Abstract
Although the liver is known to be the main site of factor VIII (FVIII) production, other organs are probably also important for the regulation of FVIII secretion. However, the study of the regulation of extrahepatic FVIII production has been hampered by the lack of definitive identification of human tissues able to secrete FVIII. Recent studies have shown that lung endothelial cells can synthesize FVIII. We therefore studied the production of FVIII by endothelial cells purified from other vascular beds. Because physiologic stress results in a rapid elevation of FVIII, we also investigated whether endothelial cells can store FVIII and secrete it after treatment with agonists. Microvascular endothelial cells from lung, heart, intestine, and skin as well as endothelial cells from pulmonary artery constitutively secreted FVIII and released it after treatment with phorbol-myristate acetate and epinephrine. By contrast, endothelial cells from the aorta, umbilical artery and umbilical vein did not constitutively secrete FVIII or release it after treatment with agonists, probably because of a lack of FVIII synthesis. Extrahepatic endothelial cells from certain vascular beds therefore appear to be an important FVIII production and storage site with the potential to regulate FVIII secretion in chronic and acute conditions.
Introduction
Although the liver is known to be the main site of factor VIII (FVIII) production, several observations suggest that other organs also are important for the regulation of FVIII synthesis. Transplantation of a normal canine liver to a dog with hemophilia A corrects plasma FVIII levels1 ; however, transplantation of the liver of a hemophilic dog to a normal animal does not change the phenotype of the recipient into that of a severely hemophilic animal.2 Similarly, transplantation of the liver of a healthy donor to a patient with hemophilia A results in normal FVIII levels,3 and transplantation of the liver of a human donor with moderate hemophilia A has not resulted in abnormal FVIII levels in the recipient.4
Extrahepatic FVIII production site(s) are probably important for the regulation of FVIII production, as suggested by the normal or increased FVIII levels during severe liver diseases, which result in low plasma levels of the other coagulation factors.5 However, the study of the regulation of extrahepatic FVIII production has been hampered by the lack of identification of human extrahepatic tissues able to produce functional FVIII6-10 and by the important difference in the regulation of plasma FVIII levels between humans and mice.11
Recent observations have shown that human lungs secrete FVIII.12 A significant production of FVIII has also been reported after transplantation of lungs from healthy dogs into hemophilic recipients.9 In agreement with these observations, endothelial cells (ECs) purified from human lung microvasculature secrete FVIII in vitro. It is, however, still unknown whether ECs from other extrahepatic human tissues are also able to secrete functional FVIII.12 We therefore studied the production of FVIII by ECs purified from different vascular beds.
We also evaluated the release of FVIII on incubation with an EC agonist, phorbol 12-myristate 13-acetate (PMA). Because acute stress associated with the release of epinephrine results in a rapid elevation of FVIII in humans,13-16 we also investigated whether ECs can secrete FVIII after treatment with this agonist.
Methods
Cell culture
Human pulmonary microvascular ECs (HPMECs), human cardiac microvascular ECs (HCMECs), human pulmonary artery ECs (HPAECs), human intestinal microvascular ECs (HIMECs), human dermal microvascular ECs (HDMECs), human aortic ECs (HAECs), human umbilical vein ECs (HUVECs), and human umbilical artery ECs (HUAECs) were purchased from ScienCell Research Laboratories.
The microvessels as defined by the manufacturer include all vessels, the diameter of which is less than 50 μm, with most vessels being between 15 and 30 μm. The ECs from the microvessels were isolated from the peripheral tissues with no obvious visible blood vessels. Each lot of cells was from an individual donor. HPMECs and HCMECs had been cryopreserved immediately after purification, whereas the other cell types were delivered frozen at passage 1 or 2. HCMECs and HUVECs at passage 2, were also purchased from Lonza.
Cells were thawed, diluted at 30 000 cells/mL, and seeded at 15 000 cells/cm2 in 96-well plates precoated with 2 μg/cm2 fibronectin (Sigma-Aldrich). After a 2-minute centrifugation at 1200 rpm, two-thirds of the medium was removed to eliminate a fraction of dimethylsulfoxide (DMSO) and replaced by fresh medium. After 24 hours, the medium was completely removed and replaced by 200 μL of fresh medium. Cultures were maintained at 37°C and 5% CO2. The medium was replaced 3 times a week for up to 3 months.
All cells, irrespective of the supplier, were cultured in EC medium containing 1% EC growth supplement and 1% penicillin/streptomycin solution (ECM) supplemented with 5% fetal bovine serum (FBS; ECM-FBS), provided by ScienCell.
For some experiments, HUVECs and HCMECs were expanded in vitro for several passages according to the manufacturer's recommendations. Cells reaching approximately 80% confluence were harvested by treatment with trypsin/EDTA (ethylenediaminetetraacetic acid) solution (ScienCell) and diluted to 8500 cells/cm2 in 6-well plates. Culture medium was replaced every 2 days.
All EC cultures were evaluated by phase-contrast microscopy for their typical “cobble stone” structure. Preparations contaminated by other cell types were discarded.
Activation of ECs
Endothelial cells were plated in microculture wells as described in “Cell culture” and kept 8 to 90 days in culture. The culture medium was removed, and the cells were washed twice with ECM. After removal of the culture medium the cells were incubated for 1 hour in 100 μL of ECM supplemented with 100 nM PMA (Sigma-Aldrich), 10 or 100 ng/mL epinephrine (STEROP Laboratory).
Measurement of FVIII:C
Determination of FVIII activity in cell culture supernatant was performed with Coatest SP Factor VIII assay from Chromogenix Instrumentation Laboratory SpA after adding 0.29% sodium citrate (Na3C3H5O(CO2)3) and 10 μg/mL polybrene. In control experiments, supernatants were incubated with 2 human monoclonal antibodies to FVIII (BO2C11,19 1.8 μg/mL; and BO2BII,20 1.8 μg/mL) before measuring residual FVIII:C. Addition of polybrene was necessary for neutralization of heparin present in the EC culture medium. Sodium citrate was added to allow the first incubation phase (FVIII activation) to occur in the absence of calcium ions. Two reference curves were prepared by diluting a human plasma pool in the cell culture medium (ECM-FBS or ECM). In control experiments, supernatants were incubated with 2 anti-FVIII human monoclonal antibodies (mAbs), BO2C1119 and BO2BII,20 both at 1.8 μg/mL, before measuring residual FVIII:C.
Coatest SP Factor VIII assay and Factor VIII Chromogenic Assay (Dade Behring) were used to measure intracellular FVIII activity in undiluted cell lysate. In control experiments, cell lysates were preincubated with 2 human monoclonal antibodies to FVIII (BO2C11,19 5 μg/mL; and BO2BII,20 5 μg/mL). Reference curves were prepared by diluting a human plasma pool in cell lysis buffers.
The limit of detection of the Coatest SP Factor VIII assay was defined as the first concentration of the reference curve above the mean plus 3 SDs of the negative controls, rounded at the first upper decimal.
Cell lysis
After removal of the culture medium and washing of the cells with Dulbecco phosphate-buffered saline 1× (DPBS; Gibco, Invitrogen), confluent EC cultures on 96-well plates were lysed by adding 200 μL/well CelLytic M cell lysis reagent (lysis buffer; Sigma-Aldrich). Alternatively, cells were lysed by 3 freeze-thaw cycles in a buffer containing 10mM Tris (tris(hydroxymethyl)aminomethane)/HCl, pH 7.5, 150mM NaCl, 1× protease inhibitor (Halt Protease Inhibitor Single-use Cocktail; Thermo Scientific) and 25 mM sucrose (freeze/thaw buffer).
Immunoassays
VWF antigen concentrations were determined by enzyme-linked immunoabsorbent assay (ELISA) with rabbit polyclonal anti–human VWF (Dako).12
FVIII:Ag was measured in ELISA with the use of the anti-FVIII mouse mAb 4H10, kindly provided by Marc Hoylaerts (KULeuven) and the peroxidase-labeled anti-FVIII antibody provided in the F8C-EIA kit (Affinity Biologicals). Microlon microtiter plates (Greiner Bio-One) were coated overnight with 7 μg/mL mAb 4H10 in glycin buffer (20mM glycine, 34mM NaCl, pH 9.2). After washing with PBS containing 0.005% Tween 80 (washing buffer), the plates were saturated for 30 minutes with 55mM Tris/HCl, 150mM NaCl, 1% bovine serum albumin, and 0.1% Tween 20 (pH 7.3). Samples diluted or not in culture medium or lysis buffer were added to the plates and incubated for 2 hours. After washing, the peroxidase-labeled anti-FVIII antibody was added to the plates for 2 hours. Reference curves were prepared in the same buffer as the samples.
Quantitative real-time polymerase chain reaction
After detaching the endothelial and CHO cells with Trypsin/EDTA solution (ScienCell) and 25% Trypsin-EDTA (Gibco, Invitrogen), respectively, followed by Trypsin Neutralization Solution (ScienCell), total RNA was isolated with the QIAGEN RNeasy mini kit followed by a first-strand cDNA preparation that used oligo dT primers and the SuperScript III First-Strand Synthesis kit (Invitrogen) for reverse transcription–polymerase chain reaction (RT-PCR), according to the manufacturer's instructions.
We used 200 ng of each cDNA sample to semiquantify the FVIII and VWF gene expression. The couple of primers, 5′-TCGAAACTAATACGACTCACTATAGGGAAGATGAAGCTTGGATCCATGAGCACACTT-TTTCTGGTGTACAGC-3′ (forward) and 5′-TTCTCGACTTGCGGCCGCATATTGTCCTGAAGCTGTAATCTG-3′ (reverse), was selected to amplify a 99-base pair (bp) region of FVIII cDNA between exons 19 and 20. Primers, 5′-TGCTGGTATGGAGTATAGGCAGTG-3′ (forward) and 5′-CCGGAATGCACGCAG G-3′ (reverse), were used to amplify a VWF fragment of 174 bp from exons 8 and 9. Transcript levels were normalized to levels of a 189-bp mRNA encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcribed with the use of primers 5′-TGGTATCGTGGAAGGACTCATGAC-3′ (forward) and 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′ (reverse).
Quantitative PCR was performed with a SYBR-green QPCR master mix (Applied Biosystems) and a 7500 Fast Real-Time PCR System (Applied Biosystems). Each experimental sample was run in duplicate with the following steps: incubation for 2 minutes at 50°C and for 10 minutes at 95°C followed by 50 cycles of 95°C for 15 seconds, 58°C annealing for 25 seconds, and elongation at 60°C for 45 seconds. After PCR a melting curve (60°C-90°C) was produced for product verification and purity.
Data were analyzed with the 7500 fast system SDS software, Version 1.4.0.25 (Applied Biosystems) and are expressed as relative gene expression (fold increase) by comparison to CHO cells with the use of the 2−ΔΔCt method.
Immunofluorescence staining
HCMECs and HUVECs at passages 1, 2, or 3 were cultured at a density of 8500 cells/cm2 on 8-well Sterile Permanox chamber slides (Nunc) precoated with 2 μg/cm2 fibronectin (Sigma-Aldrich) until they reached confluence. CHO-FVIII and CHO-VWF were plated at a similar density on the Permanox slides and were stained when they reached 50% confluence. All cells were washed with DPBS (Gibco, Invitrogen) supplemented with 0.005% Tween 20 (DPBS/Tween 20) for 5 minutes and fixed with BD Cytofix (BD Biosciences) for 20 minutes at room temperature and protected from direct light,
After washing 3 times for 10 minutes in DPBS/Tween 20, cells were permeabilized with 0.2% Triton X-100 containing 1% normal rabbit serum (Dako) for 10 minutes on ice and then washed again 3 times for 10 minutes in DPBS/Tween 20 containing 1% normal rabbit serum. Intracellular FVIII antigen was labeled with 5 μg/mL affinity-purified sheep anti–human FVIII antibody (Innovative Research Inc) diluted in a staining buffer containing 0.1% gelatin from cold fish skin (Sigma-Aldrich), 1.5M NaCl, 0.1% casein, and 10mM Tris/HCl, pH 7.2 (staining buffer) for 2 hours at room temperature. After washing 3 times for 10 minutes in staining buffer supplemented with 1% normal rabbit serum, cells were further stained with 5 μg/mL Alexa Fluor 488 FluoroNanogold rabbit anti–sheep Fab′ (Nanoprobes Inc) and 3.1 μg/mL monoclonal anti–human VWF, 82D6A3,21 conjugated to Alexa Fluor 568 for 2 hours at room temperature and protected from light. Anti–human VWF antibody, 82D6A3,21 was kindly provided by Marc Hoylaerts (KULeuven) and was conjugated to Alexa Fluor 568 (Invitrogen) according to the manufacturer's instruction. Slides were mounted with Prolong Gold Antifade reagent with DAPI (4,6 diamidino-e-phenylindole; Invitrogen) overnight at room temperature and stored at −20°C before viewing by confocal laser scanning microscopy with a Multiphoton Zeiss CLSM510 META NLO (Carl Zeiss).
Statistical analysis
A 2-sided Student t test was used to compare FVIII and VWF secretion before and after stimulation of ECs.
Results
Different microvascular and macrovascular ECs secrete FVIII
The Coatest SP Factor VIII assay was modified for the detection of low FVIII concentrations in cell culture supernatant. To avoid bias due to matrix constituents, reference curves were prepared in the medium in which the cells were maintained. Dilution of samples is required when measuring FVIII in human plasma with a chromogenic assay because the assay has to be performed with limited FVIII concentrations and because dilutions minimize heterogeneity between samples. By contrast, we only seldom needed to dilute cell culture supernatants to measure FVIII. The low FVIII concentrations ensured that limited FVIII amounts were added to the reagents of the tests. Furthermore, cell culture supernatants and reference curves contained the same amount of cell culture medium and FBS, so that there was little heterogeneity between samples. Although the presence of FBS was expected to influence the chromogenic assay, similar dilution curves were obtained in culture medium with or without FBS (supplemental Figure 1A,E,F, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The limit of detection of FVIII:C was 0.2 mU/mL in culture medium with or without FBS (supplemental Figure 1A). The specificity of the assay was confirmed by analyzing each sample in the presence and in the absence of neutralizing anti-FVIII antibodies.
Endothelial cells were seeded in microculture wells. After 1 week, FVIII accumulated in the cell culture supernatant during a 48-hour time period was measured in the modified Coatest SP Factor VIII assay as described in “Measurement of FVIII:C.” As shown in Table 1, microvascular ECs from several tissues, such as lung, intestine, skin, and heart, produced significant amounts of FVIII. HPAECs also secreted FVIII (Table 2), indicating that FVIII production is not restricted to microvascular ECs. A good parallelism was observed between reference curves prepared in culture medium and dilutions of cell culture supernatants. Supplemental Figure 1E shows parallelism between a reference curve and dilutions of a 48-hour cell culture supernatant of HCMECs. FVIII:C in the first 4 dilutions of the supernatant was calculated with the dilutions of the culture medium spiked with FVIII as reference curve. The coefficient of variation (CV) of the concentrations calculated for the first 4 dilutions of the supernatant was 20% or less, indicating that the parallelism between the reference curves and the samples was within an acceptable limit.22 Therefore, the assay provides not only a qualitative but also a quantitative estimation of FVIII concentration in cell culture supernatants. FVIII activity was completely abolished by anti-FVIII mAbs (supplemental Figure 1E), indicating the specificity of FVIII detection. In control experiments, FVIII was also detected in the supernatant of HCMECs with FVIII:Ag ELISA. FVIII:C and FVIII:Ag were 11 ± 0.7 mU/mL and 15.5 ± 0.9 mU/mL (mean ± SD; n = 40), respectively, in 48-hour cell culture supernatants of HCMECs from ScienCell and 13.1 ± 2.8 mU/mL and 15.8 ± 2.8 mU/mL (mean ± SD; n = 5), respectively, in the supernatant of HCMECs from Lonza.
FVIII and VWF production by microvascular ECs
| Cell type . | Passage no. . | FVIII:C, mU/mL . | VWF:Ag, mU/mL . |
|---|---|---|---|
| HCMEC* | 1° | 11.5 ± 0.8 (n = 5) | 74.3 ± 0.1 (n = 5) |
| HCMEC* | 1° | 11.5 ± 0.9 (n = 10) | 115.2 ± 6.1 (n = 10) |
| HCMEC† | 3°°° | 13.17 ± 2.8 (n = 5) | 124.8 ± 19.1 (n = 5) |
| HIMEC* | 2°° | 2.6 ± 0.3 (n = 10) | 16 ± 0.1 (n = 10) |
| HDMEC* | 2°° | 2.3 ± 0.2 (n = 5) | 32.8 ± 0.1 (n = 5) |
| HPMEC* | 1° | <0.2 ± 0.25 (n = 10) | 36 ± 0.1 (n = 10) |
| HPMEC* | 1° | 0.68 ± 0.11 (n = 10) | 54.9 ± 3.11 (n = 10) |
| Cell type . | Passage no. . | FVIII:C, mU/mL . | VWF:Ag, mU/mL . |
|---|---|---|---|
| HCMEC* | 1° | 11.5 ± 0.8 (n = 5) | 74.3 ± 0.1 (n = 5) |
| HCMEC* | 1° | 11.5 ± 0.9 (n = 10) | 115.2 ± 6.1 (n = 10) |
| HCMEC† | 3°°° | 13.17 ± 2.8 (n = 5) | 124.8 ± 19.1 (n = 5) |
| HIMEC* | 2°° | 2.6 ± 0.3 (n = 10) | 16 ± 0.1 (n = 10) |
| HDMEC* | 2°° | 2.3 ± 0.2 (n = 5) | 32.8 ± 0.1 (n = 5) |
| HPMEC* | 1° | <0.2 ± 0.25 (n = 10) | 36 ± 0.1 (n = 10) |
| HPMEC* | 1° | 0.68 ± 0.11 (n = 10) | 54.9 ± 3.11 (n = 10) |
Microvascular cells provided frozen by the suppliers (*ScienCell; †Lonza) were thawed and plated in 96-well plates. Cells were at passage 1(°) when provided by the supplier without any in vitro passage, and at passage 2(°°) or 3(°°°) when supplied after 1 or 2 passages in vitro, respectively. After 1 week, culture medium was completely replaced by fresh medium, and, after a further 48-hour incubation period, FVIII:C was measured with a Coatest SP FVIII assay and VWF:Ag with an ELISA. Results are expressed as the mean plus or minus SD of 5 or 10 microculture wells.
FVIII and VWF production by macrovascular ECs
| Cell type . | Passage no. . | FVIII:C, mU/mL . | VWF:Ag, mU/mL . |
|---|---|---|---|
| HPAEC* | 3°°° | 1.2 ± 0.2 (n = 10) | 94.5 ± 0.1 (n = 10) |
| HUVEC* | 2°° | <0.2 (n = 5) | 214.9 ± 0.2 (n = 5) |
| HUVEC† | 3°°° | <0.2 (n = 5) | 263.5 ± 8.5 (n = 5) |
| HUAEC* | 3°°° | <0.2 (n = 5) | 66.5 ± 0.1 (n = 5) |
| HAEC* | 2°° | <0.2 (n = 5) | 82.6 ± 0.1 (n = 5) |
| Cell type . | Passage no. . | FVIII:C, mU/mL . | VWF:Ag, mU/mL . |
|---|---|---|---|
| HPAEC* | 3°°° | 1.2 ± 0.2 (n = 10) | 94.5 ± 0.1 (n = 10) |
| HUVEC* | 2°° | <0.2 (n = 5) | 214.9 ± 0.2 (n = 5) |
| HUVEC† | 3°°° | <0.2 (n = 5) | 263.5 ± 8.5 (n = 5) |
| HUAEC* | 3°°° | <0.2 (n = 5) | 66.5 ± 0.1 (n = 5) |
| HAEC* | 2°° | <0.2 (n = 5) | 82.6 ± 0.1 (n = 5) |
Macrovascular cells provided frozen by the suppliers (*ScienCell; †Lonza) were thawed and plated in 96-well plates. Cells were at passages 2(°°) or 3(°°°) when supplied after 1 or 2 passages in vitro, respectively. After 1 week, culture medium was completely replaced by fresh medium, and, after a further 48-hour incubation period, FVIII:C was measured with Coatest SP FVIII assay and VWF:Ag with an ELISA. Results are expressed as the mean plus or minus SD of 5 or 10 microculture wells.
By contrast, HUVECs and HUAECs did not release any measurable FVIII (when using the Coatest SP Factor VIII assay). FVIII was also undetectable in the supernatant of HUVECs when using FVIII:Ag ELISA.
HUVECs, HUAECs, and HAECs, which did not secrete any measurable FVIII, produced amounts of VWF similar to ECs derived from lung arteries or cardiac microvessels that did produced FVIII (Table 1; Figure 1).
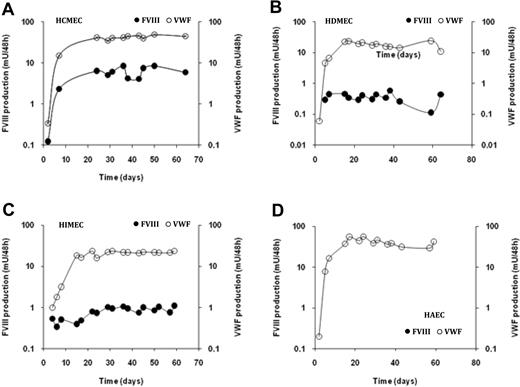
Long-term FVIII production by ECs. Endothelial cells (A, HCMECs; B, HDMECs; C, HIMECs, and D, HAECs) were plated in microculture wells. The culture medium was completely removed 3 times a week. FVIII concentrations (left y-axis; ●) and VWF:Ag (right y-axis; ○) were measured in culture supernatant collected 48 hours after replacement of the medium. Results are expressed in logarithmic scale as the mean ± SD of 5 or 10 microcultures.
Long-term FVIII production by ECs. Endothelial cells (A, HCMECs; B, HDMECs; C, HIMECs, and D, HAECs) were plated in microculture wells. The culture medium was completely removed 3 times a week. FVIII concentrations (left y-axis; ●) and VWF:Ag (right y-axis; ○) were measured in culture supernatant collected 48 hours after replacement of the medium. Results are expressed in logarithmic scale as the mean ± SD of 5 or 10 microcultures.
Long-term FVIII production by confluent ECs
Microscopic observation indicated that ECs plated in microculture wells reached confluence after a few days and then survived as a confluent monolayer for several weeks. We therefore evaluated the persistence of FVIII and of VWF secretion by ECs kept confluent at passage 1, 2, or 3 in microculture wells. Cell culture supernatant was replaced every 2 to 3 days, and concentrations of FVIII and of VWF secreted over a 48-hour time period were measured. As shown in Figure 1, a significant and stable FVIII production was detected up to 8 weeks after initiation of the cell culture. FVIII production was observed up to 4 months in some microcultures (data not shown). In addition, the production of FVIII by HCMECs remained stable after 3 further passages in vitro, strongly arguing that FVIII released in the cell culture supernatant is derived from de novo synthesis rather than from a storage pool formed before removal of the cells from human tissues. Similarly, in those culture conditions, a stable production of VWF was observed for 4 months for all EC cultures, although the levels of VWF production varied according to the origin of the cells (Figure 1).
Intracellular FVIII production
The lack of FVIII production by several EC types such as HAECs, HUVECs, and HUAECs could be due to either a secretion defect or a lack of FVIII synthesis.
We therefore measured FVIII activity and FVIII:Ag in cell extracts obtained with a lysis buffer. FVIII activity was detected in all the cells secreting FVIII but not in HAECs, HUVECs, and HUAECs (Tables 3,Table 4–5; data not shown). In additional experiments, we measured FVIII in cell extracts obtained by freeze/thawing of HUVECs and of HCMECs. With the use of this technique a significant amount of FVIII:C and FVIII:Ag was detected in HCMECs but not in HUVECs (Table 5).
Intracellular production of FVIII and VWF in microvascular ECs
| Cell type . | Passage no. . | FVIII:C, mU/well . | VWF:Ag, mU/well . |
|---|---|---|---|
| HCMEC* | 1° | 1.7 ± 0.2 (n = 10) | 15.6 ± 2.2 (n = 10) |
| HCMEC* | 2°° | 2.5 ± 0.4 (n = 5) | 20.1 ± 2 (n = 5) |
| HCMEC† | 3°°° | 3 ± 1.3 (n = 5) | 15.3 ± 4.4 (n = 5) |
| HIMEC* | 2°° | ND | ND |
| HDMEC* | 2°° | 0.3 ± 0.1 (n = 10) | 10.5 ± 0.9 (n = 10) |
| HPMEC* | 1° | 0.3 ± 0.1 (n = 10) | 11.4 ± 4.2 (n = 10) |
| HPMEC* | 1° | ND | ND |
| Cell type . | Passage no. . | FVIII:C, mU/well . | VWF:Ag, mU/well . |
|---|---|---|---|
| HCMEC* | 1° | 1.7 ± 0.2 (n = 10) | 15.6 ± 2.2 (n = 10) |
| HCMEC* | 2°° | 2.5 ± 0.4 (n = 5) | 20.1 ± 2 (n = 5) |
| HCMEC† | 3°°° | 3 ± 1.3 (n = 5) | 15.3 ± 4.4 (n = 5) |
| HIMEC* | 2°° | ND | ND |
| HDMEC* | 2°° | 0.3 ± 0.1 (n = 10) | 10.5 ± 0.9 (n = 10) |
| HPMEC* | 1° | 0.3 ± 0.1 (n = 10) | 11.4 ± 4.2 (n = 10) |
| HPMEC* | 1° | ND | ND |
Microvascular ECs were plated in 96-well plates and grown for 9 to 24 days. After removal of cell culture supernatant, cells were washed and incubated in lysis buffer. FVIII:C in the cell lysate was measured with a FVIII chromogenic assay and VWF:Ag with an ELISA. Results are expressed as the mean plus or minus SD of 5 or 10 microculture wells. Legends are as in Table 1.
ND indicates not done.
Supplied by ScienCell.
Supplied by Lonza.
Intracellular production of FVIII and VWF in macrovascular ECs
| Cell type . | Passage no. . | FVIII:C, mU/well . | VWF:Ag, mU/well . |
|---|---|---|---|
| HPAEC* | 3°°° | 1.6 ± 0.5 (n = 10) | 20.2 ± 1.9 (n = 10) |
| HUVEC* | 2°°° | < 0.2l (n = 10) | 31.6 ± 3.4 (n = 10) |
| HUAEC* | 3°°° | < 0.2 (n = 10) | 14.1 ± 1.1 (n = 10) |
| HAEC* | 2°° | < 0.2 (n = 10) | 23.6 ± 2.5 (n = 10) |
| Cell type . | Passage no. . | FVIII:C, mU/well . | VWF:Ag, mU/well . |
|---|---|---|---|
| HPAEC* | 3°°° | 1.6 ± 0.5 (n = 10) | 20.2 ± 1.9 (n = 10) |
| HUVEC* | 2°°° | < 0.2l (n = 10) | 31.6 ± 3.4 (n = 10) |
| HUAEC* | 3°°° | < 0.2 (n = 10) | 14.1 ± 1.1 (n = 10) |
| HAEC* | 2°° | < 0.2 (n = 10) | 23.6 ± 2.5 (n = 10) |
Macrovascular endothelial cells were plated in 96-well plates and grown for 9 to 24 days. After removal of cell culture supernatant, cells were washed and incubated in lysis buffer. FVIII:C in the cell lysate was measured with a FVIII chromogenic assay and VWF:Ag with an ELISA. Results are expressed as the mean plus or minus SD of 5 or 10 microculture wells. Legends are as in Table 2.
Supplied by ScienCell.
Intracellular FVIII:Ag and FVIII mRNA
| . | HCMECs . | HUVECs . |
|---|---|---|
| FVIII:Ag prepared by lysis buffer, mU/well* | 2.3 ± 0.3 (n = 5) | ND |
| FVIII:Ag prepared by F/T buffer, mU/well* | 1.3 ± 0.1 (n = 5) | ND |
| VWF:Ag prepared by lysis buffer, mU/well* | 20.1 ± 2 (n = 5) | 31.6 ± 3.4 (n = 10) |
| VWF:Ag prepared by F/T buffer, mU/well* | 7.3 ± 0.6 (n = 5) | ND |
| FVIII:C prepared by lysis buffer, mU/well* | 2.5 ± 0.4 (n = 5) | < 0.2 (n = 10) |
| FVIII:C prepared by F/T buffer, mU/well* | 0.9 ± 0.1 (n = 5) | < 0.2 (n = 10) |
| FVIII mRNA† | 94.3 ± 0.1 | 0 |
| VWF mRNA† | 394 714.5 ± 0.1 | 489 161 ± 0.2 |
| . | HCMECs . | HUVECs . |
|---|---|---|
| FVIII:Ag prepared by lysis buffer, mU/well* | 2.3 ± 0.3 (n = 5) | ND |
| FVIII:Ag prepared by F/T buffer, mU/well* | 1.3 ± 0.1 (n = 5) | ND |
| VWF:Ag prepared by lysis buffer, mU/well* | 20.1 ± 2 (n = 5) | 31.6 ± 3.4 (n = 10) |
| VWF:Ag prepared by F/T buffer, mU/well* | 7.3 ± 0.6 (n = 5) | ND |
| FVIII:C prepared by lysis buffer, mU/well* | 2.5 ± 0.4 (n = 5) | < 0.2 (n = 10) |
| FVIII:C prepared by F/T buffer, mU/well* | 0.9 ± 0.1 (n = 5) | < 0.2 (n = 10) |
| FVIII mRNA† | 94.3 ± 0.1 | 0 |
| VWF mRNA† | 394 714.5 ± 0.1 | 489 161 ± 0.2 |
ND indicates not done.
Cell extracts of 5 microculture wells were prepared either by lysis buffer or by freeze/thawing (F/T) buffer. FVIII:Ag and VWF:Ag were measured with ELISA; FVIII:C was measured with a FVIII chromogenic assay. Results are expressed as the mean plus or minus SD of 5 or 10 microculture wells.
FVIII and VWF mRNA levels in HCMECs and HUVECs are normalized to the level of corresponding genes in a control cell line with the use of the 2−ΔΔCt method. GAPDH expression served as the control gene. All assays were performed in duplicate.
FVIII mRNA was also measured by quantitative reverse transcription PCR analysis in these 2 cell types. After normalizing data to the expression of an endogenous control gene (GAPDH) and to the expression of the genes of interest in a control cell line, a significant FVIII mRNA level was detected in HCMECs but not in HUVECs (Table 5).
The lack of FVIII production by the latter cells can, therefore, be attributed to a lack of intracellular FVIII synthesis rather than to a defect in the FVIII secretion pathway.
FVIII storage pool in ECs
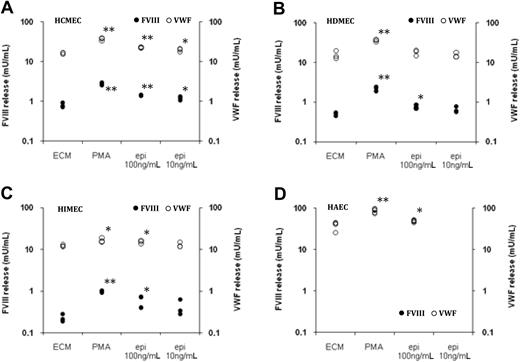
Acute physical stress, such as exercise or insulin-induced hypoglycemia, induces within 30 minutes a rapid increase of FVIII:C in plasma.13,15 This rapid increase suggests that the released FVIII is derived from a preformed storage pool rather than from de novo synthesis. To investigate whether ECs can store FVIII and release it after cell activation, confluent EC cultures were treated with PMA. As shown in Figure 2, a 1-hour treatment with PMA induced a release of FVIII of about the same order of magnitude as the spontaneous FVIII production over a 48-hour time period (Figure 1). By contrast, HAECs, HUVECs, and HUAECs did not release any FVIII after treatment with PMA (Figure 2; data not shown), in agreement with the observation that those cells do not contain measurable intracellular FVIII (Table 4).
FVIII storage and release by ECs. Endothelial cells (A, HCMECs; B, HDMECs; C, HIMECs, and D, HAECs) were plated in microculture wells and kept 8 days in culture. After washing, the cells were incubated in culture medium supplemented with 100nM PMA or epinephrine (epi) at 100 and 10 ng/mL. FVIII concentrations (left y-axis; ●) and VWF:Ag (right y-axis; ○) were measured in culture supernatant collected 1 hour after the addition of the medium. Results are expressed in logarithmic scale as FVIII and VWF concentration in individual microculture wells. **P < .001; *P < .05
FVIII storage and release by ECs. Endothelial cells (A, HCMECs; B, HDMECs; C, HIMECs, and D, HAECs) were plated in microculture wells and kept 8 days in culture. After washing, the cells were incubated in culture medium supplemented with 100nM PMA or epinephrine (epi) at 100 and 10 ng/mL. FVIII concentrations (left y-axis; ●) and VWF:Ag (right y-axis; ○) were measured in culture supernatant collected 1 hour after the addition of the medium. Results are expressed in logarithmic scale as FVIII and VWF concentration in individual microculture wells. **P < .001; *P < .05
PMA is known to induce the release of VWF stored in Weibel-Palade bodies. In agreement with those observations, all EC types tested released VWF on treatment with this agonist. HAECs, HUVECs, and HUAECs, which do not release any FVIII after stimulation with PMA, released amounts of VWF similar to that released by ECs producing FVIII (Figure 2; data not shown).
A good parallelism was observed between reference curves prepared in culture medium and dilutions of culture supernatant of cells stimulated with PMA. Supplemental Figure 1E shows parallelism between a reference curve and a culture supernatant collected 1 hour after stimulation of HCMECs with PMA. FVIII activity was completely abolished by anti-FVIII mAbs (supplemental Figure 1E). Similar data were obtained when cells were activated with PMA in ECM medium containing no FBS (supplemental Figure 1F).
To determine whether FVIII is costored in Weibel-Palade bodies, we investigated FVIII and VWF intracellular localization by immunostaining. With the use of CHO cells transfected with either FVIII or VWF expression vectors, we identified a combination of antibodies allowing costaining of FVIII and VWF with a good specificity and sensitivity. To allow for identification of potential colocalization of FVIII and VWF, the labeling for VWF was adjusted to obtain a staining with intensity similar to that of FVIII. CHO cells transfected with either FVIII or VWF expression vectors and costained for FVIII and VWF are shown in Figure 3E and F. With the use of this optimized staining protocol, FVIII was detected in the cytoplasm of HCMECs (Figure 3A-D) but not in that of HUVECs (Figure 3G-H). A fraction of FVIII was localized in vesicles that were also strongly stained for VWF, suggesting that FVIII, at least in part, is costored with VWF in Weibel-Palade bodies. However, within any given cell, the colocalization of VWF with FVIII was very heterogeneous, and only some Weibel-Palade bodies are clearly positive for FVIII.
Immunolocalization of FVIII and VWF in CHO and ECs by confocal microscopy. CHO cells transfected with an expression vector for FVIII (E) or for VWF (F), HCMECs (A-D), and HUVECs (G-H) were costained for VWF (in red) and FVIII (in green). Nuclei are counterstained with DAPI (blue) in panels D through H. Cells were analyzed by a Multiphoton Zeiss CLSM510 META NLO confocal microscope equipped with LSM 510 acquisition software, Version 4.2.SP1 (Carl Zeiss). All images are taken with a Plan-Apochromat 63×/1.4 oil DIC objective at 2× zoom (B-H) or without zoom (A). Images in panels B through D and G and H are 3D reconstructions. Top panels are (A) representative merge image of FVIII and of VWF costaining in HCMECs (bar, 10 μm); (B-C) staining of FVIII and of VWF, respectively, in HCMECs; (D) merged image of panels B and C. White arrows point out some colocalizations of FVIII and VWF (bars, 5μm). Bottom panels are CHO-FVIII (E), CHO-VWF (F), and HUVECs (G-H) costained for FVIII and VWF (bars, 5 μm).
Immunolocalization of FVIII and VWF in CHO and ECs by confocal microscopy. CHO cells transfected with an expression vector for FVIII (E) or for VWF (F), HCMECs (A-D), and HUVECs (G-H) were costained for VWF (in red) and FVIII (in green). Nuclei are counterstained with DAPI (blue) in panels D through H. Cells were analyzed by a Multiphoton Zeiss CLSM510 META NLO confocal microscope equipped with LSM 510 acquisition software, Version 4.2.SP1 (Carl Zeiss). All images are taken with a Plan-Apochromat 63×/1.4 oil DIC objective at 2× zoom (B-H) or without zoom (A). Images in panels B through D and G and H are 3D reconstructions. Top panels are (A) representative merge image of FVIII and of VWF costaining in HCMECs (bar, 10 μm); (B-C) staining of FVIII and of VWF, respectively, in HCMECs; (D) merged image of panels B and C. White arrows point out some colocalizations of FVIII and VWF (bars, 5μm). Bottom panels are CHO-FVIII (E), CHO-VWF (F), and HUVECs (G-H) costained for FVIII and VWF (bars, 5 μm).
Endothelial cells release FVIII on stimulation with epinephrine
The acute FVIII elevation in plasma after physical stress is probably mediated by epinephrine increase concomitant to physical stress.13,15 We therefore investigated whether ECs can release FVIII after activation with epinephrine. Confluent EC cultures were treated with 2 concentrations of epinephrine to match its pharmacologic concentrations.23,24 As shown in Figure 2, HCMECs, HDMECs, and HIMECs released statistically significant amounts of FVIII when stimulated with 100 ng/mL epinephrine. At 10 ng/mL the 3 types of ECs also released some FVIII, but this reached statistical significance only for HCMECs, possibly because of the variation in FVIII production between different wells and to the limited number of replicates. Measurement of FVIII:Ag in HCMECs after stimulation of the cells was in agreement with the measured FVIII activity (Table 6).
FVIII:C and FVIII:Ag secreted in the supernatant of stimulated HCMECs
| Stimuli . | FVIII:C, mU/mL . | FVIII:Ag, mU/mL . |
|---|---|---|
| PMA 100nM | 6.4 ± 0.9 (n = 8)* | 3.9 ± 0.7 (n = 8)* |
| Epinephrine 100 ng/mL | 5.3 ± 2.2 (n = 8)* | 3.2 ± 1 (n = 8)* |
| Epinephrine 10 ng/mL | 5.5 ± 2.1 (n = 8)* | 3.3 ± 0.9 (n = 8)* |
| Control | 2.9 ± 0.3 (n = 8)* | 2.2 ± 0.3 (n = 8)* |
| Stimuli . | FVIII:C, mU/mL . | FVIII:Ag, mU/mL . |
|---|---|---|
| PMA 100nM | 6.4 ± 0.9 (n = 8)* | 3.9 ± 0.7 (n = 8)* |
| Epinephrine 100 ng/mL | 5.3 ± 2.2 (n = 8)* | 3.2 ± 1 (n = 8)* |
| Epinephrine 10 ng/mL | 5.5 ± 2.1 (n = 8)* | 3.3 ± 0.9 (n = 8)* |
| Control | 2.9 ± 0.3 (n = 8)* | 2.2 ± 0.3 (n = 8)* |
Cardiac microvascular ECs were stimulated with PMA or epinephrine for 1 hour. FVIII:C and FVIII:Ag were measured in the cell supernatants with Coatest SP FVIII assay and ELISA FVIII:Ag, respectively.
P < .01.
Discussion
Extrahepatic FVIII production sites probably play a significant role in regulating FVIII levels in acute as well as in chronic conditions. However, the extrahepatic sites producing FVIII have proven to be difficult to identify, probably because of the trace FVIII concentrations.6-10 To circumvent the limitations inherent to previous approaches, we evaluated the production of FVIII by purified ECs. To guarantee the specificity of the detection, FVIII was measured with a functional assay in the presence and in the absence of high-affinity anti-FVIII mAbs.
HUVECs and HUAECs, as well as HAECs did not secrete any measurable FVIII. Interestingly, blood outgrowth ECs derived from the mononuclear fraction of peripheral human blood did not produce FVIII either (M.J. and A.L., unpublished results, January-June 2009). By contrast, HDMECs, HIMECs, HPMECs, and HCMECs as well as HPAECs produced significant amounts of FVIII. Among the cells producing FVIII, large differences were observed in the amounts of FVIII secreted, whereas all EC cultures produced similar amounts of VWF. The difference in FVIII production between cells purified from different vascular beds is therefore a further characteristic distinguishing ECs from different anatomic sites, in agreement with the many observations of important phenotypic differences between ECs from different vascular beds.25 The release of FVIII by ECs producing it was 5 to 80 times lower than that of VWF, either in basal conditions or after stimulation, which is in agreement with previous data with HPMECs12 and with the observation that most FVIII is produced by the liver, whereas VWF is produced in extrahepatic tissues.1-3
Several studies evaluated the distribution of FVIII mRNA in different tissues. In humans, FVIII mRNA was detected primarily in liver, in spleen, and lymph nodes and in lesser amounts in pancreas, kidney, and muscle.7 Other organs, such as lungs, skin, intestine, or heart, were not evaluated in this study, which prevents comparison with our observations. Interestingly, FVIII mRNA was not detected in cultured HUVECs,7 in agreement with our data. In mice, FVIII mRNA was detected in liver and kidney and at lower levels in testis and spleen. Lower levels were measured in heart, skeletal muscle, and lung.10 The detection of low levels mRNA in heart and lung is compatible with our observation that MECs isolated from these tissues produce FVIII. However, such a comparison must be taken with caution because there are clear differences in FVIII regulation between mice and humans.11
The frequent use of HUVECs as source of human ECs and the heterogeneity in FVIII production between different EC types probably explain why, with the exception of liver sinusoidal ECs, the ECs were not recognized earlier as a source of FVIII. Note, Rosenberg et al26 have analyzed FVIII secretion by HPMECs but have not detected measurable FVIII. However, the sensitivity of the FVIII functional assay used for the latter study was 6 mU/mL,26 which would not have allowed detection of FVIII production by HPMECs in our culture conditions.
Acute physical stress such as physical exercise13 or insulin-dependent hypoglycemia15 leads to the rapid secretion of FVIII, subsequent to the release of catecholamines and stimulation of β2-receptors.15,27,28 Interestingly, β2-receptor blockade also lowers sustained elevated FVIII:C in patients with deep vein thrombosis.29
The amounts of FVIII released during acute physical stress are important by comparison to the steady state production of FVIII. Given that the average FVIII level in plasma is 1 U/mL and that the half-life of FVIII is 12 hours, the steady state rate of FVIII production can be estimated at 0.042 U/mL plasma/h. By comparison, FVIII plasma levels increase by 1 to 3 U/mL within 30 minutes after epinephrine administration,28 which is at least 40 times higher than the steady state rate of FVIII production. The rapid secretion of FVIII after epinephrine administration indicates that FVIII is derived from a preformed storage pool rather than from de novo protein synthesis. However, to date, the cells able to secrete FVIII in response to catecholamines have not been identified.
FVIII elevation after epinephrine infusion is accompanied by a similar increase in VWF plasma levels, indicating that in vivo ECs respond either directly or indirectly to epinephrine. It has therefore been speculated that ECs could be a costorage site for FVIII and VWF and could release both proteins after stimulation with epinephrine. This hypothesis is indirectly supported by several in vitro observations. Rosenberg et al26 have demonstrated that HUVECs transduced with a retroviral vector encoding FVIII store FVIII with VWF in Weibel-Palade bodies and release it after treatment with strong agonists. In addition, Vischer et al24 have shown that purified ECs are able to respond directly to epinephrine by releasing VWF when the experiment is performed in the presence of an inhibitor of phosphodiesterases. Altogether, those observations suggested that ECs are a potential storage site for FVIII in addition to VWF and may release both proteins after treatment with epinephrine.
In agreement with this hypothesis, we showed that treatment of ECs from heart, intestine, or skin with PMA induces a rapid release of both FVIII and VWF. By contrast, ECs from the aorta, umbilical vein, and artery did not release measurable amounts of FVIII. The lack of FVIII release by these cells is probably because of the absence of FVIII production rather than to a defect in FVIII storage. Indeed, the cells that did not release FVIII on stimulation with agonists did not contain or spontaneously secrete any measurable FVIII. However, they released significant amounts of VWF, which indicated that the signal transduction mechanisms and the Weibel-Palade bodies were not defective.
The concomitant releases of FVIII and VWF after treatment with PMA and the high affinity of FVIII for VWF suggest that both proteins may be costored in Weibel-Palade bodies. This hypothesis is supported by the observations mentioned previously that transfection of ECs with expression vectors for wild-type FVIII or even with FVIII with partially reduced affinity for VWF result in accumulation of FVIII in Weibel-Palade bodies.30-32 In agreement with this hypothesis, FVIII was detected in the cytoplasm of HCMECs but not in that of HUVECs. A fraction of FVIII was localized in vesicles that were also strongly stained with VWF, indicating that FVIII is at least in part costored with VWF in Weibel-Palade bodies.
Previous experiments have indicated that FVIII storage sites are located in cells synthesizing it. Prolonged administration of FVIII to a patient with severe hemophilia A did not restore FVIII response to 1-deamino-8-D-arginine vasopressin, suggesting that FVIII must be endogenously produced to create a releasable pool.33 Similarly, no colocalization of FVIII and VWF was observed in HUVECS cocultured with AtT-20 cells producing FVIII.34 The long-term production of FVIII by the ECs observed in our study also argues that FVIII released in the cell culture supernatant is derived from de novo synthesis rather than from a storage pool formed before removal of the cells from human tissues.
Vischer et al24 have shown that treatment of ECs with epinephrine induces the release of VWF when the cells are treated with an inhibitor of phosphodiesterease. In our study, treatment of HCMECs, HDMECs, nd HIMECs with epinephrine led to a significant release of both FVIII and VWF in the absence of an inhibitor of phosphodiesterase.
For all types of ECs producing FVIII, the secretion of FVIII was lower after stimulation with epinephrine than with PMA. A similar observation was made for VWF. The latter data are in agreement with the report by Vischer et al35 that VWF secretion induced by cytosolic free Ca++-raising agents involves both peripheral and central Weibel-Palade bodies, whereas secretion induced by cyclic adenosine monophosphate–raising agents is limited to the release of peripheral granules. Similar mechanisms may explain the lower secretion of FVIII after activation with epinephrine than with PMA.
Although our data are consistent with the hypothesis that ECs from several tissues could release FVIII in vivo on infusion with epinephrine or after acute physical stress, it is not clear whether these cells can be responsible for the whole FVIII release observed in such circumstances. Indeed, the ratio between FVIII and VWF released in vivo in acute physical stress or after epinephrine treatment is approximately equal to 1 when FVIII and VWF are expressed in unit per milliliter, whereas in vitro this ratio is much lower.
In addition, the hypothesis of a direct release of FVIII from ECs subsequent to their activation with epinephrine is not consistent with the observation that in asplenic persons, FVIII levels do not increase after strenuous exercise or epinephrine administration.36 Accordingly, it cannot be excluded that additional sources of FVIII and indirect mechanisms controlling its release after epinephrine administration still need to be identified.
Altogether, our results indicate that extrahepatic FVIII production by ECs is not restricted to the lungs but is a property of several tissues. Microvascular or macrovascular ECs from different vascular beds constitutively secrete FVIII. Such cells also constitute a storage pool of FVIII, releasable after treatment with agonists such as PMA. This FVIII storage pool is also partially released after treatment with epinephrine, which may contribute to the rapid FVIII increase in plasma after acute physical stress.
Extrahepatic ECs therefore appear to be an important FVIII production site with the potential to regulate FVIII levels in chronic and acute conditions. These observations have potential importance to explore the mechanisms of mild/moderate hemophilia A, to develop gene therapy for hemophilia A, and to understand the mechanisms leading to high FVIII levels, which are associated with increased risks of thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Stefan Vinckier and Kristel Peeters for their help with microscopy and Jos Vermylen for discussion and comments on the manuscript.
This work was supported by the Flemish Research Foundation (grants G.0231.05 and G.0275.05), by the KULeuven Wyeth and Baxter chairs for hemophilia, by the Prof Norbert Heimburger chair for thrombosis and hemostasis (KULeuven), and by the “Excellentie Financiering KULeuven” EF/05/013.
Authorship
Contribution: T.S., M.J., and A.L. were responsible for in vitro studies; T.S., M.J., A.L., J.-M.S.-R., and K.P. contributed to the design of the study; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc Jacquemin, Center for Molecular and Vascular Biology, Herestraat 49, B-3000 Leuven, Belgium; e-mail: marc.jacquemin@med.kuleuven.ac.be.