Abstract
During erythrocyte invasion, Plasmodium falciparum merozoites use multiple receptor-ligand interactions in a series of coordinated events, but current knowledge of these interactions is limited. Using real-time imaging of invasion, we established that heparin-like molecules block early, and essential, events in erythrocyte invasion by merozoites. All P falciparum isolates tested, and parasites using different invasion pathways were inhibited to comparable levels. Furthermore, it was not possible to select for heparin-resistant parasites. Heparin-like molecules occur naturally on the surface of human erythrocytes, where they may act as receptors for binding of merozoite surface proteins. Consistent with this, we demonstrated that MSP1-42, a processed form of merozoite surface protein 1 (MSP1) involved in invasion, bound heparin in a specific manner; furthermore, binding was observed with the secondary processing fragment MSP1-33, but not MSP1-19. We defined key structural requirements of heparin-like molecules for invasion inhibition and interactions with MSP1-42. Optimal activity required a degree of sulfation more than or equal to 2, disulfation of the N-acetylglucosamine or hexuronic acid residue, and a minimum chain length of 6 monosaccharides. These findings have significant implications for understanding P falciparum invasion of erythrocytes and the development of novel therapeutics and vaccines.
Introduction
Plasmodium falciparum causes the majority of malaria morbidity and mortality world-wide and is a leading cause of childhood deaths.1 The pathogenic processes of malaria occur during blood-stage infection when merozoites invade erythrocytes using multiple receptor-ligand interactions and replicate inside them. Antigens on the merozoite surface, such as merozoite surface protein 1 (MSP1),2 are thought to mediate initial attachment, followed by apical reorientation involving apical membrane antigen 1 (AMA1)3 and possibly other antigens. Proteins contained within apical organelles, such as erythrocyte-binding antigens (EBAs) and P falciparum reticulocyte-binding homologs (PfRh), are also released to form a tight junction between the merozoite and erythrocyte membrane. After this the merozoite is propelled into the erythrocyte by an actin-myosin–like motor.2 Very few receptor-ligand interactions involved in invasion events, particularly early events, have been defined. Furthermore, specific functions have been determined for only a small number of merozoite antigens. This limited knowledge has restricted the development of approaches to block essential interactions with vaccine-induced antibodies or molecules that could be used therapeutically.
MSP1 appears to be essential for erythrocyte invasion and involved in initial attachment of merozoites to erythrocytes. MSP1 exists as a high molecular mass protein (Mr ∼ 180 kDa) and is proteolytically processed into 83-, 30-, 38-kDa, and C-terminal 42-kDa (MSP1-42) fragments4 just before egress from the schizont by protease PfSUB1.5 These remain noncovalently associated on the merozoite surface, together with MSP6 and MSP7.6,7 During invasion, PfSUB2 further processes MSP1-42 into MSP1-19 and MSP1-33 fragments.8 The latter protein along with its noncovalently linked binding partners are shed during invasion. The short C-terminal MSP1-19 fragment is anchored to the merozoite surface via a surface-expressed glycosylphosphatidyl inositol anchor and is carried inside the erythrocyte.9 Although the reason for MSP1 processing remains poorly understood, primary and secondary processing is thought to be essential for MSP1 function and invasion because MSP1-42 and MSP1-19 specific antibodies and inhibitors that block processing also inhibit invasion.10,11 MSP1-19 may function by binding to erythrocyte membrane protein band 3.12
Heparin and other sulfated polysaccharides, such as pentosan polysulfate, dextran sulfate, curdlan sulfate, and fucoidan, can inhibit blood-stage growth of P falciparum in vitro and fucoidan and dextran sulfate have antimalarial activity in mouse models of malaria.13-17 Heparan sulfate (HS) proteoglycan (structurally very similar to heparin) has been identified on the surface of erythrocytes.18 This suggests a potential role for heparin-like molecules in invasion by P falciparum merozoites; however, specific interactions have not been demonstrated, and the mechanism of action of heparin remains unknown. Other stages of the P falciparum life cycle, such as sporozoites, use heparin-like molecules as cell surface receptors for invasion.19
Heparin and HS are members of the glycosaminoglycan family and consist of repeating disaccharide units of β-glucuronic acid (GlcA) and α-N-acetylglucosamine (GlcNAc). During biosynthesis, linear polysaccharide chains are modified with N-deacetylation/N-sulfation, epimerization of GlcA to iduronic acid (IdoA), and O-sulfation at different positions.20 There is considerable heterogeneity and structural diversity in polysaccharides generated by various levels and patterns of sulfation and degree of epimerization. Heparin and HS differ in level of sulfation and proportion of GlcA and IdoA. K5 polysaccharides are heparin-like molecules isolated from the capsule of Escherichia coli K5.21 They have the same backbone structure as heparin and HS [→4)-β-D-GlcA-(1→4)-α-D-GlcNAc-(1→]n, reviewed in Rusnati et al22 and can be used for the generation of semisynthetic heparin-like polysaccharides.23 Modified K5 polysaccharides have defined levels and patterns of sulfation that facilitate studies to characterize the structural requirements of heparin/HS for specific biologic activities.
Here, we identify cellular and molecular interactions with heparin-like molecules during erythrocyte invasion by P falciparum merozoites. We found that heparin-like molecules primarily act by inhibiting early and essential events in erythrocyte invasion. MSP1-42 and MSP1-33 can bind heparin, suggesting the use of heparin-like molecules as receptors for invasion. Furthermore, we determined the key structural requirements of heparin-like molecules for invasion-inhibitory activity and interaction with MSP1-42.
Methods
Reagents
Heparin (porcine intestinal mucosa), de-N-sulfated heparin (re-N-acetylated), de-N- and de-O-sulfated heparin (re-N-acetylated), chondroitin sulfate C (CSC), heparin-agarose beads, heparin conjugated to bovine serum albumin (heparin-BSA), and lactoferrin were obtained from Sigma-Aldrich. Partially de-N-sulfated (re-N-acetylated) heparin, de-6-O-sulfated heparin, de-2-O-sulfated heparin, K5 polysaccharides derivatives with N-sulfation (N-sulfated K5 polysaccharide [K5-NS]), low and high levels of O-sulfation (low-level O-sulfated K5 polysaccharide [K5-OS-L] and high-level O-sulfated K5 polysaccharide [K5-OS-H]), low and high level of N- and O-sulfation (low-level N- and O-sulfated K5 polysaccharide [K5-NSOS-L] and high-level N- and O-sulfated K5 polysaccharide [K5-NSOS-H]), and epimerized K5 (EK5) polysaccharide derivatives with low and high levels of N- and O-sulfation (low-level N- and O-sulfated epimerized K5 polysaccharide [EK5-NSOS-L] and high-level N- and O-sulfated epimerized K5 polysaccharide [EK5-NSOS-H]) were obtained from Iduron (Paterson Institute for Cancer Research, University of Manchester). Sheep anti–rabbit–horseradish peroxidase (HRP) and sheep anti–mouse–HRP antibodies were purchased from Chemicon. Rabbit anti-BSA was obtained from Sigma-Aldrich.
Antibodies used include rabbit antibodies to MSP1-19 (recombinant P falciparum 3D7 MSP1-19 protein expressed in E coli and purified by nickel affinity chromotography24 ), EBA175,25 and MSP3,26 (from Alan Cowman, Walter and Eliza Hall Institute), MSP1-33 (Mike Blackman, National Institute of Medical Research), AMA1 (Kerstin Leykauf and Brendan Crabb, Burnet Institute), and MSP4 (Ross Coppel, Monash University). Recombinant MSP1-42 and AMA1 (expressed as His-tagged proteins in E coli)11 were provided by Carole Long (National Institutes of Health) and Robin Anders (La Trobe University); neuraminidase (Vibrio cholerae) was from Calbiochem (Merck), and chymotrypsin and trypsin were from Worthington Biochemical Corporation.
Heparin and chondroitin sulfate C oligosaccharide fragments
Heparin and CSC oligosaccharides were prepared and characterized as described.27,28 Heparin was partially digested with heparinase I, and CSC (200 mg) was partially digested with chondroitinase ABC. Fractionation of heparin oligosaccharides was performed with a Bio-GelP-6 column (1.6 × 90 cm), elution by 0.2M NH4Cl (pH 3.5). CSC oligosaccharides were fractionated on a Bio-GelP-4 column (1.6 × 90 cm), elution by 0.2M ammonium acetate. Oligosaccharide fractions were desalted and quantified by carbazole assay.29 Oligosaccharides were resuspended in phosphate-buffered saline (PBS) and filter sterilized before testing in growth inhibition assays.
Parasite culture and growth inhibition assays
P falciparum isolates were cultured as described,30 and genetic identity of isolates was confirmed by sequencing the ama1 gene. Genetic knockout lines lacking Rh1, Rh2a, EBA140, EBA175, EBA181, and MSP3 were kindly provided by Alan Cowman.2 Heparin-like molecules (Table 1) were tested for growth-inhibitory activity against P falciparum parasites using established assays.30 Samples were tested for inhibitory activity in duplicate, and cultures were incubated for 40 hours and parasitemia determined by flow cytometry (FACSCalibur; BD Biosciences). The inhibitory effects of molecules were expressed as percentage of growth compared with controls (PBS) for each experiment. Enzyme treatment of erythrocytes31 was performed with neuraminidase (67μM, 15 minutes), chymotrypsin (1 mg/mL, 45 minutes), or trypsin (100 μg/mL, 45 minutes) at 37°C, then washed, before use in growth inhibition assays. For live filming of erythrocyte invasion, parasites at 5% to 10% schizont stage were maintained in culture medium at 37°C, 6% CO2 with humidification.32 Only definite merozoite invasion events, accompanied by erythrocyte deformation, were counted.
Composition and inhibitory activity of carbohydrates
| Molecule . | Uronate type . | Uronate sulfation* (%) . | Glucosamine sulfation* (%) . | Degree of sulfation† . | IC50 . |
|---|---|---|---|---|---|
| Heparin | IdoA | 2S, 84 | NS, 95, 6S, 90 | 2.6 | 19 |
| Heparin, partially de-N-sulfated | IdoA | 2S, 84 | NS, 80, 6S, 90 | 2.5 | 74 |
| Heparin, de-N-sulfated | IdoA | 2S, 83 | NS, 8, 6S, 92 | 1.8 | >100 |
| Heparin, de-N/O-sulfated | IdoA | 0 | 0 | 0 | >100 |
| Heparin, de-6-O-sulfated | IdoA | 2S, 60 | NS, 95, 6S, 9 | 1.6 | 373 |
| Heparin, de-2-O-sulfated | IdoA | 2S, 5 | NS, 95, 6S, 88 | 1.9 | 123 |
| K5-NS | GlcA | 0 | NS, 100 | 1 | >100 |
| K5-OS-L | GlcA | 2S, 10 | 6S, 90 | 1.4 | >100 |
| K5-OS-H | GlcA | 2,3-diS, 100 | 6S,‡ 100 | 3 | 20.2 |
| K5-NSOS-L | GlcA | 2S, 10 | NS, 100, 6S, 90 | 2 | 56 |
| K5-NSOS-H | Glc A | 2S, 30, 2,3-diS, 70 | NS, 100, 6S, 100 | 3.7 | 7.4 |
| EK5-NSOS-L | IdoA/GlcA 1:1 | 2S, 10 | NS, 100, 6S, 90 | 2 | 103 |
| EK5-NSOS-H | IdoA/GlcA 1:1 | 2S, 30, 2,3-diS, 70 | NS, 100, 6S, 100 | 3.7 | 103 |
| Molecule . | Uronate type . | Uronate sulfation* (%) . | Glucosamine sulfation* (%) . | Degree of sulfation† . | IC50 . |
|---|---|---|---|---|---|
| Heparin | IdoA | 2S, 84 | NS, 95, 6S, 90 | 2.6 | 19 |
| Heparin, partially de-N-sulfated | IdoA | 2S, 84 | NS, 80, 6S, 90 | 2.5 | 74 |
| Heparin, de-N-sulfated | IdoA | 2S, 83 | NS, 8, 6S, 92 | 1.8 | >100 |
| Heparin, de-N/O-sulfated | IdoA | 0 | 0 | 0 | >100 |
| Heparin, de-6-O-sulfated | IdoA | 2S, 60 | NS, 95, 6S, 9 | 1.6 | 373 |
| Heparin, de-2-O-sulfated | IdoA | 2S, 5 | NS, 95, 6S, 88 | 1.9 | 123 |
| K5-NS | GlcA | 0 | NS, 100 | 1 | >100 |
| K5-OS-L | GlcA | 2S, 10 | 6S, 90 | 1.4 | >100 |
| K5-OS-H | GlcA | 2,3-diS, 100 | 6S,‡ 100 | 3 | 20.2 |
| K5-NSOS-L | GlcA | 2S, 10 | NS, 100, 6S, 90 | 2 | 56 |
| K5-NSOS-H | Glc A | 2S, 30, 2,3-diS, 70 | NS, 100, 6S, 100 | 3.7 | 7.4 |
| EK5-NSOS-L | IdoA/GlcA 1:1 | 2S, 10 | NS, 100, 6S, 90 | 2 | 103 |
| EK5-NSOS-H | IdoA/GlcA 1:1 | 2S, 30, 2,3-diS, 70 | NS, 100, 6S, 100 | 3.7 | 103 |
Data on sulfation levels are based on information provided by manufacturers and published data.23 The position and degree (%) of sulfation is indicated.
The average number of sulfate groups per disaccharide unit.
3-O-sulfation of the glucosamine is also present (level of sulfation is unknown).
Binding of native parasite proteins to heparin-agarose beads
Proteins were extracted from P falciparum schizonts into 1% Triton X-100 in PBS as described.33 Proteins from culture supernatants were collected by allowing highly synchronous schizonts to rupture into protein-free culture medium and cells removed by centrifugation. Heparin-agarose beads were washed twice in PBS, then blocked with 1% casein PBS overnight at 4°C. Schizont protein extracts were incubated overnight at 4°C with beads containing 0.1% casein and 200 μg/mL of test inhibitor, or PBS control (50 μL of packed beads plus 100 μL of protein supernatant). Unbound proteins in the supernatant were collected through Micro Bio-Spin Chromatography Columns (Bio-Rad) and beads washed 5 times with PBS containing 0.1% casein, 1% Triton X-100, and protease inhibitors. Bound proteins were eluted from beads with 50 μL of warmed reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Bound and unbound proteins were separated by SDS-PAGE under reducing conditions and Western blotted onto polyvinylidene difluoride membranes for probing with antibodies to detect MSP1, MSP3, MSP4, AMA1, or EBA175.
MSP1 processing inhibition assay
MSP1 processing inhibition assay was performed as described previously for AMA1 processing inhibition.34 Schizonts were purified by passing synchronized mature trophozoites (∼ 38 hours after invasion) through magnetic-activated cell sorting columns to remove uninfected erythrocytes, followed by further culture to mature parasites to the schizont stage. Purified schizonts at 107/mL were incubated for more than 12 hours until rupture had completed, with or without the addition of ethylenediaminetetraacetic acid 1mM or heparin 1 mg/mL. Merozoites were collected by centrifugation at 10 000g for 10 minutes and washed in cold PBS containing protease inhibitors. Proteins were extracted with nonreducing SDS-PAGE sample buffer, freeze thawed and sonicated, and used in Western blots.
Heparin-BSA binding assay
Recombinant merozoite antigens were coated (1 μg/mL) onto 96-well plates (Nunc Maxisorb) in PBS overnight at 4°C. Plates were washed and blocked with 1% casein, then incubated with heparin-BSA or BSA. Plates were washed, and bound heparin-BSA/BSA was detected with anti-BSA antibodies (rabbit, Sigma-Aldrich), followed by antirabbit-HRP, and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid; Sigma-Aldrich). All incubations were performed in PBS with 0.1% casein and 0.05% Tween 20, 1 hour at room temperature.
Results
Heparin inhibits initial contact and reorientation of merozoites during erythrocyte invasion
Previous studies suggested that heparin may act by inhibiting schizont rupture13,16 and/or merozoite invasion.17,35 To define the mechanism of action of heparin and heparin-like molecules, we performed time-course studies of schizont rupture, merozoite invasion, and intra-erythrocytic development of P falciparum in the presence of heparin. Parasite stages were evaluated using flow cytometry, differentiating between late-stage trophozoites, schizonts, and ring-stage parasites with ethidium bromide staining. In the presence of heparin (100 μg/mL), there was evidence of some delay, but not complete inhibition of schizont rupture compared with control (Figure 1A). In contrast, rates of merozoite invasion and ring formation were very low, suggesting that heparin acts primarily by blocking merozoite invasion (Figure 1B). No inhibition of intra-erythrocytic growth was observed (data not shown).
Heparin inhibits P falciparum merozoite invasion at initial contact events.P falciparum schizonts (3D7 isolate) in culture were monitored over time for schizont rupture (A) and formation of ring-stage (B) parasites in the presence of heparin (100 μg/mL) or PBS control. Data are mean (range) of one representative assay (2 assays were performed in duplicate). (A) There was a small, but significant, delay of schizont rupture in the presence of heparin *P = .03 for difference in schizont parasitemia for heparin versus control at 15 hours (Mann-Whitney U test). (B) Formation of new ring-stage parasites was greatly inhibited in the presence of heparin, showing that heparin acts predominately by inhibition of merozoite invasion. (C-D) Real-time microscopy of merozoite invasion. (C) Merozoite invasion (arrow) in PBS control cultures demonstrated: (1) initial contact of merozoite with erythrocyte, (2) reorientation, (3) commencement of invasion, (3-5) mechanical invasion and deformation of the erythrocyte, and (6) complete invasion and erythrocyte reformation. (D) Merozoite invasion with cultures containing heparin (100 μg/mL): (1-5) initial contact of merozoite with erythrocyte without reorientation, (6) initial contact is not sustained and merozoite detached. No invasion, reorientation, or erythrocyte deformation was observed in the presence of heparin. Time in seconds is indicated in the bottom right corner. Pictures shown are representative selections from real-time microscopy recordings using the 3D7 P falciparum line. Videos can be found in supplemental data. Microscopy was performed on a Zeiss Axiovert 200M microscope with 63×/1.4 oil (magnification/numeric aperture) objective lens, at humidified tissue culture conditions (5% CO2, 5% O2, 90% N2), 37°C, standard culture media. Images were collected by Zeiss AxioCam MRm with Zeiss Axio Vision software and processed with ImageJ.
Heparin inhibits P falciparum merozoite invasion at initial contact events.P falciparum schizonts (3D7 isolate) in culture were monitored over time for schizont rupture (A) and formation of ring-stage (B) parasites in the presence of heparin (100 μg/mL) or PBS control. Data are mean (range) of one representative assay (2 assays were performed in duplicate). (A) There was a small, but significant, delay of schizont rupture in the presence of heparin *P = .03 for difference in schizont parasitemia for heparin versus control at 15 hours (Mann-Whitney U test). (B) Formation of new ring-stage parasites was greatly inhibited in the presence of heparin, showing that heparin acts predominately by inhibition of merozoite invasion. (C-D) Real-time microscopy of merozoite invasion. (C) Merozoite invasion (arrow) in PBS control cultures demonstrated: (1) initial contact of merozoite with erythrocyte, (2) reorientation, (3) commencement of invasion, (3-5) mechanical invasion and deformation of the erythrocyte, and (6) complete invasion and erythrocyte reformation. (D) Merozoite invasion with cultures containing heparin (100 μg/mL): (1-5) initial contact of merozoite with erythrocyte without reorientation, (6) initial contact is not sustained and merozoite detached. No invasion, reorientation, or erythrocyte deformation was observed in the presence of heparin. Time in seconds is indicated in the bottom right corner. Pictures shown are representative selections from real-time microscopy recordings using the 3D7 P falciparum line. Videos can be found in supplemental data. Microscopy was performed on a Zeiss Axiovert 200M microscope with 63×/1.4 oil (magnification/numeric aperture) objective lens, at humidified tissue culture conditions (5% CO2, 5% O2, 90% N2), 37°C, standard culture media. Images were collected by Zeiss AxioCam MRm with Zeiss Axio Vision software and processed with ImageJ.
The mechanism of action of heparin was further defined using real-time microscopy of invasion. In control parasite cultures, schizont rupture and stepwise invasion of erythrocytes by merozoites were readily observed as reported previously32,36-38 (Figure 1C; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Briefly, we observed initial merozoite contact with the erythrocyte surface resulting in its rapid and brief oscillatory deformation, followed by merozoite reorientation and internalization. Subsequently, the erythrocyte underwent deformation, or echinocytosis, followed by a gradual recovery of its biconcave shape over several minutes. In the presence of heparin (100 μg/mL), schizont rupture occurred and merozoites appeared to disperse normally, further indicating that heparin does not act by inhibiting schizont rupture. Initial contact between merozoites and the erythrocyte surface was also observed; however, no oscillatory deformation of the erythrocyte was seen and contact between erythrocytes and merozoites was not sustained with merozoites dissociating after various lengths of time (Figure 1D; supplemental Figure 1B). No successful invasion events were observed; additionally, reorientation of merozoites and echinocytosis of erythrocytes were not clearly seen. The total filming time of control parasites was 5442 seconds covering 13 separate schizont rupture events, with 21 confirmed invasion events. Total filming time of cultures with heparin was 4529 seconds over 15 separate events, with no invasion events. These studies clearly established that heparin acts early in invasion by inhibiting the ability of the merozoite to sustain contact with the erythrocyte surface.
Heparin inhibits essential erythrocyte invasion events
Heparin inhibited 6 genetically different lines tested (3D7, W2mef, D10, E8B, XIE, HCS3; data not shown). P falciparum is known to use different pathways for invasion of erythrocytes, determined by different receptor-ligand interactions. Pathways can be partially defined by their sensitivity to enzyme treatment (eg, neuraminidase, chymotrypsin, and trypsin) of erythrocyte surface receptors and can be broadly classified into 2 groups based on the use of sialic acid (SA) on the erythrocyte surface by parasite ligands. Parasite lines W2mef and 3D7 were used as representative of SA-dependent and SA-independent invasion phenotypes,31 respectively, and were equally inhibited by heparin, whereas the related compound CSC was noninhibitory (Figure 2A). We next measured inhibition of merozoite invasion by heparin using erythrocytes treated with enzymes to selectively remove subsets of erythrocyte invasion receptors. With 3D7 parasites, the inhibitory activity of heparin was equivalent when tested for inhibition of merozoite invasion into neuraminidase-, trypsin-, or chymotrypsin-treated, compared with untreated erythrocytes (Figure 2B), suggesting heparin inhibits essential step(s) common to all invasion pathways. Heparin also gave similar inhibition of invasion of W2mef into chymotrypsin- and trypsin-treated erythrocytes (Figure 2C). Of note, heparin effectively inhibited invasion of merozoites into neuraminidase-treated erythrocytes in both 3D7wt (Figure 2B) and by W2mefEBA175KO (data not shown) parasites, indicating that heparin does not act by interfering with the binding of parasite ligands to negatively charged SA on the erythrocyte surface.
Heparin inhibits essential erythrocyte invasion events. (A) Heparin effectively inhibited merozoite invasion of P falciparum lines W2mef (sialic acid–dependent invasion phenotype) and 3D7 (sialic acid–independent invasion phenotype) at comparable levels. CSC was noninhibitory against all lines tested; data are excluded from panels B through D for clarity. (B) Heparin had similar inhibitory activity against invasion of 3D7 P falciparum into neuraminidase-, chymotrypsin-, or trypsin-treated erythrocytes versus untreated erythrocytes. (C) Heparin efficiently inhibited invasion of W2mef P falciparum into chymotrypsin- and trypsin-treated erythrocytes compared with untreated. (D) 3D7 parasites were selected once or twice for invasion of erythrocytes in the presence of heparin 100 μg/mL. The ability of heparin to inhibit erythrocyte invasion by merozoites was comparable between the parent parasites and heparin-selected parasites. All data are mean growth ± SEM, expressed as percentage of control (PBS; 3 assays in duplicate), except for panel B mean ± growth range, expressed as percentage of control (PBS; 2 assays in duplicate).
Heparin inhibits essential erythrocyte invasion events. (A) Heparin effectively inhibited merozoite invasion of P falciparum lines W2mef (sialic acid–dependent invasion phenotype) and 3D7 (sialic acid–independent invasion phenotype) at comparable levels. CSC was noninhibitory against all lines tested; data are excluded from panels B through D for clarity. (B) Heparin had similar inhibitory activity against invasion of 3D7 P falciparum into neuraminidase-, chymotrypsin-, or trypsin-treated erythrocytes versus untreated erythrocytes. (C) Heparin efficiently inhibited invasion of W2mef P falciparum into chymotrypsin- and trypsin-treated erythrocytes compared with untreated. (D) 3D7 parasites were selected once or twice for invasion of erythrocytes in the presence of heparin 100 μg/mL. The ability of heparin to inhibit erythrocyte invasion by merozoites was comparable between the parent parasites and heparin-selected parasites. All data are mean growth ± SEM, expressed as percentage of control (PBS; 3 assays in duplicate), except for panel B mean ± growth range, expressed as percentage of control (PBS; 2 assays in duplicate).
In the presence of 100 μg/mL heparin, very low parasitemias were detected after one cycle of replication. It was possible that this represented parasites that were invading using an alternative pathway resistant to heparin inhibition. To test this possibility, we recultured parasites (3D7) that were present after a 48-hour incubation with heparin until a high parasitemia was obtained, and these parasites were again treated with heparin for 48 hours and surviving parasites were recultured. When the parent and the heparin-selected (1 and 2 cycles of selection) parasites were tested in growth-inhibition assays, the sensitivity to heparin was found to be the same; there was no evidence that we were able to select for increased resistance to heparin reflecting parasites using an alternative invasion pathway (Figure 2D). Furthermore, higher concentrations of heparin completely inhibited invasion (data not shown).
MSP1-42 and MSP1-33 bind to heparin
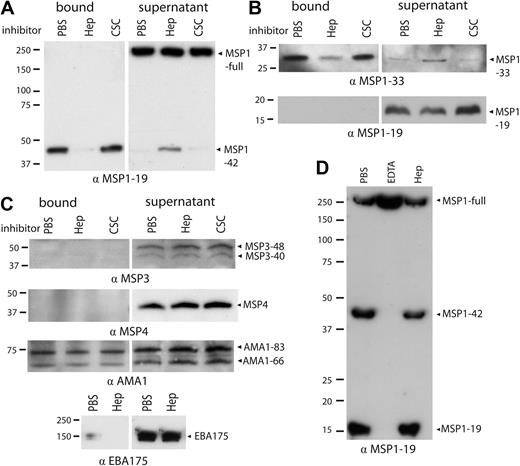
Having shown that heparin inhibited essential and early interactions of merozoite invasion, we next sought to identify merozoite antigens that bind to heparin during invasion. The major merozoite surface protein MSP1 was considered a probable target as this protein is thought to be essential for invasion and involved in initial invasion events. Protein extracts of P falciparum schizonts (3D7 line) containing MSP1 were incubated with heparin-agarose beads, and bound proteins were eluted from beads and identified by Western blotting. Using this approach, we consistently identified, in repeat experiments, a MSP1-positive band in the bead-eluted fraction that had an Mr of approximately 40 kDa; this represents MSP1-42 (Figure 3A; PBS control). The same band was readily detected in protein extracts before incubation with beads (data not shown) but was greatly depleted from the supernatant after incubation with heparin-agarose beads. The binding of MSP1-42 to heparin-agarose beads was effectively inhibited by soluble heparin, but not by the related glycosaminoglycan CSC, which does not inhibit erythrocyte invasion; this suggested that binding was specific. In contrast, full-length MSP1 and MSP1-19 were detected in schizont protein extracts, but there was little or no detectable protein in fractions eluted from beads and little evidence of depletion from the supernatant after incubation with beads, suggesting that they do not bind heparin (Figure 3A-B). MSP1 exists as 2 main allelic forms, 3D7 and FVO.39 Sequence analysis showed that both contain several putative heparin-binding–like motifs represented by xBBxBx,40 where B is a basic residue and x is a hydrophobic residue (supplemental Figure 2A). Furthermore, proteins extracted from parasites with the FVO allelic type (E8B isolate) also bound heparin in a specific manner (supplemental Figure 2B).
Native MSP1-42 and MSP1-33, but not full-length MSP1, MSP1-19, or other merozoite proteins, bind to heparin. Merozoite proteins were extracted from whole schizonts (3D7 isolate) and incubated with heparin-agarose beads with and without soluble inhibitors. (A) MSP1-42 bound to immobilized heparin in the presence of control (PBS) or soluble CSC, and MSP1-42 was depleted from the supernatant after incubation. In the presence of soluble heparin, binding of MSP1-42 was inhibited and was not depleted from the supernatant. Full-length MSP1 (MSP1-full) did not bind to beads and was only present in the supernatant. (B) MSP1-33 from culture supernatant bound heparin, being present in the bead-bound fraction and depleted from the supernatant. In the presence of soluble heparin, MSP1-33 was not able to bind and was predominately found in the supernatant (top panel). MSP1-19 did not bind to beads and was only present in the supernatant fraction (bottom panel). (C) Other merozoite antigens MSP3, MSP4, AMA1, and EBA175 showed little or no binding to heparin-agarose. For MSP3 and MSP4, no protein was seen in the bound fraction. AMA1 showed nonspecific binding to heparin-agarose beads that was not inhibited by the addition of soluble heparin. For EBA175, the majority of the protein was seen in the supernatant after incubation with heparin-agarose. Binding of merozoite proteins was tested in the presence of control (PBS), soluble heparin, or CSC. (D) Heparin did not inhibit proteolytic processing of MSP1. Purified schizonts were incubated with PBS, ethylenediaminetetraacetic acid (1mM), or heparin (1 mg/mL) until complete rupture of schizonts had occurred, and merozoites were then collected. In the presence of control (PBS) and heparin, processed forms of MSP1 (MSP1-42 and MSP1-19) were observed, in addition to full-length MSP1. Heparin did not significantly reduce the appearance of MSP1-42 or MSP1-19 compared with PBS control. Ethylenediaminetetraacetic acid prevented processing of full-length MSP1 to MSP1-42 and MSP1-19 fragments, indicating inhibition of processing. For all figures, the antibody used in the Western blot is indicated at the bottom of the panel (all antibodies were raised in rabbits). Molecular weight markers are indicated to the left, and proteins are indicated with arrows. Results shown are representative examples taken from multiple assays.
Native MSP1-42 and MSP1-33, but not full-length MSP1, MSP1-19, or other merozoite proteins, bind to heparin. Merozoite proteins were extracted from whole schizonts (3D7 isolate) and incubated with heparin-agarose beads with and without soluble inhibitors. (A) MSP1-42 bound to immobilized heparin in the presence of control (PBS) or soluble CSC, and MSP1-42 was depleted from the supernatant after incubation. In the presence of soluble heparin, binding of MSP1-42 was inhibited and was not depleted from the supernatant. Full-length MSP1 (MSP1-full) did not bind to beads and was only present in the supernatant. (B) MSP1-33 from culture supernatant bound heparin, being present in the bead-bound fraction and depleted from the supernatant. In the presence of soluble heparin, MSP1-33 was not able to bind and was predominately found in the supernatant (top panel). MSP1-19 did not bind to beads and was only present in the supernatant fraction (bottom panel). (C) Other merozoite antigens MSP3, MSP4, AMA1, and EBA175 showed little or no binding to heparin-agarose. For MSP3 and MSP4, no protein was seen in the bound fraction. AMA1 showed nonspecific binding to heparin-agarose beads that was not inhibited by the addition of soluble heparin. For EBA175, the majority of the protein was seen in the supernatant after incubation with heparin-agarose. Binding of merozoite proteins was tested in the presence of control (PBS), soluble heparin, or CSC. (D) Heparin did not inhibit proteolytic processing of MSP1. Purified schizonts were incubated with PBS, ethylenediaminetetraacetic acid (1mM), or heparin (1 mg/mL) until complete rupture of schizonts had occurred, and merozoites were then collected. In the presence of control (PBS) and heparin, processed forms of MSP1 (MSP1-42 and MSP1-19) were observed, in addition to full-length MSP1. Heparin did not significantly reduce the appearance of MSP1-42 or MSP1-19 compared with PBS control. Ethylenediaminetetraacetic acid prevented processing of full-length MSP1 to MSP1-42 and MSP1-19 fragments, indicating inhibition of processing. For all figures, the antibody used in the Western blot is indicated at the bottom of the panel (all antibodies were raised in rabbits). Molecular weight markers are indicated to the left, and proteins are indicated with arrows. Results shown are representative examples taken from multiple assays.
Around the time of invasion into the erythrocyte, MSP1-42 is further processed with shedding of MSP1-33 from the merozoite surface. To investigate whether the MSP1-33 fragment could bind heparin, culture supernatants containing MSP1-33 were incubated with heparin-agarose beads. Like MSP1-42, MSP1-33 was consistently found in the bead-eluted bound fraction, although depleted from the culture supernatant. Binding was inhibited with heparin (Figure 3B). Several other merozoite proteins, extracted from schizonts, did not appear to bind to heparin. EBA175 did not bind heparin-agarose beads and was consistently observed in the supernatant after incubation of schizont protein extracts with beads (Figure 3C). EBA175 binds to SAs, which are also negatively charged like heparin. MSP3 and MSP4 did not bind to heparin-agarose beads and were found only in the supernatant (Figure 3C). Furthermore, heparin inhibited a D10-MSP3-knockout line to the same extent as parental D10 (data not shown); this line also has a disrupted function of MSP9 (ABRA).26 AMA1 did not bind heparin in a specific manner; although some AMA1 was found eluted from beads, this binding was nonspecific as it was not inhibited by soluble heparin (Figure 3C). Parasite isolates with genetic disruption of EBA140, EBA175, EBA181, PfRh1, and PfRh2a were inhibited by heparin at the same level as the parental isolate (data not shown).
To test whether heparin may act by inhibiting secondary processing of MSP1-42 to MSP1-33 and MSP1-19, purified schizonts were incubated with heparin at a concentration in excess of that known to be inhibitory (1 mg/mL), and we evaluated levels of MSP1-42 and MSP1-19 in merozoites after schizont rupture compared with controls. Heparin appeared unable to inhibit MSP1-42 processing to MSP1-19; there were comparable amounts of MSP1-42 and MSP1-19 seen in heparin (1 mg/mL) and control treated samples (Figure 3D). Ethylenediaminetetraacetic acid (positive control) inhibited processing as expected.41
Binding of MSP1-42 to heparin was further examined by testing binding of recombinant MSP1-42 and MSP1-19 to heparin conjugated to BSA (heparin-BSA). BSA alone was used as a negative control and lactoferrin, a known heparin-binding protein, as a positive control. Recombinant MSP1-42, but not MSP1-19 or AMA1, had significant heparin-binding activity (Figure 4A). The binding of lactoferrin to heparin-BSA was dose-dependent and saturated at concentrations of heparin-BSA more than 2.5 μg/mL, and binding was inhibited by heparin, but not CSC (data not shown); this validated the use of this assay in studying heparin-binding interactions. Binding of heparin-BSA to MSP1-42 was similarly dose-dependent and saturated with a heparin-BSA concentration of 1 μg/mL (Figure 4B). MSP1-42 binding was inhibited in a concentration-dependent manner by heparin, but not by CSC (Figure 4C).
Recombinant MSP1-42 binds to heparin. (A) Recombinant merozoite antigens were tested for binding to heparin-BSA (5 μg/mL) or BSA alone (5 μg/mL). Binding to lactoferrin was used as a positive control and to normalize binding between assays. Data are mean binding ± SEM expressed as percentage binding of lactoferrin (3 assays in duplicate). Heparin-BSA had similar growth-inhibitory activity against P falciparum in vitro as heparin, whereas BSA was not inhibitory (data not shown). (B) Binding of heparin-BSA to MSP1-42 was concentration-dependent and saturable at low concentrations. Data are normalized to percentage binding of lactoferrin to heparin-BSA at 5 μg/mL. (C) Binding of heparin-BSA to MSP1-42 was inhibited with soluble heparin but not CSC. Data are expressed as the proportion of binding observed in the absence of inhibitors. Data are mean ± SEM (3 assays in duplicate).
Recombinant MSP1-42 binds to heparin. (A) Recombinant merozoite antigens were tested for binding to heparin-BSA (5 μg/mL) or BSA alone (5 μg/mL). Binding to lactoferrin was used as a positive control and to normalize binding between assays. Data are mean binding ± SEM expressed as percentage binding of lactoferrin (3 assays in duplicate). Heparin-BSA had similar growth-inhibitory activity against P falciparum in vitro as heparin, whereas BSA was not inhibitory (data not shown). (B) Binding of heparin-BSA to MSP1-42 was concentration-dependent and saturable at low concentrations. Data are normalized to percentage binding of lactoferrin to heparin-BSA at 5 μg/mL. (C) Binding of heparin-BSA to MSP1-42 was inhibited with soluble heparin but not CSC. Data are expressed as the proportion of binding observed in the absence of inhibitors. Data are mean ± SEM (3 assays in duplicate).
The level and pattern of sulfation and chain length of heparin-like molecules determine their invasion inhibitory activity
To further understand the molecular basis of interactions with heparin-like molecules during merozoite invasion of erythrocytes, a range of heparin-like molecules with defined structural features and oligosaccharides of defined length were tested as inhibitors of invasion (Figure 5; Table 1).
Structure of heparin and related molecules. (A) Heparin: the major disaccharide unit of heparin is 4-linked α-iduronic acid (IdoA) and 4-linked α-glucosamine (GlcNAc), in which IdoA is 2-O-sulfated and GlcNAc is 6-O- and de-acetyl-N-sulfated. Heparin can be modified by selective de-N-sulfation and/or de-O-sulfation. (B) K5 polysaccharide: this has the same backbone as the precursor to heparin and can be modified by addition of sulfate groups at indicated positions (arrows) and by epimerization of GlcA to IdoA (indicated in gray). This results in polysaccharides with various sulfation levels and patterns and structural features.
Structure of heparin and related molecules. (A) Heparin: the major disaccharide unit of heparin is 4-linked α-iduronic acid (IdoA) and 4-linked α-glucosamine (GlcNAc), in which IdoA is 2-O-sulfated and GlcNAc is 6-O- and de-acetyl-N-sulfated. Heparin can be modified by selective de-N-sulfation and/or de-O-sulfation. (B) K5 polysaccharide: this has the same backbone as the precursor to heparin and can be modified by addition of sulfate groups at indicated positions (arrows) and by epimerization of GlcA to IdoA (indicated in gray). This results in polysaccharides with various sulfation levels and patterns and structural features.
Testing variously desulfated heparins indicated the importance of both N- and O-sulfation for P falciparum inhibitory activity. Completely desulfated (de-N/O-sulfated; degree of sulfation [DOS] = 0) and de-N-sulfated heparin (DOS = 1.8) had no activity, and partially de-N-sulfated heparin (DOS = 2.5), de-6-O-sulfated (DOS = 1.6), and de-2-O-sulfated (DOS = 1.9) had reduced inhibitory activity (50% inhibitory concentration [IC50] = 74, 373, and 123 μg/mL, respectively; Table 1; Figure 6A). These results show the importance of both N- and O-sulfation for maximum inhibitory activity. Comparison of fully de-N-sulfated heparin (DOS = 1.8; IC50 > 100 μg/mL) and partially de-N-sulfated heparin (DOS = 2.5; IC50 = 74 μg/mL) further indicated the importance of sulfation level for inhibition, with higher activity observed with an increasing sulfate content. De-2-O-sulfated heparin had greater inhibitory activity than de-6-O-sulfated heparin, which may reflect their differences in sulfate content (DOS = 1.9 and 1.6, respectively) or a preference for 6-O- rather than 2-O-sulfation for activity.
Structural requirements of heparin-like molecules for invasion-inhibitory activity. A panel of modified heparins and K5 polysaccharides was tested for inhibition of invasion. (A) Growth-inhibitory activity of selectively desulfated heparins against P falciparum blood stage parasites. (B) Growth-inhibitory activity of K5 polysaccharide derivatives with low (K5-OS-L) and high (K5-OS-H) levels of O-sulfation. (C) Inhibitory activity of K5 polysaccharides with low (K5-NSOS-L) and high (K5-NSOS-H) levels of N- and O-sulfation, compared with the same molecules that have GlcA partially epimerized to IdoA (EK5; ratio of GlcA/IdoA is 50:50). (D) The degree of sulfation (d.o.s.; number of sulfate groups per disaccharide unit) of heparin and heparin-like molecules was compared with the IC50 (μg/mL). A DOS more than or equal to 2 (dashed line) was required for inhibitory activity. Noninhibitory molecules were plotted as having an IC50 of 500 μg/mL. Calculated IC50 values for all molecules are shown in Table 1. (E) Inhibitory activity of heparin and CSC oligosaccharides of different chain lengths (2-16 monosaccharide units), tested at 100 μg/mL. Data are mean growth ± SEM (3 assays in duplicate).
Structural requirements of heparin-like molecules for invasion-inhibitory activity. A panel of modified heparins and K5 polysaccharides was tested for inhibition of invasion. (A) Growth-inhibitory activity of selectively desulfated heparins against P falciparum blood stage parasites. (B) Growth-inhibitory activity of K5 polysaccharide derivatives with low (K5-OS-L) and high (K5-OS-H) levels of O-sulfation. (C) Inhibitory activity of K5 polysaccharides with low (K5-NSOS-L) and high (K5-NSOS-H) levels of N- and O-sulfation, compared with the same molecules that have GlcA partially epimerized to IdoA (EK5; ratio of GlcA/IdoA is 50:50). (D) The degree of sulfation (d.o.s.; number of sulfate groups per disaccharide unit) of heparin and heparin-like molecules was compared with the IC50 (μg/mL). A DOS more than or equal to 2 (dashed line) was required for inhibitory activity. Noninhibitory molecules were plotted as having an IC50 of 500 μg/mL. Calculated IC50 values for all molecules are shown in Table 1. (E) Inhibitory activity of heparin and CSC oligosaccharides of different chain lengths (2-16 monosaccharide units), tested at 100 μg/mL. Data are mean growth ± SEM (3 assays in duplicate).
To further understand the relative importance of N- versus O-sulfation, sulfation pattern, and the overall level of sulfation, modified K5 polysaccharides with known sulfation levels and patterns were tested for inhibitory activity (Figure 5; Table 1). K5 polysaccharides with N-sulfation only (K5-NS, DOS = 1) or low levels of O-sulfation (K5-OS-L, DOS = 1.4) had no growth inhibitory activity (Figure 6B). In contrast, highly O-sulfated K5 (K5-OS-H; DOS = 3) had significant activity (IC50 = 20.2 μg/mL) despite lacking N-sulfation. This indicates that N-sulfation is not an absolute requirement for activity, in contrast to the prior finding of the lack of inhibitory activity of de-N-sulfated heparin (Figure 6A).42 K5 polysaccharides with both N- and O-sulfation (K5-NSOS-L, DOS = 2; and K5-NSOS-H, DOS = 3.7) had substantial activity (IC50 = 55.7 and 7.4 μg/mL, respectively; Figure 6C). K5-NSOS-H was the most inhibitory of all molecules tested. The greater inhibitory activity of K5-NSOS-H compared with K5-NSOS-L suggests that higher levels of sulfation enhance activity. The greater inhibition by K5-NSOS-H compared with K5-OS-H, which have similar O-sulfation levels, suggests that the presence of N-sulfate groups increases inhibitory activity.
K5 polysaccharide contains GlcA only, whereas heparin contains mainly IdoA; the inhibitory activity of sulfated K5 polysaccharides suggested that IdoA was not essential for activity. To investigate this, we tested epimerized K5 (EK5) polysaccharide derivatives. EK5-NSOS-L and EK-NSOS-H were prepared from K5-NSOS-L and K5-NSOS-H, respectively, and contain an estimated 50:50 of IdoA to GlcA but maintain the same sulfation level (supplier's information; Table 1). Both epimerized K5 derivatives had significantly less inhibitory activity compared with their parent GlcA-containing polysaccharides (Figure 6C; Table 1). This suggests that IdoA is not required for optimal activity, and there is a preference for GlcA.
To further understand the importance of sulfation level and pattern for inhibitory activity of heparin-like molecules, we compared the inhibitory activity of molecules to their DOS (Figure 6D; Table 1). This showed that inhibitory activity requires a threshold level of sulfation of DOS more than or equal to 2, with a correlation between DOS and IC50 (Spearman ρ = −0.87, P < .001). However, there was no significant correlation between sulfation level and IC50 if only analyzing inhibitory molecules with IC50 less than 400 (Spearman ρ = −0.63, P = .1); this suggests that sulfation pattern is important for activity and not just the overall level of sulfation. Comparing the inhibitory activity of different molecules (Table 1) suggests that sulfation at both N and O positions is not an absolute requirement but that sulfate groups must be spatially positioned on the same monosaccharide residue (ie, hexuronic acid or GlcNAc) of each disaccharide for optimal activity. Inhibitory molecules (heparin, K5-OS-H, K5-NSOS-L, and K5-NSOS-H) have this property, whereas less inhibitory molecules (de-2-O-sulfated heparin, de-N-sulfated heparin, and K5-OS-L) do not.
To investigate the importance of chain length for inhibitory activity, heparin and CSC oligosaccharides (2-16 residues in length) were tested for inhibition of P falciparum growth. A minimum of 6 monosaccharide units of heparin was needed for inhibitory activity (Figure 6E), and 8mer or higher oligosaccharides had greater activity (IC50 = 79, 67, and 67 μg/mL, for 6mer, 8mer, and 10mer, respectively; data not shown). CSC oligosaccharides had little or no inhibitory activity compared with heparin oligosaccharides of the same length.
Structural features of heparin-like molecules for binding MSP1-42
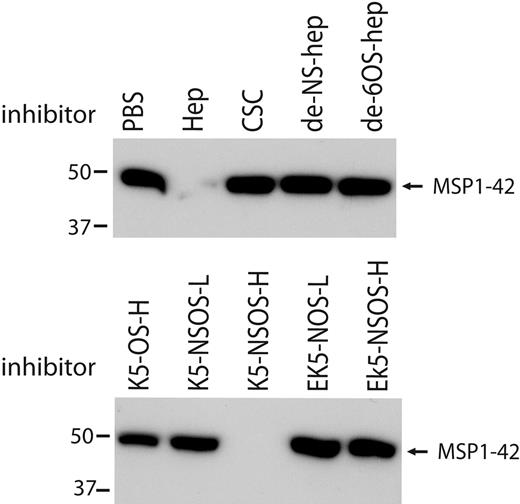
To understand the structural requirements of heparin for binding to MSP1-42, modified heparins and K5 polysaccharides were tested for inhibition of MSP1-42 binding to heparin. Binding of MSP1-42 was not inhibited by de-N-sulfated heparin or de-6-O-sulfated heparin (Figure 7), demonstrating a dependence on sulfation for binding. Binding of MSP1-42 was inhibited by soluble K5-NSOS-H and, to a lesser degree, by K5-OS-H. There was very little or no inhibition of binding by K5-NSOS-L, EK5-NSOS-L, or EK5-NSOS-H (Figure 7). MSP1-42 binding to heparin is therefore not only dependent on level of sulfation, but also the pattern of sulfation, with specific spatial positions of sulfate groups probably important for binding. The binding-inhibition activity of the polysaccharides closely reflected their activity in cellular inhibition assays, further suggesting that heparin binding to MSP1-42 is probably, at least in part, mediating the growth-inhibitory effect of heparin and heparin-like molecules.
Structural requirements of heparin-like molecules for binding of MSP1-42. Binding of MSP1-42 to heparin-agarose beads was inhibited by heparin but not by de-N-sulfated (de-NS-) and de-6O-sulfated (de-6OS-) heparin. Binding of MSP1-42 was also inhibited by K5 polysaccharides with high-level N- and O-sulfation (K5-NSOS-H), but not by other K5 polysaccharides having different sulfation levels or patterns, or by EK5 polysaccharides. Heparin-agarose bound proteins were detected by Western blot using antibodies to MSP1-19. K5-NSOS-H was the most inhibitory molecule in parasite invasion inhibition assays, whereas the other molecules had little or no invasion-inhibitory activity.
Structural requirements of heparin-like molecules for binding of MSP1-42. Binding of MSP1-42 to heparin-agarose beads was inhibited by heparin but not by de-N-sulfated (de-NS-) and de-6O-sulfated (de-6OS-) heparin. Binding of MSP1-42 was also inhibited by K5 polysaccharides with high-level N- and O-sulfation (K5-NSOS-H), but not by other K5 polysaccharides having different sulfation levels or patterns, or by EK5 polysaccharides. Heparin-agarose bound proteins were detected by Western blot using antibodies to MSP1-19. K5-NSOS-H was the most inhibitory molecule in parasite invasion inhibition assays, whereas the other molecules had little or no invasion-inhibitory activity.
Discussion
In this study, we have demonstrated that heparin-like molecules inhibit early and essential events in invasion. We have defined their timing and mechanism of action, and established that a processed form of the major merozoite protein MSP1 has heparin-binding activity and appears to be the target of heparin-like molecules in mediating invasion inhibition. In addition, we have determined key structural features of heparin-like molecules required for inhibitory activity and binding MSP1-42.
We clearly demonstrated that heparin inhibits the early events in invasion using live video microscopy. Over multiple experiments, we observed merozoites make contact with erythrocytes in the presence of heparin; however, this contact was reversible and not sustained. We never observed successful invasion or events downstream of initial attachment, such as apical reorientation or erythrocyte deformation. This inhibitory phenotype was clearly distinct to the phenotype recently described for inhibition of AMA1 function, where initial oscillatory deformation of the erythrocyte, merozoite reorientation, and echinocytosis were observed without successful invasion.38 Tracking the effect of heparin on schizont rupture and ring formation further supported the observation that heparin-like molecules act by inhibiting erythrocyte invasion. Previously, it was reported that heparin may act by blocking schizont rupture.13,16 Although we showed that heparin can cause some delay in schizont rupture, the modest effect observed was not sufficient to explain inhibition of parasite replication.
Several observations suggest that heparin-like molecules inhibit essential invasion events. The identification of essential interactions during invasion is important when considering potential targets for drug and vaccine development. Of further significance, heparin inhibited parasite isolates using different invasion pathways. The ability of P falciparum isolates to use alternative invasion pathways appears to be mediated by differential expression and use of EBA and PfRh ligands43 and is thought to be one mechanism of immune invasion used by P falciparum.31 These findings suggest that the EBA and PfRh proteins are not the major targets of heparin-like molecules and that the inhibitory activity of heparin is not mediated by blocking the binding of EBAs to negatively charged SA residues on erythrocyte receptors. EBA and PfRh ligands are thought to play a role in tight-junction formation,2 an event downstream of initial attachment and reorientation; therefore, the timing and mechanism of action of heparin inhibition are not consistent with it acting by inhibiting the function of EBAs and PfRhs. Furthermore, parasites lacking specific EBA and PfRh genes were inhibited to the same degree as parental parasites.
Consistent with the timing and mechanism of action of heparin-mediated inhibition of invasion, we found that MSP1-42, a processed form of MSP1, bound to heparin in a specific manner. Other merozoite antigens (MSP3, MSP4, AMA1, and EBA175) did not bind. Only MSP1-42, and not full-length MSP1 or MSP1-19, bound heparin, suggesting that primary processing of MSP1 is required for binding activity. MSP1-33 also bound heparin, suggesting that the binding of MSP1-42 to heparin is at least partly mediated through this region. MSP1 is known to exist in complex with other antigens MSP644 and MSP7.45 Although this study has not formally excluded the role of these antigens in the binding of MSP1-42 to heparin, if MSP6 or MSP7 were predominantly mediating the binding of MSP1 to heparin, it would be expected that full-length MSP1would bind to heparin to similar degrees, which it did not. Furthermore, the binding of MSP1-42 was confirmed using recombinant MSP1-42. Further studies testing inhibition of heparin binding using antibodies to different MSP1 constructs and alleles may aid the identification of specific interactions and could be valuable in informing vaccine development. Heparin-like molecules are known to be present on the surface of reticulocytes and erythrocytes18 where they may be bound by MSP1-42 during invasion. Our findings suggest a model in which the primary processing of full-length MSP1 to generate MSP1-42 allows the correct conformation for binding to heparin-like molecules on erythrocytes. On invasion, secondary processing would disrupt binding from this receptor by shedding MSP1-33, and MSP1-19 would be carried into the erythrocyte possibly bound to band 3. Multiple studies suggest that MSP1 is essential for invasion and is consistent with our findings that heparin-like molecules inhibit essential interactions during invasion. MSP1-42 remains an important target of vaccine development.46 Further characterization of the interaction between heparin and MSP1-42 to identify a specific binding site may be valuable in vaccine approaches to generate antibodies with potent inhibitory activity. There may be other interactions between merozoite proteins and heparin-like molecules that are also important during invasion. It was recently reported that PfRh547 and BAEBL/EBA14048 have some heparin-binding activity. However, further studies are needed to confirm the specificity and relevance of these interactions, and EBA140 appears to function predominantly by binding glycophorin C.2,43 Live imaging revealed that inhibition of invasion by heparin occurred before tight-junction formation when the EBAs and PfRh proteins are thought to act. Furthermore, EBA140-knockout parasite lines showed comparable and complete inhibition by heparin in our studies. Binding of surface antigens may help to establish initial contact, and then binding of other ligands, possibly PfRh5 or EBA140, may be involved in subsequent invasion steps. Although these ligands may play some role in the inhibitory activity of heparin, it seems probable that the major inhibitory activity occurs at initial contact steps.
Our studies clearly show that the overall level of sulfation is crucial for the inhibitory activity of heparin-like molecules and a DOS more than or equal to 2 (ie, 2 or more sulfate groups per disaccharide unit) are required for substantial activity. The pattern of sulfation is also important, and comparing inhibitory and noninhibitory molecules suggests that sulfate groups need to be present on the same monosaccharide residue of each disaccharide unit for optimal activity. Furthermore, a chain length of more than or equal to 6 monosaccharide units was the minimum length required for substantial activity, and there is a preference for GlcA rather than IdoA. GlcA is thought to convey greater rigidity to the polysaccharide structure than IdoA.
The identification of highly inhibitory molecules may be useful in the development of therapeutic inhibitors; glycosaminoglycans and sulfated polysaccharides (eg, heparin, chondroitin sulfate, pentosan polysulfate) are used clinically in other diseases. Although polysaccharides are highly heterogeneous, it is technically possible to prepare oligosaccharides with defined compositions that may have much greater activity. This approach using structure/function relationships as the basis for drug design was successful in the development of a pentasaccharide anticoagulant fondaparinux, based on the antithrombin-binding motif of heparin.49 Heparin is used clinically as an anticoagulant; however, the structural requirements for anticoagulant activity differ from those for optimal antimalarial activity identified here. The most inhibitory molecule we identified, K5-NSOS-H, has little anticoagulant activity.22,23,50 In addition, IdoA is required for optimal anticoagulant activity, whereas antimalarial activity was greater with GlcA. Presently, all licensed antimalarials act by inhibiting the intra-erythrocytic development of parasites. Our studies demonstrate the potential for therapeutic approaches that target merozoite invasion.
In conclusion, we have clearly identified the mechanism and timing of inhibitory action of heparin-like molecules, determined key structural features for activity and identified MSP1-42 as a heparin-binding protein and the probable target of inhibitory molecules. These findings have significant implications for understanding the mechanisms of P falciparum invasion of erythrocytes, identifying parasite proteins that could be targets of novel therapies or vaccines and developing novel antimalarials inhibitors based on the structure and activity of heparin-like molecules.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Carole Long for providing recombinant MSP1-42 protein, Dr Robin Anders for recombinant AMA1, Dr Alan Cowman for providing antibodies to EBA175 and MSP3 and knockout parasite lines, Dr Mike Blackman for providing antibodies to MSP1-33, Ross Coppel for antibodies to MSP4, Kerstin Leykauf and Brendan Crabb for antibodies to AMA1, Dr Brendan Crabb for helpful discussions and suggestions, Dr Mirja Hommel and Dr Freya Fowkes for comments on the manuscript, and the Red Cross Blood Bank (Melbourne) for providing erythrocytes and serum for parasite culture.
This work was supported by the National Health and Medical Research Council of Australia (Project grant and Career Development Award to J.G.B.), Postgraduate Research Fellowships (J.S.R.), Independent Research Institutes (Infrastructure Support Scheme Grant to the Walter and Eliza Hall Institute), the Australian Government (Australian Postgraduate Award to M.J.B.), University of Melbourne (top-up award to M.J.B.), the Victorian State Government (Operational Infrastructure Support Grant to the Walter and Eliza Hall Institute), and the Wellcome Trust (W.C.).
Authorship
Contribution: M.J.B. and J.G.B. designed the study, analyzed and interpreted the results, performed experiments, and wrote the manuscript; J.S.R. contributed to study design, interpreted the results, performed experiments, and contributed to writing the manuscript; P.R.G. designed the experiment and specific experimental expertise, interpreted the results, and contributed to writing the manuscript; and W.C. generated reagents, contributed to study design, interpreted the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James G. Beeson, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria 3050, Australia; e-mail: beeson@wehi.edu.au.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal