Abstract
The reduced folate carrier (RFC) is involved in the transport of methotrexate (MTX) across the cell membrane. The RFC gene (SLC19A1) is located on chromosome 21, and we hypothesized that the RFC80 G>A polymorphism would affect outcome and toxicity in childhood leukemia and that this could interact with chromosome 21 copy number in the leukemic clone. A total of 500 children with acute lymphoblastic leukemia treated according to the common Nordic treatment protocols were included, and we found that the RFC AA variant was associated with a 50% better chance of staying in remission compared with GG or GA variants (P = .046). Increased copy numbers of chromosome 21 appear to improve outcome also in children with GA or GG variant. In a subset of 182 children receiving 608 high-dose MTX courses, we observed higher degree of bone marrow toxicity in patients with the RFC AA variant compared with GA/GG variants (platelet 73 vs 99/105 × 109/L, P = .004, hemoglobin 5.6 vs 5.9/6.0 mmol/L, P = .004) and a higher degree of liver toxicity in patients with RFC GG variant (alanine aminotransferase 167 vs 127/124 U/L, P = .05). In conclusion, the RFC 80G>A polymorphism interacts with chromosome 21 copy numbers and affects both efficacy and toxicity of MTX.
Introduction
Pharmacogenetics influence the risk of relapse in childhood acute lymphoblastic leukemia (ALL).1 Methotrexate (MTX) is one of the most widely used drugs in these patients,2-5 and many genetic polymorphisms may influence MTX pharmacokinetics and dynamics.6 MTX is a folic acid antagonist with increased affinity for its target enzymes when polyglutamated intracellularly. Furthermore, the polyglutamated MTX (MTX-glu(n)) is intracellularly retained far longer than the administered monoglutamated MTX. MTX and MTX-glu(n) exert their effect by inhibiting enzymes essential for thymidylate synthesis and de novo purine synthesis, which will affect DNA synthesis and cellular proliferation.7,8 The first step, cellular uptake, involves the reduced folate carrier (RFC).9,10 A polymorphism, 80G>A (rs1051266, His27Arg), in the RFC gene (SLC19A1) has been identified with an allele frequency of 0.48.11,12 Functional studies of the polymorphism have produced somewhat ambiguous results. Whetstine et al13 found no significant differences in MTX uptake between RFC-allele variants in transfected cells, whereas Baslund et al demonstrated increased MTX uptake in cells from healthy persons with the AA variant.14 The RFC gene is located at chromosome 21, and there appears to be a direct relationship between copy number of chromosome 21 and the risk of toxicity after MTX therapy, as patients with Down syndrome show more treatment-related toxicity. Furthermore, hyperdiploid B-precursor ALL patients, more than 90% of whom have 3 or 4 copies of chromosome 21, have a low relapse rate and generate significantly higher intracellular levels of MTX-glu(n) in their leukemic cells compared with nonhyperdiploid patients.15-17 These findings are likely to reflect enhanced intracellular transport of MTX, because it has been shown that the RFC gene is expressed at significantly higher levels in hyperdiploid lymphoblasts than in nonhyperdiploid lymphoblasts.18,19
Studies of rheumatoid arthritis patients in MTX monotherapy have, like the functional study of MTX uptake by Baslund et al,14 demonstrated better MTX efficacy in AA variants.20,21 However, only 2 studies have previously investigated the effect on outcome in childhood ALL: Rocha et al22 could not demonstrate any significant effect on cure rate in 246 children with ALL, whereas Laverdiere et al11 in a study of 204 patients found a reduced relapse rate for patients with the G-allele. However, a different MTX treatment strategy was used in the latter study, and neither of the 2 studies explored the impact of chromosome 21 copy number.11,22
In this nationwide study comprising 500 children with ALL, we have investigated the influence of RFC (SLC19A1) polymorphism on the risk of relapse and of post high-dose MTX (HDMTX) toxicity as well as the interactions with chromosome 21 copy number.
Methods
Patients
A total of 563 children aged 1 to 15 years were diagnosed with nonmature B-cell or T-cell leukemia in Denmark from January 1992 to January 2007 (246 girls, 317 boys; aged 1.1-14.9 years; median: 4.5 years). At a median follow-up time of 7.9 years (50% range: 4.1-12.1 years), 452 (80%) are still in first remission, whereas 74 (13%) patients have relapsed within 0.2 to 8.3 years from the diagnosis (median: 2.6 years). Of the 74 relapses, 17 involved the central nervous system (CNS), 12 of which were isolated. In first remission, 5 patients died (1%) and 5 developed a second malignancy (1%; Table 1). Twenty-two patients died before the first HDMTX was given (4%), and 5 patients did not achieve remission during induction treatment and thus changed protocol before the first HDMTX (1%).
Patients and studies
| . | Patients . | No events . | Relapse . | Died before first HDMTX . | Change of protocol before first HDMTX . | SMN . | Dead in CR . |
|---|---|---|---|---|---|---|---|
| All patients | 563 | 452 | 74 | 22 | 5 | 5 | 5 |
| All patients treated at Rigshospitalet | 238 | 192 | 31 | 6 | 3 | 3 | 3 |
| Outcome study | 500 | 422 | 70 | 0 | 0 | 5 | 3 |
| Toxicity study, first HDM course | 123 | 110 | 15 | 1 | |||
| Toxicity study, all HDM courses | 182 | 156 | 22 | 2 | 2 |
| . | Patients . | No events . | Relapse . | Died before first HDMTX . | Change of protocol before first HDMTX . | SMN . | Dead in CR . |
|---|---|---|---|---|---|---|---|
| All patients | 563 | 452 | 74 | 22 | 5 | 5 | 5 |
| All patients treated at Rigshospitalet | 238 | 192 | 31 | 6 | 3 | 3 | 3 |
| Outcome study | 500 | 422 | 70 | 0 | 0 | 5 | 3 |
| Toxicity study, first HDM course | 123 | 110 | 15 | 1 | |||
| Toxicity study, all HDM courses | 182 | 156 | 22 | 2 | 2 |
HDMTX indicates high-dose methotrexate; SMN, secondary malignancy; and CR, complete remission.
A total of 63 patients were excluded from the study because of a lack of DNA material or poor quality of DNA in the specimens (n = 47), change of protocol (n = 5), or death (n = 11) before the first HDMTX course. The remaining 500 patients included in this study (ie, 89% of those potentially eligible in Denmark during the study period; Table 2) were treated according to the Nordic Society of Pediatric Haematology and Oncology 1992 (NOPHO-92) protocol (n = 295) or NOPHO-2000 (n = 205), either with a low-risk (n = 350) or high-risk (n = 150) protocol. Less than 5% of the patients were of other ethnic origin than Nordic white race.
Patient and blood donor characteristics in relation to reduced folate carrier 80G>A polymorphism
| . | RFC GG variant . | RFC GA variant . | RFC AA variant . |
|---|---|---|---|
| Sex (fraction) | |||
| Male | 90 (0.3) | 136 (0.5) | 60 (0.2) |
| Female | 70 (0.3) | 106 (0.5) | 38 (0.2) |
| Median age, y (range) | 4.5 (1.1-14.8) | 4.5 (1.1-14.9) | 4.2 (1.6-14.6) |
| Phenotype; B/T lineage, no. (fraction) | 137 (0.3)/23 (0.4) | 206 (0.5)/36 (0.5) | 89 (0.2)/9 (0.1) |
| Risk; High/low, no. (fraction) | 45 (0.3)/115 (0.3) | 80 (0.5)/162 (0.5) | 25 (0.2)/73 (0.2) |
| Protocol; NOPHO-92/NOPHO-2000, no. (fraction) | 98 (0.3)/62 (0.3) | 131 (0.5)/111 (0.5) | 66 (0.2)/32 (0.2) |
| Chr21 copy no. higher than 2/2, no. (fraction) | 44 (0.4)/92 (0.3) | 55 (0.4)/152 (0.5) | 27 (0.2)/59 (0.2) |
| Relapse, CNS only/CNS plus other relapse involvements | 23 (0.3)-3 (0.3)/2 (0.4) | 39 (0.6)-7 (0.6)/3 (0.6) | 8 (0.1)-1 (0.1)/0 |
| Secondary malignancy, no. (fraction) | 1 (0.2) | 3 (0.6) | 1 (0.2) |
| Bone marrow transplantation, no. (fraction) | 6 (0.4) | 4 (0.2) | 6 (0.4) |
| Died in remission, no. (fraction) | 2 (0.7) | 1 (0.3) | 0 |
| t(12;21) in high/low risk, no. (fraction) | 3 (0.3)/19 (0.3) | 7 (0.6)/31 (0.5) | 2 (0.1)/14 (0.2) |
| Red cell transfusion, min (fraction)/max (range) | 51 (0.3)/56 (1-147) | 93 (0.5)/64 (1-324) | 35 (0.2)/64 (24-416) |
| Platelets transfusion, min (fraction)/max (range) | 38 (0.3)/41 (3-144) | 68 (0.5)/40 (2-216) | 25 (0.2)/64 (8-208) |
| Excluded patients with genotype, no. (fraction) | |||
| Death before first HDMTX | 3 (0.3) | 6 (0.5) | 2 (0.2) |
| Change of protocol before first HDMTX | 2 (0.4) | 2 (0.4) | 1 (0.2) |
| . | RFC GG variant . | RFC GA variant . | RFC AA variant . |
|---|---|---|---|
| Sex (fraction) | |||
| Male | 90 (0.3) | 136 (0.5) | 60 (0.2) |
| Female | 70 (0.3) | 106 (0.5) | 38 (0.2) |
| Median age, y (range) | 4.5 (1.1-14.8) | 4.5 (1.1-14.9) | 4.2 (1.6-14.6) |
| Phenotype; B/T lineage, no. (fraction) | 137 (0.3)/23 (0.4) | 206 (0.5)/36 (0.5) | 89 (0.2)/9 (0.1) |
| Risk; High/low, no. (fraction) | 45 (0.3)/115 (0.3) | 80 (0.5)/162 (0.5) | 25 (0.2)/73 (0.2) |
| Protocol; NOPHO-92/NOPHO-2000, no. (fraction) | 98 (0.3)/62 (0.3) | 131 (0.5)/111 (0.5) | 66 (0.2)/32 (0.2) |
| Chr21 copy no. higher than 2/2, no. (fraction) | 44 (0.4)/92 (0.3) | 55 (0.4)/152 (0.5) | 27 (0.2)/59 (0.2) |
| Relapse, CNS only/CNS plus other relapse involvements | 23 (0.3)-3 (0.3)/2 (0.4) | 39 (0.6)-7 (0.6)/3 (0.6) | 8 (0.1)-1 (0.1)/0 |
| Secondary malignancy, no. (fraction) | 1 (0.2) | 3 (0.6) | 1 (0.2) |
| Bone marrow transplantation, no. (fraction) | 6 (0.4) | 4 (0.2) | 6 (0.4) |
| Died in remission, no. (fraction) | 2 (0.7) | 1 (0.3) | 0 |
| t(12;21) in high/low risk, no. (fraction) | 3 (0.3)/19 (0.3) | 7 (0.6)/31 (0.5) | 2 (0.1)/14 (0.2) |
| Red cell transfusion, min (fraction)/max (range) | 51 (0.3)/56 (1-147) | 93 (0.5)/64 (1-324) | 35 (0.2)/64 (24-416) |
| Platelets transfusion, min (fraction)/max (range) | 38 (0.3)/41 (3-144) | 68 (0.5)/40 (2-216) | 25 (0.2)/64 (8-208) |
| Excluded patients with genotype, no. (fraction) | |||
| Death before first HDMTX | 3 (0.3) | 6 (0.5) | 2 (0.2) |
| Change of protocol before first HDMTX | 2 (0.4) | 2 (0.4) | 1 (0.2) |
There was no difference in the distribution of sex and genotype (P = .7). Among the healthy blood donors, there were 38 (0.36), 54 (0.51), and 14 (0.13) men in the RFC GG, GA, and AA variant groups, respectively, and 22 (0.23), 54 (0.56), and 20 (0.21) women, respectively.
NOPHO indicates Nordic Society of Pediatric Haematology and Oncology; CNS, central nervous system; and HDMTX, high-dose methotrexate.
At the time of diagnosis, 65% of samples were drawn. To ensure that leukemic DNA did not differentiate from germline DNA, we compared RFC genotypes in 42 patients with samples taken at time of diagnosis and after remission was achieved; 14 pairs of samples (of which 4 samples were from patients with hyperdiploid clone involving chromosome 21) in each RFC variant group and found no mismatch. The Ethics Committee of Copenhagen (journal number 01-259108) as well as the Danish Data Protection Authority (journal number 2005-41-4808) approved the study design and protocol. Toxicity studies were conducted in children treated only at Rigshospitalet, where HDMTX data were available. Blood samples from 202 healthy donors were used to compare RFC variant frequencies (Table 2).
Therapy
The children were divided into treatment groups based on risk assessments according to the NOPHO protocol.23 The children were considered high risk if any of the following parameters were present: white blood count 50 × 109/L or higher, T-lineage ALL, the presence of CNS or testicular leukemia, translocations t(9;22)(q34;q11) or t(4;11)(q21;q23), lymphomatous leukemia or mediastinal lymphoma, and/or a poor treatment response (≥ 25% blasts in bone marrow day 15 or ≥ 5% blasts in bone marrow day 29).
HDMTX
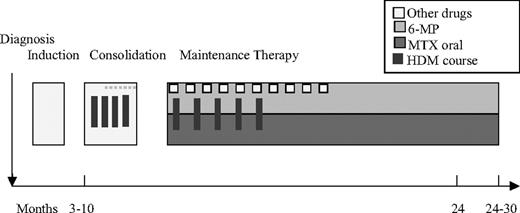
Children with low-risk ALL (Figure 1) received 5 g/m2 HDMTX courses 3 to 4 times during the consolidation period, with an interval of 14 to 28 days, and 5 times during maintenance therapy with an interval of approximately 8 weeks. Leucovorine rescue 15 mg/m2 was given at 6-hour intervals from hour 36 (NOPHO 2000: 42 hours) after the start of each HDMTX course until plasma-MTX less than 200 nmol/L.24
Low-risk MTX therapy. A punctured line indicates that not all patients received this specific dose. In maintenance therapy, 6-mercaptopurine (6-MP) and MTX are administered in low oral doses. MTX (8-12 mg) was administered intrathecally 6 times before consolidation.
Low-risk MTX therapy. A punctured line indicates that not all patients received this specific dose. In maintenance therapy, 6-mercaptopurine (6-MP) and MTX are administered in low oral doses. MTX (8-12 mg) was administered intrathecally 6 times before consolidation.
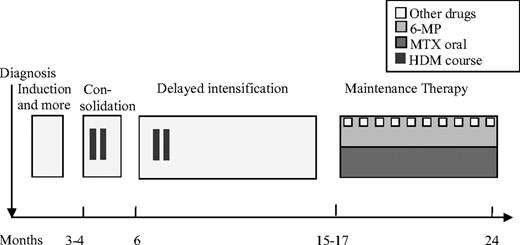
Children with high-risk ALL (Figure 2) received 8 g/m2 HDMTX courses 2 to 4 times during the consolidation period with an interval of at least 42 days. The initial leucovorine rescue dose at hour 36 was 50 mg/m2 (NOPHO 2000: 15 mg/m2) followed by leucovorine rescue (15 mg/m2) at 6-hour intervals until plasma-MTX less than 200 nmol/L.24 Intrathecal MTX (8-12 mg) was administered during all HDMTX courses in both low- and high-risk leukemia protocols.
High-risk MTX therapy. A punctured line indicates that not all patients received this specific dose. In maintenance therapy, 6-mercaptopurine (6-MP) and MTX were administered in low oral doses. MTX (8-12 mg) was administered intrathecally 6 times before consolidation.
High-risk MTX therapy. A punctured line indicates that not all patients received this specific dose. In maintenance therapy, 6-mercaptopurine (6-MP) and MTX were administered in low oral doses. MTX (8-12 mg) was administered intrathecally 6 times before consolidation.
RFC genotyping and MTX
Genomic DNA was extracted and purified by NaCl and ethanol precipitation from 1 to 5 mL of ethylenediaminetetraacetic acid–stabilized blood. Allelic discrimination was carried out to detect the RFC80 G>A polymorphism using fluorogenic 3′-minor groove-binding probes in an end-point polymerase chain reaction assay on an ABI 7500 Fast platform (Applied Biosystems). Primers and probes were as described in an earlier study.14 The method was validated using the BigDye terminator V1.1 cycle sequencing kit and Sanger sequencing on ABI 3730. All reagents were purchased from Applied Biosystems. To measure MTX, a photometric assay was used.
Validation of genotyping assay
Blood donor RFC frequencies were in agreement with Hardy-Weinberg equilibrium, and sequencing of 3 samples with each RFC variant (n = 9) showed identical results with the allelic discrimination method.
Pharmacokinetic and toxicity after HDMTX
The lowest platelet and hemoglobin values within a month after HDMTX or before the next HDMTX treatment (if given earlier) were used to quantify the degree of myelosuppression. The highest value of plasma alanine aminotransferase (ALAT) during the same time period was used as a marker of liver toxicity. Samples drawn 20 to 24 hours after administration of HDMTX were used for plasma MTX measurements.
Karyotype results
Only G-band karyotyping was mandatory in the ALL-92 protocol, but the ALL-2000 protocol also required directed analysis by fluorescent in situ hybridization and/or reverse-transcriptase polymerase chain reaction for translocation t(9;22)(q34;q11) (BCR-ABL) and for 11q23/MLL aberrations. Furthermore, many leukemic samples have been explored by comparative genomic hybridization and spectral karyotyping.25 All cytogenetic results are scrutinized annually by the NOPHO cytogenetic working group and described according to International System for Human Cytogenetic Nomenclature 1995.26 Karyotype results were available for 429 of the included patients and were used to classify patients according to their chromosome 21 copy number.
Statistics
SAS Software Version 9.1.3 was used for the statistical analysis. Two-sided P values less than .05 were considered significant. Survival analyses were performed with a basic time scale defined by the date of diagnosis. The duration of event-free survival was defined as the time from diagnosis until the date of relapse, death, or the development of a second malignancy (whichever first) or the last known follow-up for event-free survivors. Because the RFC genotype may reflect relapse, risk of toxic death, and secondary malignancy (SMN), all of these events are included in the survival analysis. Bone marrow transplantation and protocol failure or changes of protocol (NOPHO guidelines 1992 or 2000) were classified as censuring events. Patients in first remission were followed until July 30, 2008. Survival probabilities were estimated using the Kaplan-Meier method. Univariate Cox regression and multivariate Cox regression analysis with stepwise backward selection (variables: phenotype, protocol, and enhanced number of chromosome 21) with stratification by risk group were used to identify potential risk factors for an event. Model assumptions, including the proportionality assumption, were assessed using conventional methods
A general linear model was used for pharmacokinetic analyses after the first HDMTX. Repeated measurements (mixed model analysis), with the order of HDMTX courses as a random effect, were used to explore toxicity and pharmacokinetics after all HDMTX courses. Each low-risk patient had from 1 to 9 HDMTX courses and each high-risk patients had from 1 to 3 courses. In the statistical tests, all data were logarithmically transformed and adjustment for low/high-risk group was applied. χ2 tests were used for tests of independence to determine RFC polymorphism and risk of developing leukemia.
Results
Risk of developing leukemia
We found no significant differences in the RFC allele frequencies between the 516 patients and the 200 blood donors (P = .44; Table 2), and patients' RFC frequencies were in agreement with Hardy-Weinberg equilibrium.
Event risk by RFC genotype
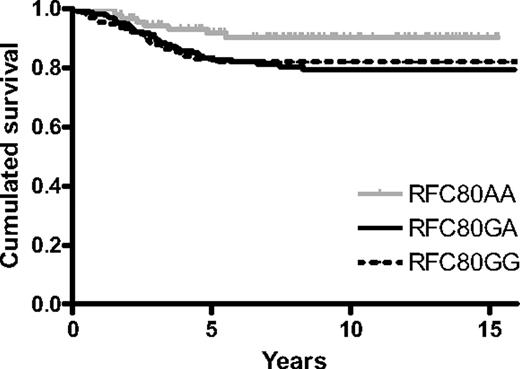
The risk of developing an event was 50% lower for the RFC variant AA patients than for the AG/GG variant patients (hazard ratio, 0.5 [95% CI: 0.25-1.00]; P = .046; Figure 3). Although not statistically significant, patients with the AA variant had an approximately 75% lower risk of developing a CNS relapse than RFC GA/GG variants when including all CNS relapses (n = 16 relapses; P = .20) and a 60% lower risk when including only isolated CNS relapses (n = 11 relapses; P = .38).
RFC polymorphism and outcome. The Kaplan-Meier survival curve is shown to visualize the difference between AA variant and GG/GA variants (log-rank P = .046).
RFC polymorphism and outcome. The Kaplan-Meier survival curve is shown to visualize the difference between AA variant and GG/GA variants (log-rank P = .046).
RFC and chromosome 21 copy numbers
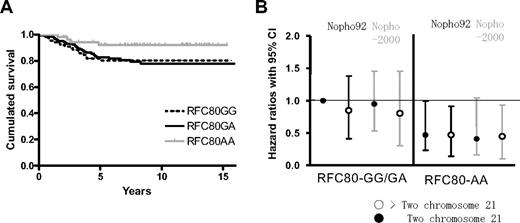
To investigate the interaction between the RFC variants and chromosome 21 copy numbers, 429 patients for whom cytogenetic information was available, were divided into groups with a normal chromosome 21 copy number (n = 303) or an increased copy number (n = 126). Among patients with 2 copies of chromosome 21 in their leukemic clone, the 59 patients with the RFC80 AA variant had a 64% reduced risk of relapse compared with the 244 patients with the RFC80 GA/GG variants (P = .048; hazard ratio, 0.36 [95% CI: 0.13-0.99]; Figure 4). In contrast, the 126 patients with 3 or more copies of chromosome 21 in their leukemic clone showed no significant differences in the risk of relapse between the RFC80 variant groups (P = .80), and exclusion of patients with Down syndrome (n = 2) did not change this result.
RFC polymorphism, outcome, and relation to chromosome 21. (A) Outcome in patients with a normal copy number of chromosome 21. Patients with an increased copy number of chromosome 21 (expected higher MTX influx) had no significant differences among RFC variants (P = .94; data not shown). (B) Different hazard ratios compared with patients with a normal copy number of chromosome 21 and RFC80 GA/GG variant according to the NOPHO 92 protocol.
RFC polymorphism, outcome, and relation to chromosome 21. (A) Outcome in patients with a normal copy number of chromosome 21. Patients with an increased copy number of chromosome 21 (expected higher MTX influx) had no significant differences among RFC variants (P = .94; data not shown). (B) Different hazard ratios compared with patients with a normal copy number of chromosome 21 and RFC80 GA/GG variant according to the NOPHO 92 protocol.
Multivariate analysis
In a backward stepwise Cox regression model adjusted for risk group, phenotype, protocol, and chromosome 21 copy numbers, the RFC AA variant still had a better prognosis (P = .10), but only risk group (hazard ratio, 2.83 [95% CI: 1.83-4.47]) had a significant effect on outcome (Table 3).
Cox regression analysis
| Covariate . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | |
| RFC80 AA versus GA/GG | 0.50 (0.25-1.00) | .046 | 0.56 (0.28-1.13) | .10† |
| Phenotype, pre B versus T* | 0.97 (0.51-1.87) | .94 | .94‡ | |
| Copy no. of Chr21 higher than 2 versus 2 | 0.80 (0.46-1.39) | .44 | .71‡ | |
| Risk group, high versus low | 2.97 (1.90-4.63) | < .001 | 2.86 (1.83-4.47) | < .001 |
| Protocol, 2000 versus 1992 | 0.90 (0.55-1.48) | .68 | .32‡ | |
| Covariate . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | |
| RFC80 AA versus GA/GG | 0.50 (0.25-1.00) | .046 | 0.56 (0.28-1.13) | .10† |
| Phenotype, pre B versus T* | 0.97 (0.51-1.87) | .94 | .94‡ | |
| Copy no. of Chr21 higher than 2 versus 2 | 0.80 (0.46-1.39) | .44 | .71‡ | |
| Risk group, high versus low | 2.97 (1.90-4.63) | < .001 | 2.86 (1.83-4.47) | < .001 |
| Protocol, 2000 versus 1992 | 0.90 (0.55-1.48) | .68 | .32‡ | |
CI indicates confidence interval.
Low-risk patients were excluded from the model because no T-ALL were assigned to low-risk therapy.
If only relapse was considered as an event, then P = .13.
P value of covariate if included in the final model.
Toxicity after high-dose methotrexate
To investigate the impact of RFC80 polymorphism on toxicity after HDMTX, the highest level of ALAT and the nadir of neutrophils, platelets, and hemoglobin within a month after HDMTX or before the next HDMTX (if earlier) were available for 182 patients. Low-risk patients in the AA variant group had significantly lower platelets (P = .004/.002) and hemoglobin nadirs (P = .004/.001) compared with the GA and GG variant groups, and the levels of ALAT were significantly higher in the GG variant group compared with the GA and AA variant groups (P = .030/.049; Table 4). The same trends were seen in high-risk patients, although they did not reach statistical significance.
Nadir of platelets, hemoglobin, and lymphocytes and highest ALAT value after high-dose methotrexate and relation to RFC80 polymorphism in low-risk ALL patients
| Reduced folate carrier, genotype . | AA . | GA . | GG . | Ratio (95% CI) . | P . |
|---|---|---|---|---|---|
| Platelets, ×109/L | |||||
| Patients | 29 | 72 | 39 | ||
| HDMTX courses | 116 | 279 | 153 | ||
| Mean (geometric) | 73 | 99 | 105 | ||
| CI | 55-95 | 78-125 | 81-136 | ||
| GG versus AA | 1.44 (1.14-1.82) | .002 | |||
| GA versus AA | 1.36 (1.10-1.68) | .004 | |||
| GG versus GA | 1.06 (.88-1.28) | .546 | |||
| Time to count recovery (> 150) after first HDMTX in days, geometric mean | 3.4 | 2.4 | 2.4 | ||
| Hemoglobin, mmol/L | |||||
| Patients | 29 | 72 | 39 | ||
| HDMTX courses | 116 | 279 | 153 | ||
| Mean (geometric) | 5.6 | 5.9 | 6.0 | ||
| CI | 5.4-5.8 | 5.7-6.1 | 5.8-6.2 | ||
| GG versus AA | 1.06 (1.02-1.10) | .001 | |||
| GA versus AA | 1.05 (1.01-1.08) | .004 | |||
| GG versus GA | 1.01 (.98-1.04) | .395 | |||
| Neutrophils, ×109/L | |||||
| Patients | 28 | 72 | 38 | ||
| HDMTX courses | 111 | 262 | 149 | ||
| Mean (geometric) | 0.7 | 0.8 | 0.8 | ||
| CIl | 0.6-0.9 | 0.7-1.0 | 0.7-0.9 | ||
| GG versus AA | 1.07 (.86-1.32) | .551 | |||
| GA versus AA | 1.11 (.92-1.35) | .286 | |||
| GG versus GA | .96 (.81-1.14) | .652 | |||
| Time to count recovery (> 0.5) after first HDMTX in days, geometric mean | 4.4 | 3.4 | 2.4 | ||
| ALAT, U/L | |||||
| Patients | 28 | 71 | 38 | ||
| HDMTX courses | 102 | 227 | 132 | ||
| Mean (geometric) | 123.8 | 127.0 | 167.1 | ||
| CI | 86-177 | 93-174 | 119-236 | ||
| GG versus AA | 1.35 (1.00-1.82) | .049 | |||
| GA versus AA | 1.02 (.78-1.34) | .851 | |||
| GG versus GA | 1.31 (1.03-1.69) | .030 |
| Reduced folate carrier, genotype . | AA . | GA . | GG . | Ratio (95% CI) . | P . |
|---|---|---|---|---|---|
| Platelets, ×109/L | |||||
| Patients | 29 | 72 | 39 | ||
| HDMTX courses | 116 | 279 | 153 | ||
| Mean (geometric) | 73 | 99 | 105 | ||
| CI | 55-95 | 78-125 | 81-136 | ||
| GG versus AA | 1.44 (1.14-1.82) | .002 | |||
| GA versus AA | 1.36 (1.10-1.68) | .004 | |||
| GG versus GA | 1.06 (.88-1.28) | .546 | |||
| Time to count recovery (> 150) after first HDMTX in days, geometric mean | 3.4 | 2.4 | 2.4 | ||
| Hemoglobin, mmol/L | |||||
| Patients | 29 | 72 | 39 | ||
| HDMTX courses | 116 | 279 | 153 | ||
| Mean (geometric) | 5.6 | 5.9 | 6.0 | ||
| CI | 5.4-5.8 | 5.7-6.1 | 5.8-6.2 | ||
| GG versus AA | 1.06 (1.02-1.10) | .001 | |||
| GA versus AA | 1.05 (1.01-1.08) | .004 | |||
| GG versus GA | 1.01 (.98-1.04) | .395 | |||
| Neutrophils, ×109/L | |||||
| Patients | 28 | 72 | 38 | ||
| HDMTX courses | 111 | 262 | 149 | ||
| Mean (geometric) | 0.7 | 0.8 | 0.8 | ||
| CIl | 0.6-0.9 | 0.7-1.0 | 0.7-0.9 | ||
| GG versus AA | 1.07 (.86-1.32) | .551 | |||
| GA versus AA | 1.11 (.92-1.35) | .286 | |||
| GG versus GA | .96 (.81-1.14) | .652 | |||
| Time to count recovery (> 0.5) after first HDMTX in days, geometric mean | 4.4 | 3.4 | 2.4 | ||
| ALAT, U/L | |||||
| Patients | 28 | 71 | 38 | ||
| HDMTX courses | 102 | 227 | 132 | ||
| Mean (geometric) | 123.8 | 127.0 | 167.1 | ||
| CI | 86-177 | 93-174 | 119-236 | ||
| GG versus AA | 1.35 (1.00-1.82) | .049 | |||
| GA versus AA | 1.02 (.78-1.34) | .851 | |||
| GG versus GA | 1.31 (1.03-1.69) | .030 |
P values are adjusted for repeated measurements.
ALAT indicates alanine aminotransferase; CI, confidence interval; and HDMTX, high-dose methotrexate.
Pharmacokinetics
To explore the pharmacokinetic impact of the RFC80 polymorphism, we analyzed 182 patients, all treated at Rigshospitalet, with plasma MTX levels measured at 20 to 24 hours after initiation of the HDMTX infusion (n = 608 HDMTX courses). A total of 123 patients had MTX measurements available from their first HDMTX. These patients did not differ significantly from the remaining total study cohort with respect to sex, treatment protocol, immunophenotype, or relapse rate. Patients with the GG variant had lower end-of-infusion plasma MTX levels (Table 5) than the other variant groups both at the first HDMTX course (P = .037; 123 patients stratified for low/high-risk ALL) and after all HDMTX courses (P = .012; 608 HDMTX courses; 182 patients).
RFC80 polymorphism and plasma levels of methotrexate after HDMTX (nmol/L)
| . | First HDMTX course, 123 patients, GG versus GA/AA, P = .037, n = 123 . | All HDMTX courses, 182 patients, GG versus GA/AA, P = .012, n = 608 . | ||||
|---|---|---|---|---|---|---|
| GG, n = 37 . | GA, n = 58 . | AA, n = 28 . | GG, n = 173 . | GA, n = 307 . | AA, n = 128 . | |
| Low risk | 82 207 | 89 554 | 90 300 | 67 818 | 79 585 | 79 074 |
| High risk | 91 524 | 122 684 | 148 458 | 100 160 | 105 373 | 157 403 |
| . | First HDMTX course, 123 patients, GG versus GA/AA, P = .037, n = 123 . | All HDMTX courses, 182 patients, GG versus GA/AA, P = .012, n = 608 . | ||||
|---|---|---|---|---|---|---|
| GG, n = 37 . | GA, n = 58 . | AA, n = 28 . | GG, n = 173 . | GA, n = 307 . | AA, n = 128 . | |
| Low risk | 82 207 | 89 554 | 90 300 | 67 818 | 79 585 | 79 074 |
| High risk | 91 524 | 122 684 | 148 458 | 100 160 | 105 373 | 157 403 |
ALAT indicates alanine aminotransferase; ALL, acute lymphoblastic leukemia; CI, confidence interval; and HDMTX, high-dose methotrexate.
Discussion
This study not only indicates a significant impact of RFC 80G>A polymorphism on MTX treatment efficacy in childhood ALL but also, as importantly, emphasizes that these results can be fully appreciated only within the karyotypic background of the leukemic cells.
Other studies have explored variations in the RFC expression in leukemic cells19 and demonstrated that low expression of RFC is related to a poor outcome in childhood leukemia.27 Furthermore, Belkov et al showed that higher mRNA levels of RFC were related to the chromosome 21 copy number,18 and Jansen et al suggested that higher mRNA levels was related to the RFC80 A variant and to the chromosome 21 copy number.28 This is in conformity with both the results in the study by Baslund et al,14 in which the RFC80 AA variant takes up MTX more efficiently, and the clinical results of the present study, in which higher numbers of chromosome 21 seem to neutralize the effect of RFC variants.
In contrast to the present study, Laverdiere et al11 found an association between variant A-alleles and a worse outcome in childhood leukemia. However, in their study only one 4-g/m2 HDMTX course and 30 mg/m2 per week after induction were given instead of the 4 to 9 courses of 5 or 8 g/m2 HDMTX courses and 20 mg/m2 per week after induction used in the NOPHO protocols. Furthermore, the present study includes twice as many patients and the median follow up time is much longer (7.9 vs 4 years), which is important because relapses tend to come late in many low-risk ALL patients such as the high-hyperdiploid cases and those with t(12;21).25
However, we confirmed the association between the RFC GG variant and lower MTX plasma concentrations reported by Laverdiere et al.11 Furthermore, we found that low-risk patients had more post-HDMTX liver toxicity (higher ALAT levels) in the GG variant group compared with patients with GA/AA variants. The biologic background for this association is unclear. However, under normal conditions high levels of folic acid polyglutamates are stored in liver cells. Because the gene dosage of A-alleles would be expected to correlate with increased levels of folic acid polyglutamates in liver cells, this could protect the cells from MTX toxicity. In addition, higher folic acid polyglutamate levels in liver cells in A-alleles may also decrease the influx of MTX into the hepatocytes, where a significant fraction of MTX during HDMTX is eliminated, which could explain why patients with A-allele have a slower elimination of MTX and thus higher MTX plasma concentrations.
A new study by de Jonge et al29 has shown a correlation between variants in the RFC80 polymorphism and the etiology of leukemia, and although it was not the purpose of this study, we looked at the correlation but found no connection between the RFC allele frequencies and etiology of childhood leukemia.
Conclusion
This study emphasizes the need to add pharmacogenetic profiling to the already extensive biologic exploration of childhood ALL, because both these and previously published data30 support that individual variations in genes that influence the disposition of anticancer agents may be as important for the risk of relapse as the genetic aberrations of the leukemic clone. Although this study showed significant association between RFC genotypes and outcome, these results need to be validated in an independent patient cohort. Further studies looking at the interaction between pharmacogenetic polymorphisms and risk of treatment failure are needed to determine whether adjusting the drug dosing according to genotype will increase the efficacy of the antileukemic treatment. Such studies are ongoing in the study group, and some are now integrated into the NOPHO ALL2008 protocol.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are thankful for the skillful technical assistance from the staff of the Childhood Cancer Laboratory, Rigshospitalet. We are indebted to Mr Isaac Austin for linguistic corrections and to all patients for their participation in this study.
This study was supported by grants from the Danish Childhood Cancer Foundation, the Otto Christensen Foundation, and the University Hospital Rigshospitalet. K.S. holds the Childhood Cancer Foundation Research Professorship in Pediatric Oncology.
Authorship
Contribution: J.G., K.S., C.P., K.D., and B.L. designed the study; J.G. performed experiments, analyzed results, performed statistical analysis, and wrote the paper; I.J.C. performed statistical analysis and validated statistics; B.L., H.S., S.R., and N.C. provided clinical data on the patients; and all authors commented on and approved the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jannie Gregers, Division of Molecular Genetic Diagnostics 4111, Department of Clinical Biochemistry, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; e-mail: jgregers@rh.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal