Abstract
The high prevalence of hemoglobin S (HbS) in Africa and hemoglobin C (HbC) in parts of West Africa is caused by the strong protection against severe falciparum malaria during childhood. Much less is known about the effect of HbS and especially HbC on Plasmodium falciparum infection, uncomplicated malaria, and anemia. A total of 1070 children from the Ashanti Region, Ghana, were enrolled at the age of 3 months and visited monthly until 2 years of age. The effects of the β-globin genotype on the age-dependent incidence of malaria, levels of parasitemia, and hemoglobin as well as physical development were analyzed by population-averaged models. Infants with HbAS were protected from uncomplicated malaria (P < .005) and anemia (P < .001), had lower age-adjusted parasite densities (P < .001), and higher age-adjusted hemoglobin levels compared with children with the HbAA genotype (P = .004). In contrast, HbAC carriers had lower hemoglobin levels (P < .033) and were not protected against malaria or anemia. Notably, infants with HbAS were also significantly protected against stunting compared with carriers of HbAA or HbAC. This indicates differing mechanisms of protection against malaria of HbAS and HbAC and might help to understand why HbC is restricted to distinct areas of West Africa.
Introduction
Every year malaria kills more than 1 million children less than 5 years of age in sub-Saharan Africa. The high frequency of red blood cell polymorphisms in areas presently or historically endemic for malaria has led to the hypothesis that they might provide a survival advantage.1-3 The hemoglobin variants HbS (β6Glu → Val) and HbC (β6Glu → Lys) have reached carrier frequencies of up to 30% in some parts of Africa.4 HbS serves as the paradigm for balanced polymorphisms: whereas persons homozygote for HbS (HbSS) have sickle cell disease with an increased mortality, heterozygote carriers (HbAS, sickle cell trait) are asymptomatic and protected from severe malaria.5,6 A few studies have demonstrated a reduced risk for uncomplicated malaria in children with the sickle cell trait,6-8 and it was recently demonstrated that this may lead to a lower frequency of stunting in children with the HbAS genotype.9 HbAS carriers have reduced parasite densities compared with carriers of the β-globin wild-type (HbAA),8,10-12 whereas a protective effect against asymptomatic Plasmodium falciparum infection per se is subject to controversy.6,8,10
Inhibition of parasite growth and reduced invasion into HbS erythrocytes have been demonstrated in vitro.13,14 It has been assumed that this is partly caused by a reduction of the hemoglobin solubility under low oxygen tension14,15 and increased sickling of parasitized cells with an enhanced elimination by the reticuloendothelial system.15-17 Recently, it has been suggested that HbAS causes a reduced cytoadherence of parasitized red blood cells18 and that it enhances immune responses against P falciparum–infected erythrocytes7,19
In contrast to HbS, the distribution of HbC is restricted to distinct areas in West Africa4,20 ; and as opposed to HbSS, the HbCC genotype only results in a mild clinical phenotype.21 It has been demonstrated that HbC protects from severe malaria as well22-25 and hypothesized that HbC may replace HbS in the future.23,26 The mechanism of selection for and protection by HbC remains unclear, and there is some controversy on an effect on parasitemia.22,27,28 In vitro, parasite replication in HbAC erythrocytes equals that in HbAA erythrocytes,29 whereas a lower multiplication has been observed in HbCC cells.30 It has been assumed that the disability of HbCC red cells to lyse and to release merozoites is responsible for this phenomenon.31 Recent data show an abnormal expression of PfEMP-1 on the surface of erythrocytes with HbAC, resulting in reduced cytoadherence.32
Several studies analyzed the effects of HbS and HbC on the risk of severe malaria and, in vitro, on potential mechanisms of protection.6,10-18,22-25 However, data on possible effects of HbS and HbC on mild malaria and parasitemia, in particular from early childhood, when acquired immunity has not yet developed, are missing. While severe malaria can be studied in case-control or hospital-based cross-sectional studies, an analysis of the influence of HbS/HbC on the risk of P falciparum infection and uncomplicated malaria is only possible in longitudinal studies, such as the one presented here.
The aim of this study was to analyze the influence of HbAS and HbAC genotypes on the incidence of parasitemia, uncomplicated malaria, anemia, and possible effects on stunting, a marker for childhood development and a risk factor for childhood mortality,33 during the first 2 years of life.
Methods
Study area
A cohort of 1070 children from the rural Afigya Sekyere district in the Ashanti region of Ghana was followed from January 2003 to September 2005. The area is holoendemic for malaria with perennial malaria transmission and seasonal peaks. The predominant parasite species is P falciparum (> 95%), and main vectors are from the Anopheles gambiae complex and A funestus.
Participants
Infants 3 months of age (± 4 weeks tolerance accepted) with permanent residency in one of 9 selected villages in the study area were recruited over the period of one full year. The aim and principles of the study were explained, and informed consent was signed or thumb printed by the caregiver in accordance with the Declaration of Helsinki. The cohort was originally enrolled in the frame of a trial on Intermittent Preventive Treatment in Infants.34 Ethical approval for the clinical trial was given by the Committee of Human Research, Publications and Ethics, School of Medical Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, and the Ärztekammer Hamburg, Hamburg, Germany. Briefly, participants were recruited at regular sessions of the World Health Organization Expanded Program of Immunization. The investigational products contained either 250 mg of sulfadoxine/12.5 mg of pyrimethamine or placebo (Roche Pharmaceuticals) and were blister-packed and precoded after block-randomization (blocks of 10). The first dose was given to 3-month-old infants. The second and third doses were administered at 9 and 15 months of age, respectively.
Study procedures
Study participants were followed up by the study team in monthly intervals for 21 months until the age of 2 years, with passive case detection between active visits. On every visit, the study physician performed a clinical examination and a standardized assessment of the medical history. Trained technicians did heel-prick or finger-prick blood sampling for malaria parasites (thick and thin film) and hemoglobin measurements at each contact. In addition to the active and passive case detection by the study team, parents were free to visit the surrounding health facilities if their child was ill. To enable monitoring of any treatment given on such occasions, costs were reimbursed if a receipt was presented. Any relevant medical information documented in the child's weighing card and hospital files was assessed on a regular basis. Medical treatment was free of charge, and hospital fees were covered during the complete follow-up. Episodes of uncomplicated malaria were treated with chloroquine or amodiaquine/artesunate (from April 2004) according to national guidelines.
Children with severe malaria, as defined by World Health Organization,35 were sent to a hospital and treated according to hospital guidelines. According to local guidelines, children with hemoglobin levels less than 8 g/dL received supplementation of oral ferrous-sulfate solution with a target dose of 3 to 6 mg/kg daily. Oral iron supplementation was given to 833 children (82.6%) during 2554 visits. The need for iron application reflected the occurrence of anemia episodes among the different genotypes. Causes of death were ascertained by verbal autopsy.
Laboratory procedures
Venous blood samples and films were processed and analyzed in the project laboratory at the Kumasi Center for Collaborative Research in Tropical Medicine.
Parasite densities.
Thin and thick blood films were air-dried, Giemsa-stained, and examined by light microscopy with 1000× oil- immersion lens by 2 independent slide readers. Malaria parasite count was done until 200 leukocytes were counted; if less than 10 parasites were found, assessment was extended to 500 leukocytes. Parasite densities were calculated by assuming a total leukocyte count of 8000/μL. In case of divergent parasite positivity/negativity, parasite density (ratio > 3.0), or parasite species, slides were reexamined and final parasitemia was defined as the median parasite densities of positive results.
Hemoglobin level.
Hemoglobin was measured photometrically (Hemocue Ltd) during follow-up visits.
Anthropometric data.
The length of each child was assessed at recruitment and subsequently in 3-monthly intervals, with measurements performed on the same standardized length board for all children.
DNA preparation.
DNA was preserved by addition of an equivalent volume of 8M urea to the blood sample. Parallel purification of genomic DNA from whole blood was performed in a 96-well format with magnetic beads (NucleoMag 96 Blood; Macherey-Nagel). Extracted DNA was quantitated and adjusted to a concentration of 5 ng/μL by fluorescence dye binding to DNA and fluorescence measurement at a microplate reader (PicoGreen dsDNA Quantitation Assay and Kit; Invitrogen).
Genotyping of β-globin.
Genotyping was performed after the end of the study or in case of recurrent severe anemia or other reasonable suspicion of sickle cell anemia. The results were made available to the study participants. Parents were counseled and children with homozygote genotype HbSS were referred to a specialized clinic. High-throughput postamplification genotyping was performed using fluorescent melting curve assays on 384-well microplate formats in a homogeneous system (LightTyper; Roche Diagnostics). All reagents were added at the beginning of the reaction, and there was no sample manipulation between amplification and genotyping. High-resolution melting curves were achieved during slow and continuous heating through fluorescent resonance energy transfer reaction. Genotyping was performed automatically, and each sample gave a score reflecting the similarity of the genotype to the standards provided. The first exon of the β-globin gene was amplified under the following conditions: 4 pmol/μL of the primer Hb-F (5′ACATTTGCTTCTGACACAAC3′), 10 pmol/μL of the primer Hb-R (5′GCCCAGTTTCTATTGGTCTCC3′), denaturation 2 minutes at 94°C, 45 cycles (15 seconds at 94°C, 30 seconds at 63°C, ramp to 75°C 0.5°C/second, 75°C for 2 seconds), elongation 2 minutes at 72°C, and final denaturation 2 minutes at 94°C. The probes and their concentrations for assessment of the melting curves were 4 pmol/μL Hb-anchor (5′Cy5-GTTACTGCCCTGTGGGGCAAGGTGAACGTGGATGA-Ph3′) and 4 pmol/μL Hb-sensor (5′-CTCCTGTGGAGAAGTCTGC-Fl3′). Peaks of the differential melting curves (range, 35°C to 80°C, 0.1°C/second) were at 55°C (HbA), 50°C (HbC), and 62°C (HbS).
Clinical definitions
According to the study protocol, anemia was defined as a hemoglobin concentration less than 7.5 g/dL. Results did not differ if 8 g/dL was used as a cut-off. An episode of malaria was defined as fever (temperature ≥ 38.0°C or fever during the preceding 48 hours reported by mothers without being asked), accompanied by asexual P falciparum parasitemia of more than 500 parasites/μL. According to the algorithm proposed by Smith et al,36 this case definition yielded the highest sensitivity and specificity (> 90%) for attribution of fever episodes to malaria among all events of fever. Children were not considered to be at risk of malaria for 21 days after confirmed malaria episodes or after presumptive antimalarial therapy; children were not considered to be at risk for anemia for 21 days after a preceding episode of anemia, iron administration, or antimalarial therapy. Severe malaria was defined according to World Health Organization.35 A total of 97% of the observed malaria episodes were uncomplicated.
Statistical analyses
Data exploration and final analysis were carried out with STATA/SE (Version 10.1; StataCorp). P values less than .05 were considered significant. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to estimate the risks for outcomes with categorical exposures. Bivariate Mantel-Haenszel analyses were performed to assess possible interactions and confounding of exposures. Nonconditional logistic regression was conducted for multivariate analyses. Coefficients were log-transformed into ORs and their 95% confidence intervals. Effect modification through interaction between β-globin genotypes and α+-thalassemia was assessed by Wald tests. Likelihood ratio tests were performed to measure how well the models accounted for the outcomes and whether excluding single variables affected the quality of the model. Incidence rate ratio (IRR) and hazard risk ratio (HRR) were calculated with Poisson regression and Cox regression, respectively, and adjusted for the treatment arm.
To analyze the effect of β-globin genotypes on the age-dependent dynamics of parasitemia, hemoglobin levels and stunting the between-group effects for the β-globin genotypes were assessed by population-averaged models estimated by generalized estimation equation (GEE). A Gaussian variable distribution was assumed and an exchangeable correlation structure was used for the analyses of parasitemia and hemoglobin. To analyze the influence of the β-globin genotypes on the age-dependent risk of stunting an autoregressive correlation structure of the order 5 was used, and missing values for anthropometric data between 2 existing follow-up visits were interpolated by calculating the mean of the 2 flanking visits (in total, 6606 anthropometric measurements and 364 interpolated values).
In case of continuous variables, t tests were performed if the parameters were normally distributed, and Kruskal-Wallis tests were carried out if the parameters were non-normally distributed. The assumption of Hardy-Weinberg equilibrium was tested in controls based on a z statistic, and a κ value was calculated.
Results
Cohort characteristics
Genotyping results of the β-globin gene were available in 1010 of 1070 participants (5.6% missing). Of these, 852 could be followed until the age of 23 months. Nineteen children were lost to follow-up resulting from death (1.8%). During the observation time, 19 561 active visits, 940 passive visits, and 209 hospital admissions were documented. The total time of follow-up under observation was 1725 person-years. Baseline characteristics of the cohort stratified for the β-globin genotype are given in Table 1. No significant differences were found in persons with genotyping failures. The genotype frequencies of HbAS and HbAC were 10.9% and 10.5%, respectively. The frequencies of the genotypes HbCC, HbCS, and HbSS were less than 1.5%, resulting in a weak statistical power to reject the null hypothesis of no difference. Consequently, most analyses focus on the comparison of HbAA, HbAS, and HbAC.
Basic characteristics at recruitment, stratified for β-globin genotypes
| β-Globin genotype . | HbAA (n = 774) . | HbAC (n = 106) . | HbCC (n = 2) . | HbAS (n = 110) . | HbCS (n = 12) . | HbSS (n = 6) . | Total (n = 1010) . |
|---|---|---|---|---|---|---|---|
| Genotype frequency, % | 76.6 | 10.5 | 0.2 | 10.9 | 1.2 | 0.6 | 100 |
| Male:female | 51:49 | 50:50 | 50:50 | 46:54 | 42:58 | 50:50 | 50:50 |
| Ethnicity, n (%) | |||||||
| Akan | 682 (88.1) | 80 (75.5) | 2 (100) | 95 (86.4) | 9 (75.0) | 4 (66.7) | 872 (86.3) |
| Northerners | 86 (11.1) | 25 (23.6) | 0 (0.0) | 15 (13.6) | 3 (25.0) | 1 (16.7) | 130 (12.9) |
| Others | 6 (0.8) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (0.8) |
| Mosquito protection,* % | 381 (54.6) | 46 (48.4) | 1 (50.0) | 43 (44.3) | 7 (63.6) | 2 (50.0) | 480 (52.9) |
| Born in the rainy season,† % | 365 (47.2) | 53 (50.0) | 1 (50.0) | 48 (43.6) | 5 (41.7) | 3 (50.0) | 475 (47.0) |
| Mother's age, y (mean ± SD) | 27.2 ± 6.3 | 28.1 ± 6.2 | 30.3 ± 5.5 | 27.1 ± 6.1 | 27.1 ± 5.5 | 26.1 ± 7.2 | 27.3 ± 6.3 |
| β-Globin genotype . | HbAA (n = 774) . | HbAC (n = 106) . | HbCC (n = 2) . | HbAS (n = 110) . | HbCS (n = 12) . | HbSS (n = 6) . | Total (n = 1010) . |
|---|---|---|---|---|---|---|---|
| Genotype frequency, % | 76.6 | 10.5 | 0.2 | 10.9 | 1.2 | 0.6 | 100 |
| Male:female | 51:49 | 50:50 | 50:50 | 46:54 | 42:58 | 50:50 | 50:50 |
| Ethnicity, n (%) | |||||||
| Akan | 682 (88.1) | 80 (75.5) | 2 (100) | 95 (86.4) | 9 (75.0) | 4 (66.7) | 872 (86.3) |
| Northerners | 86 (11.1) | 25 (23.6) | 0 (0.0) | 15 (13.6) | 3 (25.0) | 1 (16.7) | 130 (12.9) |
| Others | 6 (0.8) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (0.8) |
| Mosquito protection,* % | 381 (54.6) | 46 (48.4) | 1 (50.0) | 43 (44.3) | 7 (63.6) | 2 (50.0) | 480 (52.9) |
| Born in the rainy season,† % | 365 (47.2) | 53 (50.0) | 1 (50.0) | 48 (43.6) | 5 (41.7) | 3 (50.0) | 475 (47.0) |
| Mother's age, y (mean ± SD) | 27.2 ± 6.3 | 28.1 ± 6.2 | 30.3 ± 5.5 | 27.1 ± 6.1 | 27.1 ± 5.5 | 26.1 ± 7.2 | 27.3 ± 6.3 |
Use of bednets or window screens.
Months from May to October.
Allele frequencies of the β-globin variants HbA, HbS, and HbC were 87.3%, 6.6%, and 6.0%, respectively. Distribution of the HbAC and HbAS genotypes did not significantly deviate from the Hardy-Weinberg equilibrium (P > .30).
Effect of HbAS and HbAC on the incidence of falciparum malaria
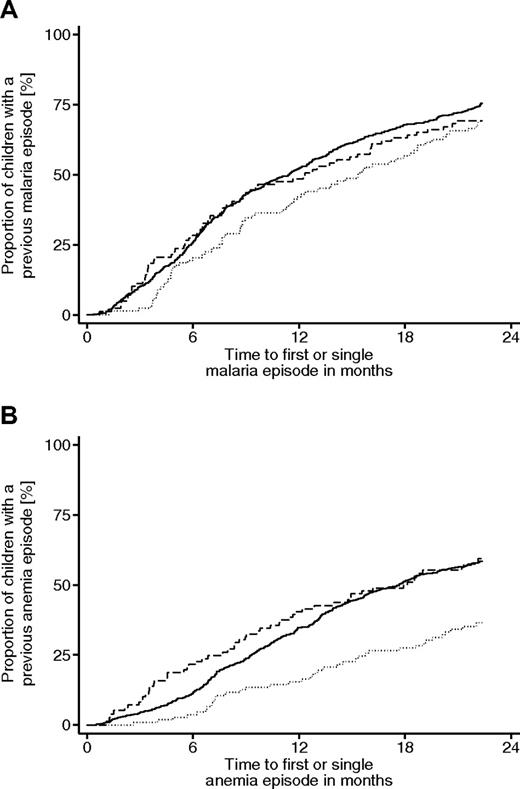
The majority of malaria episodes were detected by active case detection (93.2%). The prevalence of uncomplicated malaria at recruitment was 1.8% and, probably because of the low number, did not significantly differ between the various β-globin genotypes (Table 2). During the time of follow-up, the incidence rate of uncomplicated malaria was 22% lower in the group of HbAS carriers compared with children with HbAA (IRR = 0.78; 95% CI, 0.66-0.92, P < .005). HbAC carriers, however, were at a similar risk for uncomplicated malaria as HbAA carriers (IRR = 1.08; 95% CI, 0.93-1.26, P = .31) but differed significantly from HbAS carriers (P = .003; Table 3). Notably, the power to detect a difference of incidence rates was similar for the group with HbAS and HbAC carriers. Considering a model with statistical interaction between β-globin and α+-thalassemia, a weak trend for an antagonistic epistatic effect against the protection by HbAS was seen (IRR = HbAS plus αα/αα = 0.76; 95% CI, 0.63-0.93; IRR HbAS plus α/αα = 0.82; 95% CI, 0.58-1.16). In contrast, there was an agonistic effect on HbAC toward a susceptibility for malaria episodes (IRR HbAC plus αα/αα = 0.98; 95% CI, 0.82-1.18; IRR HbAC plus α/αα = 1.43; 95% CI, 1.11-1.84). As a tendency, the time to a first or single malaria episode was longer in children with HbAS genotype compared with those with the HbAA genotype (HRR = 0.79; 95% CI, 0.62-1.02, P = .075; Figure 1A).
Parasitologic and hematologic parameters at recruitment (3 months of age)
| . | HbAA (n = 773)* . | HbAC (n = 106) . | HbCC (n = 2) . | HbAS (n = 110) . | HbCS (n = 12) . | HbSS (n = 6) . | Total (n = 1009)* . |
|---|---|---|---|---|---|---|---|
| Positive parasitemia, n (%) | 120 (15.5) | 17 (16.0) | 0 (0.0) | 9 (8.2)† | 1 (8.3) | 1 (16.7) | 148 (14.7) |
| Asymptomatic parasitemia, n (%) | 103 (13.3) | 15 (14.2) | 0 (0.0) | 8 (8.3)‡ | 1 (8.3) | 1 (16.7) | 128 (12.7) |
| Parasitemia, geomean (95%CI) | 853 (619-1177) | 566 (209-1530) | — | 312 (70-1382)§ | 760 | 80 | 753 (561-1010) |
| Parasitemia in asymptomatic children, geomean (95%CI) | 709 (503-999) | 446 (160-1248) | — | 257 (49-1337) | — | — | 620 (454-847) |
| Malaria, % | 15 (1.9) | 2 (1.9) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 18 (1.8) |
| Anemia, %‖ | 12 (1.6) | 3 (2.8) | 1 (50.0) | 0 (0.0) | 4 (33.3) | 2 (33.3) | 22 (2.2) |
| Hemoglobin, g/dL, median (quartiles) | 10.4 (9.5-11.2) | 10.0 (9.2-11.0)¶ | 8.7 (7.4-10.0) | 10.5 (9.7-11.2) | 9.1 (7.4-9.9)# | 9.2 (7.4-10.0)** | 10.4 (9.5-11.2) |
| . | HbAA (n = 773)* . | HbAC (n = 106) . | HbCC (n = 2) . | HbAS (n = 110) . | HbCS (n = 12) . | HbSS (n = 6) . | Total (n = 1009)* . |
|---|---|---|---|---|---|---|---|
| Positive parasitemia, n (%) | 120 (15.5) | 17 (16.0) | 0 (0.0) | 9 (8.2)† | 1 (8.3) | 1 (16.7) | 148 (14.7) |
| Asymptomatic parasitemia, n (%) | 103 (13.3) | 15 (14.2) | 0 (0.0) | 8 (8.3)‡ | 1 (8.3) | 1 (16.7) | 128 (12.7) |
| Parasitemia, geomean (95%CI) | 853 (619-1177) | 566 (209-1530) | — | 312 (70-1382)§ | 760 | 80 | 753 (561-1010) |
| Parasitemia in asymptomatic children, geomean (95%CI) | 709 (503-999) | 446 (160-1248) | — | 257 (49-1337) | — | — | 620 (454-847) |
| Malaria, % | 15 (1.9) | 2 (1.9) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 18 (1.8) |
| Anemia, %‖ | 12 (1.6) | 3 (2.8) | 1 (50.0) | 0 (0.0) | 4 (33.3) | 2 (33.3) | 22 (2.2) |
| Hemoglobin, g/dL, median (quartiles) | 10.4 (9.5-11.2) | 10.0 (9.2-11.0)¶ | 8.7 (7.4-10.0) | 10.5 (9.7-11.2) | 9.1 (7.4-9.9)# | 9.2 (7.4-10.0)** | 10.4 (9.5-11.2) |
— indicates not applicable.
One sample was missing at recruitment visit. Two children with parasitemia < 500 parasites/μL and fever.
HbAS versus HbAA (OR = 0.48; 95% CI, 0.21-0.99, P = .041).
HbAS versus HbAA (OR = 0.51; 95% CI, 0.21-1.09, P = .073).
HbAS versus HbAA (Wilcoxon test, P < .05).
Hemoglobin less than 7.5 g/dL.
HbAC versus HbAA (Wilcoxon test, P < .02).
HbCS versus HbAA (Wilcoxon test, P < .001).
HbSS versus HbAA (Wilcoxon test, P < .05).
Incidence of uncomplicated falciparum malaria and anemia stratified for hemoglobin genotypes
| β-Globin genotype . | Malaria . | Anemia . | ||||||
|---|---|---|---|---|---|---|---|---|
| Events . | PYAR . | Rate (per year) . | IRR (95%CI) . | Events . | PYAR . | Rate (per year) . | IRR (95% CI) . | |
| HbAA | 1327 | 1108.7 | 1.2 | 1 | 787 | 1217.7 | 0.6 | 1 |
| HbAC | 193 | 149.1 | 1.3 | 1.08 (0.93-1.26)* | 121 | 163.2 | 0.7 | 1.15 (0.95-1.39) |
| HbCC | 0 | 3.4 | 0.0 | — | 3 | 3.4 | 0.9 | 1.38 (0.44-4.28) |
| HbAS | 153 | 164.3 | 0.9 | 0.78 (0.66-0.92)† | 59 | 179.7 | 0.3 | 0.51 (0.39-0.66)‡ |
| HbCS | 7 | 16.4 | 0.4 | 0.36 (0.17-0.75)§ | 18 | 17.3 | 1.0 | 1.61 (1.01-2.57) |
| HbSS | 4 | 7.6 | 0.5 | 0.44 (0.17-1.18) | 40 | 7.0 | 5.7 | 8.84 (6.43-12.1)‖ |
| β-Globin genotype . | Malaria . | Anemia . | ||||||
|---|---|---|---|---|---|---|---|---|
| Events . | PYAR . | Rate (per year) . | IRR (95%CI) . | Events . | PYAR . | Rate (per year) . | IRR (95% CI) . | |
| HbAA | 1327 | 1108.7 | 1.2 | 1 | 787 | 1217.7 | 0.6 | 1 |
| HbAC | 193 | 149.1 | 1.3 | 1.08 (0.93-1.26)* | 121 | 163.2 | 0.7 | 1.15 (0.95-1.39) |
| HbCC | 0 | 3.4 | 0.0 | — | 3 | 3.4 | 0.9 | 1.38 (0.44-4.28) |
| HbAS | 153 | 164.3 | 0.9 | 0.78 (0.66-0.92)† | 59 | 179.7 | 0.3 | 0.51 (0.39-0.66)‡ |
| HbCS | 7 | 16.4 | 0.4 | 0.36 (0.17-0.75)§ | 18 | 17.3 | 1.0 | 1.61 (1.01-2.57) |
| HbSS | 4 | 7.6 | 0.5 | 0.44 (0.17-1.18) | 40 | 7.0 | 5.7 | 8.84 (6.43-12.1)‖ |
PYAR indicates person-years at risk; and —, not applicable.
Trend of an agonistic epistatic effect by α+-thalassemia toward susceptibility: IRR HbAC plus αα/αα = 0.98; 95% CI, 0.82-1.18; IRR HbAC plus α/αα = 1.43; 95% CI, 1.11-1.84.
P < .005 (Poisson regression). Relative risk estimate significantly different from that of HbAC (test of interaction, z = 2.83, P < .005). Trend of an antagonistic epistatic effect by α+-thalassemia against protection: IRR HbAS plus αα/αα = 0.76; 95% CI, 0.63-0.93; IRR HbAS plus α/αα = 0.82, CI 0.58-1.16.
P < .001 (Poisson regression). Relative risk estimate significantly different from that of HbAC (test of interaction, z = 4.91, P < .001).
P < .01 (Poisson regression).
P < .001 (Poisson regression).
Time to first or single episode of malaria and anemia stratified for β-globin genotypes. (A-B) Kaplan-Meier plots for failures of children between the age of 3 and 24 months (± 4 weeks). Solid line represents HbAA; dotted line, HbAS; and dashed line, HbAC. (A) Cumulative proportion of children with a first or single uncomplicated malaria episode. The difference between the group with HbAA genotype and the HbAS genotype was not significant (P < .08 by Breslow test). (B) Cumulative proportion of children with a first or single episode of anemia. The difference between the group with the HbAA genotype and the HbAS genotype was significant (P < .001 by Breslow test).
Time to first or single episode of malaria and anemia stratified for β-globin genotypes. (A-B) Kaplan-Meier plots for failures of children between the age of 3 and 24 months (± 4 weeks). Solid line represents HbAA; dotted line, HbAS; and dashed line, HbAC. (A) Cumulative proportion of children with a first or single uncomplicated malaria episode. The difference between the group with HbAA genotype and the HbAS genotype was not significant (P < .08 by Breslow test). (B) Cumulative proportion of children with a first or single episode of anemia. The difference between the group with the HbAA genotype and the HbAS genotype was significant (P < .001 by Breslow test).
Influence of HbAS and HbAC on P falciparum parasitemia
At the age of 3 months, 14.7% of the infants were infected with P falciparum and the odds for infection were 52% lower in those with HbAS trait (8.2%) compared with those with the β-globin wild-type HbAA (OR = 0.48; 95% CI, 0.21-0.99, P = .041; Table 2). In contrast, the infection prevalence was similar in children with HbAA (15.5%) and HbAC (16.0%).
To analyze the age-adjusted effect of β-globin genotypes on the level of P falciparum parasitemia, the follow-up times were stratified in blocks of 180 days. The maximum parasitemias within each of these 180-day strata and their geomean in the study group were calculated. Then, the effect of genotypes on the age-stratified geomeans was analyzed in a GEE to fit a generalized linear model for the longitudinal data. Parasitemia geomeans were calculated from symptomatic and asymptomatic episodes during active and passive visits.
Geomean parasitemia was lowest in children between the age of 60 and 240 days (HbAA, 1640 parasites/μL; HbAS, 711 parasites/μL; HbAC, 1215 parasites/μL) and increased continuously over the observation period until an age older than 600 days (HbAA, 4012 parasites/μL; HbAS, 3048 parasites/μL; HbAC, 5584 parasites/μL; Figure 2A). According to the GEE model, in children with the HbAS genotype, the age-adjusted parasite density was reduced by 50.8% compared with those with the HbAA genotype (P < .001). In children with the HbAC genotype, the parasite density was not significantly reduced (P = .16).
Dependency of parasitemia and hemoglobin levels on age and β-globin genotypes in children between the ages of 3 and 24 months (± 4 weeks). ● represents HbAA; ■, HbAS; and ♦, HbAC. (A) Age-adjusted geomean of maximum parasitemia during 180-day intervals. Children with the HbAS genotype had significant lower log-transformed parasite densities compared with those with the HbAA genotype (GEE model: HbAA vs HbAS, P < .001). (B) Age-adjusted mean of minimum hemoglobin levels during 180-day intervals. Compared with children with the HbAA genotype, those with HbAS had significantly higher hemoglobin levels (GEE: HbAA vs HbAS, P < .003) and those with HbAC had significantly lower hemoglobin levels (GEE: HbAA vs HbAC, P < .02)
Dependency of parasitemia and hemoglobin levels on age and β-globin genotypes in children between the ages of 3 and 24 months (± 4 weeks). ● represents HbAA; ■, HbAS; and ♦, HbAC. (A) Age-adjusted geomean of maximum parasitemia during 180-day intervals. Children with the HbAS genotype had significant lower log-transformed parasite densities compared with those with the HbAA genotype (GEE model: HbAA vs HbAS, P < .001). (B) Age-adjusted mean of minimum hemoglobin levels during 180-day intervals. Compared with children with the HbAA genotype, those with HbAS had significantly higher hemoglobin levels (GEE: HbAA vs HbAS, P < .003) and those with HbAC had significantly lower hemoglobin levels (GEE: HbAA vs HbAC, P < .02)
Influence of HbAS and HbAC on the incidence of anemia
Children with the HbAS genotype were protected from anemia compared with those with HbAA (IRR = 0.51; 95% CI, 0.39-0.66, P < .001). In contrast, carriage of HbAC was not associated with protection from anemia (IRR = 1.15; 95% CI, 0.95-1.39, P = .16). Accordingly, the hazards for the first or a single anemia episode were significantly reduced in children with the HbAS genotype (HRR = 0.50; 95% CI, 0.35-0.70, P < .001), but not in those with HbAC (Figure 1B).
Influence of HbAS and HbAC on the hemoglobin level
The age-adjusted mean of the minimum hemoglobin levels in 180-day intervals was stratified for the β-globin genotype (Figure 2B). According to the resulting GEE, persons with the HbAS genotype had slightly but significantly higher age-adjusted hemoglobin levels during the first 19 months of life compared with children with the HbAA genotype (mean hemoglobin difference, 0.3 g/dL; 95% CI, 0.1-0.5, P = .004). In contrast, carriers of HbAC had significantly lower hemoglobin levels (difference, −0.2 g/dL; 95% CI, −0.4 to −0.03, P = .033).
Influence of HbAS and HbAC on risk of severe malaria, hospital admissions, and death
In total, 52 cases of severe malaria were observed. The majority of these cases were caused by anemia or hyperparasitemia and were similarly distributed in children with HbAA, HbAC, and HbAS. During the observation period, there were 3390 visits to health facilities that were not included in the analyses. This might have led to an underestimation of the malaria incidence in the study area. In addition, this also bears the risk of a reporting bias if the distribution of β-globin genotypes among these visits were different from that among the malaria episodes documented by the study team. However, this was not the case: protection of children with HbAS against visits to other health facilities was similar to protection against malaria episodes (IRR = 0.76; 95% CI, 0.67-0.86, P < .001), whereas HbAC carriers were not protected (IRR = 1.01; 95% CI, 0.90-1.12, P < .86).
The time to the first or single hospital admission was longer in children with the HbAS genotype compared with HbAA (HRR = 0.52; 95% CI, 0.28-0.95, P = .036). Hazards of hospital admission for children with HbAC did not differ from those with HbAA (HRR = 1.01; 95% CI, 0.63-1.63, P = .96). Of the 209 hospital admissions, 64 were probably caused by malaria. However, the accuracy of these diagnoses is uncertain, and the sample was too small to make any meaningful analysis with regard to the different β-globin genotypes.
According to verbal autopsies, 11 deaths were possibly caused by malaria. Eight occurred in carriers of HbAA, the other 3 in carriers of HbAC. None of the children with HbAS died. The sample is too small to achieve statistical significance (P = .118).
Effect of HbAS and HbAC on child growth
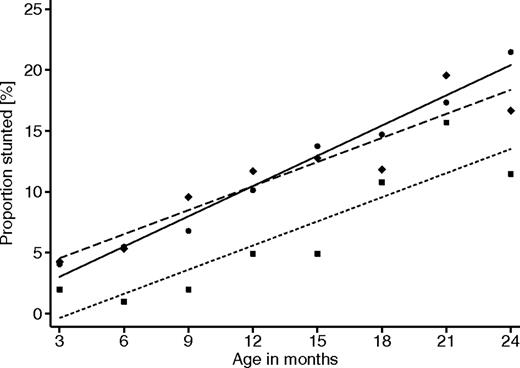
The risk of stunting as a benchmark for chronic malnutrition is associated with the number of malaria and anemia episodes during infancy. As previously demonstrated,9 the risk of stunting was significantly lower in carriers of the HbAS genotype compared with carriers of HbAA (OR = 0.56; 95% CI, 0.33-0.96, P = .035). Children with the HbAC genotype, on the other hand, showed a similar risk as those with HbAA (OR = 0.93; 95% CI, 0.58-1.50, P = .77; Figure 3).
Proportion of children stunted with increasing age, stratified for β-globin genotypes. ● represents HbAA; ■, HbAS; and ♦, HbAC. GEE analyses with adjustment for within-group effects, between-group effects, and age dependency were performed with autoregressive correlation structure of order 5 and assumption of binomial variable distribution. HbAA versus HbAS (OR = 0.56; 95% CI, 0.33-0.96, P = .035). HbAA versus HbAC (OR = 0.93; 95% CI, 0.58-1.50, P = .77).
Proportion of children stunted with increasing age, stratified for β-globin genotypes. ● represents HbAA; ■, HbAS; and ♦, HbAC. GEE analyses with adjustment for within-group effects, between-group effects, and age dependency were performed with autoregressive correlation structure of order 5 and assumption of binomial variable distribution. HbAA versus HbAS (OR = 0.56; 95% CI, 0.33-0.96, P = .035). HbAA versus HbAC (OR = 0.93; 95% CI, 0.58-1.50, P = .77).
HbSS, HbCS, and HbCC
Children with HbCS and HbCC were clinically inconspicuous, whereas several children with HbSS were incidentally diagnosed resulting from recurrent anemia. All of the children with HbSS, HbCS, and HbCC survived; however, 1 child with HbSS and 2 with HbCS were lost to follow-up for unknown reasons. As expected, children with HbSS and HbCS had significantly lower age-adjusted hemoglobin levels (data not shown) and a higher risk of anemia compared with carriers of HbAA (Table 3). The hemoglobin levels of the 2 children with HbCC were similar to carriers of HbAA. Compared with carriers of HbAA, the risk of malaria was lower in children with HbCS (IRR = 0.36; 95% CI, 0.17-0.75) and, as a trend, in carriers of HbSS (IRR = 0.44; 95% CI, 0.17-1.18; Table 3). Severe malarial anemia was diagnosed in 2 children with HbSS. However, in areas with high prevalence of parasitemia, patients with sickle cell anemia and asymptomatic parasitemia may often be misdiagnosed as severe malarial anemia. No episodes of malaria and only one episode of infection with low parasitemia (96 parasites/μL) were observed in the 2 children with HbCC who, on the other hand, had an increased number of contacts to health facilities (IRR = 2.37; 95% CI, 1.46-3.81, P < .001).
Discussion
There are numerous publications on the strong protection of the HbAS trait against severe falciparum malaria,5,6,12,25,37 and there is evidence for less extensive protective effect mediated by the HbC traits.22-25 Whereas some studies show that HbAS also protects against mild malaria,6-8 less is known about the effects of HbC traits on the incidence of mild malaria, P falciparum infection, and anemia, particularly in young children. Whereas a case-control study and family-based analyses from Burkina Faso suggested a protective effect of HbC against uncomplicated malaria,23,28 some other studies could not confirm a protection against asymptomatic parasitemia or anemia.22,38 Because high coprevalence of HbS and HbC alleles only exists in a few areas, HbAS and HbAC have hardly been compared in the same study, and longitudinal studies, especially in young children, are completely lacking.
To overcome this gap, a cohort study was performed in the Ashanti Region of Ghana where the prevalences of HbAS and HbAC are comparably high (> 10%). The study group presented here was suited to analyze the influence of HbAS and HbAC on the risk of uncomplicated malaria episodes for several reasons. First, at the age of 3 to 24 months, children are decreasingly protected by maternal antibodies and acquired immunity has not yet fully developed. Second, the similar frequencies of HbAS and HbAC resulted in a similar statistical power to detect possible effects. Third, the longitudinal study design with active and passive follow-up allows an adequate calculation of malaria incidence rates and the control of several confounding factors.
The results show that infants with an HbAS genotype: (1) were protected against uncomplicated malaria, (2) had lower age-dependent P falciparum parasite densities, (3) had a decreased incidence of anemia and higher age-dependent hemoglobin levels, most probably as a consequence of protection against episodes of high parasitemia and, indirectly, anemia, HbAS carriers, (4) had a reduced risk for hospital admissions, and (5) were significantly protected against stunting during the first 2 years of life. In contrast, children with an HbAC genotype were not significantly protected against any of these conditions and even had slightly decreased age-dependent hemoglobin levels.
The observed lower age-dependent parasite density and lower risk for uncomplicated malaria in children with the HbAS trait is in accordance with some previous epidemiologic studies.8,10,12,37 Lower levels of parasitemia indicate that HbS directly or indirectly affects invasion, presence, or proliferation of parasites during the erythrocytic cycle. Actually, experiments demonstrated a reduced invasion of erythrocytes,14 an inhibition of parasite growth,13,15,16 enhanced elimination of infected erythrocytes by the reticuloendothelial system,16,17,39 reduced cytoadherence,18 and other immune mechanisms.7,19 Another hypothesis is that the decline of HbF might be slower in HbAS carriers.40 In addition, an innate retention of young, ring-infected erythrocytes in the spleen has recently been demonstrated.41 This effect might be greater in HbAS carriers possibly leading to lower parasite densities.
In agreement with a previous report, children with HbAS had increased levels of hemoglobin compared with carriers of HbAA.42 The decreasing positive effect of HbAS on hemoglobin levels after the first 19 months may be explained by the growing impact of acquired immunity in children with all β-globin genotypes. This observation is also in agreement with an innate mechanism of protection and argues against a significant influence of HbAS on the development of acquired immunity as has been hypothesized.7 However, both the age of the study populations and the endemicity of malaria differ between these studies. The impact of HbAS on the development of acquired immunity has been shown to be dependent on age and transmission pattern in the study area,19 which might explain why such an effect could not be observed in the current study.
Although in the present study the HbAS trait significantly protected against uncomplicated malaria, high parasitemia, and anemia, HbAC carriers had the same risk as children with the HbAA wild-type. A reduction of parasite densities in persons with HbAC genotype has been found in a few epidemiologic studies,27,28 but other observations and in vitro data argue against such an effect.22,29 Indeed, it seems to be unlikely that a simple reduction of parasite growth or multiplication can sufficiently explain the protection from severe malaria in children with HbC as observed in some studies.22-25 Evidence for an involvement of HbC in the immune response to infected erythrocytes has been provided by the observation of an enhanced IgG production.19,43 In addition, clustering of erythrocyte band 3 protein44 and abnormal PfEMP1 expression in HbAC erythrocytes32 may prime recognition by antibodies and may lead to reduced cytoadherence and enhanced elimination. Furthermore, recent laboratory work suggests that red blood cells containing HbC have a modified topography of surface proteins that reduce parasite adhesiveness.45
Whereas these in vitro data demonstrate several mechanisms of protection common to both HbAS and HbAC, the results of the present study, together with other in vivo findings,22,23,25 suggest that the effects of HbS and HbC on the risk for malaria may differ. Although not completely transferable to the situation in uncomplicated malaria, 2 clinical studies on severe malaria support the hypothesis of a different protective effect of HbS and HbC traits. Whereas protection by HbC was selective for cerebral malaria, HbS protected against all phenotypes of severe malaria.22,25 A further study showed a protective effect against severe malaria mediated by HbC but did not differentiate between phenotypes.23 However, one study showed a selective protection by HbC against severe anemia at borderline significance,24 but not against cerebral malaria. A selective protection from cerebral malaria could be explained by a specific role of HbAC erythrocytes in sequestration through adhesion to endothelial cells.32,45 It has been shown that protection against malaria severe malaria mediated by HbCC is significantly stronger than that by HbAC.23 Data on protection against severe malaria by HbAC have led to conflicting results. This may partly be the result of an only marginal effect of the heterozygote genotype and thus an insufficient sample size of most studies to detect such an effect. Even though this study was not designed to analyze the effect of homozygote genotypes, it was noted that high levels of parasitemia were not seen in children with HbCC. This supports the hypothesis of strong protection against malaria in the HbCC homozygous children. It cannot be excluded that HbAC has weak effects that could not be detected as a result of the study design with tight monthly follow-ups that equalize the study arms.
Further support for the hypothesis that HbS, but not HbC, reduces the cumulative parasite load in the first years of life comes from our observation that only HbS protects from anemia and stunting. The observed tendency toward a delay of the first episode of malaria in children with HbAS is in accordance with a recent study from Mali.46 The additional observation of a significant delay of the first anemia episode implies that studies addressing anemia should include the genotyping of hemoglobin variants. A time delay effect on stunting is conceivable but was not seen when measuring body length every 3 months (data not shown).
A major cause of anemia in children from sub-Saharan Africa is a recurrent and chronic parasitemia, and chronic anemia again is one of the major causes of stunting and morbidity in young children in sub-Saharan Africa.47 Thus, the indirect protection against stunting in children by HbAS might provide a further indirect survival advantage. The observation of a lower risk of malaria but more severe manifestations in carriers of HbSS corresponds to recent data from Tanzania.48
Although this study was not designed to address rare events, such as severe disease, we nevertheless observed a significant reduction of the risk of hospital admissions in children with HbAS probably mediated by the protective effect against malaria and not through protection against other diseases.6 This, and the observation that only carriers of HbAA and HbAC died from malaria, further supports the thesis of selective protection against cerebral malaria by HbC and a general protection by HbS.
Similar to earlier observations,25,49 a trend of an antagonistic epistatic effect between heterozygote α+-thalassemia and HbAS was found. A tendency toward higher susceptibility was detected between heterozygote α+-thalassemia and HbAC also supporting the hypothesis that protection against malaria by HbAS and HbAC is mediated by different mechanisms. Because of the low number of persons heterozygous for both the α-globin deletion and for the HbS or HbC mutation (23 and 27, respectively), these results were not robust and need to be interpreted with appropriate caution.
The observation that the HbC trait protects selectively from cerebral malaria may also partly explain the focal distribution of HbC in distinct regions of West Africa, with notably mesoendemic to hyperendemic malaria endemicity and, therefore, a high incidence of cerebral malaria.2,50 Accordingly, a significant survival advantage can only be expected in these areas with high risk of cerebral malaria. The HbS trait, on the other hand, with a general protection against malarial infection25 should create a survival advantage in all endemic areas. The high prevalence of HbC in our holoendemic study area is at least partly the result of strong migration from northern Ghana.
Our data show that protective effects of HbAS against parasitemia, mild malaria, and anemia already exist in very young children and are probably mediated by an inhibition of parasite growth and multiplication, or by the innate removal by the spleen of a proportion of the circulating parasite population after each multiplication cycle. It can be concluded that a general health advantage for these children starts in the first months of life. Obviously, the advantage mediated by the HbAC variant acts differently and may rather be specific for mechanisms associated with cerebral malaria. The results demonstrate that the survival advantage mediated by 2 different amino acid exchanges at the same position of the β-globin gene may be the result of different pathophysiologic mechanisms.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all participants, their parents and guardians for participating in the study, the field workers and staff of the Kumasi Centre for Collaborative Research in Tropical Medicine for dedicated assistance, W. Loag for data management, and C. Bevilacqua for external audit.
This work was supported by the Volkswagen Foundation (grant I/79562) and the German Academic Exchange Service (C.K., R.K., and S.A.). Sanofi-Aventis donated Artesunate tablets for treatment of uncomplicated malaria episodes.
Authorship
Contribution: B.K. and C.K. collected data, performed statistical analysis, and wrote the first draft of the manuscript; R.K. collected data and was involved in the writing of the manuscript; M.A.-A., P.A.-T., B.T., S.A., and I.L. collected data and coordinated the study; C.E. performed the genotype analysis; O.A. was the principle investigator of the study; and J.M. designed the study, performed statistical analysis, and was involved in the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benno Kreuels, Infectious Disease Epidemiology, Bernhard Nocht Institute for Tropical Medicine, Bernhard-Nocht-Strasse 74, D-20359 Hamburg, Germany; e-mail: kreuels@bni-hamburg.de.
References
Author notes
B.K. and C.K. contributed equally to this study


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal