Abstract
Natural killer (NK) cells were identified by their ability to kill target cells without previous sensitization. However, without an antecedent “arming” event, NK cells can recognize, but are not equipped to kill, target cells. How NK cells become armed in vivo in healthy hosts is unclear. Because latent herpesviruses are highly prevalent and alter multiple aspects of host immunity, we hypothesized that latent herpesvirus infection would arm NK cells. Here we show that NK cells from mice latently infected with Murid herpesvirus 4 (MuHV-4) were armed as evidenced by increased granzyme B protein expression, cytotoxicity, and interferon-γ production. NK-cell arming occurred rapidly in the latently infected host and did not require acute viral infection. Furthermore, NK cells armed by latent infection protected the host against a lethal lymphoma challenge. Thus, the immune environment created by latent herpesvirus infection provides a mechanism whereby host NK-cell function is enhanced in vivo.
Introduction
Natural killer (NK) cells were originally identified and distinguished from other cytotoxic lymphocytes by their capacity for “natural” cytotoxicity, the ability to kill tumor target cells in the absence of previous sensitization.1,2 These cells are now appreciated as an important line of defense against infectious pathogens and tumors and mediate resistance via 2 main functions: cytotoxicity and cytokine production.3-5 Killing of virus-infected and tumor target cells by NK cells depends primarily on granule exocytosis—the release of perforin and granzymes into the cytotoxic synapse, resulting in the apoptotic death of the target.6,7 Interferon-γ (IFN-γ) produced by NK cells shapes the emerging adaptive immune response and is required for effective defense against tumors, viruses, and bacteria.8 These responses are initiated rapidly, before the development of adaptive immunity.
Over the past few decades many germline-encoded receptors on NK cells have been identified that recognize appropriate target cells by sensing the loss of major histocompatibility complex (MHC) class I molecules (“missing self”) as well as stimulatory ligands that are up-regulated on tumor and virus-infected cells. These NK-cell receptors integrate and transmit signals that may result in the rapid triggering of an NK response.9,10 In addition, events that result in NK-cell tolerance to self, via signaling through inhibitory NK receptors, have been delineated.10,11 However, for the triggering of an NK cell to lead to an effective cytotoxic response, the NK cell must be appropriately prepared with cytotoxic effector proteins. This aspect of NK-cell biology, arming for optimal effector function, is a form of activation distinct from NK receptor–mediated triggering. Resting NK cells from specific pathogen-free (SPF) laboratory mice illustrate this concept: they express abundant perforin and granzyme (Gzm) B mRNA but minimal perforin and GzmB proteins and are ineffective killers directly ex vivo.12 Efficient cytotoxicity by these cells requires arming (also termed “priming”) to induce the translation of perforin and GzmB mRNA into protein.12 Here, arming refers to an enhanced NK-cell functional capacity in general, as opposed to a specific theory of NK-cell education.11 The arming of murine NK cells for potent cytotoxicity can be induced in vitro by cytokines (eg, interleukin-2 [IL-2] or IL-15), or by activated accessory cells that stimulate NK cells in response to acute infection,12-14 but the mechanisms whereby these arming events occur in the healthy host are unclear. In contrast to mice, most NK cells from the peripheral blood (CD56dim) of healthy humans are armed with preformed perforin and Gzm proteins within cytotoxic granules and mediate killing directly ex vivo.15 This finding suggests that NK cells from SPF laboratory mice are lacking a critical arming event present in healthy humans and provides an experimental system to elucidate the events that result in NK-cell arming in vivo.
Several circumstances are known to enhance NK-cell function, but they do not explain the presence in the healthy host of armed NK cells with the capacity to kill target cells on first encounter. Cytokines present in an acute inflammatory environment are a well-known stimulus for NK-cell activation and enhance NK cytotoxicity.3,16 NK cells responding to acute viral infection acquire potent cytotoxicity, but the NK compartment typically returns to its baseline state as the adaptive immune response suppresses viral replication.3,5 In addition, chronic infection of laboratory mice with persistently replicating lymphocytic choriomeningitis virus Armstrong strain leads to prolonged enhanced cytotoxicity by NK cells.17 But because lymphocytic choriomeningitis virus infection in humans is uncommon and never chronic, the broad applicability of these findings is uncertain. Thus, the events that arm NK cells for efficient cytotoxicity in vivo remain unknown.
We hypothesized that latent infection with herpesviruses, a family of viruses with high prevalence in humans, may contribute to NK-cell arming in vivo. This hypothesis was formed on the basis of the previous observation that latent herpesviruses have multiple stimulatory effects on the host immune system.18,19 We tested this idea using Murid herpesvirus 4 (MuHV-4, also known as murine gammaherpesvirus-68), a natural pathogen in wild mice20 that is closely related to the human viruses Kaposi sarcoma–associated herpesvirus and Epstein-Barr virus. MuHV-4, like all herpesviruses, establishes life-long latent infection (carriage of the viral genome in latently infected cells punctuated by intermittent reactivation) in its hosts. We found that latent infection with MuHV-4 armed NK cells as shown by multiple alterations in NK-cell biology.
Methods
Mice and viruses
C57BL/6J and B6.RAG1−/− mice (8-12 weeks of age) were used in accordance with the guidelines of and with the approval of Washington University's Animal Studies Committee. MuHV-4 (WUSM strain) and O73.stop were passaged on NIH 3T12 fibroblasts before intranasal inoculation of ketamine-xylazine–anesthetized mice with 104 plaque-forming units in 30 μL of RPMI-1640 with 10% heat-inactivated fetal bovine serum, 1× penicillin/streptomycin, 10mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1× nonessential amino acids, 1× l-glutamine, and 1× sodium pyruvate (medium).
Cell isolation, staining, and stimulation
Peritoneal cells were harvested by lavage with 10 mL of medium. Viable cell numbers and percentages of NK1.1+CD3− lymphocytes were determined on pooled samples from 2 to 5 mice by the use of trypan blue exclusion and flow cytometry. Staining for flow cytometry made use of anti-CD3 (145-2C11), NK1.1 (PK136), IFN-γ (XMG1.2), CD107a (1D4B), and FasL (MFL3; all from BD Biosciences); NKp46 (29A1.4), NKG2D (CX5), and TRAIL (N2B2; all from eBioscience); and GzmB (GB12; from Caltag) monoclonal antibodies. Ex vivo stimulation was performed with rmIL-12 (10 ng/mL), IL-15 (100 ng/mL; R&D Systems), and plate-bound anti-NK1.1 for 8 hours, with the last 7 hours in the presence of brefeldin A.
Adoptive transfers and tumor challenge
Recipient mice were injected intraperitoneally with 2.4 to 3.0 × 106 carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen)–labeled B6.RAG1−/− splenocytes 3 days before lavage and recovery of peritoneal cells for analysis of GzmB. NK-cell depletion experiments used 0.1 mg of anti-NK1.1 (PK136) or isotype-control monoclonal antibody (MARD10) generated in parallel at the Washington University antibody production facility.
Degranulation and cytotoxicity assays
Peritoneal effector cells were plated with targets at the indicated ratios in 96-well V-bottom plates and incubated for 2 hours at 37°C before analysis. Control wells contained varying effector cell numbers without targets. For cytotoxicity assays, peritoneal cells were enriched for nonadherent cells by plating on non-TC–treated plastic at 37°C for 1 hour followed by negative selection (NK-cell isolation kit; Miltenyi Biotec), resulting in an effector cell population that was 5% to 16% NK1.1+ CD3−. Effector cells were incubated with RMA-S target cells for 4 hours followed by flow cytometric identification of killed targets by 7-amino-actinomycin D.12
Statistical analysis
Two-tailed t tests, Kaplan-Meier analyses (log rank comparisons), and correlation coefficient calculations were performed by the use of GraphPad Prism software (GraphPad).
Results
MuHV-4 latency arms NK cells for cytotoxicity
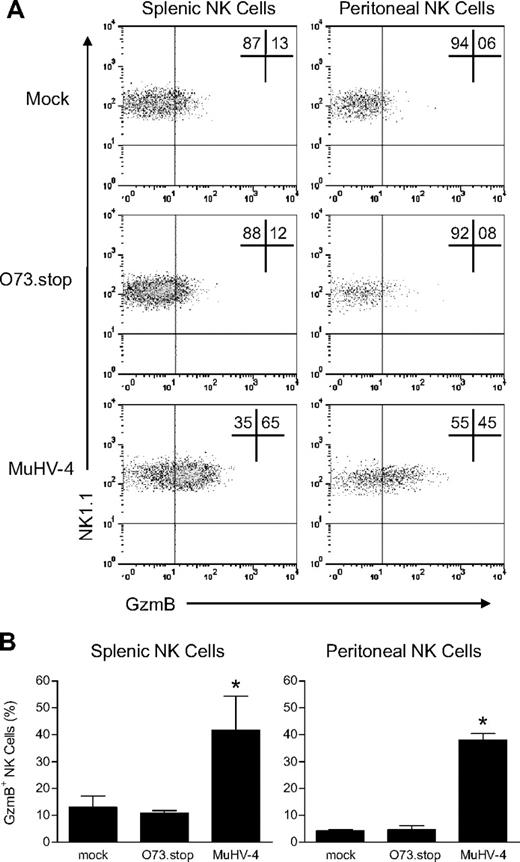
Acute replication of MuHV-4 in SPF laboratory mice is controlled within 13 to 16 days of inoculation.21 Latent infection, established by intranasal inoculation of the virus at least 28 days before analysis, was associated with significantly increased GzmB protein expression in NK cells from the spleen and peritoneal cavity (Figure 1) as well as the liver and lymph nodes (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In contrast, we detected no difference in the expression of tumor necrosis factor-related apoptosis-inducing ligand and Fas ligand, proteins involved in alternative NK-cell cytotoxicity pathways (supplemental Figure 2). Latent infection also was associated with an increase in the absolute number of NK cells in the peritoneal cavity but a decrease in the spleen (supplemental Table 1).
Latent infection with MuHV-4 arms NK cells with GzmB protein. Mice were inoculated with media (mock), MuHV-4, or latency-defective MuHV-4 (O73.stop) 31 days before flow cytometric analysis of GzmB expression in NK1.1+CD3− NK cells from the spleen and peritoneal cavity. (A) Representative density plots demonstrating GzmB expression within NK1.1+CD3− NK cells. (B) Pooled data (10 mice per condition) showing the mean ± SD percentage of NK cells that express GzmB protein. These data are representative of 4 independent experiments, *P < .009 (MuHV-4 vs O73.stop or mock).
Latent infection with MuHV-4 arms NK cells with GzmB protein. Mice were inoculated with media (mock), MuHV-4, or latency-defective MuHV-4 (O73.stop) 31 days before flow cytometric analysis of GzmB expression in NK1.1+CD3− NK cells from the spleen and peritoneal cavity. (A) Representative density plots demonstrating GzmB expression within NK1.1+CD3− NK cells. (B) Pooled data (10 mice per condition) showing the mean ± SD percentage of NK cells that express GzmB protein. These data are representative of 4 independent experiments, *P < .009 (MuHV-4 vs O73.stop or mock).
Because the increase in the absolute number of NK cells that were armed with GzmB was most pronounced in the peritoneal cavity, a prominent site of MuHV-4 latency, we focused further investigations on this compartment. In addition to elevated GzmB protein expression, there was an increased propensity of peritoneal NK cells from latently infected mice to degranulate, as measured by the appearance of lysosomal-associated membrane protein 1 (CD107a) on their surface after triggering with YAC-1 target cells (Figure 2A). This finding was not attributable to an increase in the NKG2D-activating NK receptor because there was no difference in expression between latently infected mice and control mice (supplemental Figure 2). Furthermore, as expected on the basis of the changes in GzmB protein levels and enhanced degranulation, NK cells from latently infected mice exhibited greater cytotoxicity against tumor targets ex vivo (Figure 2B). These data demonstrate that latent herpesvirus infection arms the granule-exocytosis pathway in NK cells.
Latent infection with MuHV-4 enhances NK-cell degranulation and cytotoxicity. (A) Latent infection with MuHV-4 increases NK-cell degranulation. Peritoneal cells from mock, O73.stop, or MuHV-4 latently infected mice were cultured ex vivo with YAC-1 target cells at the indicated target:effector ratios for 2 hours, followed by flow cytometric analysis of CD107a expression on the surface of NK1.1+CD3− NK cells. Control mice were incubated without targets. Bars represent the mean ± SD of 5 to 10 mice per group. *P < .032 (MuHV-4 compared with mock or O73.stop). These data are representative of 2 independent experiments with 15 to 20 total mice per condition. (B) Latent infection with MuHV-4 induces cytotoxic activity by NK cells. Peritoneal cells from control (naive) mice and mice infected 28 days previously with MuHV-4 were enriched for NK cells and then coincubated with RMA-S target cells for 4 hours at the indicated effector:target ratios before analysis of target cell death by 7-AAD. *P < .003 (MuHV-4 vs control). These data are representative of 2 independent experiments with 20 to 30 mice per group per experiment.
Latent infection with MuHV-4 enhances NK-cell degranulation and cytotoxicity. (A) Latent infection with MuHV-4 increases NK-cell degranulation. Peritoneal cells from mock, O73.stop, or MuHV-4 latently infected mice were cultured ex vivo with YAC-1 target cells at the indicated target:effector ratios for 2 hours, followed by flow cytometric analysis of CD107a expression on the surface of NK1.1+CD3− NK cells. Control mice were incubated without targets. Bars represent the mean ± SD of 5 to 10 mice per group. *P < .032 (MuHV-4 compared with mock or O73.stop). These data are representative of 2 independent experiments with 15 to 20 total mice per condition. (B) Latent infection with MuHV-4 induces cytotoxic activity by NK cells. Peritoneal cells from control (naive) mice and mice infected 28 days previously with MuHV-4 were enriched for NK cells and then coincubated with RMA-S target cells for 4 hours at the indicated effector:target ratios before analysis of target cell death by 7-AAD. *P < .003 (MuHV-4 vs control). These data are representative of 2 independent experiments with 20 to 30 mice per group per experiment.
To determine whether latent infection was required for NK-cell arming, we used an engineered MuHV-4 mutant (O73.stop) that is severely defective in its ability to establish latency.22,23 O73.stop undergoes productive acute replication, elicits a normal humoral immune response (data not shown and Barton et al18 ), and activates NK cells during acute infection indistinguishably from wild-type MuHV-4 (supplemental Figure 3). However, 28 days after infection with O73.stop, there was no increase in GzmB protein expression or degranulation by NK cells (Figures 1–2). This result provides genetic evidence that latent infection, and not simply the residual effects of acute viral infection, is required for the observed changes in NK cells.
MuHV-4 latency enhances production of IFN-γ by NK cells
NK cells are also an important source of IFN-γ during the early immune response. As expected, NK cells from control and latently infected mice did not produce IFN-γ protein spontaneously when immediately evaluated ex vivo with the use of intracellular flow cytometry. However, after stimulation ex vivo, a greater percentage of NK cells from the peritoneal cavity of latently infected mice produced IFN-γ compared with NK cells from control mice (Figure 3). This was evident whether the NK cells were stimulated with cytokines (IL-12 and IL-15) or through ligation of the activating receptor NK1.1. Interestingly, splenic NK cells from latently infected mice did not demonstrate an increased propensity to produce IFN-γ compared with control mice (data not shown). Thus, latent MuHV-4 infection increases the fraction of peritoneal NK cells with the capacity to rapidly produce IFN-γ when stimulated with innate cytokines or through activating NK-cell receptors.
NK cells from mice latently infected with MuHV-4 have increased capacity to produce IFN-γ protein. Peritoneal cells from latently infected or control (naive or mock-infected) mice were stained immediately after harvest (No Cx) or after culture ex vivo with phosphate-buffered saline (PBS), IL-12 plus IL-15, or plate-bound antibodies against NK1.1 (Anti-NK1.1). (A) Representative flow cytometric density plots showing IFN-γ expression in NK cells. (B) Pooled data from 10 mice per group showing the mean ± SD percentage of NK cells that express IFN-γ. These data are representative of 3 independent experiments with 10 mice per group per experiment. *P <.001 (MuHV-4 vs control).
NK cells from mice latently infected with MuHV-4 have increased capacity to produce IFN-γ protein. Peritoneal cells from latently infected or control (naive or mock-infected) mice were stained immediately after harvest (No Cx) or after culture ex vivo with phosphate-buffered saline (PBS), IL-12 plus IL-15, or plate-bound antibodies against NK1.1 (Anti-NK1.1). (A) Representative flow cytometric density plots showing IFN-γ expression in NK cells. (B) Pooled data from 10 mice per group showing the mean ± SD percentage of NK cells that express IFN-γ. These data are representative of 3 independent experiments with 10 mice per group per experiment. *P <.001 (MuHV-4 vs control).
Acute MuHV-4 infection is not required for GzmB expression
The latent phase of herpesvirus infection is preceded by acute infection, which may also affect NK-cell activation. Indeed, equivalent NK-cell activation was observed during acute infection (day 7) with O73.stop or wild-type MuHv-4 (supplemental Figure 3). However, as demonstrated previously in this work, only wild-type MuHV-4 infection was capable of inducing alterations in NK cells after the acute viral infection had been cleared. To determine whether any aspect of acute infection was required for the observed alterations in the NK compartment, we injected CFSE-labeled RAG1−/− splenocytes (a source of naive NKcells) into the peritoneal cavity of latently infected or control mice. After 72 hours, endogenous and adoptively transferred NK cells were analyzed for GzmB protein expression. Both adoptively transferred NK cells (CFSE+) and endogenous NK cells (CFSE−) from latently infected recipients displayed increased GzmB protein expression compared with control mice (Figure 4A-B). Furthermore, the endogenous and adoptively transferred NK cells in latently infected mice had similar increases in GzmB protein expression (Figure 4B-C). Adoptively transferred cells did not exhibit dilution of CFSE (Figure 4 and data not shown), signifying that proliferation was not required for NK arming in a latently infected host. Thus, short-term exposure to the environment created by latent MuHV-4 was sufficient to increase GzmB protein levels in NK cells.
NK cells express increased GzmB protein after short-term exposure to the latent MuHV-4 environment in vivo. Latently infected mice (28 days after infection with MuHV-4) were injected intraperitoneally with CFSE-labeled splenocytes from B6.RAG1−/− donors as a source of naive NK cells. Mock-infected and naive recipient mice were used as controls. At 72 hours peritoneal cells were harvested and analyzed for CFSE and GzmB expression in NK1.1+CD3− NK cells. (A) Representative flow cytometric density plots illustrating the expression of GzmB protein in transferred (CFSE+) versus endogenous (CFSE−) NK cells. (B) Pooled data from 2 independent experiments showing the mean ± SD percentage of NK cells that express GzmB. *P = .027 and **P < .001 (MuHV-4 vs control, 11-14 mice per group). (C) Correlation of GzmB protein expression between transferred versus endogenous NK cells in each mouse. Each dot represents one mouse. Correlation coefficient (r2) = 0.9 in latently infected hosts.
NK cells express increased GzmB protein after short-term exposure to the latent MuHV-4 environment in vivo. Latently infected mice (28 days after infection with MuHV-4) were injected intraperitoneally with CFSE-labeled splenocytes from B6.RAG1−/− donors as a source of naive NK cells. Mock-infected and naive recipient mice were used as controls. At 72 hours peritoneal cells were harvested and analyzed for CFSE and GzmB expression in NK1.1+CD3− NK cells. (A) Representative flow cytometric density plots illustrating the expression of GzmB protein in transferred (CFSE+) versus endogenous (CFSE−) NK cells. (B) Pooled data from 2 independent experiments showing the mean ± SD percentage of NK cells that express GzmB. *P = .027 and **P < .001 (MuHV-4 vs control, 11-14 mice per group). (C) Correlation of GzmB protein expression between transferred versus endogenous NK cells in each mouse. Each dot represents one mouse. Correlation coefficient (r2) = 0.9 in latently infected hosts.
Latency-induced arming enhances NK-cell activity in vivo
To determine whether MuHV-4-induced arming of NK cells had an impact on their function in vivo, we challenged latently infected mice with the T-cell lymphoma RMA-S. These tumor cells are syngeneic with C57BL/6 hosts, express minimal MHC class I, and are cleared by activated NK cells.24 After injection of RMA-S, the majority of control mice developed ascites and succumbed to the tumor, whereas latently infected mice were protected (Figure 5A). Moreover, MuHV-4–induced protection was dependent on NK cells because the depletion of NK cells from latently infected mice eliminated the antitumor effect (Figure 5B). Survival of latently infected mice was associated with clearance of the RMA-S cells because tumor cells in the peritoneal fluid were readily detected when NK cells were depleted but were never detected in animals whose NK cells were left intact (supplemental Table 2). Depletion of NK cells from latently infected mice caused no deaths in the absence of RMA-S (data not shown), demonstrating that RMA-S cells were required for the observed mortality. Because T cells are not known to support MuHV-4 growth in vivo and RMA-S cells do not support MuHV-4 replication in vitro (data not shown), direct killing of the tumor cells by replicating virus does not explain these results. Thus, MuHV-4–induced arming of NK cells has a functional consequence in vivo: protection from a lethal lymphoma challenge.
NK cells in latently infected mice protect against a lethal RMA-S lymphoma challenge. (A) Mice were inoculated with medium (mock), MuHV-4, or latency-defective MuHV-4 (O73.stop) 29 days before injection with 103 RMA-S cells intraperitoneally and then followed for survival. These data are pooled from 3 independent experiments with a total of 20 mice per group. *P < .001 (MuHV-4 vs either control). (B) Mice were inoculated with MuHV-4 35 days before injection with anti-NK1.1 or control monoclonal antibody (mAb). One day later, all animals were challenged with RMA-S as in panel A. Antibody injections were continued every 6 to 8 days for the duration of the experiment. These data are pooled from 2 independent experiments with a total of 10 mice per group. *P < .001 (anti-NK1.1 vs control).
NK cells in latently infected mice protect against a lethal RMA-S lymphoma challenge. (A) Mice were inoculated with medium (mock), MuHV-4, or latency-defective MuHV-4 (O73.stop) 29 days before injection with 103 RMA-S cells intraperitoneally and then followed for survival. These data are pooled from 3 independent experiments with a total of 20 mice per group. *P < .001 (MuHV-4 vs either control). (B) Mice were inoculated with MuHV-4 35 days before injection with anti-NK1.1 or control monoclonal antibody (mAb). One day later, all animals were challenged with RMA-S as in panel A. Antibody injections were continued every 6 to 8 days for the duration of the experiment. These data are pooled from 2 independent experiments with a total of 10 mice per group. *P < .001 (anti-NK1.1 vs control).
Discussion
How NK cells become prepared for rapid and potent responses to appropriate targets remains an intriguing and open question in NK-cell biology. In this report, we demonstrate that latent infection with a herpesvirus results in armed NK cells with an enhanced capacity for both cytotoxicity and IFN-γ production. Moreover, this NK-cell arming confers protection against RMA-S lymphoma challenge, indicating that the NK-cell modulation observed ex vivo has important significance to the host in vivo. Our data, in which we used a genetic mutant (O73.stop) of MuHV-4 that fails to establish latency after the acute phase of infection, indicate that acute infection is not responsible for the observed NK-cell arming. These experiments were extended by an adoptive transfer model that shows that naive NK cells acquire GzmB protein within 72 hours of exposure to the latently infected environment, signifying that NK cells require no aspect of acute infection to become armed in the latently infected host. This adoptive transfer result also suggests that NK cells that develop or that traffic to the site of latency after the resolution of acute MuHV-4 infection may also be armed during this chronic phase of infection. Thus, our data offer a potential mechanism whereby NK cells become armed for potent and rapid responses in the absence of a clinically evident acute infectious insult.
For decades mice have been used as a model for human NK-cell biology; however, clear differences in the functional status of resting human versus mouse NK cells have not been explained. The major subset of human NK cells in peripheral blood and spleen (CD56dim) express GzmB and perforin protein and mediate NK cytotoxicity against MHC class I low K562 targets directly ex vivo.15 It is notable that not all human NK cells are armed for immediate cytotoxicity. CD56bright human NK cells constitute a minor subset of peripheral blood NK cells (but a major subset in the lymph nodes and tonsil) and are postulated to be developmentally immature and functionally distinct from CD56dim NK cells.15,25 Similar to SPF mouse NK cells, CD56bright NK cells have minimal GzmB and perforin protein and require ex vivo cytokine activation to become potent cytotoxic effectors.
Thus, clear parallels exist in the lack of cytotoxic effector proteins and killing potential between the majority of resting mouse NK cells and a selected subset of resting human NK cells. What accounts for the difference in arming status between resting NK cells from the SPF mouse and human CD56dim NK cells in the peripheral blood and spleen? Our data suggest that a critical event that occurs in healthy humans to arm NK cells for potent cytotoxic capacity is lacking in SPF mice and that this process may contribute to human NK-cell function. In humans, herpesviruses are highly prevalent and are not associated with clinically evident disease in the majority of those infected.26 Because MuHV-4 infection in mice shares important properties with Epstein-Barr virus infection in humans, we speculate that this mode of NK-cell arming may occur in healthy people. Of note, CD56dim NK cells with cytotoxic capacity have been identified in umbilical cord blood,27 suggesting that multiple pathways may lead to functionally competent human NK cells. Thus, some of the differences between human and SPF laboratory mouse NK-cell function may reflect a disparity in immune activation induced by chronic virus infection, as opposed to an intrinsic difference in the NK cells themselves. More broadly, these results, along with previous findings18 in which the authors demonstrated that herpesvirus infection alters susceptibility to bacterial infection, indicate that the activation state of the innate immune system needs to be taken into account when using mice to model immune responses against pathogens and tumors.
Our experiments examining the arming of NK cells, as well as previous experiments that uncovered heightened resistance against Listeria monocytogenes during herpesvirus latency,18 made use of the latency-defective MuHV-4 mutant O73.stop. This virus replicates to normal22,28 or near-normal levels23 during acute infection but is severely defective in its ability to persist in the latent state. NK-cell activation is equivalent for wild-type MuHV-4 and O73.stop during acute infection. Thus, this mutant afforded us the opportunity to address whether viral persistence per se, as opposed to residual effects from the acute infection, is required for the alterations in the host immune system. In both sets of experiments, mice infected with O73.stop had immunologic phenotypes similar to uninfected control mice, indicating that the latent stage of infection is required for prolonged alterations in host immunity. These experiments, however, leave important questions to address. Because O73.stop cannot persist in the host, by definition it lacks the potential to reactivate. Therefore, it is possible that viral reactivation events (productive or abortive, both of which are encompassed in latency as we use the term here) provide the driving force behind the observed immune activation in the host.19 Future experiments that use MuHV-4 mutants with specific defects in the ability to reactivate will allow us to dissect the contribution of these components of chronic herpesvirus infection to immune modulation during latency.
The potential benefits of herpesvirus latency in humans, including taking into account the morbidity and mortality associated with these infections, have been previously discussed.18,29,30 Our current findings define in greater detail the effects of MuHV-4 latency on innate immunity in the mouse and are consistent with previous observation of enhanced immunity against bacterial infection.18,30 Whether these effects contribute to the activation status of the innate immune system of healthy humans, and thus alter the risk:benefit ratio of herpesvirus infection in people, has yet to be determined.
What aspects of the immune environment created by MuHV-4 latency are responsible for arming NK cells in vivo? Although the precise signals or factors in latently infected mice that directly arm NK cells remain to be elucidated, certain features of latent infection and NK-cell activation point to potential candidates. Importantly, the finding that MuHV-4 latency can elicit different responses from NK cells in the spleen compared with the peritoneal cavity (supplemental Table 1) highlights the impact of the local immune environment in determining the exact arming effect of latent infection. Because latent MuHV-4 is predominantly found in B cells in the spleen31 and macrophages in the peritoneal cavity,26 the type of cell harboring the latent virus may contribute to these tissue-specific arming effects. Previous authors12 have identified IL-15/IL-15R signaling as central to the arming of murine NK cells for potent cytotoxicity in vitro via induction of GzmB and perforin protein translation. In addition, dendritic cell trans-presentation of IL-15 after acute infection or TLR ligand administration also has been shown to arm NK cells for enhanced cytotoxicity.14 Other cytokines augment NK-cell cytotoxicity, including IL-2, IL-12, IL-18, IL-21, and IFN-α, providing additional candidates to evaluate in vivo. Interestingly, both target-triggered NK degranulation and target cell death were increased after coculture of tumor targets with armed NK cells. This result suggests that the granule-exocytosis pathway is affected at multiple levels, and not only through the translation of cytotoxic effector molecules, by this process. Although these issues remain to be clarified in future studies, our findings identify MuHV-4 latency as an important in vivo mechanism resulting in armed NK cells.
IFN-γ produced by NK cells during an innate immune response is an important functional counterpart to NK-cell cytotoxicity.3,8 NK cells armed in latently infected mice did not spontaneously produce IFN-γ protein. However, ex vivo stimulation with a well-described monokine combination (IL-12 + IL-15) or ligation of the NK1.1 activating receptor resulted in IFN-γ protein production by a significantly greater number of armed peritoneal NK cells compared with controls. The authors of a previous study32 have identified constitutive transcription of IFN-γ mRNA early in NK-cell development without effective translation into protein, suggesting a posttranscriptional mode of IFN-γ regulation in NK cells. The precise mechanisms mediating this posttranscriptional regulation remain uncertain.33 Thus, although our findings may reflect enhanced IFN-γ transcription or mRNA stability, a more likely explanation is an alteration at the level of posttranscriptional control. Alternatively, changes in NK-cell signaling intermediates may increase the likelihood of producing IFN-γ after cytokine-receptor or activating NK-cell receptor ligation. Further comparison of peritoneal and splenic NK cells in MuHV-4 latently infected mice may provide clues to the molecular mechanisms underlying the increased capacity to produce IFN-γ protein.
The authors of recent reports34,35 suggest that NK cells have intrinsic memory-like properties such that particular NK functions are enhanced by previous stimulation. In a study by Cooper et al,34 NK cells that were previously stimulated with the proinflammatory cytokines IL-12 and IL-18 and then adoptively transferred into mice produced IFN-γ more efficiently than naive NK cells upon restimulation. This augmented IFN-γ response was intrinsic to the NK cell and passed on to NK-cell progeny after proliferation. Notably, GzmB expression and cytotoxic capacity were not enhanced in these memory-like NK cells. After infection with MCMV, Sun et al35 reported an intrinsic enhancement of the Ly49H+ NK-cell response to Ly49H-ligand (virally encoded m157) using adoptive transfer models. This study showed that NK cells with an augmented ability to expand and produce IFN-γ in response to m157 persisted for months after MCMV infection. Similar to the results of Cooper et al,34 there was no reported enhancement of NK-cell cytotoxic effector molecules or cytotoxicity by Ly49H+“memory” NK cells, although limited evidence that these memory-like NK cells degranulated more efficiently in response to tumor targets was presented. Furthermore, because specific activating receptor-ligand–dominated NK-cell responses occur rarely in nature, how this relates to less specific (but much more common) NK cells responses is uncertain.
What is the relationship between NK cells armed by MuHV-4 latency and memory-like NK cells? Although both types of NK cells have an enhanced capacity for IFN-γ production, only the NK cells armed by latent MuHV-4 demonstrate clear increases in GzmB protein expression and cytotoxicity. NK-cell arming occurred within 72 hours in the latently infected mouse and was clearly separated from any NK-cell activation induced during acute viral infection. Thus, in our study armed NK cells depended on the extrinsic immune environment created during MuHV-4 latency, providing a distinct and general mechanism whereby NK cells acquire greater functional capacity without having been present during a specific response (eg, to MCMV) or host exposure to an acute inflammatory insult. This contrasts to the requirement for direct, previous, and potent activation for generation of memory-like NK cell. It remains a possibility that MuHV-4 latency not only arms NK cells for enhanced immediate cytotoxicity and IFN-γ production as we have demonstrated but also results in an intrinsic change in the NK cell itself. Regardless, NK-cell arming through alteration of the immune environment by a latent herpesvirus provides a general host mechanism to prepare innate NK cells for an optimal functional response.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank P. Keyel for technical advice on the degranulation assay; L. Mitchell in the Washington University Division of Rheumatology antibody production facility for technical assistance; and M. Cooper, E. Barton, A. French, M. Colonna, and W. Yokoyama for insightful discussion.
Support for this work came from the NIH (K08AI079011 to D.W.W.; R01CA074730, R01CA096511 to H.W.V., R01DK49786 to T.J.L., and K08HL093299 to T.A.F.), the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (U54 AI057160 to H.W.V.), the Abbott Scholar Award (to D.W.W.), the Damon Runyon Cancer Research Foundation (to T.A.R.), the Edward Mallinckrodt, Jr. Foundation (to T.A.F.), and the HHMI Physician-Scientist Early Career Award (to T.A.F.). Flow cytometry data were acquired in the Siteman Cancer Center High Speed Cell Sorting Core.
National Institutes of Health
Authorship
Contribution: D.W.W. and T.A.F. designed and performed experiments, analyzed data, and wrote the manuscript; C.R.K., S.E.S, T.A.R., and J.C. performed experiments; J.E.P. designed experiments and analyzed data; T.J.L. and H.W.V. provided materials and contributed to experimental design; and all authors commented on data and conclusions before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.W.W. is Division of Rheumatology, Gundersen Lutheran Medical Foundation, La Crosse, WI.
Correspondence: Todd A. Fehniger, MD/PhD, Department of Internal Medicine, Division of Oncology, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: tfehnige@wustl.edu.