradication.
The inability to dissociate GVL and GVH responses stems in part from the fact that alloreactive donor T cells are equally capable of trafficking to sites of disease as well as target organs, such as the colon, liver, and skin.9,10 Although GVHD is a systemic disease, there is a restricted set of organs (ie, skin, liver, and gastrointestinal tract) that are typically involved during the acute phase of this disease, and involvement of these organs is responsible for most of the tissue damage and attendant morbidity. The ability to selectively inhibit the capability of donor T cells to mediate pathologic damage in these specific target organs without interfering with the ability of these same cells to traffic to sites of underlying disease is a potential strategy that might allow for the separation of GVL and GVH effects. To that end, we have recently shown that interleukin-23 (IL-23) has a critical role in the pathophysiology of GVHD in the colon.11 In the absence of donor antigen-presenting cell (APC) secretion of IL-23, there is a profound and selective reduction in the severity of GVHD in this organ. This is attributable to a significant decrease in proinflammatory cytokine production, lipopolysaccharide (LPS) levels, and expansion of TH1 cells within the colon microenvironment. Prior studies have also shown that the colon, in particular, is a source of significant morbidity and mortality when it becomes involved during GVHD.12 Moreover, the colon is not a typical sanctuary for leukemia, which tends to reside in the bone marrow (BM) and secondary lymphoid tissues, although extramedullary sites can be involved in some patients.13,14 The purpose of these studies, therefore, was to determine whether blockade of IL-23 signaling and the subsequent conferred protection in the colon could attenuate GVHD without loss of the GVL effect.
Methods
Mice
C57BL/6 (B6) (H-2b), Balb/cJ (H-2d), and FVB (H-2q) mice were bred in the Animal Resource Center at the Medical College of Wisconsin (MCW) or purchased from The Jackson Laboratory. IL-23p19 knockout mice (IL-23−/−)15 on a B6 background were kindly provided by Dr Nico Ghilardi (Genentech) and bred at MCW. All animals were housed in the American Association for Laboratory Animal Care–accredited Animal Resource Center of the Medical College of Wisconsin. Experiments were all carried out under protocols approved by the MCW Institutional Animal Care and Use Committee. Mice received regular mouse chow and acidified tap water ad libitum.
Leukemia/lymphoma models
To examine the GVL effect in a hematologic malignancy model with rapid disease kinetics, A20 cells of Balb/c background (H-2d) were obtained from ATCC. In some studies, luciferase-transfected A20 cells (A20-luc) were used to allow for visualization of tumor dissemination. A20-luc cells were obtained by transfecting this cell line with firefly luciferase using Amaxa Nucleofector technology (Lonza Basel). Briefly, 5 × 106 A-20 cells were resuspended in 100 μL of nucleofection buffer, mixed with 5 μg of a plasmid encoding firefly luciferase, and then transfected using Amaxa Nucleofector program T-020. Cells were then grown under limiting dilution conditions in complete RPMI medium containing 10% fetal bovine serum and G418 (Geneticin) at a concentration of 800 μg/mL. Stable luciferase-expressing clones were identified by addition of the substrate D-luciferin (1 μg/well) to individual wells and then visualizing them using an IVIS Imaging system (Xenogen). To examine the GVL effect in a leukemia with more indolent kinetics, a previously described inducible model of chronic myelogenous leukemia (CML) was used.16 Transgenic mice in which a tetracycline (tet)-controlled transactivator was placed under the control of the murine stem cell leukemia gene 3′ enhancer [SCLtTA mice (H-2q)] were crossbred to transgenic TRE-BCR-ABL mice (H-2q), which expressed the bcr/abl oncogene to create double transgenic SCLtTA x BCR-ABL mice (ie, CML mice). Induction of bcr-abl expression by withdrawal of tet (0.5 g/L) from the drinking water results in activation of the transgene and subsequent leukemogenesis.
BMT
Bone marrow (BM) was flushed from donor femurs and tibias with Dulbecco modified media (Dulbecco modified Eagle medium, Invitrogen) and passed through sterile mesh filters to obtain single-cell suspensions. In some experiments, BM was T cell–depleted (TCD) in vitro with anti-Thy1.2 monoclonal antibody plus low-toxicity rabbit complement (C-6 Diagnostics). The hybridoma for 30-H12 (anti-Thy1.2, rat IgG2b) antibody was purchased from ATCC. Host mice were conditioned with total body irradiation administered as a single exposure at a dose rate of 84 cGy using a Shepherd Mark I Cesium Irradiator (J.L. Shepherd and Associates). Irradiated recipients received a single intravenous injection in the lateral tail vein of BM with or without added spleen cells in a total volume of 0.4 mL.
Reagents
Anti-IL-23 (p19) antibody (clone MB379-490) is a mouse IgG1 antibody that has been previously described.17 Animals received a weekly dose of 2 mg intraperitoneally for 4 consecutive weeks beginning on the day of transplantation. Antibody was resuspended in phosphate-buffered saline before injection. Mouse IgG1 (clone TC31.27F11; Schering Biopharma) was used as a control and administered at the same dose and schedule as p19 antibody.
Flow cytometry
Spleens and thymi were obtained from chimeras at defined intervals after transplantation, processed into single-cell suspensions, and stained for 2-color analysis. Monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used to assess cell populations in mice. PE–anti-CD8 (clone CT-CD8a, rat IgG2a) was obtained from Caltag. FITC–anti-Thy1.2 (clone 30-H12, rat IgG2b), FITC–anti-H-2Kb (clone AF6–88.5, mouse IgG2a), FITC–anti-Gr-1 (clone RB6–8C5, rat IgG2b), PE–anti-CD4 (clone GK1.5, rat IgG2b), PE–anti-TCRαβ (clone H57–597, hamster IgG), PE–anti-CD11b (clone M1/170, rat IgG2b), and PE-CD45R (clone RA3–6B2, rat IgG2a) were all purchased from BD Biosciences PharMingen. Cells were analyzed on a FACSCalibur flow cytometer with CellQuest software (BD Biosciences). Data were analyzed using FlowJo (TreeStar).
Cytokine analysis
To assess cytokine concentration in supernatants from colonic tissues, small pieces of colon (∼ 5 mm of mid-colon) were isolated and weighed under aseptic conditions, as previously described.11 Colon explants were then cultured overnight in complete RPMI 1640 (Invitrogen) at 37°C. After centrifugation to pellet debris, culture supernatants were analyzed for proinflammatory cytokines (IL-1β, tumor necrosis factor-α, IL-6, IL-17, granulocyte colony-stimulating factor, IL-12, and interferon-γ) using the multiplex cytokine Bio-Rad assay system. Cytokine levels were normalized to the weight of the colon explants. All samples were run in duplicate.
Histologic analysis
Representative samples of liver, colon, spleen, kidney, small intestines, mesenteric lymph nodes, and lung were obtained from transplanted recipients and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin. A semiquantitative scoring system was used to account for histologic changes consistent with GVHD in the colon, liver, and lung as previously described.11 Data are presented as individual GVHD target organ scores as well as a composite score from all 3 tissues. All slides for GVHD analysis were coded and read in a blinded fashion. Images were visualized with an Olympus BX45 microscope. Image acquisition was performed with an Olympus DP70 digital camera and software package.
In vivo and ex vivo BLI
For in vivo bioluminescence imaging (BLI), mice were given an intraperitoneal injection of luciferin (150 mg/kg body weight) and then anesthetized with isoflurane gas using a Xenogen XGI Gas Anesthesia System and imaged using the IVIS Imaging system (Xenogen) to assess bioluminescence 10 minutes after injection of the substrate. In some experiments, to image specific tissues for tumor involvement, mice were injected with an additional dose of luciferin (150 mg/kg body weight by intraperitoneal injection). Five minutes later, animals were killed. Selected tissues were harvested from animals and then imaged. Tissue processing and imaging were conducted within a period of 3 minutes to minimize signal decay. Imaging data were analyzed and quantified with Living Image Software (Xenogen).
Real-time quantitative PCR
Liver, spleen, and colon samples were harvested and immediately snap-frozen in liquid nitrogen for RNA extraction. Total RNA was extracted from frozen samples using TRIzol reagent (Invitrogen). Real-time quantitative polymerase chain reaction (PCR) was performed using Rotor-Gene 3000 (Corbett Research), BCR-ABL primers (5′-GCTGCAGATGCTGACCAACTC-3′, 5′-TCCAACGAGCGGCTTCAC-3′), c-ABL primers (5′-GGCTGGTGAAGAAGAACGAG-3′, 5′-GAAGGAGAGGCAGTGACCTG), and QuantiTect SYBR Green PCR Master Mix (QIAGEN) according to the manufacturer's instructions. Synthesis of first-strand cDNA from 1 μg of RNA per animal was accomplished with random primers and Superscript III (Invitrogen) according to the manufacturer's instructions. Primers were purchased from Integrated DNA Technologies. Specificity for all quantitative PCR reactions was verified by melting curve analysis. Data were analyzed with Rotor-Gene 3000 software Version 6.1 using the cycle threshold for quantification. Relative gene expression data (fold change) between samples was accomplished using the mathematic model described by Pfaffl.18
Statistics
Group comparisons of cell populations, peripheral blood counts, cytokine levels, pathology scores, and gene expression data were performed using the Mann-Whitney U test. Survival curves were constructed using the Kaplan-Meier product limit estimator and compared using the log-rank rest. A P value less than or equal to .05 was deemed to be significant in all experiments.
Results
Transplantation with IL-23−/− marrow grafts results in reduction in GVHD mortality without loss of the GVL effect
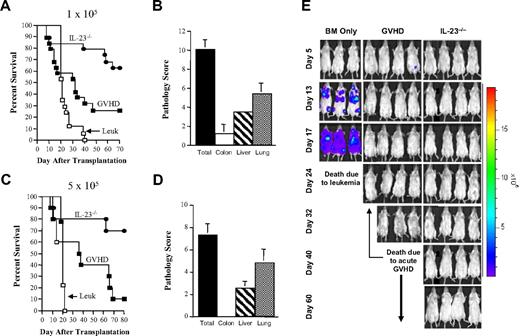
Experiments were conducted to determine the effect that absence of donor APC-derived IL-23 production had on the GVL effect in allogeneic bone marrow transplantation (BMT) recipients. Cohorts of Balb/c mice were lethally irradiated and then intravenously injected with 105 A20 cells 1 day before transplantation to allow for dissemination of tumor cells. The next day, groups of animals were then reconstituted with either B6 TCD BM alone or TCD BM and spleen cells from B6 or IL-23−/− donors. Mice transplanted with TCD BM alone all died of disease progression within 40 days after transplantation (Figure 1A). Mice transplanted with B6 BM and spleen cells had clinical evidence of GVHD (ie, weight loss, ruffled fur, and diarrhea), and the majority of these animals died by day 70 (overall survival, 26%). In contrast, transplantation with IL-23−/− marrow grafts significantly prolonged survival (63% at day 70, P = .028 compared with GVHD controls). Pathologic examination of surviving animals reconstituted with IL-23−/− BM and spleen cells revealed near-complete protection from GVHD in the colon but not in the liver or lung, consistent with our prior observations11 (Figure 1B). Notably, there was no evidence of lymphoma in any of these tissue sites. To more fully characterize the potency of the GVL effect, we repeated these studies using a 5-fold higher tumor cell dose (5 × 105). A significant prolongation of survival was observed in animals transplanted with marrow grafts from IL-23−/− donors compared with GVHD or lymphoma controls (Figure 1C). The colon was again noted to be protected from pathologic damage in contrast to other GVHD target tissues (Figure 1D). To further delineate the GVL response, A20 cells were transfected with the luciferase gene (A20-luc), permitting the visualization of tumor cell migration within the transplant recipient. A20-luc cells were detectable within 2 weeks after transplantation in the liver, lung, and nodal sites in tumor control animals (Figure 1E). In contrast, both GVHD control mice and those transplanted with IL-23−/− marrow grafts had no evidence of lymphoma throughout the 60-day observation period, demonstrating that the GVL effect was not compromised in these animals.
Transplantation of IL-23−/− marrow grafts reduces GVHD severity without loss of the GVL effect. (A-B) Lethally irradiated (900 cGy) Balb/c mice were administered 1 × 105 A20 cells and then the next day transplanted with either B6 TCD BM alone (10 × 106; □, n = 17; Leuk), B6 TCD BM plus B6 spleen cells (■, n = 19; GVHD), or IL-23−/− TCD BM and spleen cells (●, n = 19). Spleen cell inoculums were adjusted so that the dose of T cells (0.6 × 106) was equivalent in both groups of mice in all experiments. Survival is shown in panel A, whereas pathologic scores from surviving mice transplanted with IL-23−/− marrow grafts (n = 12) 80 to 84 days after transplantation are shown in panel B. Data are individual GVHD target organ scores as well as the composite score (lung, liver, and colon) and are derived from cumulative results of 4 experiments. (C-D) Lethally irradiated Balb/c mice were administered 5 × 105 A20 cells and then transplanted the next day with B6 TCD BM alone (□, n = 9), B6 TCD BM plus B6 spleen cells (■, n = 10), or IL-23−/− TCD BM and spleen cells (●, n = 10). Survival is shown in panel C, whereas pathologic scores of surviving mice transplanted with IL-23−/− marrow grafts 82 to 110 days after transplantation are shown in panel D. Data are derived from cumulative results from 2 experiments. (E) Whole body distribution of A20-luc cells over the first 60 days after transplantation using in vivo BLI is shown in animals transplanted with B6 BM only, B6 BM and spleen cells, or IL-23−/− BM and spleen cells. Data are representative of one of 3 experiments.
Transplantation of IL-23−/− marrow grafts reduces GVHD severity without loss of the GVL effect. (A-B) Lethally irradiated (900 cGy) Balb/c mice were administered 1 × 105 A20 cells and then the next day transplanted with either B6 TCD BM alone (10 × 106; □, n = 17; Leuk), B6 TCD BM plus B6 spleen cells (■, n = 19; GVHD), or IL-23−/− TCD BM and spleen cells (●, n = 19). Spleen cell inoculums were adjusted so that the dose of T cells (0.6 × 106) was equivalent in both groups of mice in all experiments. Survival is shown in panel A, whereas pathologic scores from surviving mice transplanted with IL-23−/− marrow grafts (n = 12) 80 to 84 days after transplantation are shown in panel B. Data are individual GVHD target organ scores as well as the composite score (lung, liver, and colon) and are derived from cumulative results of 4 experiments. (C-D) Lethally irradiated Balb/c mice were administered 5 × 105 A20 cells and then transplanted the next day with B6 TCD BM alone (□, n = 9), B6 TCD BM plus B6 spleen cells (■, n = 10), or IL-23−/− TCD BM and spleen cells (●, n = 10). Survival is shown in panel C, whereas pathologic scores of surviving mice transplanted with IL-23−/− marrow grafts 82 to 110 days after transplantation are shown in panel D. Data are derived from cumulative results from 2 experiments. (E) Whole body distribution of A20-luc cells over the first 60 days after transplantation using in vivo BLI is shown in animals transplanted with B6 BM only, B6 BM and spleen cells, or IL-23−/− BM and spleen cells. Data are representative of one of 3 experiments.
Antibody blockade of IL-23 results in the selective protection of the colon during GVHD
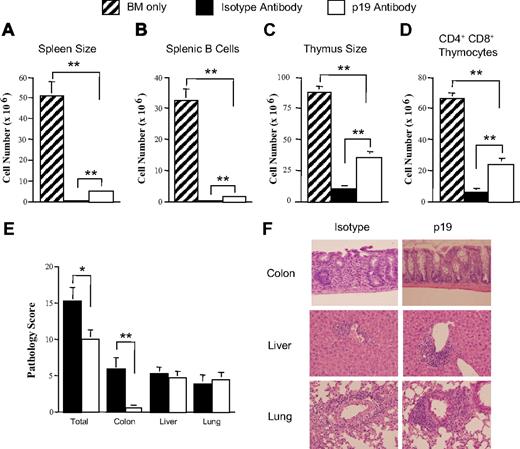
Studies were then conducted to determine whether a more clinically relevant strategy of antibody blockade of IL-23 signaling was efficacious in protecting mice from GVHD without loss of a GVL effect. Because the efficacy of antibody blockade in preventing GVHD has not been previously examined, we performed experiments to determine whether antibody administration yielded the same outcome as observed with transplantation of marrow grafts from transgenic IL-23−/− donors. Lethally irradiated Balb/c mice were transplanted with B6 BM alone or together with B6 spleen cells to induce GVHD. Cohorts of mice transplanted with BM and spleen cells were then administered either isotype control or p19-specific antibody once weekly beginning the day of transplantation. This antibody targets the unique p19 subunit of IL-23, which is not shared with IL-12.19 Mice in all groups were killed 32 to35 days after transplantation and examined for the extent of immune reconstitution and GVHD in target organs (lung, liver, and colon). We observed that there was a significant increase in splenic cellularity, overall number of splenic B cells, thymus size, and absolute number of double-positive thymocytes in p19 antibody-treated animals versus isotype control mice (Figure 2A-D), although mean values were significantly lower than in BM control animals. Moreover, there was a significant reduction in overall pathology scores in animals treated with p19 versus isotype antibody (10.1 ± 1.3 vs 15.4 ± 1.8, P = .03; Figure 2E). This was solely attributable to a reduction in the severity of colonic GVHD (0.7 ± 0.3 vs 6.0 ± 1.6, P < .01) as there was no difference in pathology scores in either the lung or liver between these groups of mice (Figure 2E-F).
Antibody blockade of IL-23 significantly enhances immune reconstitution and results in selective GVHD protection in the colon. (A-D) Lethally irradiated Balb/c mice were transplanted with B6 BM alone (n = 5) or B6 BM and spleen cells adjusted to yield 0.225 to 0.4 × 106 T cells. Cohorts of animals transplanted with adjunctive spleen cells were then treated with either isotype control (n = 11) or p19 antibody (n = 15). Spleen cellularity (A), total number of splenic B cells (B), thymus size (C), absolute number of double-positive (CD4+CD8+) thymocytes (D), and pathologic damage in GVHD target tissues (colon, liver, and lung; E) in mice 3 to 4 weeks after transplantation are shown. Data are derived from cumulative results from 3 experiments and are presented as the mean ± SEM. (F) Histology of colon, liver, and lung from representative Balb/c recipients 3 to 4 weeks after transplantation with B6 BM and spleen cells and then treated with either isotype control or p19 antibody. Colon in isotype-treated mouse shows inflammation in the lamina propria, extensive crypt cell destruction, and goblet cell depletion, whereas colon in p-19-treated animal has normal-appearing mucosa with no inflammatory infiltration and preserved goblet cell content. Livers in both mice show infiltration in the portal triads with mononuclear and granulocytic cells. Lungs demonstrate peribronchial and/or perivascular cuffing attributable to mononuclear and granulocytic cells along with associated interstitial inflammation. Images were obtained with an Olympus DP70 digital camera and an Olympus BX45 microscope with a 20×/0.5 NA lens. *P ≤ .05. **P < .01.
Antibody blockade of IL-23 significantly enhances immune reconstitution and results in selective GVHD protection in the colon. (A-D) Lethally irradiated Balb/c mice were transplanted with B6 BM alone (n = 5) or B6 BM and spleen cells adjusted to yield 0.225 to 0.4 × 106 T cells. Cohorts of animals transplanted with adjunctive spleen cells were then treated with either isotype control (n = 11) or p19 antibody (n = 15). Spleen cellularity (A), total number of splenic B cells (B), thymus size (C), absolute number of double-positive (CD4+CD8+) thymocytes (D), and pathologic damage in GVHD target tissues (colon, liver, and lung; E) in mice 3 to 4 weeks after transplantation are shown. Data are derived from cumulative results from 3 experiments and are presented as the mean ± SEM. (F) Histology of colon, liver, and lung from representative Balb/c recipients 3 to 4 weeks after transplantation with B6 BM and spleen cells and then treated with either isotype control or p19 antibody. Colon in isotype-treated mouse shows inflammation in the lamina propria, extensive crypt cell destruction, and goblet cell depletion, whereas colon in p-19-treated animal has normal-appearing mucosa with no inflammatory infiltration and preserved goblet cell content. Livers in both mice show infiltration in the portal triads with mononuclear and granulocytic cells. Lungs demonstrate peribronchial and/or perivascular cuffing attributable to mononuclear and granulocytic cells along with associated interstitial inflammation. Images were obtained with an Olympus DP70 digital camera and an Olympus BX45 microscope with a 20×/0.5 NA lens. *P ≤ .05. **P < .01.
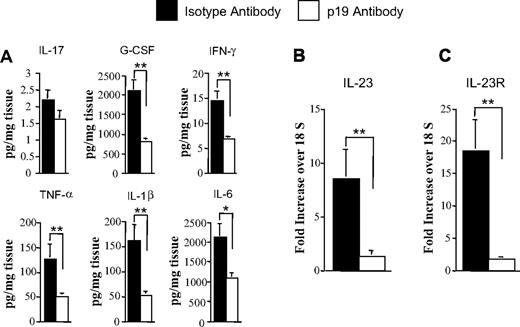
We also observed a significant reduction in proinflammatory cytokine production in the colons of p19 versus isotype control-treated animals as further confirmation of the protective effect within this organ (Figure 3A). Specifically, granulocyte colony-stimulating factor, interferon-γ, tumor necrosis factor-α, IL-1β, and IL-6 levels were all reduced in the colons of animals that received p19 antibody administration. In previous studies, we observed that transplantation with IL-23−/− marrow grafts results in significant decreases in IL-23 and IL-23R mRNA levels by inhibiting the proinflammatory feedback loop that perpetuates GVHD in the colon microenvironment.11 We therefore examined whether antibody blockade had a similar effect on these gene expression levels. Mice that were administered p19 as opposed to isotype control antibody had a significant reduction in IL-23 and IL-23R mRNA levels in the colon (Figure 3B-C). Collectively, these studies demonstrated that antibody blockade of IL-23 signaling was effective at preventing GVHD in the colon microenvironment.
Antibody blockade of IL-23 attenuates proinflammatory cytokine production and decreases gene expression of IL-23 and IL-23R in the colon microenvironment. (A) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells adjusted to yield 0.225 to 0.4 × 106 T cells. Cohorts of mice were then treated with either isotype control (n = 11) or p19 antibody (n = 15) once weekly. Mice were killed 32 to 35 days after transplantation, and colon explant tissue was assayed for levels of proinflammatory cytokines by multiplex. Data are derived from cumulative results from 3 experiments and are the mean amount of cytokine divided by the weight of cultured colon tissue ± SEM. (B-C) RNA was extracted from colon tissues obtained from animals transplanted in panel A that were treated with either isotype control or p19 antibody. Gene expression of IL-23 (B) or IL-23R (C) mRNA levels was analyzed by real-time quantitative PCR as described in “Real-time quantitative PCR.” Data are normalized for 18S ribosomal RNA and presented as fold increase over 18S RNA ± SEM. Data are cumulative results from 3 independent experiments for mice treated with either isotype control or p19 antibody. *P ≤ .05. **P < .01.
Antibody blockade of IL-23 attenuates proinflammatory cytokine production and decreases gene expression of IL-23 and IL-23R in the colon microenvironment. (A) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells adjusted to yield 0.225 to 0.4 × 106 T cells. Cohorts of mice were then treated with either isotype control (n = 11) or p19 antibody (n = 15) once weekly. Mice were killed 32 to 35 days after transplantation, and colon explant tissue was assayed for levels of proinflammatory cytokines by multiplex. Data are derived from cumulative results from 3 experiments and are the mean amount of cytokine divided by the weight of cultured colon tissue ± SEM. (B-C) RNA was extracted from colon tissues obtained from animals transplanted in panel A that were treated with either isotype control or p19 antibody. Gene expression of IL-23 (B) or IL-23R (C) mRNA levels was analyzed by real-time quantitative PCR as described in “Real-time quantitative PCR.” Data are normalized for 18S ribosomal RNA and presented as fold increase over 18S RNA ± SEM. Data are cumulative results from 3 independent experiments for mice treated with either isotype control or p19 antibody. *P ≤ .05. **P < .01.
Antibody blockade of IL-23 allows for preservation of GVL reactivity and protection from GVHD
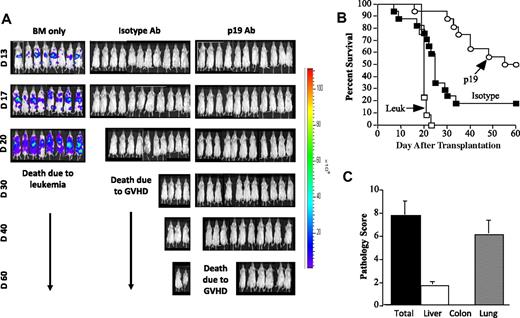
We then examined whether antibody administration in tumor-bearing animals could protect mice from lethal GVHD without loss of an underlying GVL response. Lethally irradiated Balb/c mice were administered 5 × 105 A20-luc cells 1 day before transplantation, and the next day cohorts of animals were transplanted with either B6 BM cells alone or together with B6 spleen cells to induce GVHD. Mice that received adjunctive spleen cells were then administered either isotype control or p19 antibody on the schedule as described in “Reagents.” Serial BLI studies again demonstrated evidence of lymphoma in animals transplanted with BM alone but no evidence of disease in isotype or p19-treated mice at any of the time points examined (Figure 4A). Because of the increased GVHD-related mortality in isotype antibody-treated mice, however, there was a significant increase in overall survival for animals that were administered p19 antibody (50% vs 18% at day 60, P = .001; Figure 4B). Pathologic examination of GVHD target organs in p19-treated mice demonstrated that there was no significant tissue damage in the colon attributable to GVHD (Figure 4C) similar to what was observed with the transplantation of transgenic donor IL-23−/− T cells (Figure 1). Histologic analysis also did not reveal evidence of tumor cells in these tissue sites (data not shown).
Antibody blockade of IL-23 signaling results in a reduction in GVHD without abrogation of the GVL effect. (A) Whole body distribution of A20-luc cells over the first 60 days after transplantation using in vivo BLI is shown in animals transplanted with B6 BM only, B6 BM and spleen cells treated with isotype control antibody, or B6 BM and spleen cells treated with p19 antibody. Data are derived from 2 experiments. (B) Lethally irradiated Balb/c mice were administered 5 × 105 A20-luc+ tumor cells and the next day were transplanted with either B6 BM cells alone (□, n = 13) or together with B6 spleen cells adjusted to yield 0.225 to 0.4 × 106 T cells. Mice that received adjunctive spleen cells were then administered either isotype control (■, n = 17) or p19 antibody (○, n = 19) on the schedule as described in “Reagents.” Survival is shown. Data are cumulative results derived from 3 independent experiments. (C) Pathologic scores in individual GVHD target organs and composite score of all 3 tissues from surviving mice transplanted with p19 antibody are shown. Data are presented as mean ± SEM.
Antibody blockade of IL-23 signaling results in a reduction in GVHD without abrogation of the GVL effect. (A) Whole body distribution of A20-luc cells over the first 60 days after transplantation using in vivo BLI is shown in animals transplanted with B6 BM only, B6 BM and spleen cells treated with isotype control antibody, or B6 BM and spleen cells treated with p19 antibody. Data are derived from 2 experiments. (B) Lethally irradiated Balb/c mice were administered 5 × 105 A20-luc+ tumor cells and the next day were transplanted with either B6 BM cells alone (□, n = 13) or together with B6 spleen cells adjusted to yield 0.225 to 0.4 × 106 T cells. Mice that received adjunctive spleen cells were then administered either isotype control (■, n = 17) or p19 antibody (○, n = 19) on the schedule as described in “Reagents.” Survival is shown. Data are cumulative results derived from 3 independent experiments. (C) Pathologic scores in individual GVHD target organs and composite score of all 3 tissues from surviving mice transplanted with p19 antibody are shown. Data are presented as mean ± SEM.
A20 cells fail to traffic to the colon during tumor progression
An optimal GVL response requires that donor-derived effector cells be capable of eradicating disease in all involved tissue sites. Therefore, a question that arose from these studies was whether decreased GVHD severity in the colon, as a consequence of IL-23 blockade, might compromise the GVL response in this tissue site and thereby result in the colon serving as a sanctuary site for subsequent systemic recurrence. To address this issue, we first elucidated whether this question could be addressed in the A20 model by examining the organ-specific migration of A20 cells after transplantation in the absence of an alloresponse. Lethally irradiated Balb/c mice were administered 5 × 105 A20-luc cells and the next day transplanted with B6 BM cells. Serial BLI studies were done, and animals were killed when BLI demonstrated widespread lymphomatous dissemination (Figure 5A). Examination of individual organs from these mice confirmed that A20-luc cells were readily discernible in the liver, lung, and spleen (Figure 5B). There were also scattered foci of activity present in the mid and upper portions of the small intestines. Notably, however, no signal was detectable in the colons of these animals. Pathologic examination of these same tissues was then performed to confirm the presence of disease and verify specific tissue involvement. These studies demonstrated the presence of widespread lymphomatous infiltrates in the parenchyma of the liver, lung, and spleen (Figure 5C). Tumor involvement was also evident in the small intestines where it appeared to originate in Peyer patches extending down into villi as well as in nodal tissue that was adjacent to the duodenum. Notably, histopathologic examination demonstrated no evidence of disease in the colon. Thus, the fact that A20 cells did not appear to traffic to the colon precluded the use of this model to determine whether organ-specific protection as a consequence of blockade of IL-23 signaling would compromise the GVL response.
A20 leukemia cells do not traffic to the colon. (A) Lethally irradiated Balb/c mice were administered 5 × 105 A20-luc cells and then transplanted the next day with B6 BM cells. Serial BLI studies were performed, and mice were killed 20 days after transplantation when leukemic dissemination was widespread. (B) BLI of individual organs, specifically the spleen (S), liver (L), lung (Lu), small intestines (SI), stomach (St), and colon from a representative mouse with disseminated A20 leukemia demonstrating tumor involvement in the spleen, liver, lung, stomach, and small intestines but not in the colon. (C) Pathologic examination of the same tissues that were imaged in panel B. In the liver, there is extensive leukemic infiltration around the portal triads (arrows). Leukemia cells are present in the interstitium of the lung and exhibit perivascular cuffing (arrows), and there is effacement of the red and white pulp by leukemia cells in the spleen. A20 cells are also present in the Peyer patches of the small intestines and extend into adjacent villi. Nodal tissue (solid arrow) that was contiguous to the duodenum (hatched arrow, Brunner glands) was observed to be replaced with leukemia cells as well. Leukemia was not detectable in the colon. One representative animal of 11 individual mice is shown in panels B and C. Images were obtained with an Olympus DP70 digital camera and an Olympus BX45 microscope with a 40×/0.75 NA lens.
A20 leukemia cells do not traffic to the colon. (A) Lethally irradiated Balb/c mice were administered 5 × 105 A20-luc cells and then transplanted the next day with B6 BM cells. Serial BLI studies were performed, and mice were killed 20 days after transplantation when leukemic dissemination was widespread. (B) BLI of individual organs, specifically the spleen (S), liver (L), lung (Lu), small intestines (SI), stomach (St), and colon from a representative mouse with disseminated A20 leukemia demonstrating tumor involvement in the spleen, liver, lung, stomach, and small intestines but not in the colon. (C) Pathologic examination of the same tissues that were imaged in panel B. In the liver, there is extensive leukemic infiltration around the portal triads (arrows). Leukemia cells are present in the interstitium of the lung and exhibit perivascular cuffing (arrows), and there is effacement of the red and white pulp by leukemia cells in the spleen. A20 cells are also present in the Peyer patches of the small intestines and extend into adjacent villi. Nodal tissue (solid arrow) that was contiguous to the duodenum (hatched arrow, Brunner glands) was observed to be replaced with leukemia cells as well. Leukemia was not detectable in the colon. One representative animal of 11 individual mice is shown in panels B and C. Images were obtained with an Olympus DP70 digital camera and an Olympus BX45 microscope with a 40×/0.75 NA lens.
Leukemia cells are present in the colon in transplantation recipients with CML
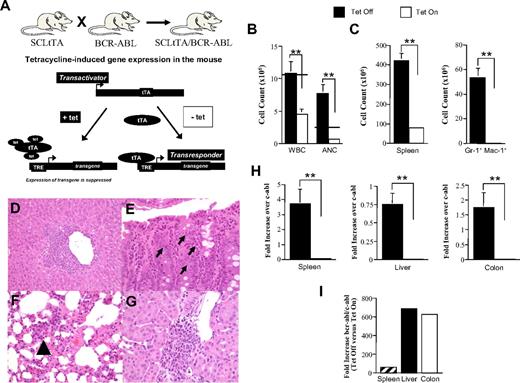
We therefore used an alternative, clinically relevant model of CML in which disease induction by way of bcr/abl expression is regulated by the presence or absence of tet in the drinking water (Figure 6A). Another aspect of this model is that tumor growth is much more indolent, which allowed us to determine whether the separation of GVH and GVL responses by interruption of IL-23 signaling was affected by disease kinetics. To first determine the migration of leukemia cells in this model, BM cells from FVB CML mice that had been maintained on tet to prevent induction of bcr/abl, along with TCD B6 BM cells, were transplanted into lethally irradiated normal FVB animals. One cohort of mice was kept off tet, whereas the second was maintained on tet throughout the following transplantation course to serve as a control group. Approximately 7 weeks after transplantation, mice off tet had a statistically significant increase in their total white blood cell count (WBC) compared with control animals (Figure 6B). This was attributable almost completely to the marked increase in the absolute number of neutrophils (ANC), which is a hallmark of CML. To assess the extent of leukemic involvement in specific organs, mice were killed 52 to 57 days after BMT. Overall spleen size was significantly greater in leukemic versus nonleukemic mice (mean 422.9 × 106 vs 83.2 × 106, P < .001; Figure 6C). Immunophenotyping of the spleen revealed an increased number of granulocytes (Gr-1+ Mac-1+) in mice taken off tet relative to those maintained on tet (53.7 ± 7.1 vs 0.29 ± 0.05 × 106, P < .001). Histologic analysis of colon, lung, kidney, and liver tissues demonstrated the presence of granulocytic infiltrates in all 4 organs (Figure 6D-G), consistent with the leukemia infiltration that is observed in human CML. To confirm that neutrophils present in the colon were of leukemic origin, real-time PCR analysis for the bcr/abl oncogene was performed. These studies demonstrated that there was a statistically significant increase in bcr/abl mRNA levels in the spleen, liver, and colon of leukemic animals (Figure 6H). When the ratio of bcr-abl/c-abl mRNA levels in these same tissues was examined, we observed that there was a 60-fold increase in the spleen and 600-fold increases in the liver and colon of mice off tet compared with animals maintained on tet (Figure 6I). These data confirmed that leukemia cells in this model migrated to both hematopoietic and nonhematopoietic tissue sites, including the colon.
Leukemia cells are present in the colon in transplantation recipients with CML. (A) Schematic illustrating tet-induced repression of bcr/abl gene expression obtained after crossing ScltTA with bcr/abl mice to produce double-transgenic CML animals. tet binds to the tetracycline-controlled transactivator (tTA), which prevents binding of tTA to the tetracycline response element (TRE) resulting in repression of bcr/abl transcription. In the absence of tet, the tTA is able to bind to the TRE, and there is expression of bcr/abl. (B) Lethally irradiated (1000 cGy) FVB mice were transplanted with TCD B6 BM (10 × 106) and non-TCD BM (10 × 106) cells from CML (SCLTta × BCR/ABL) animals. One cohort was maintained on tet (n = 10), whereas the second was removed from tet after transplantation (n = 8). Mice in both groups were bled 48 to 52 days after transplantation. Peripheral blood was analyzed for total WBC and ANC. Horizontal lines indicate the upper limit of normal for WBC and ANC. (C) Mice in similarly transplanted cohorts (n = 9 or 10/group) were killed 52 to 57 days after BMT, and spleen size and the absolute number of Gr-1+ Mac-1+ cells were calculated. (D-G) Histology of representative mouse with underlying CML that was killed 83 days after BMT. (D) Granulocytic infiltrate surrounding a central vein in the liver. (E) Granulocytes infiltrating the crypts in the colon (arrows indicate eosinophils). (F) Immature granulocytic precursors within the interstitium of the lung parenchyma (arrow). (G) Perivascular granulocytic infiltrate in the kidney. (H-I) Lethally irradiated FVB mice were transplanted with TCD B6 BM and non-TCD BM cells from CML mice. One cohort was maintained on tet (n = 15), whereas the second group was placed on normal drinking water without tet (n = 12). Mice were killed 50 days after transplantation, and spleen, liver, and colon tissues were obtained from each animal to determine bcr/abl mRNA levels by real-time quantitative PCR. Data are presented as fold increase over c-abl in panel H. The fold increase of bcr/abl over c-abl expression in the spleen, liver, and colon of animals not on tet versus those on tet is shown in panel I. Data are the mean ± SEM. **P < .01. Panels D through G: Images were obtained with an Olympus DP70 digital camera and an Olympus BX45 microscope with a 40×/0.75 NA lens.
Leukemia cells are present in the colon in transplantation recipients with CML. (A) Schematic illustrating tet-induced repression of bcr/abl gene expression obtained after crossing ScltTA with bcr/abl mice to produce double-transgenic CML animals. tet binds to the tetracycline-controlled transactivator (tTA), which prevents binding of tTA to the tetracycline response element (TRE) resulting in repression of bcr/abl transcription. In the absence of tet, the tTA is able to bind to the TRE, and there is expression of bcr/abl. (B) Lethally irradiated (1000 cGy) FVB mice were transplanted with TCD B6 BM (10 × 106) and non-TCD BM (10 × 106) cells from CML (SCLTta × BCR/ABL) animals. One cohort was maintained on tet (n = 10), whereas the second was removed from tet after transplantation (n = 8). Mice in both groups were bled 48 to 52 days after transplantation. Peripheral blood was analyzed for total WBC and ANC. Horizontal lines indicate the upper limit of normal for WBC and ANC. (C) Mice in similarly transplanted cohorts (n = 9 or 10/group) were killed 52 to 57 days after BMT, and spleen size and the absolute number of Gr-1+ Mac-1+ cells were calculated. (D-G) Histology of representative mouse with underlying CML that was killed 83 days after BMT. (D) Granulocytic infiltrate surrounding a central vein in the liver. (E) Granulocytes infiltrating the crypts in the colon (arrows indicate eosinophils). (F) Immature granulocytic precursors within the interstitium of the lung parenchyma (arrow). (G) Perivascular granulocytic infiltrate in the kidney. (H-I) Lethally irradiated FVB mice were transplanted with TCD B6 BM and non-TCD BM cells from CML mice. One cohort was maintained on tet (n = 15), whereas the second group was placed on normal drinking water without tet (n = 12). Mice were killed 50 days after transplantation, and spleen, liver, and colon tissues were obtained from each animal to determine bcr/abl mRNA levels by real-time quantitative PCR. Data are presented as fold increase over c-abl in panel H. The fold increase of bcr/abl over c-abl expression in the spleen, liver, and colon of animals not on tet versus those on tet is shown in panel I. Data are the mean ± SEM. **P < .01. Panels D through G: Images were obtained with an Olympus DP70 digital camera and an Olympus BX45 microscope with a 40×/0.75 NA lens.
Transplantation with IL-23−/− marrow grafts results in reduction in GVHD mortality without loss of the GVL effect in CML mice
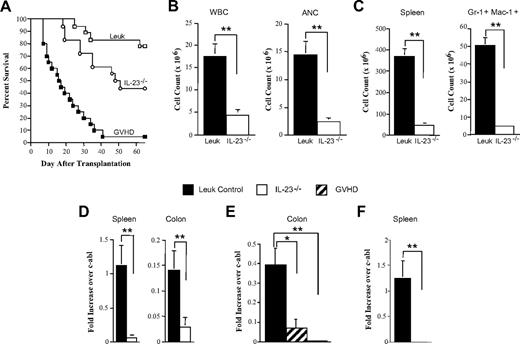
To determine whether GVL and GVH responses could be dissociated in this chronic model of leukemia and whether an antileukemic response could be mounted in the colon, lethally irradiated FVB mice were transplanted with a combination of TCD B6 or IL-23−/− (10 × 106) and non-TCD bcr/abl (10 × 106) BM cells alone or together with spleen cells adjusted to yield a T-cell dose of 3.5 × 106 αβ T cells from B6 or IL-23−/− animals. Because of the indolent nature of this chronic leukemia, the majority of leukemic animals transplanted with BM cells alone survived until day 90 (Figure 7A). This was in contrast to mice transplanted with adjunctive B6 spleen cells where nearly all animals died of GVHD. Transplantation with IL-23−/− BM along with IL-23−/− spleen cells resulted in significantly enhanced survival compared with GVHD control mice (44% vs 5%, P < .001). To further assess the antileukemia response, similarly transplanted mice were bled 5 to 7 weeks after transplantation. Leukemia control animals had significant increases in WBC and ANC consistent with CML, whereas the WBC and ANC in recipients of IL-23−/− marrow grafts were significantly lower and within normal limits (Figure 7B). The overall spleen cellularity and total number of splenic Gr-1+ Mac-1+ cells were also higher in leukemia control animals versus mice reconstituted with mature T cells from IL-23−/− donors (Figure 7C). To provide definitive confirmation that a GVL response had occurred in surviving mice transplanted with IL-23−/− marrow grafts, we performed real-time PCR analysis for the bcr/abl oncogene from RNA extracted from tissues that had been previously shown to harbor disease (Figure 6). Compared with leukemia control animals, there was a dramatic reduction in bcr/abl transcripts in the spleen and colon of animals transplanted with IL-23−/− grafts when assessed 32 days after transplantation (Figure 7D). To further define the potency of the GVL response in the colon, which was the site of maximal protection, we performed additional studies to quantify bcr/abl transcript levels in recipients of IL-23−/− grafts versus GVHD controls. In these experiments, the donor T-cell dose was attenuated so that GVHD control mice would survive a sufficient time for analysis. These studies demonstrated a significant reduction in bcr/abl transcripts in the colons of both groups relative to leukemia controls, but no difference was observed between recipients of IL-23−/− grafts compared with GVHD control mice. These results indicated that the selective GVHD protection in the colon did not appear to compromise a localized antileukemia response (Figure 7E). Finally, to assess the durability of the GVL effect in this model, we then examined transplanted animals 80 days after BMT. Examination of the spleen, which is the site of maximal leukemia burden (Figure 6), demonstrated persistent leukemia in control animals but no detectable disease in the spleens of recipients of IL-23−/− grafts (Figure 7F). Collectively, these results demonstrated that transplantation with marrow grafts from IL-23−/− donors was effective in reducing GVHD without loss of the GVL effect in a clinically relevant leukemia model with more indolent disease kinetics.
Transplantation of IL-23−/− marrow grafts reduces GVHD severity without loss of the GVL effect in a chronic leukemia model. (A) Lethally irradiated FVB mice were transplanted with TCD B6 and non-TCD bcr/abl BM cells alone (□, n = 18; Leuk), TCD B6, and non-TCD bcr/abl BM cells plus B6 spleen cells adjusted to yield a T-cell dose of 3.5 × 106 αβ T cells (■, n = 20; GVHD) or TCD IL-23−/− and non-TCD bcr/abl BM cells plus IL-23−/− spleen cells adjusted to yield the same T-cell dose (○, n = 18). Overall survival is shown. (B-C) Lethally irradiated FVB mice were transplanted with a combination of TCD B6 and non-TCD bcr/abl BM cells alone (n = 15; Leuk) or together with IL-23−/− spleen cells (n = 15; IL-23−/−). Mice in both groups were bled 32 to 50 days after transplantation. WBC and ANC are shown. Data are cumulative results from 3 experiments. (C) Similarly transplanted animals (n = 9/group) were killed 35 to 42 days after BMT. Spleen cellularity and the absolute number of splenic Gr-1+ Mac-1+ cells are shown. Data are cumulative results from 2 experiments and are the mean ± SEM. (D) Fold increase in bcr/abl mRNA levels over c-abl in the spleen or colon of lethally irradiated FVB mice transplanted with TCD IL-23−/− and non-TCD bcr/abl BM cells alone (n = 9) or together with IL-23−/− spleen cells (n = 9) analyzed 32 days after transplantation. Data are cumulative results from 2 experiments and are the mean ± SEM. (E) Lethally irradiated FVB mice were transplanted with TCD B6 and non-TCD bcr/abl BM cells alone (n = 8), TCD B6 and non-TCD bcr/abl BM cells plus B6 spleen cells adjusted to yield a T-cell dose of 3 × 106 αβ T cells (n = 3), or TCD IL-23−/− and non-TCD bcr/abl BM cells plus IL-23−/− spleen cells adjusted to yield the same T-cell dose (n = 5). Mice were killed 28 to 29 days after transplantation, and the fold increase of bcr/abl mRNA levels over c-abl in the colon is shown. (F) Lethally irradiated FVB mice were transplanted with a combination of TCD IL-23−/− and non-TCD bcr/abl BM cells alone (n = 4) or together with IL-23−/− spleen cells adjusted to yield 3.5 × 106 αβ T cells (n = 5). Mice were killed 80 days after transplantation, and spleen tissues were obtained from each animal to determine bcr/abl mRNA levels. Data are presented as fold increase over c-abl. *P ≤ .05. **P < .01.
Transplantation of IL-23−/− marrow grafts reduces GVHD severity without loss of the GVL effect in a chronic leukemia model. (A) Lethally irradiated FVB mice were transplanted with TCD B6 and non-TCD bcr/abl BM cells alone (□, n = 18; Leuk), TCD B6, and non-TCD bcr/abl BM cells plus B6 spleen cells adjusted to yield a T-cell dose of 3.5 × 106 αβ T cells (■, n = 20; GVHD) or TCD IL-23−/− and non-TCD bcr/abl BM cells plus IL-23−/− spleen cells adjusted to yield the same T-cell dose (○, n = 18). Overall survival is shown. (B-C) Lethally irradiated FVB mice were transplanted with a combination of TCD B6 and non-TCD bcr/abl BM cells alone (n = 15; Leuk) or together with IL-23−/− spleen cells (n = 15; IL-23−/−). Mice in both groups were bled 32 to 50 days after transplantation. WBC and ANC are shown. Data are cumulative results from 3 experiments. (C) Similarly transplanted animals (n = 9/group) were killed 35 to 42 days after BMT. Spleen cellularity and the absolute number of splenic Gr-1+ Mac-1+ cells are shown. Data are cumulative results from 2 experiments and are the mean ± SEM. (D) Fold increase in bcr/abl mRNA levels over c-abl in the spleen or colon of lethally irradiated FVB mice transplanted with TCD IL-23−/− and non-TCD bcr/abl BM cells alone (n = 9) or together with IL-23−/− spleen cells (n = 9) analyzed 32 days after transplantation. Data are cumulative results from 2 experiments and are the mean ± SEM. (E) Lethally irradiated FVB mice were transplanted with TCD B6 and non-TCD bcr/abl BM cells alone (n = 8), TCD B6 and non-TCD bcr/abl BM cells plus B6 spleen cells adjusted to yield a T-cell dose of 3 × 106 αβ T cells (n = 3), or TCD IL-23−/− and non-TCD bcr/abl BM cells plus IL-23−/− spleen cells adjusted to yield the same T-cell dose (n = 5). Mice were killed 28 to 29 days after transplantation, and the fold increase of bcr/abl mRNA levels over c-abl in the colon is shown. (F) Lethally irradiated FVB mice were transplanted with a combination of TCD IL-23−/− and non-TCD bcr/abl BM cells alone (n = 4) or together with IL-23−/− spleen cells adjusted to yield 3.5 × 106 αβ T cells (n = 5). Mice were killed 80 days after transplantation, and spleen tissues were obtained from each animal to determine bcr/abl mRNA levels. Data are presented as fold increase over c-abl. *P ≤ .05. **P < .01.
Discussion
The GVL effect is one of the most potent demonstrations of effective adoptive immunotherapy. In both clinical and experimental BMT, however, the GVL response is often coexpressed with GVHD. This is because both GVL and GVH reactivity are largely concurrent systemic responses that are mediated primarily by donor T cells. In the current study, we demonstrate that GVHD can be significantly attenuated without a corresponding loss in the GVL effect by using a strategy designed to regionally protect one of the major GVHD target organs. These data indicate that the localized protection of the colon that occurs as a consequence of blockade of IL-23 signaling does not abrogate a systemically directed GVL effect. Rather, regional protection of the colon allows for eradication of leukemia while reducing the severity of GVHD in the colon. These results also serve to extend our previous report,11 which showed that IL-23 plays a critical role in the pathophysiology of GVHD in the colon serving as a bridge between conditioning regimen-induced mucosal injury and LPS translocation and subsequent proinflammatory cytokine production and GVHD-associated pathologic damage.
One of the major factors that has been shown to influence the GVL effect is the type and stage of leukemia for which the transplantation is conducted. A number of studies have demonstrated that the antileukemia effect associated with GVHD differs in patients with acute myelogenous leukemia, acute lymphoblastic leukemia, and CML.4,20 For that reason, to determine whether GVH and GVL reactivity could be effectively dissociated by blockade of IL-23 signaling, we examined this question using tumor models with very different biologic characteristics. The A20 lymphoma is a rapidly dividing, B cell–derived tumor that typically causes death within 4 weeks in nearly all recipients transplanted with BM cells alone. In contrast, the second model we used is a CML model in which induction of the bcr/abl oncogene results in the gradual accumulation of malignant cells, and leukemic cell death occurs in only a minority of similarly reconstituted animals because of the slow kinetics of the disease. In both models, we observed that mice could be cured of their disease without dying of GVHD by the inhibition of IL-23 signaling through either genetic or antibody-based approaches. To establish that animals were indeed cured of disease, we performed sensitive methods for tumor detection using BLI plus pathologic analysis to assess for persistent lymphoma cells in mice challenged with A20 cells, and real-time quantitative PCR to quantitate for bcr/abl transcripts in the CML model. The latter, in particular, has been shown to be a powerful tool for assessing disease status after allogeneic SCT and predicting for leukemia recurrence.21-23 In both instances, a potent GVL response was observed in mice that were otherwise protected from lethal GVHD. We should emphasize, however, that these studies do not exclude the possibility that blockade of IL-23 signaling might attenuate the GVL effect if tumor doses were escalated to even higher doses.
The selective targeting of the gastrointestinal tract is an attractive strategy for the separation of GVL and GVH responses. First, numerous studies have demonstrated that the gastrointestinal tract has a critical role in GVHD biology.11,12,18 Mucosal damage induced by the conditioning regimen coupled with inflammation occurring as a consequence of migration of donor T cells into the colon microenvironment induces the overproduction of proinflammatory cytokines.11,12,24 Moreover, the leakage of LPS across damaged mucosal surfaces further enhances inflammatory cytokine production, which serves to perpetuate GVHD and also exacerbate systemic manifestations of the disease.25-27 IL-23, in particular, has been shown to play a pivotal role in GVHD-induced pathologic damage of the gastrointestinal tract. Initial studies in nontransplantation models demonstrated that IL-23 was important in the pathobiology of inflammatory bowel disease.28-31 More recently, we have shown that the absence of donor APC-derived IL-23 secretion significantly attenuates GVHD severity selectively in the colon but not in other tissues sites, such as the liver or lung.11 When considered within the context of leukemia-bearing animals, the colon is generally not considered to be a major site of leukemia compared with nodal and medullary tissues. Thus, one would predict that the selective abrogation of GVHD in the colon might not significantly compromise an otherwise systemically directed GVL response. This premise was shown to be valid in the A20 model in which tumor cells did not appear to disseminate to the colon during the progression of disease (Figure 3).
Although disease recurrence after allogeneic BMT most often occurs in the BM, extramedullary relapse in the absence of, or concurrent with, BM recurrence has also been reported in a number of studies.13,14,32,33 In the latter instances, it has been speculated that these extramedullary tissues, by virtue of their location, are protected from GVL-reactive donor T cells and thereby serve as sanctuary sites for subsequent systemic relapse. Furthermore, studies in patients with recurrent disease who receive donor lymphocyte infusions, whose efficacy is solely dependent on a GVL effect, have also shown that extramedullary relapse is a cause of treatment failure.34 Thus, these studies support the premise that, under certain circumstances, there are “protected” tissue sites in which leukemia cells are able to reside and thereby escape from immune-mediated eradication. For that reason, we considered that the selective GVHD protection afforded the colon might reduce the potency of the local GVL effect and serve as a potential sanctuary site for recurrent disease under conditions in which leukemia did disseminate to the colon. To address this question, we used a model analogous to human CML in which disease was shown to be widely disseminated in multiple tissue sites, including the colon (Figure 6). These studies demonstrated that blockade of IL-23 signaling did not compromise the GVL effect in this organ as sensitive molecular testing revealed a significant reduction in the number of bcr/abl transcripts. Indeed, we observed no significant difference in transcripts when recipients of IL-23−/− grafts were compared with GVHD control animals (Figure 7). Because eradication of CML has been shown in humans to be critically dependent upon donor T cells,35,36 one can infer from these studies that the attenuation of GVHD in the colon did not abrogate the ability of donor-derived T cells to mediate an antileukemia effect. Moreover, we observed the near-complete eradication of bcr/abl transcripts in the spleen (Figure 7), which is a primary site of leukemia in this model when examined 3 months after transplantation. Thus, the GVL response appeared to be durable as well.
The targeting of IL-23 is also of interest from the standpoint that this cytokine has been postulated to play an important role in tumorigenesis. In the absence of IL-23, Langowski et al37,38 demonstrated that mice are almost completely resistant to tumor formation in a chemical carcinogenesis model. The authors surmised that IL-23 prevents the induction of a fully functional cytotoxic T-cell response as well as alters the tumor microenvironment by recruiting myeloid cells with suppressive capabilities and promoting angiogenesis through induction of other soluble factors. A more recent report by Kortylewski et al39 also demonstrated that IL-23 induces a suppressive immune environment that favors tumor growth. Notably, these studies were performed in solid tumors, so whether this premise is valid in hematologic malignancies, which are the major indication of allogeneic SCT, remains to be determined. Moreover, not all studies have validated these conclusions.40,41 Nonetheless, if IL-23 does indeed contribute to leukemogenesis, the simultaneous ability to both prevent GVHD and inhibit leukemia cell growth would enhance the clinical appeal of this strategy. Further studies are needed to determine whether blockade of IL-23 signaling has direct antileukemic effects or is able to reverse a suppressive immune environment in the absence of a concurrent GVH response.
In conclusion, these studies demonstrate that the regional GVHD protection in the colon, which occurs as a consequence of blockade of IL-23 signaling, does not abrogate a functional GVL response. Furthermore, the induced GVL effect is durable and does not appear to be constrained by the kinetic profile of the underlying leukemia. Given the primacy of the gastrointestinal tract in the pathophysiology of GVHD, these results suggest that this strategy may be a viable clinical approach to reduce GVHD severity, yet preserve antileukemia reactivity, in patients undergoing allogeneic SCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants HL064603, HL081650, and DK083358) and the Midwest Athletes Against Childhood Cancer Fund (Milwaukee, WI).
National Institutes of Health
Authorship
Contribution: R.D. designed and performed research, analyzed data, and wrote the paper; R.K. performed all pathologic analyses; M.J.H. designed the primers and assisted with real-time quantitative PCR studies; H.S. performed research; D.C and C.S.H. provided vital reagents; and W.R.D. designed and supervised research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William R. Drobyski, Bone Marrow Transplant Program, 9200 W Wisconsin Ave, Milwaukee, WI 53226; e-mail: wdrobysk@mcw.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal