Abstract
CD20 is an important target for the treatment of B-cell malignancies, including non-Hodgkin lymphoma as well as autoimmune disorders. B-cell depletion therapy using monoclonal antibodies against CD20, such as rituximab, has revolutionized the treatment of these disorders, greatly improving overall survival in patients. Here, we report the development of GA101 as the first Fc-engineered, type II humanized IgG1 antibody against CD20. Relative to rituximab, GA101 has increased direct and immune effector cell-mediated cytotoxicity and exhibits superior activity in cellular assays and whole blood B-cell depletion assays. In human lymphoma xenograft models, GA101 exhibits superior antitumor activity, resulting in the induction of complete tumor remission and increased overall survival. In nonhuman primates, GA101 demonstrates superior B cell–depleting activity in lymphoid tissue, including in lymph nodes and spleen. Taken together, these results provide compelling evidence for the development of GA101 as a promising new therapy for the treatment of B-cell disorders.
Introduction
Rituximab, a type I chimeric IgG1 anti-CD20 antibody, has revolutionized the management and treatment of B-cell malignancies, increasing the median overall survival of patients with many of these diseases.1 In combination with chemotherapy, it has significantly improved response rates and progression-free and overall survival of patients with diffuse large B-cell lymphoma (DLBCL) or follicular lymphoma.1,2 Rituximab treatment has also benefited patients with other diseases amenable to B-cell depletion therapy, including B-cell chronic lymphocytic leukemia (B-CLL) and rheumatoid arthritis.2,3 Nevertheless, relapse is a common occurrence, for example, in B-CLL, and there remains a need for treatments that delay the onset of relapse without increasing toxicity.1 To this end, various therapeutic approaches are being explored, including new chemotherapies, small molecules, antibody-drug conjugates, and the use of alternative B-cell targets. However, in contrast to the situation with rituximab, the clinical benefit of these therapies remains to be demonstrated. In addition, many of these agents exhibit poor safety and tolerability profiles or necessitate the use of more complex treatment regimens.
Thus far, CD20 has been the most effective unconjugated antibody target for the treatment of B-cell malignancies. An alternative and complementary approach is to generate new unconjugated CD20 antibodies with enhanced functional activities that may lead to superior efficacy. Three types of functional activities of anti-CD20 antibodies have been described: signaling in target cells on CD20 binding leading to growth inhibition and (nonclassic) apoptosis (referred to as “direct cell death”), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC) mediated by cells displaying Fcγ receptors (FcγRs), such as FcγRIIIa-expressing NK cells and macrophages.4,5
Anti-CD20 antibodies with different functions may be generated either (1) by selecting antibodies that bind to a different CD20 epitope, which bind in an alternative mode or with changed affinity, resulting in altered intensity or type of functional mechanism; or (2) by engineering the Fc region of the antibody to enhance immune effector functions. The epitope and/or binding mode have been shown to dictate 2 major types of CD20 antibody effector function profiles, termed type I or type II.5-7 Although both types I and II antibodies bind bivalently to CD20, they form distinct complexes with CD20, as inferred from the fact that the B-cell surface can accommodate approximately double the number of type I antibodies compared with type II. Type I antibodies stabilize CD20 on lipid rafts, leading to stronger C1q binding and potent induction of CDC. However, this binding mode triggers only low levels of direct cell death. In contrast, type II antibodies do not stabilize CD20 in lipid rafts and thus exhibit reduced binding to C1q and lower levels of CDC, but they potently induce direct cell death.5 The majority of CD20 antibodies, including rituximab, veltuzumab,8 ocrelizumab,9 and ofatumumab,10 are of type I, whereas the prototype type II antibody is the murine antibody B1 (tositumomab).11
The Fc region of rituximab plays a critical role in triggering the cellular events that lead to B-cell elimination in vivo.7,12,13 This region of the molecule can interact with complement protein C1q and FcγRs to trigger CDC and ADCC, respectively. Direct cell death mediated by rituximab does not involve the Fc region directly but could potentially be enhanced by Fc-mediated crosslinking via the C1q complex and FcγRs.5 Alternative type I CD20 antibodies have been generated, including ofatumumab,14,15 AME-133,16 and a hexavalent anti-CD20 antibody.17 However, superior maximal efficacy over rituximab, that is, efficacy at the saturation point of the dose-response curve, has not been shown for any of these antibodies, and their clinical efficacy compared with rituximab remains to be demonstrated.
Our aim was to engineer a novel unconjugated agent against CD20 that displayed enhanced activity. To this end, we report the first Fc-engineered type II CD20 humanized IgG1 antibody, GA101. This manuscript describes the engineering of the variable and Fc regions of GA101 and presents its in vitro and in vivo activity profiles.
Methods
Antibodies
The murine anti-CD20 antibody H299 (B1) was obtained from Beckmann Coulter. Because of its aggregate content, the monomeric fraction was isolated using size exclusion chromatography. Commercial-grade rituximab was obtained from Hoffmann La Roche. GA101 was humanized by grafting the complementarity-determining region sequences from the murine antibody B-ly1 onto the following human frameworks: the VH1-10 plus the JH4 human germline sequences, and the VK-2-40 plus the JK4 human germline sequences, for the heavy and light chains, respectively. GA101 was expressed from stable Chinese hamster ovary (CHO) K1 cell lines engineered to constitutively overexpress the heavy and light chains of GA101 as well as recombinant wild-type β-1,4-N-acetyl-glucosaminyltransferase III and wild-type Golgi α-mannosidase II,18-21 using the glutamine synthetase expression system (Lonza Biologics). GA101 was produced using a fed-batch fermentation process using the engineered CHO cells in a chemically defined animal component-free medium and was subsequently purified by protein A and ion-exchange chromatographic techniques. The identity and monomer content (> 98%) of the isolate were analytically confirmed. Fab′ and F(ab)′2 fragments of GA101 and rituximab were generated via digestion with papain or pepsin, respectively, according to standard procedures.
NHL cell lines
Cell lines were obtained from DSMZ or ATCC; WSU-DLCL2 from Wayne State University, Detroit, MI; and OCI-LY cell lines from the Ontario Cancer Institute, Toronto, ON; and cultured according to the standard protocol recommendations.
Scatchard plot analysis
Aliquots of 2 × 105 SU-DHL4 cells were seeded into V-bottom plates, and Eu-labeled antibodies were added in different concentrations. Cells were washed, and the pellet was resuspended in enhancer solution, transferred into a black 96-well plate, and placed onto a shaker for 10 minutes. Release of coupled Eu was analyzed on a BMG PheraStar reader (ex337/em615).
Annexin V/PI FACS assay
Phosphatidylserine (PS) exposure and cell death were assayed by flow cytometric analysis (FACScan, BD Biosciences) of annexin V (ie, Annexin-V-FLOUS Staining Kit; Roche Applied Science) and propidium iodide (PI)–stained cells. Simultaneous application of PI or 7-amino-actinomycin D as a DNA stain enabled the discrimination between live and dead cells. In general, 3 × 105 cells were seeded into 24-well plates and were either untreated (control) or treated with 10 μg/mL isotype control or antibody for 24 to 72 hours. Annexin V/PI staining was performed according to the manufacturer's instructions.
For CD40 stimulation of hCD20 cells from transgenic mice, red blood cells (RBCs) were removed by hypotonic ammonium-chloride-potassium (ACK) lysis, and untouched splenic B cells were purified by anti-CD43–mediated depletion of non-B cells (MACS). B cells were incubated in conditioned complete RPMI 1640 medium containing 10% fetal bovine serum for 2 hours (with or without 10 μg/mL mitogenic stimulation) and either anti-CD40 (BD Biosciences PharMingen) or anti-IgM Fab2 (Jackson ImmunoResearch Laboratories) antibodies. This was followed by 36 hours of anti-CD20 stimulation (GA101 or rituximab 10 μg/mL). As positive controls for the induction of cell death, the following agents were used: 100nM staurosporine (Sigma-Aldrich) for mitochondrial-mediated apoptosis, or 1 μg/mL anti-CD95 Fas (BD Biosciences PharMingen) for the extrinsic induction of apoptosis.
FACS analysis of binding stoichiometry
To compare the binding mode of type I and type II antibodies, fluorescence-activated cell sorter (FACS) binding curves were generated by direct immunofluorescence using Cy5-conjugated rituximab and GA101, respectively. A total of 5 × 105 cells per sample were stained for 30 minutes at 4°C in a final volume of 200 μL and washed in culture medium. PI staining was used to identify and exclude dead cells. Measurements were performed using the FACSArray (BD Biosciences); PI fluorescence levels were quantified using Far Red A and Cy5 using the Red-A settings.
Western blot analysis of Triton X-100–soluble proteins
Aliquots of 5 × 106 Ramos cells per sample were incubated at 37°C for 30 minutes in complete culture medium either alone or supplemented with 10 μg/mL antibody or 10 μg/mL isotype control. After centrifugation at 500g for 10 minutes, cells were treated on ice with 200 μL of lysis buffer consisting of 1% Triton X-100 in Tris-buffered saline (50mM Tris-HCl, 150mM NaCl, pH 7.5), 1mM ethylenediaminetetraacetic acid, 1mM phenylmethylsulfonyl fluoride, and 1mM Na3VO4 plus a cocktail of protease inhibitors. Cells were left on ice for 30 minutes to lyse and centrifuged at 15 000g for 20 minutes (detergent-insoluble pellet). Samples were run on 4% to 12% Bis-Tris gels and transferred onto polyvinylidene difluoride membranes. Anti-Lyn (sc-15) rabbit monoclonal and anti-CD71 (transferrin receptor, sc-32272) mouse monoclonal antibodies were purchased from Santa Cruz Biotechnology. Mouse anti–human CD20 detection antibody for Western blotting was obtained from Dako North America (clone L26).
Immunofluorescence
Colocalization of CD20 antibodies with cholera toxin subunit B (CTB) on Ramos cells was visualized using the Vybrant Lipid Raft Labeling Kit (Invitrogen) according to the manufacturer's instructions using confocal microscopy. CTB binds to the pentasaccharide chain of plasma membrane ganglioside GMI, which selectively partitions into membrane microdomains called “lipid rafts.”
CDC assay
Cells were plated in AIM-V medium at a density of 50 000 cells/well into flat-bottom 96-well plates. Diluted antibody was added 10 minutes before addition of the serum/complement preparation to the cells, and the suspension kept at room temperature. Rabbit serum (rabbit complement MA; Cederlane Labs) was diluted in 1 mL of cytotoxicity medium plus 2 mL AIM-V to the final concentration and added to the cell suspension, and incubated for 2 hours at 37°C/5%CO2. Release of LDH activity (Roche Applied Science) into the supernatant was used as a readout, relative to maximum lysis (0.66% Triton X-100) and spontaneous lysis levels (without antibody).
ADCC assay
Raji cells were collected, washed, and resuspended in culture medium, stained with freshly prepared calcein AM (Invitrogen) at 37°C for 30 minutes, and plated on a round-bottom 96-well plate (at a density of 30 000 cells/well). The respective antibody dilution was added and incubated for 10 minutes before contact with human effector cells (peripheral blood mononuclear cells [PBMCs]). Effector and target cells at a ratio of 25:1 were coincubated for 4 hours. The retention of calcein in the remaining live cells was used as a readout as previously described.20
Autologous whole blood B-cell depletion assay
Blood from healthy volunteer donors was collected and an aliquot placed into FACS tubes. Subsequently, 20 μL of antibody dilution was added, and the tubes were mixed gently and incubated at 37°C for 24 hours in a humidified cell incubator. A 50-μL aliquot of the blood was stained with anti-CD45 (lymphocyte population), anti-CD3 (T cells), and anti-CD19 (B cells). FACS lysis solution (BD Biosciences) was added to deplete erythrocytes and fix cells before analysis with a flow cytometer. Results were evaluated by displaying 20 000 cells in the CD45-positive lymphocyte gate. CD3-positive T cells and CD19-positive B-cell populations were gated. Evaluation of relative B-cell depletion was performed using the B-/T-cell ratio with the antibody-untreated samples set as 100% B cells (equivalent to 0% B-cell depletion). B-/T-cell ratio = number of B cells/number of T cells; percentage of B-cell depletion = 100 − ([100/B-/T-cell ratio in sample without antibody] × [B-/T-cell ratio in sample containing antibody]).
In cases where the B-/T-cell ratio could not be applied because the studied agent (alemtuzumab) not only depleted B cells but also T cells, B-cell depletion was determined based on absolute B-cell numbers quantified using TruCount tubes (BD Biosciences) containing a mixture of anti-CD3-fluorescein isothiocyanate (BD Biosciences), anti–CD19-phycoerythrin (BD Biosciences), and anti–CD45-phycoerythrin-Cy5 (BD Biosciences; 10 μL each). After 15 minutes of incubation at room temperature in the dark, 300 μL of BD FACSLysis Solution (BD Biosciences) was added. The samples were measured using a FACSCalibur machine (Software BD CellQuestPro). Estimation of relative B-cell depletion was performed based on the absolute B-cell counts. The B-cell numbers obtained with the antibody-untreated samples were set as 100% B cells (equivalent to 0% B-cell depletion).
NHL xenograft studies in SCID beige mice
Female SCID beige mice, 4 to 5 weeks of age at arrival, were maintained under specific pathogen-free conditions according to guidelines. The experimental study protocol was reviewed and approved by the Roche Group ethical committee. Continuous health monitoring was carried out on a regular basis. Animals were monitored daily for clinical symptoms and detection of adverse effects. Throughout the experimental period, the body weight of animals was recorded twice weekly, and tumor volume was measured by caliper after staging. Three weeks after cell transplantation with established subcutaneous SU-DHL4 tumors (250 mm3), animals were randomized into treatment groups of 10 animals each and treated with 1, 10, and 30 mg/kg of the respective antibody (every 7 days, 3 times, intravenously).
For the second-line treatment study, animals with established subcutaneous SU-DHL4 xenografts were treated with first-line rituximab (30 mg/kg, every 7 days, intravenously) as a single agent (days 22-35). Animals with xenografts progressing after first-line rituximab treatment (750 mm3) were subsequently randomized and reassigned to the following treatment groups of 10 animals each with weekly dosing (from days 35-60) with the following agents: vehicle, rituximab (30 mg/kg every 7 days), or GA101 (30 mg/kg every 7 days).
For the disseminated Z138 model, 10 × 106 Z138 MCL cells were injected intravenously per animal into the tail vein in 200 μL of Aim V cell culture medium (Invitrogen). Treatment was initiated in a blinded fashion 29 days after intravenous injection of tumor cells followed by a weekly application of 10 mg/kg GA101 (every 7 days, 6 times, intraperitoneally). Treatment began at the day of randomization approximately 4 weeks after cell transplantation with 10 animals per group. Antibodies and the corresponding vehicle as a single agent were given intraperitoneally at a dose of 10 mg/kg. Animals were monitored daily for clinical symptoms and detection of adverse effects. Study endpoints were visible disease, including scruffy fur, impaired locomotion, and hind leg paralysis.
Analysis of B-cell depletion in cynomolgus monkeys (Macaca fascicularis)
The efficacy of GA101 in depleting B cells in cynomolgus monkeys was compared with that of rituximab in groups of 3 animals each. GA101 (2 × 10 and 30 mg/kg) was compared with rituximab (2 × 10 mg/kg) and vehicle after 2 intravenous doses administered on days 0 and 7 to male and female cynomolgus monkeys (n = 3 per group; 1 female, 2 males). The experimental study protocol was reviewed and approved by the Roche Group ethical committee. Total leukocytes from sodium heparin-collected peripheral blood were isolated by Ficoll buffy coat preparation. Residual RBCs were removed using a buffered ammonium chloride lysing solution. Lymph node and spleen tissue samples were weighed and then homogenized into single-cell suspension, lysed of residual RBCs, and filtered of debris. The resulting leukocyte cell preparations were washed and resuspended in an appropriate volume of Dulbecco phosphate-buffered saline supplemented with 1% fetal bovine serum. Absolute cell numbers for a given cell population were determined as the percentage of total leukocytes (based on cytometric surface marker detection) and the total cell counts of the sample corrected for volume or weight of starting material.
Results
Engineering of novel antibody variable regions and characterization of the type II CD20 antibody, GA101
GA101 was derived by humanization and further engineering of the parental murine IgG1-κ antibody B-ly1.22 This antibody mediates a degree of homotypic aggregation, a characteristic of type II antibodies, and did not stabilize CD20 in Triton X-100–resistant lipid rafts as it would have been expected for a classic type II antibody.11 cDNAs encoding variable heavy (VH) and light (VL) chain regions were cloned from the B-ly1 hybridoma, and their complementarity-determining regions were grafted onto human VH and VL acceptor frameworks.23 Different human frameworks were tested, and the resulting humanized antibody variants were compared for CD20 binding in human lymphoma cells. Those variants with complete identity to the human germline VH and VL framework sequences and with high binding affinity to human CD20 were selected for further analysis, including GA101. The affinity (KD value) of GA101 for human CD20 was determined to be approximately 4.0nM according to Scatchard analysis of binding experiments using SU-DHL4 non-Hodgkin lymphoma (NHL) cells and labeled GA101, whereas a KD value of approximately 4.5nM was obtained for rituximab. Binding experiments using Cy5-labeled GA101 and rituximab revealed that both antibodies compete for binding to B-cell CD20 and that GA101 recognizes a distinct but overlapping epitope compared with rituximab (manuscript in preparation).24
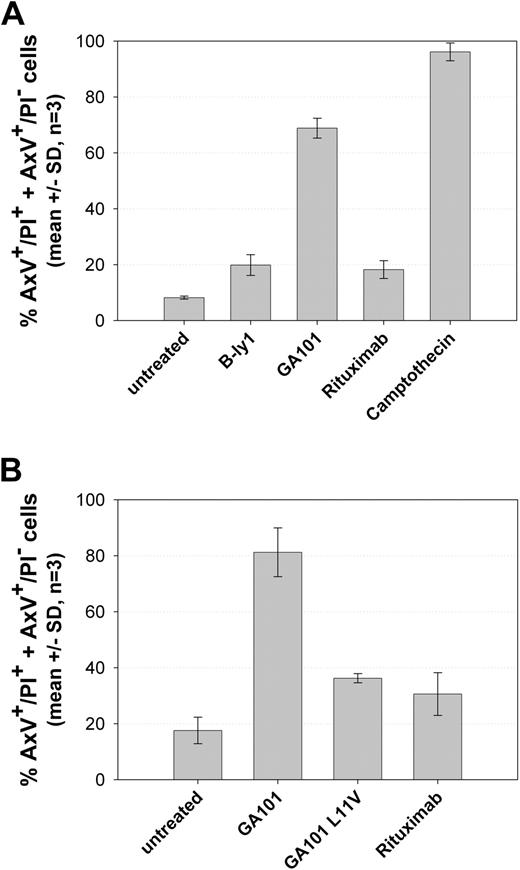
Because our goal was to obtain a functional type II, humanized CD20 antibody, the resulting variants were further screened for their ability to induce direct cell death in human B-cell lymphoma cells in vitro. Effective antibody variants were identified using the annexin V/PI assay, some of which showed a large gain of function compared with the parental murine B-ly1 antibody. The most significant structural distinction between high- and low-activity variants was a sequence alteration in the elbow-hinge region, an area known to affect the flexibility of the Fab′ and F(ab)′2 domains.25 The more active variants, including GA101, harbored a human germline VH framework 1 with a valine residue at Kabat position 11 instead of the leucine present in the murine donor antibody. Figure 1 shows the results of the annexin V/PI assay for GA101 compared with rituximab. GA101 treatment resulted in higher PS exposure and cell death compared with rituximab (Figure 1A). To test whether this activity required the modified elbow-hinge residue, a GA101-variant was generated with the key residue on Kabat position 11 remutated back to the original leucine. This variant displayed a large loss of activity and decreased cell death induction to levels similar to rituximab (Figure 1B), despite maintaining its high binding affinity for CD20.
GA101 induces superior PS exposure and cell death induction compared with rituximab in the annexin V/PI FACS assay. Z138 NHL cells were seeded and treated with 10 μg/mL GA101 or rituximab for 24 hours. The graphs depict the mean percentage of total annexin V–positive, PI-negative (AnnV+) cells and annexin V/PI double-positive cells (AnnV+/PI+; n = 3). (A) Cell death induction by GA101 compared with B-ly1, camptothecin, and the type I anti-CD20 antibody rituximab. (B) Cell death induction by GA101 can be reduced to the level of rituximab by reintroducing the L11V mutation in the elbow-hinge region of the antibody.
GA101 induces superior PS exposure and cell death induction compared with rituximab in the annexin V/PI FACS assay. Z138 NHL cells were seeded and treated with 10 μg/mL GA101 or rituximab for 24 hours. The graphs depict the mean percentage of total annexin V–positive, PI-negative (AnnV+) cells and annexin V/PI double-positive cells (AnnV+/PI+; n = 3). (A) Cell death induction by GA101 compared with B-ly1, camptothecin, and the type I anti-CD20 antibody rituximab. (B) Cell death induction by GA101 can be reduced to the level of rituximab by reintroducing the L11V mutation in the elbow-hinge region of the antibody.
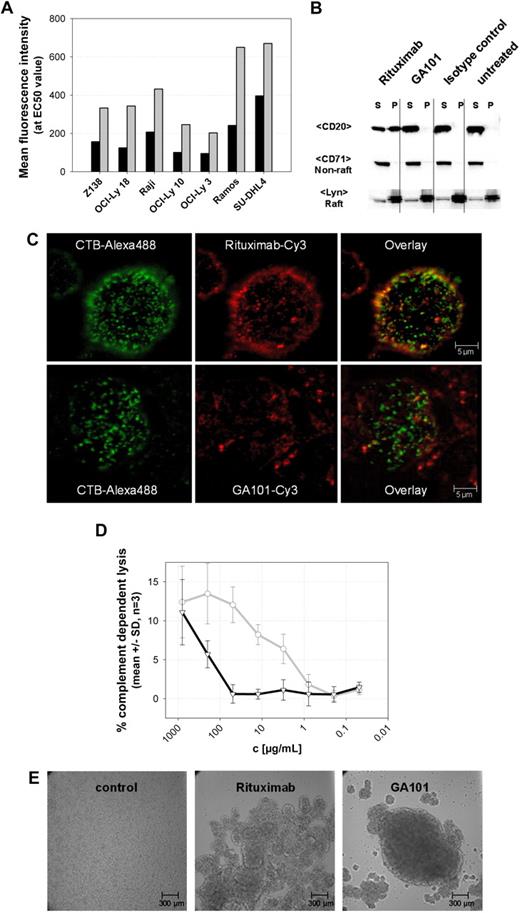
Further analysis of GA101 revealed that it exhibited characteristics typical of a type II antibody6,7,26,27 (Figure 2). Figure 2A through D shows the results of GA101 characterization using various assays compared with the type I antibody rituximab. At saturating antibody concentrations, GA101 bound to B cells at levels approximately half those of rituximab (Figure 2A). Unlike GA101, rituximab stabilizes CD20 molecules into Triton X-100–resistant lipid rafts on the surface of B cells (Figure 2B-C). Type I antibody-CD20 complexes on lipid rafts bind more strongly to C1q, leading to higher levels of CDC compared with type II antibodies.7 Consistent with this, GA101 displays reduced CDC relative to rituximab (Figure 2D). Typical of type II CD20 antibodies, GA101 induced stronger homotypic aggregation of B cells in vitro (Figure 2E). Interestingly, mutating the elbow-hinge valine of GA101 back to the parental murine leucine residue leads to partial loss of its type II characteristics, as seen by increased maximal binding to B cells, higher CDC activity, reduced homotypic B-cell aggregation, and decreased induction of direct cell death (data not shown).
GA101 exhibits characteristics typical of a type II anti-CD20 antibody. (A) Fluorescence intensity at the EC50 value (half-maximal binding) of GA101 compared with rituximab. Titration of a panel of NHL cell lines with Cy-5–labeled GA101 (■) shows that only half the amount of antibody is bound to CD20 on the cells, compared with Cy-5 labeled rituximab (▩) at the EC50 concentration. (B) GA101 does not mobilize CD20 into lipid rafts. On binding of GA101 to CD20 in Ramos cells, CD20 is mainly found in the Triton X-100–soluble fraction (S) and not in the Triton X-100–insoluble pellet (P) representing lipid rafts (top row). In contrast, binding of rituximab resulted in the distribution of CD20 into the Triton X-100–insoluble pellet fraction (S; top row). The distribution of Lyn as a typical lipid raft marker and CD71 as a nonlipid raft marker is not affected (bottom 2 rows). (C) Confocal microscopy: Ramos cells were stained for 30 minutes at 37°C with Cy-3–labeled rituximab or GA101 (red fluorescence, middle panel) and costained with the Alexa 488-labeled lipid raft marker cholera toxin subunit B (CTB-Alexa 488, green fluorescence, left panel), which binds to the membrane ganglioside GMI in lipid rafts. The overlay (right panel) confirms that rituximab binding to CD20 results in accumulation of CD20 clusters in lipid rafts as shown by colocalization with CTB-Alexa 488 (yellow fluorescence), whereas CD20 molecules do not redistribute to lipid rafts on binding of GA101 and do not colocalize with CTB-Alexa 488. Pictures were captured with a Leica TCS SP2 confocal microscope with an HCX PL APO CS 63.0×/1.32 OIL UV objective (numeric aperture 1.32) in glycerol and aquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Metamorph Version 7.0r3 and Jasc Paint Shop Pro. (D) CDC assays (LDH release) with Z138 mantle cell lymphoma cells. In the presence of physiologic concentrations of human unspecific IgG (10 mg/mL RedImmune), the type II anti-CD20 antibody GA101 (black) mediates greatly reduced CDC induction compared with rituximab (gray; n = 3). (E) GA101 induces rapid and pronounced homotypic aggregation of SU-DHL4 cells, whereas rituximab induces only a weak aggregation. Pictures were taken 24 hours after the addition of antibody. Pictures were captured with a Zeiss Axiovert 135 microscope with a Zeiss Fluar 5×/0.25 objective (numeric aperture 0.25) in RPMI 1640, 10% FCS, 2mM L-Glutamin on non fixed Ramos cells and a Cool SNAP K4 camera (Visitron Systems GmbH). Image acquisition and manipulation were performed with Metamorph Version 7.0r3 and Jasc Paint Shop Pro.
GA101 exhibits characteristics typical of a type II anti-CD20 antibody. (A) Fluorescence intensity at the EC50 value (half-maximal binding) of GA101 compared with rituximab. Titration of a panel of NHL cell lines with Cy-5–labeled GA101 (■) shows that only half the amount of antibody is bound to CD20 on the cells, compared with Cy-5 labeled rituximab (▩) at the EC50 concentration. (B) GA101 does not mobilize CD20 into lipid rafts. On binding of GA101 to CD20 in Ramos cells, CD20 is mainly found in the Triton X-100–soluble fraction (S) and not in the Triton X-100–insoluble pellet (P) representing lipid rafts (top row). In contrast, binding of rituximab resulted in the distribution of CD20 into the Triton X-100–insoluble pellet fraction (S; top row). The distribution of Lyn as a typical lipid raft marker and CD71 as a nonlipid raft marker is not affected (bottom 2 rows). (C) Confocal microscopy: Ramos cells were stained for 30 minutes at 37°C with Cy-3–labeled rituximab or GA101 (red fluorescence, middle panel) and costained with the Alexa 488-labeled lipid raft marker cholera toxin subunit B (CTB-Alexa 488, green fluorescence, left panel), which binds to the membrane ganglioside GMI in lipid rafts. The overlay (right panel) confirms that rituximab binding to CD20 results in accumulation of CD20 clusters in lipid rafts as shown by colocalization with CTB-Alexa 488 (yellow fluorescence), whereas CD20 molecules do not redistribute to lipid rafts on binding of GA101 and do not colocalize with CTB-Alexa 488. Pictures were captured with a Leica TCS SP2 confocal microscope with an HCX PL APO CS 63.0×/1.32 OIL UV objective (numeric aperture 1.32) in glycerol and aquired with Leica Confocal Software Version 2.61. Image manipulation was performed with Metamorph Version 7.0r3 and Jasc Paint Shop Pro. (D) CDC assays (LDH release) with Z138 mantle cell lymphoma cells. In the presence of physiologic concentrations of human unspecific IgG (10 mg/mL RedImmune), the type II anti-CD20 antibody GA101 (black) mediates greatly reduced CDC induction compared with rituximab (gray; n = 3). (E) GA101 induces rapid and pronounced homotypic aggregation of SU-DHL4 cells, whereas rituximab induces only a weak aggregation. Pictures were taken 24 hours after the addition of antibody. Pictures were captured with a Zeiss Axiovert 135 microscope with a Zeiss Fluar 5×/0.25 objective (numeric aperture 0.25) in RPMI 1640, 10% FCS, 2mM L-Glutamin on non fixed Ramos cells and a Cool SNAP K4 camera (Visitron Systems GmbH). Image acquisition and manipulation were performed with Metamorph Version 7.0r3 and Jasc Paint Shop Pro.
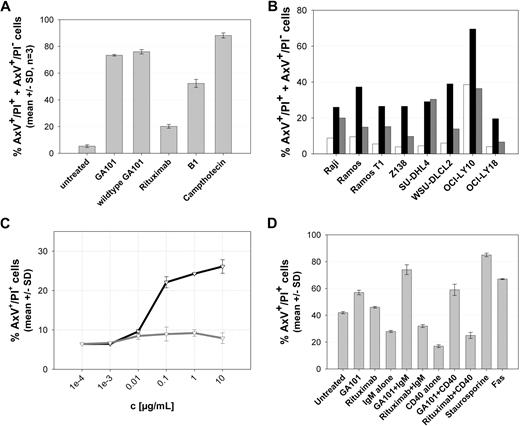
We further investigated the type II character of GA101 by comparing it with B1, the murine prototype type II CD20 antibody that has so far demonstrated the most pronounced in vitro direct cell death-inducing activity among type I and type II CD20 antibodies of the IgG isotype6,28 (Figure 3). GA101 exhibited more pronounced type II in vitro characteristics compared with B1, with respect to direct cell death induction in Z138 MCL cells (Figure 3A). As described previously for B1,6,7,28 this activity was mediated by the F(ab)′2 but not the Fab′ antibody fragment of GA101, and could not be blocked by caspase inhibitors (data not shown). The superior ability of GA101 in inducing direct cell death and PS exposure was also observed in a panel of NHL cell lines of different origin (Figure 3B). In addition, GA101 showed superior growth inhibition of B-cell lymphoma cell lines, as tested in Raji and SUDHL-4 cells (data not shown). Lymphoma cells as well as normal human peripheral blood B cells were sensitive to GA101 when incubated with GA101 in the absence of plasma and of immune effector cells in vitro (Figure 3C). Finally, manipulation of the activation status of B cells using standard mitogens, such as anti-IgM and anti-CD40 antibodies, resulted in increased sensitivity to GA101 of B cells from human CD20 transgenic mice (Figure 3D).
The type II anti-CD20 antibody GA101 mediates superior direct cell death induction in normal and malignant B cells. (A) GA101 induces PS exposure and cell death in the annexin V/PI FACS assay at levels superior to those induced by the type II anti-CD20 antibody B1 and rituximab. Z138 NHL cells were seeded and treated with 10 μg/mL GA101, nonglycoengineered GA101 (wt GA101), B1, rituximab, or camptothecin as control for 24 hours. The graph shows the mean percentage of total annexin V–positive cells, that is, annexin V/PI double-positive (AxV+/PI+) and annexin V–positive, PI-negative cells (AxV+/PI−; n = 3). (B) NHL cells were seeded and either left untreated (□) or treated with 10 μg/mL GA101 (■) or rituximab (▩), respectively, for 72 hours. The graph shows the percentage of total annexin V–positive cells for a cell line panel of 3 Burkitt lymphoma, 4 DLBCL, and 1 MCL cell lines from 1 representative experiment. (C) GA101 (black curve) induces increased cell death compared with rituximab in purified human nonmalignant B cells isolated from 2 healthy donors (gray curve), as measured by annexin V/PI double-positive staining at 36 hours. All measurements were performed in duplicate; the mean of replicate samples from multiple donors (n = 2) and SD are shown. (D) Murine purified B cells from human CD20 transgenic mice were treated as indicated for 36 hours ex vivo with GA101 or rituximab either with or without prior mitogenic stimulation by IgM or CD40L, and cell death induction was measured by annexin V/PI staining as described in “Annexin V/PI FACS assay.” Two animals were used per stimulation. All measurements were performed in duplicate; the mean of replicate samples from multiple animals (n = 2) and SD are shown.
The type II anti-CD20 antibody GA101 mediates superior direct cell death induction in normal and malignant B cells. (A) GA101 induces PS exposure and cell death in the annexin V/PI FACS assay at levels superior to those induced by the type II anti-CD20 antibody B1 and rituximab. Z138 NHL cells were seeded and treated with 10 μg/mL GA101, nonglycoengineered GA101 (wt GA101), B1, rituximab, or camptothecin as control for 24 hours. The graph shows the mean percentage of total annexin V–positive cells, that is, annexin V/PI double-positive (AxV+/PI+) and annexin V–positive, PI-negative cells (AxV+/PI−; n = 3). (B) NHL cells were seeded and either left untreated (□) or treated with 10 μg/mL GA101 (■) or rituximab (▩), respectively, for 72 hours. The graph shows the percentage of total annexin V–positive cells for a cell line panel of 3 Burkitt lymphoma, 4 DLBCL, and 1 MCL cell lines from 1 representative experiment. (C) GA101 (black curve) induces increased cell death compared with rituximab in purified human nonmalignant B cells isolated from 2 healthy donors (gray curve), as measured by annexin V/PI double-positive staining at 36 hours. All measurements were performed in duplicate; the mean of replicate samples from multiple donors (n = 2) and SD are shown. (D) Murine purified B cells from human CD20 transgenic mice were treated as indicated for 36 hours ex vivo with GA101 or rituximab either with or without prior mitogenic stimulation by IgM or CD40L, and cell death induction was measured by annexin V/PI staining as described in “Annexin V/PI FACS assay.” Two animals were used per stimulation. All measurements were performed in duplicate; the mean of replicate samples from multiple animals (n = 2) and SD are shown.
Fc engineering and Fcγ receptor-dependent functions
In addition to an engineered variable region conferring type II CD20 binding, GA101 also harbors a glycoengineered Fc segment. Glycoengineering was accomplished by producing the antibody in CHO cells, which overexpressed the recombinant glycosylation enzymes β-1,4-N-acetyl-glucosaminyltransferase III and Golgi α-mannosidase II, leading to accumulation of antibody glycoforms containing bisected, complex, nonfucosylated oligosaccharides attached to asparagine 297 in the Fc region.29,30 The glycoengineered antibody binds with increased affinity to FcgRIII, an activating Fc receptor displayed by immune effectors, such as NK cells and macrophages.29
Two dimorphic variants at position 158 of human FcγRIIIa are known: a more common, lower-affinity form containing phenylalanine (FcγRIIIa-158F) and another variant with a valine residue at the same position. GA101 and rituximab were compared by surface plasmon resonance for their binding affinity to these 2 variants of human FcγRIIIa. GA101 bound with higher affinity to both FcγRIIIa-158V and -158F (KD of 55 and 270nM, respectively), compared with rituximab (KD of 660 and 2000nM, respectively). Increased binding affinity of GA101 to FcγRIIIa translated into an increased induction of ADCC relative to rituximab, as demonstrated in vitro in ADCC assays using Raji lymphoma cells as targets and human PBMCs as effectors (Figure 4A). Both ADCC potency and efficacy were higher with GA101, and this was also maintained in the presence of an excess of nonspecific human IgG at physiologic concentrations, as present in human blood; under these conditions, rituximab showed no activity whereas that of GA101 was only partially inhibited, presumably because of the enhanced FcR binding (Figure 4B).
Superior ADCC and B cell–depleting activities of GA101 compared with rituximab. GA101 exhibits a more potent ADCC-inducing ability than rituximab, both in the presence and absence of nonspecific human IgG. Representative ADCC assay with Raji cells as target cells and NK cells from human PBMCs (F/V 158) as effector cells (calcein release, E/T ratio = 20:1) in the absence (A) and presence (B) of physiologic concentrations of nonspecific human IgG (20 mg/mL RedImune; n = 3). In the absence of nonspecific IgG, GA101 (black) was approximately 35-fold more potent in terms of EC50 values than rituximab (gray) at inducing ADCC. In the presence of nonspecific IgG, GA101 (black) still exhibited significant ADCC-inducing activity, whereas that of rituximab (gray) was completely abolished. (C). Enhanced B cell–depletion activity of GA101, as demonstrated in a whole blood B cell–depletion assay with whole blood from a healthy donor (CD16 genotype F158/F158). Representative results for the depletion of CD19-positive B cells are depicted here. GA101 (black) was approximately 25-fold more potent in terms of EC50 values and 1.9-fold more effective (in terms of absolute B-cell depletion) compared with rituximab (gray). Evaluation of relative B-cell depletion was performed using the B-/T-cell ratio set to 0% for untreated control samples (n = 4). (D) Representative whole blood B cell–depletion assay with whole blood from a B-CLL patient. GA101 (black) was more effective at depleting B cells compared with rituximab (gray) and the CD52 antibody alemtuzumab (dotted line). Because alemtuzumab also depletes T cells, the evaluation of relative B-cell depletion was performed based on the absolute B-cell counts. The B-cell numbers were then set to 100% for maximal depletion, and results were compared between the antibodies (n = 4).
Superior ADCC and B cell–depleting activities of GA101 compared with rituximab. GA101 exhibits a more potent ADCC-inducing ability than rituximab, both in the presence and absence of nonspecific human IgG. Representative ADCC assay with Raji cells as target cells and NK cells from human PBMCs (F/V 158) as effector cells (calcein release, E/T ratio = 20:1) in the absence (A) and presence (B) of physiologic concentrations of nonspecific human IgG (20 mg/mL RedImune; n = 3). In the absence of nonspecific IgG, GA101 (black) was approximately 35-fold more potent in terms of EC50 values than rituximab (gray) at inducing ADCC. In the presence of nonspecific IgG, GA101 (black) still exhibited significant ADCC-inducing activity, whereas that of rituximab (gray) was completely abolished. (C). Enhanced B cell–depletion activity of GA101, as demonstrated in a whole blood B cell–depletion assay with whole blood from a healthy donor (CD16 genotype F158/F158). Representative results for the depletion of CD19-positive B cells are depicted here. GA101 (black) was approximately 25-fold more potent in terms of EC50 values and 1.9-fold more effective (in terms of absolute B-cell depletion) compared with rituximab (gray). Evaluation of relative B-cell depletion was performed using the B-/T-cell ratio set to 0% for untreated control samples (n = 4). (D) Representative whole blood B cell–depletion assay with whole blood from a B-CLL patient. GA101 (black) was more effective at depleting B cells compared with rituximab (gray) and the CD52 antibody alemtuzumab (dotted line). Because alemtuzumab also depletes T cells, the evaluation of relative B-cell depletion was performed based on the absolute B-cell counts. The B-cell numbers were then set to 100% for maximal depletion, and results were compared between the antibodies (n = 4).
As a type II antibody, GA101 displays reduced CDC compared with type I antibodies. It was therefore of interest to compare the total B cell–depleting activity of GA101 in whole blood to better mimic in vivo conditions. The assay incorporated both FcγR-displaying effector cells, including NK cells, monocytes, and neutrophils and human complement. The sum of immune effector functions, such as ADCC and antibody-mediated cellular phagocytosis, CDC, and effector (cell)–independent mechanisms, such as direct cell death induction, could thus be measured. GA101 was significantly more potent than rituximab at depleting B cells in whole blood from 10 healthy donors. Representative results are shown in Figure 4C. Compared with rituximab, GA101 exhibited 10- to 25-fold greater potency and was 1.5- to 2.5-fold more effective in terms of absolute B-cell depletion. The efficacy of whole blood B-cell depletion by GA101 was significantly higher (∼ P < .001) than that of rituximab in all samples tested. These findings were confirmed using malignant B cells from a chronic lymphocytic leukemia (B-CLL) patient (Figure 4D). In the latter case, the majority of cells in the assay were target B cells, and GA101 was also superior to the CD52 antibody alemtuzumab at depleting B-CLL cells. In addition, GA101 showed superior efficacy in this assay compared with a glycoengineered variant of rituximab (data not shown).
Efficacy in human lymphoma xenograft models
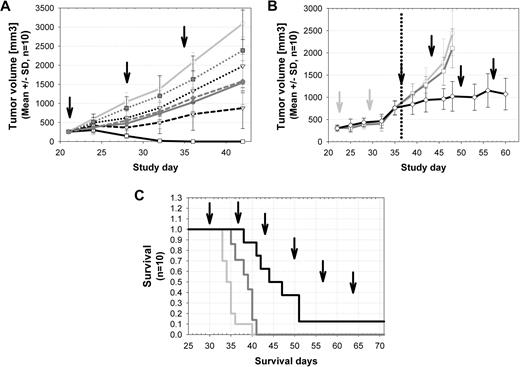
GA101 demonstrated in vivo efficacy superior to rituximab in various human lymphoma xenograft models. Both antibodies were first compared in a staged, aggressive DLBCL model using human SUDHL-4 cells subcutaneously injected in SCID beige mice. Therapy began when tumors were established and rapidly growing. Rituximab inhibited tumor growth more effectively at 10 mg/kg versus 1 mg/kg; however, the higher dose of 30 mg/kg did not result in increased efficacy of rituximab, and tumor regression was not observed at any dose. In contrast, GA101 showed a dose-dependent increase in efficacy in the range of 1 to 30 mg/kg and resulted in complete tumor regression in all animals and lasting tumor eradication in 9 of 10 animals at the highest dose of 30 mg/kg and in 1 of 10 animals at a dose of 10 mg/kg (Figure 5A).
Superior antitumor efficacy of GA101 compared with rituximab in human lymphoma xenograft models. (A) Established subcutaneous SU-DHL4 (DLBCL) tumors (250 mm3; n = 10 per group) were treated with 1 mg/kg (dotted lines), 10 mg/kg (short dashed lines), and 30 mg/kg (solid lines) GA101 (every 7 days, 3 times, intravenously; black) compared with identical doses of rituximab (dark gray) and vehicle control (light gray). GA101 treatment resulted in a dose-dependent inhibition of tumor growth that was superior to that of rituximab. A total of 10 of 10 mice showed complete tumor remission and 9 of 10 mice showed long-term survival (> 90 days; cure) after treatment with 30 mg/kg GA101; and 1 of 10 mice showed complete tumor remission after treatment with 10 mg/kg GA101. In the rituximab-treated groups, no complete tumor remission was observed. Data are mean ± SD. (B) Established subcutaneous SU-DHL4 xenografts (n = 10 per group) were treated with rituximab (30 mg/kg every 7 days, intravenously) as single-agent first-line therapy (days 22-35). Xenografts progressing under first-line rituximab treatment (30 mg/kg every 7 days) were subsequently randomized and reassigned to the following treatment groups with weekly dosing (from days 35-60): vehicle (gray curve), rituximab (30 mg/kg every 7 days; dark gray curve), or GA101 (30 mg/kg every 7 days; black curve). SU-DHL4 tumor progression (advanced xenografts; 750 mm3) was effectively controlled through the use of GA101 as a second-line therapy, whereas rituximab treated-tumors remained refractory. (C) Treatment of the aggressive orthotopic disseminated Z138 (MCL) model was initiated 29 days after intravenous injection of tumor cells (n = 10 per group). Treatment with 10 mg/kg GA101 (every 7 days, 6 times, intravenously; black line) resulted in increased overall and median survival, compared with 10 mg/kg rituximab treatment (dark gray line; P < .008) and vehicle control (light gray line). ↑ indicates the treatment time points.
Superior antitumor efficacy of GA101 compared with rituximab in human lymphoma xenograft models. (A) Established subcutaneous SU-DHL4 (DLBCL) tumors (250 mm3; n = 10 per group) were treated with 1 mg/kg (dotted lines), 10 mg/kg (short dashed lines), and 30 mg/kg (solid lines) GA101 (every 7 days, 3 times, intravenously; black) compared with identical doses of rituximab (dark gray) and vehicle control (light gray). GA101 treatment resulted in a dose-dependent inhibition of tumor growth that was superior to that of rituximab. A total of 10 of 10 mice showed complete tumor remission and 9 of 10 mice showed long-term survival (> 90 days; cure) after treatment with 30 mg/kg GA101; and 1 of 10 mice showed complete tumor remission after treatment with 10 mg/kg GA101. In the rituximab-treated groups, no complete tumor remission was observed. Data are mean ± SD. (B) Established subcutaneous SU-DHL4 xenografts (n = 10 per group) were treated with rituximab (30 mg/kg every 7 days, intravenously) as single-agent first-line therapy (days 22-35). Xenografts progressing under first-line rituximab treatment (30 mg/kg every 7 days) were subsequently randomized and reassigned to the following treatment groups with weekly dosing (from days 35-60): vehicle (gray curve), rituximab (30 mg/kg every 7 days; dark gray curve), or GA101 (30 mg/kg every 7 days; black curve). SU-DHL4 tumor progression (advanced xenografts; 750 mm3) was effectively controlled through the use of GA101 as a second-line therapy, whereas rituximab treated-tumors remained refractory. (C) Treatment of the aggressive orthotopic disseminated Z138 (MCL) model was initiated 29 days after intravenous injection of tumor cells (n = 10 per group). Treatment with 10 mg/kg GA101 (every 7 days, 6 times, intravenously; black line) resulted in increased overall and median survival, compared with 10 mg/kg rituximab treatment (dark gray line; P < .008) and vehicle control (light gray line). ↑ indicates the treatment time points.
A more aggressive, second-line therapy setting was also tested using the SUDHL-4 DLBCL model. All animals received weekly first-line treatment with rituximab at a dose of 30 mg/kg. When the tumors reached a prespecified size of approximately 750 mm3, animals were randomized into 3 groups for second-line treatment with rituximab (30 mg/kg), GA101 (30 mg/kg), or vehicle. Tumors continued to grow rapidly in the rituximab- and vehicle-treated groups, whereas GA101 treatment was able to control tumor growth (Figure 5B). Notably, GA101 treatment resulted in tumor stasis in the presence of rituximab (> 300 μg/mL in plasma), which competes with GA101 for CD20 binding. The superior efficacy of GA101 was further demonstrated in an advanced, disseminated mantle cell lymphoma model using Z138 MCL cells in SCID beige mice (Figure 5C). GA101 treatment demonstrated superior efficacy both in terms of median and overall survival compared with rituximab (Figure 5C).
In vivo depletion of normal B cells in peripheral blood and in lymphoid organs
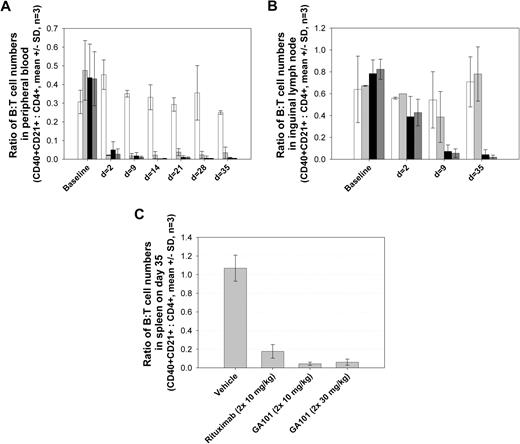
GA101 exhibited potent B cell–depleting activity in nonhuman primates, both in peripheral blood as well as in lymphoid tissue B cells (Figure 6). Cynomolgus monkeys were treated on days 1 and 8 with rituximab (10 mg/kg), GA101 (one group at 10 mg/kg and another at 30 mg/kg), or vehicle control. Both antibodies efficiently depleted B cells from peripheral blood (Figure 6A); however, B-cell depletion by GA101 was greater in spleen, and particularly in lymph nodes, where B-cell depletion is typically more problematic (Figure 6B-C). Preliminary data suggest that memory B cells and long-lived plasma cells are spared from depletion, thus leaving humoral immunity and memory intact (data not shown).
Superior B-cell depletion in cynomolgus monkeys with GA101 treatment compared with rituximab. The efficacy of GA101 at depleting B cells in cynomolgus monkeys was compared with that of rituximab in groups of 3 animals. GA101 (2 × 10 mg/kg, ■; and 30 mg/kg,  ) was compared with rituximab (2 × 10 mg/kg,
) was compared with rituximab (2 × 10 mg/kg,  ) and vehicle (□) after 2 intravenous doses administered on days 0 and 7 to male and female cynomolgus monkeys (n = 3 per group; 1 female, 2 males). Peripheral blood and lymph node B-cell numbers were evaluated at baseline (day −7) and on the indicated days by flow cytometric analysis. B-cell numbers were evaluated in the spleens of the treated animals on day 35. (A) Mean B-cell numbers expressed as B-/T-cell ratios in peripheral blood of cynomolgus monkeys treated with GA101 and rituximab. (B) Mean B-cell numbers expressed as B-/T-cell ratios in the lymph nodes of cynomolgus monkeys treated with GA101 and rituximab. (C) Mean B-cell numbers expressed as B-/T-cell ratios in the spleens of cynomolgus monkeys treated with GA101 and rituximab on day 35. GA101 treatment resulted in statistically superior depletion of total B cells from lymph nodes, compared with rituximab, from days 9 to 35, with a decrease in B-cell numbers of more than 95%. Data are mean ± SD.
) and vehicle (□) after 2 intravenous doses administered on days 0 and 7 to male and female cynomolgus monkeys (n = 3 per group; 1 female, 2 males). Peripheral blood and lymph node B-cell numbers were evaluated at baseline (day −7) and on the indicated days by flow cytometric analysis. B-cell numbers were evaluated in the spleens of the treated animals on day 35. (A) Mean B-cell numbers expressed as B-/T-cell ratios in peripheral blood of cynomolgus monkeys treated with GA101 and rituximab. (B) Mean B-cell numbers expressed as B-/T-cell ratios in the lymph nodes of cynomolgus monkeys treated with GA101 and rituximab. (C) Mean B-cell numbers expressed as B-/T-cell ratios in the spleens of cynomolgus monkeys treated with GA101 and rituximab on day 35. GA101 treatment resulted in statistically superior depletion of total B cells from lymph nodes, compared with rituximab, from days 9 to 35, with a decrease in B-cell numbers of more than 95%. Data are mean ± SD.
Superior B-cell depletion in cynomolgus monkeys with GA101 treatment compared with rituximab. The efficacy of GA101 at depleting B cells in cynomolgus monkeys was compared with that of rituximab in groups of 3 animals. GA101 (2 × 10 mg/kg, ■; and 30 mg/kg,  ) was compared with rituximab (2 × 10 mg/kg,
) was compared with rituximab (2 × 10 mg/kg,  ) and vehicle (□) after 2 intravenous doses administered on days 0 and 7 to male and female cynomolgus monkeys (n = 3 per group; 1 female, 2 males). Peripheral blood and lymph node B-cell numbers were evaluated at baseline (day −7) and on the indicated days by flow cytometric analysis. B-cell numbers were evaluated in the spleens of the treated animals on day 35. (A) Mean B-cell numbers expressed as B-/T-cell ratios in peripheral blood of cynomolgus monkeys treated with GA101 and rituximab. (B) Mean B-cell numbers expressed as B-/T-cell ratios in the lymph nodes of cynomolgus monkeys treated with GA101 and rituximab. (C) Mean B-cell numbers expressed as B-/T-cell ratios in the spleens of cynomolgus monkeys treated with GA101 and rituximab on day 35. GA101 treatment resulted in statistically superior depletion of total B cells from lymph nodes, compared with rituximab, from days 9 to 35, with a decrease in B-cell numbers of more than 95%. Data are mean ± SD.
) and vehicle (□) after 2 intravenous doses administered on days 0 and 7 to male and female cynomolgus monkeys (n = 3 per group; 1 female, 2 males). Peripheral blood and lymph node B-cell numbers were evaluated at baseline (day −7) and on the indicated days by flow cytometric analysis. B-cell numbers were evaluated in the spleens of the treated animals on day 35. (A) Mean B-cell numbers expressed as B-/T-cell ratios in peripheral blood of cynomolgus monkeys treated with GA101 and rituximab. (B) Mean B-cell numbers expressed as B-/T-cell ratios in the lymph nodes of cynomolgus monkeys treated with GA101 and rituximab. (C) Mean B-cell numbers expressed as B-/T-cell ratios in the spleens of cynomolgus monkeys treated with GA101 and rituximab on day 35. GA101 treatment resulted in statistically superior depletion of total B cells from lymph nodes, compared with rituximab, from days 9 to 35, with a decrease in B-cell numbers of more than 95%. Data are mean ± SD.
Discussion
The use of monoclonal anti-CD20 antibodies, such as rituximab for the treatment of B-cell malignancies, has greatly improved overall patient survival. Our goal was to refine and augment the efficacy of CD20 antibody therapy by engineering a novel CD20 antibody with enhanced B cell–depleting activity. Here, we describe the generation and characterization of GA101, the first Fc-engineered type II CD20 antibody to be brought to clinical development.30 Although type II antibodies exhibit lower C1q binding and CDC compared with type I, our aim was to capitalize both on the direct and immune effector cell-mediated induction of B-cell death mediated by type II antibodies and further enhance the latter via Fc engineering while retaining its type II B-cell signaling properties. Here, we compare the in vitro and in vivo properties of GA101 to those of rituximab, a well-characterized type I antibody, and demonstrate the superior in vivo efficacy of the unconjugated CD20 type II IgG1 antibody GA101 versus rituximab.
The efficacy of GA101 was superior to that of rituximab in all parameters tested in this study. GA101 exhibited enhanced B cell–depleting properties in the lymphoid organs of cynomolgus monkeys, and in aggressive human B-cell lymphoma xenograft models. In addition to increased effects on B cells, an Fc-engineered type II anti-CD20 antibody may provide further advantages over type I antibodies. First, the complement-related effects characteristic of type I antibodies may have only limited efficacy in vivo because of overexpression of complement-resistance factors on target cells and to in vivo depletion of complement proteins.4 Second, C1q binding to the type I antibody Fc region interferes with FcγR binding and may decrease ADCC.31 Finally, it has been recently reported that type II anti-CD20 antibody complexes persist for longer periods of time on the B-cell surface compared with type I,32 increasing the accessibility of the Fc region to immune effector cells at the target site in vivo, which may result in increased levels of ADCC.
An additional factor that contributes to the increased ADCC induction by GA101 is the higher binding affinity of the antibody to FcγRIIIa receptors achieved via Fc glycoengineering. The relevance of Fc-FcγRIIIa binding affinity has been demonstrated in various retrospective pharmacogenomic studies, which show a correlation between a genetic dimorphism affecting the affinity of human FcγRIIIa therapeutic IgG1 antibodies and the response rates and progression-free survival after antibody therapy. Approximately 15% of patients are homozygous for a high-affinity form of the receptor, displaying a valine residue at position 158, whereas the rest of the population carries the allele coding for a low-affinity receptor variant with a phenylalanine residue in that position.4 Rituximab therapy of follicular lymphoma patients homozygous for the high-affinity allele is associated with significantly higher response rates and progression-free survival.33,34 Similar studies have found the same correlation for other IgG1 antibodies, including trastuzumab in the treatment of metastatic breast cancer35 and cetuximab in the therapy of colorectal carcinoma patients.36 Here, we demonstrate that GA101 binds to both FcγRIIIa variants with an affinity higher than that of rituximab for the high-affinity receptor isoform.
In summary, we have developed a new, Fc-glycoengineered type II anti-CD20 antibody with in vivo efficacy superior to that of rituximab, a widely used type I anti-CD20 therapeutic antibody with clearly demonstrated clinical benefits for patients with various B-cell malignancies. Several properties of GA101 may contribute to its higher in vivo efficacy. First, GA101 exerts stronger direct B-cell death induction. Although similar observations have been reported for B1, the prototype type II CD20 antibody,6,28 GA101 exhibits more pronounced induction of direct cell death compared with B1. However, the molecular mechanism underlying caspase-independent cell death induction on binding of GA101 to CD20 remains to be elucidated. Similar effects have been described for other antibodies that target lymphocytic antigens, such as HLA-DR,37 CD47,38 and CD37.39 In addition to direct cell death, the observed increase in PS exposure may facilitate phagocytosis of targeted cells.40 GA101 also exhibits increased FcγRIIIa-binding affinities, resulting in increased ADCC and higher B cell–depleting activity in whole blood. The less pronounced down-modulation of CD20 by type II antibodies32 may facilitate this activity.
Experiments to elucidate the contributions of these different biologic functions to the superior in vivo efficacy of GA101 are ongoing. Studies using nonglycoengineered GA101 suggest that its striking in vivo potency in subcutaneous xenograft models can be attributed primarily to the type II CD20 binding mode. Relative to murine model systems, an additional contribution of enhanced ADCC mediated by NK cells is possible in humans. Although different effector functions may be dominant depending on the nature of the target, we are encouraged by the superior efficacy of GA101 in all ex vivo and in vivo models studied to date.
In conclusion, our results provide preclinical evidence for GA101 as a potential new therapy for the treatment of B-cell disorders. This novel type II anti-CD20 antibody may provide a valuable treatment alternative for those disorders amenable to B-cell depletion therapy. A phase 1/2 clinical trial of GA101 for the treatment of patients with relapsed/refractory CD20-positive B-cell malignancies has recently yielded encouraging preliminary safety and efficacy results in this difficult-to-treat patient population.30 Because of its superior B cell–depleting activity in lymphoid tissues, GA101 may provide an effective treatment alternative for autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythomatosus, or immune thrombocytic purpura, where rituximab has shown some benefit.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all coworkers contributing to the identification and characterization of GA101 as well as the members of the GA101 project team.
Authorship
Contribution: E.M., P.B., S.M., U.P., C. Schmidt, S.H., R.G., C.G., A.N., E.v.P., C.F., P. Sondermann, C.J., P. Strein, G.F., T.F., C. Schüll, S.B., J.D.P., C.D.N., and K.D. designed and performed research and analyzed data; M.J.S.D. designed research, contributed vital reagents, and analyzed data; S.P. contributed a vital new reagent; and C.K. and P.U. designed the research, analyzed the data, and wrote the article.
Conflict-of-interest disclosure: E.M., P.B., U.P., C. Schmidt, R.G., C.G., A.N., E.v.P., S.M., C.F., P. Sondermann, and P.U. are employees of GlycArt Biotechnology AG (a member of Roche Glycart AG); P. Strein, G.F., T.F., C. Schüll, S.B., and C.K. are employees of Roche Diagnostics GmbH; J.D.P., C.D.N., and K.D. are employees of Inflammation Discovery, Roche Glycart AG. The remaining authors declare no competing financial interests.
Correspondence: Pablo Umaña, Roche Glycart Biotechnology AG, Wagistrasse 18, CH-8952 Schlieren-Zürich, Switzerland; e-mail: pablo.umana@roche.com.