Surface IgM (sIgM) has a key influence on the clinical behavior of chronic lymphocytic leukemia (CLL). We now report that it exists in 2 forms with different N-glycosylation patterns in the μ-constant region. One glycoform is similar to normal B cells in bearing mature complex glycans common to most cell-surface glycoproteins. The other is an immature mannosylated form more characteristic of μ chains in the endoplasmic reticulum. Unmutated CLL (U-CLL) expresses a higher proportion of mannosylated surface μ chains than mutated CLL. Normal B cells express only the mature glycoform but can express the immature form after persistent engagement of sIgM, suggesting that glycan modification is a consequence of antigen exposure. CLL cells express variable proportions of the mannosylated form and can revert to the mature form after incubation in vitro. Both glycoforms are able to signal after sIgM engagement in vitro, leading to enhanced tyrosine phosphorylation. These findings support the concept that CLL cells are continuously exposed to antigen in vivo, driving the N-glycosylation pattern of expressed sIgM toward a mannosylated form, especially in U-CLL. Strikingly, this is reminiscent of follicular lymphoma, where mannosylated Ig is expressed constitutively via N-glycosylation sites in the variable region, suggesting a functional asset for this glycoform.
Introduction
The B-cell receptor (BCR) is critical for the survival of normal B cells1 and is retained by the majority of B-cell malignancies, including chronic lymphocytic leukemia (CLL). It comprises the surface Ig (sIg) in association with the Igα/β heterodimer (CD79a/b), thereby combining antigen recognition with signal transduction.2,3 During antigen-driven maturation, the variable (V) regions of sIg accumulate somatic mutations, which allow antigen selection of high-affinity Ig for eventual production of antibody and memory B cells.4 B-cell tumors can develop at multiple stages of differentiation, and these vary widely in their clinical manifestation.5 In CLL, the importance of the maturational status of the B cell of origin in determining clinical behavior is particularly clear. Two disease subsets have been defined which differ according to whether the Ig V genes have or have not undergone somatic hypermutation.6,7 Unmutated CLL (U-CLL) appears to derive from a B cell before initiation of mutation, and has a relatively poor prognosis. In contrast, mutated CLL (M-CLL) derives from a postfollicular B cell that has accumulated mutations, and has a relatively good prognosis. This distinction has informed clinical practice and has led to a description of several other associated features useful for prognostic assessment.8
However, the biology which determines the differential behavior of the 2 subsets remains largely unknown. Involvement of the BCR is indicated by the observation that, after engagement of the surface IgM (sIgM) with anti-μ antibody, the ability to phosphorylate p72Syk and other intermediates of the signaling pathway is more evident in U-CLL than in M-CLL.9,–11 The protein tyrosine kinase, ZAP-70, is also preferentially expressed in U-CLL.11,–13 The functional consequences of expression include increased activation of BCR-associated signaling molecules, such as Syk, extracellular signal–related kinase (ERK), BLNK, PLC-γ, and Akt.14,15 Although it appears that the kinase activity is not required for BCR stimulation in CLL, the adaptor function of ZAP-70 could have a role, possibly modulating events which terminate the response.16 The implication is therefore that BCR signaling is facilitated in U-CLL, and this could affect tumor cell behavior.
Another feature influencing BCR signaling is the level of expression of sIgM, and this is clearly higher in U-CLL than in M-CLL.9,11 Interestingly, expression in both subsets is increased by simple incubation in vitro, which apparently reverses a down-modulation that has occurred in vivo.11 A role for antigen in selecting and possibly driving CLL had been suspected from the asymmetric usage of VH and VL genes,6,17,18 and is supported by the finding that CLL-derived sIgM can bind to autoantigens and to some bacterial antigens.19,–21 Although both these features are more evident in U-CLL, they can also be found in some cases of M-CLL.21
Activation of CLL cells in vivo is indicated by up-regulation of surface markers22 and by an apparently constitutively increased phosphorylation of ERK1/2.23 If interaction with antigen is occurring, as is suggested by the reversible anergy, the consequences could be to generate an anergic phenotype. The question then concerns the status of the sIgM, some of which appears to be down-modulated.11 A previous study examined the total cellular μ chains (surface and intracellular) and reported that there appeared to be an intrinsic block on transport of nascent IgM to the cell surface in CLL. It was suggested that this could be due to a failure to assemble with the Igα/β heterodimer (CD79a/b).24,25 A disordered biosynthetic pathway for sIgM, however, may not be intrinsic but could reflect anergy, as has been found in mouse models.26
Biosynthesis of IgM includes addition of glycans to specific sites in the heavy chain.27 This process begins in the endoplasmic reticulum (ER), where core highly mannosylated oligosaccharides are added to asparagine residues within acceptor sequence motifs.28 For the majority of normal B-cell surface or secreted glycoproteins, glycan chains are modified during transit through the cell, with further “mature” complex glycans added in the Golgi stacks.29 Because of this compartmentalization, it has been assumed that the “immature” glycoform indicates ER-associated IgM, whereas the “mature” form is likely to be expressed at the cell surface or in secreted IgM.
We have focused on the sIgM in CLL and have found that the μ chain exists in 2 forms with distinct N-glycosylation patterns. One resembles that of normal B cells with a mature type; the other is mannosylated in a manner more characteristic of immature intracellular IgM. Surprisingly, both reach the cell surface and can mediate signal transduction. This pattern can be mimicked in normal B cells after ligation of sIgM. In CLL, therefore, antigen engagement is likely to have resulted in modulation of sIgM so that the mannosylated form is expressed. The fact that this glycoform is more highly expressed in U-CLL points to a potential differential benefit for the tumor cell.
Methods
Patient and healthy donor material
Approval was obtained by the Southampton and South West Hampshire Research Ethics Committee. Informed consent was provided in accordance with the Declaration of Helsinki. Blood was obtained from 34 patients with typical CLL who attended the hematology outpatient clinics at the Royal Bournemouth Hospital, Hammersmith Hospital, Leicester Royal Infirmary, Portsmouth Hospital, Southampton General Hospital, and the Royal Wolverhampton Hospitals. Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep gradient centrifugation (Axis-Shield Diagnostics) washed, and cryopreserved in RPMI 1640 medium containing 2mM glutamine, 1% sodium pyruvate, 1% nonessential amino acids, 10% DMSO, and 15% fetal calf serum (FCS). Samples were thawed in complete RPMI 1640 medium supplemented with 2mM glutamine, 1% sodium pyruvate, 1% nonessential amino acids, and 10% FCS. Cells were pelleted by centrifugation, resuspended in complete medium, and counted. Cells were allowed to recover by incubation for 1 hour at 37°C. Normal B cells were isolated from the blood of healthy donors using the B Cell Isolation Kit II according to the manufacturer's protocol (Miltenyi Biotec).
IGHV gene sequence analysis
IGHV gene mutational status was determined as previously described.6,11 An IGHV leader primer mix and a Cμ100 primer were used to amplify heavy-chain genes from cDNA.30 All nucleotide sequences were aligned to the ImMunoGeneTics (IMGT) directory,31 and mutational status was determined using a 98% cutoff.6
Flow cytometry
Phenotypic status of the CD19+CD5+ CLL cells was determined by flow cytometry, using rabbit F(ab′)2 anti-μ antibody (Dako, Inverness Medical), anti-CD38 (clone HB7; BD Biosciences), and anti–ZAP-70 (clone 2F3.2; Millipore), as described previously.11,32 Data were acquired on a BD FACSCalibur and analyzed with BD CellQuest Pro analysis software (BD Biosciences). Samples with at least 30% CD38+ and 20% ZAP-70+ tumor cells were designated positive for CD38 or ZAP-70, respectively. To measure the percentages of memory cells among the IgM+ population of B cells of healthy donors, we used anti-CD19 (clone HIB19), anti-CD27 (clone O323; both from BioLegend Europe BV) and rabbit F(ab′)2 anti-μ antibody (Dako).
Biotinylation of cell-surface proteins
Cell-surface proteins were biotinylated using the Cell Surface Protein Isolation kit (Pierce) according to the manufacturer's protocol. Briefly, freshly isolated CLL cells (5 × 107 per reaction) or normal B cells (5 × 106 per reaction) were incubated in phosphate-buffered saline (PBS) with 0.5 mg/mL sulfo-NHS-SS-biotin at 4°C. After 30 minutes, the reaction was terminated using Quenching solution (Pierce), and cell viability was determined using trypan blue exclusion (mean viability 96%). Cells were washed twice in ice-cold PBS and lysed by sonication in Lysis Buffer (Pierce) with added solution of SIGMAFAST Protease Inhibitor Tablets (Sigma-Aldrich). Cells were centrifuged at 12 000g, and the supernatant was mixed with Immobilized NeutrAvidin Gel (Pierce) and incubated at room temperature for 60 minutes. The NeutrAvidin Gel was then washed 4 times in Wash Buffer (Pierce), and biotinylated proteins were eluted by boiling in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 50mM dithiothreitol (DTT). Proteins were resolved by SDS-PAGE and analyzed using anti-μ (Jackson ImmunoResearch Laboratories). Images were collected using the Fluor-S MultiImager and quantified using Fluor-S software Quantity One Version 4.6.3 (Bio-Rad). Reproducibility was assessed by repeating the analyses of 3 single samples, with results obtained being very close (mean of 9% variation). In 2 additional cases analyzed, we compared fresh and frozen/thawed samples and also obtained very close results (< 5% variation).
Glycosidase treatment
Glycosylation of cell-surface or total μ chains was analyzed by digestion using endoglycosidase H (EndoH) or peptide:N-glycosidase F (PNGase). For analysis of cell-surface μ chains, digestions were performed using an aliquot of NeutrAvidin Gel–bound biotinylated protein (isolated as described) resuspended in enzyme buffer. For analysis of total μ chains, cell lysates were prepared using Lysis buffer (Pierce). Reactions were performed in a total volume of 40 μL with 1000 U of EndoH or PNGase (both from New England Biolabs). After incubation at 37°C for 90 minutes, samples were diluted with SDS-PAGE sample buffer, heated at 95°C for 3 minutes, and analyzed by immunoblotting using anti-μ antibody.
Analysis of tyrosine phosphorylation after treatment with anti-μ
After incubation with goat F(ab′)2 anti-μ (Southern Biotechnology Associates) 20 μg/mL, cells were collected by centrifugation and lysed using Brij-97 lysis buffer.10 Cell lysates were precleared for 30 minutes with protein G–coated Sepharose beads (Amersham Pharmacia Biotech), followed by incubation with 2 μg of antiphosphotyrosine-specific antibody 4G10 (Upstate Biotechnology) for 60 minutes at 4°C. Packed protein G–Sepharose beads (15 μL) blocked with 5% (wt/vol) bovine serum albumin (BSA) were added to the sample and incubated at 4°C. After 60 minutes, the beads were washed 5 times with cold lysis buffer and boiled in SDS-PAGE sample buffer. The precipitated proteins were separated by SDS-PAGE and analyzed by immunoblotting.
Re-expression of sIg after incubation in vitro or after engagement of sIgM
The spontaneous re-expression of sIgM by untreated CLL cells after 24, 48, and 72 hours' incubation was measured by flow cytometry as previously described.11 sIgM expression and glycosylation status were analyzed by SDS-PAGE after 48 hours of incubation. To analyze glycosylation status after engagement of sIgM, CLL cells or normal B cells were incubated with 20 μg/mL goat F(ab′)2 anti-μ (Southern Biotechnology Associates) or isotype control antibody for 24 hours. Viability was measured by annexin-V/propidium iodide staining, and viable cells were isolated by centrifugation on Lymphoprep before analysis of sIgM by biotinylation.
Statistics
When comparing CLL subgroups, P values were calculated using the Student t test (2-tailed, 99% confidence interval [CI], paired or unpaired where appropriate). Statistical analyses were performed using GraphPad Prism software.
Results
Cases of CLL and features of tumor cells
The clinical background of the patients with CLL is indicated in Table 1. Tumor cells from 34 patients, defined by the IGHV mutational status (19 M-CLL and 15 U-CLL), were isolated and analyzed by fluorescence-activated cell sorter (FACS) for CD19, CD5, CD38, ZAP-70 (Table 1), and sIgM. The proportion of contaminating normal B cells in the samples was determined using anti-CD19 and anti-CD5 antibodies and found to be 1% or less (data not shown). Because we were analyzing surface μ chains, we restricted the study to those patients with an sIgM mean fluorescence intensity (MFI) of greater than 20. This equalized the 2 subsets in terms of the mean levels of expression.
CLL stage and tumor cell features
| Patient ID no. . | Binet stage at diagnosis . | Zap70, % . | CD38, % . | IGHV mutational status . |
|---|---|---|---|---|
| 177 | A | 0 | 0 | M |
| 244* | A | 16 | 2 | M |
| 259 | A | 2 | 4 | M |
| 264 | A | 88 | 20 | M |
| 269 | B | 1 | 1 | M |
| 317 | A | 9 | 0 | M |
| 326 | B | 8 | 2 | M |
| 327 | A | 46 | 42 | M |
| 333* | A | 1 | 1 | M |
| 344 | A | 31 | 60 | M |
| 357 | A | 5 | 36 | M |
| 374 | A | 0 | 3 | M |
| 379 | A | 0 | 1 | M |
| 399 | A | 2 | 0 | M |
| 406 | A | 1 | 0 | M |
| 413 | A | 3 | 3 | M |
| 416 | A | 0 | 7 | M |
| 420 | A | 1 | 100 | M |
| 418 | C | 11 | 67 | M |
| 255 | C | 25 | 8 | U |
| 284* | B | 48 | 12 | U |
| 288* | A | 61 | 71 | U |
| 291 | NA | 88 | 18 | U |
| 293* | A | 29 | 16 | U |
| 305* | C | 65 | 9 | U |
| 315 | A | 15 | 8 | U |
| 328 | A | 16 | 23 | U |
| 350 | A | 63 | 95 | U |
| 376 | A | 9 | 63 | U |
| 383 | A | 17 | 24 | U |
| 385 | A | 11 | 36 | U |
| 394 | A | 25 | 96 | U |
| 409 | B | 77 | 56 | U |
| 415 | A | 1 | 51 | U |
| Patient ID no. . | Binet stage at diagnosis . | Zap70, % . | CD38, % . | IGHV mutational status . |
|---|---|---|---|---|
| 177 | A | 0 | 0 | M |
| 244* | A | 16 | 2 | M |
| 259 | A | 2 | 4 | M |
| 264 | A | 88 | 20 | M |
| 269 | B | 1 | 1 | M |
| 317 | A | 9 | 0 | M |
| 326 | B | 8 | 2 | M |
| 327 | A | 46 | 42 | M |
| 333* | A | 1 | 1 | M |
| 344 | A | 31 | 60 | M |
| 357 | A | 5 | 36 | M |
| 374 | A | 0 | 3 | M |
| 379 | A | 0 | 1 | M |
| 399 | A | 2 | 0 | M |
| 406 | A | 1 | 0 | M |
| 413 | A | 3 | 3 | M |
| 416 | A | 0 | 7 | M |
| 420 | A | 1 | 100 | M |
| 418 | C | 11 | 67 | M |
| 255 | C | 25 | 8 | U |
| 284* | B | 48 | 12 | U |
| 288* | A | 61 | 71 | U |
| 291 | NA | 88 | 18 | U |
| 293* | A | 29 | 16 | U |
| 305* | C | 65 | 9 | U |
| 315 | A | 15 | 8 | U |
| 328 | A | 16 | 23 | U |
| 350 | A | 63 | 95 | U |
| 376 | A | 9 | 63 | U |
| 383 | A | 17 | 24 | U |
| 385 | A | 11 | 36 | U |
| 394 | A | 25 | 96 | U |
| 409 | B | 77 | 56 | U |
| 415 | A | 1 | 51 | U |
CLL indicates chronic lymphocytic leukemia; NA, not available; M, mutated; and U, unmutated.
Patients treated during the course of the disease but were untreated for 3 months prior to blood collection (n = 6).
Analysis of the N-glycan status of surface μ chains
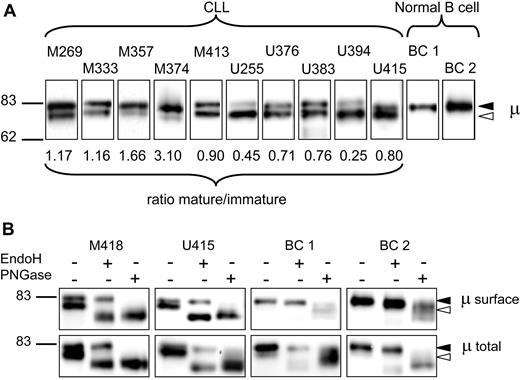
Biotinylated sIgM was purified and separated by SDS-PAGE, and μ chains were detected by Western blotting with anti-μ antibody. Figure 1A shows results from 10 representative samples derived from either M-CLL (5 samples) or U-CLL (5 samples), and 2 samples from normal purified B cells (BC1 and BC2). It is clear that all the CLL samples contain 2 bands, of variable proportions. The size of the faster (lower) band (approximately 78 kDa) corresponds to the so-called immature glycoform of μ chains, which is a precursor of the fully N-glycosylated form.24,25 This form is generally confined to the intracellular compartment. The slower (upper) band (82 kDa) corresponds to the “mature” glycoform expressing fully N-glycosylated complex glycans characteristic of cell-surface glycoproteins.29 In all cases the bands were sharp, indicating that glycan addition led to either form but not to a wide range of intermediates. The ratio of mature to immature forms in the 34 CLL samples was calculated from the densitometric analysis and is indicated below the sample panel in Figure 1A. It clearly varies widely between cases. In contrast, surface μ chains from normal B cells are seen as a single band corresponding to the slower, mature form (Figure 1A). It should be noted that the IgM+ B cells from healthy donor BC2 contained 43% of CD27+ B cells. Because only a single μ chain band was observed, this indicates that both naive and memory B cells in the blood express sIgM of the mature form.
Analysis of μ chains expressed by surface IgM of CLL and normal B cells. (A) Surface proteins were purified from normal B cells and chronic lymphocytic leukemia (CLL) samples, and μ chains were analyzed by immunoblotting. Results are shown from 2 healthy donors (BC1 and BC2) and 10 CLL samples (5 M-CLL, 5 U-CLL), representative of a total of 34 CLL samples. The ratio of expression of the 2 glycoforms of μ chain (mature-immature) is shown for each CLL sample. The immature form (◁) and the mature form (◀) of μ chain are indicated in panels A and B. (B) Purified surface proteins or total cell lysates were treated with EndoH or PNGase or left untreated and analyzed by immunoblotting. Results from 2 healthy donors (BC1 and BC2) representative of a total of 5 independent experiments, and 2 CLL samples representative of a total of 6 independent experiments are shown.
Analysis of μ chains expressed by surface IgM of CLL and normal B cells. (A) Surface proteins were purified from normal B cells and chronic lymphocytic leukemia (CLL) samples, and μ chains were analyzed by immunoblotting. Results are shown from 2 healthy donors (BC1 and BC2) and 10 CLL samples (5 M-CLL, 5 U-CLL), representative of a total of 34 CLL samples. The ratio of expression of the 2 glycoforms of μ chain (mature-immature) is shown for each CLL sample. The immature form (◁) and the mature form (◀) of μ chain are indicated in panels A and B. (B) Purified surface proteins or total cell lysates were treated with EndoH or PNGase or left untreated and analyzed by immunoblotting. Results from 2 healthy donors (BC1 and BC2) representative of a total of 5 independent experiments, and 2 CLL samples representative of a total of 6 independent experiments are shown.
Analysis of the nature of the added glycans
To investigate glycan profiles, the biotinylated sIgM was treated with EndoH, which removes only mannosylated N-glycans, or with PNGase, which removes all attached N-glycans. The results on 2 representative CLL samples (Figure 1B) show that the faster immature μ chain band in both cases is susceptible to EndoH, with enzyme treatment increasing mobility to that seen with PNGase. Similar results were obtained in a further 4 samples (1 U-CLL and 3 M-CLL; data not shown). The relative levels of the 2 forms remained the same (within 10% variation) after treatment with EndoH. The data indicate that all the N-glycans on the immature faster form are mannosylated. The slower, mature form is only susceptible to PNGase, as expected. The sIgM μ chains from normal B cells (2 representative of 5 samples) are not significantly affected by EndoH but are cleaved by PNGase, indicating that only the mature form is present (Figure 1B top panels).
The total μ chains in lysates (Figure 1B bottom panels) include both surface and intracellular glycoforms. In the CLL samples, the inclusion of the intracellular form increased the proportion of EndoH-susceptible immature μ chains. Again, PNGase removes all the N-glycans from both forms. For the normal B cells, a small amount of a faster band was visible, consistent with the presence of the immature precursor form, known to be ER-associated (Figure 1B). This was susceptible to EndoH, as expected. In summary, the surprising finding was that the immature form appeared to be present at the surface of CLL cells. It appears therefore that sIgM of CLL samples comprises both the mature form with complex N-glycans and an immature form similar to the mannosylated IgM normally located in the ER.
Consistency of glycan status
The ratios of mature to immature glycan were highly variable between patients. To analyze variability in patients over time, we were able to investigate 3 patients at different time points. A total of 2 patients (M177 and M259) had received no treatment during this period, and 1 patient (M244) had received fludaribine. The results for repeat samples collected over the following periods of disease duration showed highly consistent ratios (in parentheses): M177, 3 months (0.61, 0.60); M244, 44 months (1.39, 1.35); and M259, 10 months (1.48, 1.51). This remarkable consistency suggests that the phenomenon may be a constant feature of the tumor.
Correlation of glycan status with prognostic factors
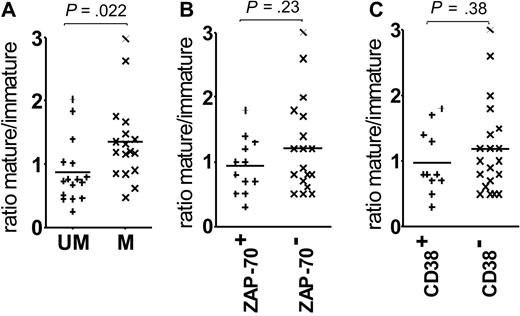
Comparison of U-CLL and M-CLL revealed a higher relative level of the immature form (lower ratio) in U-CLL (P = .022; Figure 2A). There is a parallel trend for a lower ratio in patients expressing ZAP-70 or CD38, but this does not reach statistical significance (Figure 2B-C). The consistency of the glycan profile of the surface μ chains and the correlation with mutational status suggested a relation to disease subsets; therefore, we probed the function of the 2 glycoforms.
Correlations of the surface μ chain mature-immature ratios with prognostic markers. The graphs show the association between surface μ chain glycoforms ratio and (A) VH gene mutational status, (B) ZAP-70 expression, and (C) CD38 expression. Horizontal bars indicate mean values. Statistically significant differences between the groups are shown (t test, 2-tailed, unpaired, 99% CI).
Correlations of the surface μ chain mature-immature ratios with prognostic markers. The graphs show the association between surface μ chain glycoforms ratio and (A) VH gene mutational status, (B) ZAP-70 expression, and (C) CD38 expression. Horizontal bars indicate mean values. Statistically significant differences between the groups are shown (t test, 2-tailed, unpaired, 99% CI).
Functional analysis of the immature or mature forms of sIgM
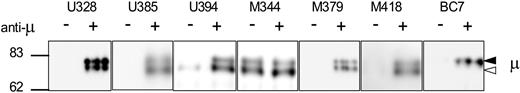
Engagement of sIgM by anti-μ leads initially to phosphorylation of Igα/Igβ and then to phosphorylation of further signaling intermediates, in both CLL and in normal B cells.4,14 To investigate the functional ability of the 2 forms of sIgM, CLL cells or normal B cells were treated with anti-μ, and the total cellular phosphorylated proteins were isolated by immunoprecipitation using the specific antiphosphotyrosine monoclonal antibody (MoAb) 4G10. After gel separation, μ chains were detected by Western blotting with anti-μ. Figure 3 shows 2 bands in all CLL cases after treatment with anti-μ, indicating that both immature and mature μ chains were associated with phosphorylated proteins. Importantly, this confirms that the immature form is found at the cell surface, and indicates that this form as well as the mature form is able to mediate signaling after engagement of the BCR (Figure 3). As expected, the mature form of the μ chain also associated with phosphorylated protein in normal B cells after treatment with anti-μ (Figure 3).
Analysis of μ chain–associated phosphoprotein after treatment with anti-μ. Normal B cells (BC7) and CLL cells were treated with anti-μ (+) or isotype control (−) for 2 minutes. Immunoprecipitations were performed with antiphosphotyrosine antibody 4G10, and μ chain was analyzed by immunoblotting. Immunoblots show results from 1 healthy donor and 6 CLL samples. The immature form (◁) and the mature form (◀) of μ chain are indicated.
Analysis of μ chain–associated phosphoprotein after treatment with anti-μ. Normal B cells (BC7) and CLL cells were treated with anti-μ (+) or isotype control (−) for 2 minutes. Immunoprecipitations were performed with antiphosphotyrosine antibody 4G10, and μ chain was analyzed by immunoblotting. Immunoblots show results from 1 healthy donor and 6 CLL samples. The immature form (◁) and the mature form (◀) of μ chain are indicated.
In some patients (4 of 10), illustrated for U394 and M344 (Figure 3), a band of phosphorylation-associated μ chain was detectable in samples not exposed to anti-μ. This provides suggestive evidence of constitutive BCR-mediated signaling in some patients with CLL. Phosphorylation appears to be associated with the immature glycoform in U394, but was evident in both glycoforms in M344.
The signal competence of both glycoforms of sIgM in CLL is consistent with the fact that the level of signaling, as measured by [Ca2+]i flux, showed no correlation with the ratio of mature to immature forms of surface μ chains (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In this cohort, because patients expressing very low levels of surface μ chain were not included (approximately 10% of M-CLL and approximately 5% of U-CLL), the previously reported trend for a higher frequency of responders in U-CLL10,11 was not evident (supplemental Figure 1B). It appears therefore that the ratio (Figure 2A) may be a more discriminating feature between U-CLL and M-CLL than the level of signaling in vitro.
Re-expression of the mature form of sIgM by CLL cells incubated in vitro
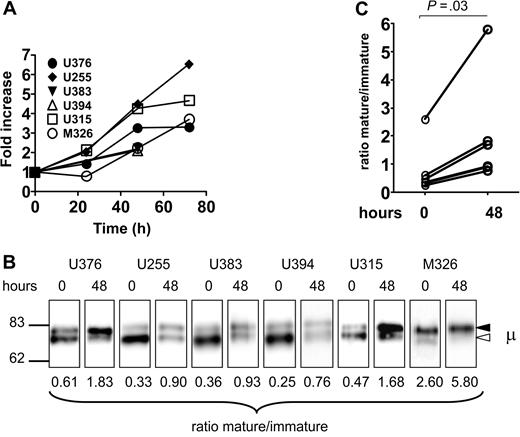
We showed previously that CLL cells can recover expression of sIgM after incubation in vitro.11 To determine the effect of this re-expression on the relative levels of immature and mature forms, we investigated samples in which recovery was detectable by flow cytometry after 48 hours. Increased expression is shown for 6 patients with CLL in Figure 4A. The relative levels of mature versus immature forms of surface μ chains were then assessed, with the results shown in Figure 4B and 4C. In each patient, there was an increase in the ratio over the 48-hour period due to an apparently selective increase of the mature form (top band). This indicates that incubation in vitro restores expression of sIgM mainly via the mature form.
Analysis of sIgM and μ chain glycoform expression after incubation of CLL samples in vitro. (A) CLL samples were incubated in vitro and the expression of sIgM was analyzed by flow cytometry. The graph shows the fold increase of sIgM (MFI), set at 1.0 at time 0, in the 6 samples. (B) Surface proteins were purified from the CLL samples at time 0 and after 48 hours of incubation in vitro, and μ chains were analyzed by immunoblotting. The immature form (◁) and the mature form (◀) of μ chain are indicated. (C) The graph shows the glycoform ratios at time 0 and after 48 hours of incubation. The difference between ratios at 1 and 48 hours are statistically significant (t test, 2-tailed, paired, 99% CI).
Analysis of sIgM and μ chain glycoform expression after incubation of CLL samples in vitro. (A) CLL samples were incubated in vitro and the expression of sIgM was analyzed by flow cytometry. The graph shows the fold increase of sIgM (MFI), set at 1.0 at time 0, in the 6 samples. (B) Surface proteins were purified from the CLL samples at time 0 and after 48 hours of incubation in vitro, and μ chains were analyzed by immunoblotting. The immature form (◁) and the mature form (◀) of μ chain are indicated. (C) The graph shows the glycoform ratios at time 0 and after 48 hours of incubation. The difference between ratios at 1 and 48 hours are statistically significant (t test, 2-tailed, paired, 99% CI).
Expression of the immature form of surface μ chains follows engagement of sIgM
CLL cells.
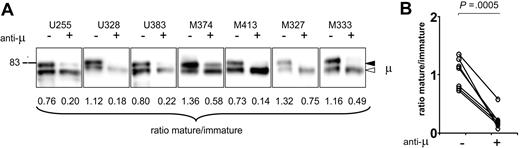
The reciprocal experiment was to investigate the effect of continuous engagement of the sIgM of CLL cells by long-term exposure to anti-μ in vitro. We were concerned that this would affect viability, but levels after 24 hours were 77% (mean value) in 7 patients analyzed, close to that observed in cells treated with an isotype-matched IgG (mean value of 76%). All samples were separated on Lymphoprep before analysis. After 24 hours, it would be expected that the majority of the sIgM will have undergone endocytosis as observed previously.11 However, most of the residual surface μ chains of 7 of 7 patients appeared to be of the immature form (Figure 5A-B) as shown by the decreased ratios. Patients with U-CLL and M-CLL behaved similarly. It appears therefore that engagement of sIgM leads to a selective loss of the mature form, with continued expression of the immature form. The next question was whether this was a CLL-specific feature, or if it represented the response of all B cells to antigen.
Analysis of μ chain glycoform expression in CLL cells after treatment with anti-μ. (A) Surface proteins were purified from 7 CLL samples treated in vitro with anti-μ (+) or isotype control (−) for 24 hours; μ chains were analyzed by immunoblotting. The immature form (◁) and the mature form (◀) of μ chain are indicated. (B) The graph shows the glycoform ratios in anti-μ–treated (+) or isotype control–treated (−) CLL samples measured after 24 hours of incubation in vitro. The difference between ratios between + or − treatments is statistically significant (t test, 2-tailed, paired, 99% CI).
Analysis of μ chain glycoform expression in CLL cells after treatment with anti-μ. (A) Surface proteins were purified from 7 CLL samples treated in vitro with anti-μ (+) or isotype control (−) for 24 hours; μ chains were analyzed by immunoblotting. The immature form (◁) and the mature form (◀) of μ chain are indicated. (B) The graph shows the glycoform ratios in anti-μ–treated (+) or isotype control–treated (−) CLL samples measured after 24 hours of incubation in vitro. The difference between ratios between + or − treatments is statistically significant (t test, 2-tailed, paired, 99% CI).
Normal B cells.
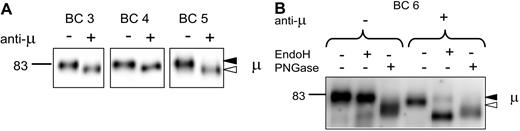
Normal B cells from blood express only the mature fully N-glycosylated form of sIgM (Figure 1A). Samples from 4 healthy donors (BC3, BC4, BC5, and BC6) were selected for study of the effects of treatment with anti-μ. Donors BC3, BC4, and BC6 were also analyzed for expression of CD27+ B cells by flow cytometric analysis and were found to contain 48%, 51%, and 19%, respectively, of this subset in the IgM+ population. All expressed only the mature form of sIgM (Figure 6A), again indicating that this is a feature of both naive and CD27+ memory B-cell subsets. To assess the effect of engagement of the sIgM on the glycan profile, normal B cells were incubated with anti-μ for 24 hours. Viability in this case was 90% compared with 76% treated with isotype-matched control Ig. All the samples of normal B cells showed similar results. Of these, 3 are illustrated in Figure 6A (BC3, BC4, and BC5), where it is clear that all the surface μ chains appear to have been replaced by the faster immature form. In a further sample (BC6), susceptibility to EndoH confirmed this, with the surface μ chains of cells treated with anti-μ, but not with control Ig, becoming susceptible (Figure 6B).
Analysis of μ chain glycoform expression in normal B cells after treatment with anti-μ. (A) Surface proteins were purified from normal B cells of 3 healthy donors treated in vitro with anti-μ (+) or isotype control (−) for 24 hours; μ chains were analyzed by immunoblotting. Data are representative of a total of 5 experiments. (B) Surface proteins from another healthy donor were purified and treated as in panel A for 24 hours. Proteins were then exposed to EndoH or PNGase or left untreated and analyzed by immunoblotting. The immature form (◁) and the mature form (◀) of μ chain are indicated in panels A and B.
Analysis of μ chain glycoform expression in normal B cells after treatment with anti-μ. (A) Surface proteins were purified from normal B cells of 3 healthy donors treated in vitro with anti-μ (+) or isotype control (−) for 24 hours; μ chains were analyzed by immunoblotting. Data are representative of a total of 5 experiments. (B) Surface proteins from another healthy donor were purified and treated as in panel A for 24 hours. Proteins were then exposed to EndoH or PNGase or left untreated and analyzed by immunoblotting. The immature form (◁) and the mature form (◀) of μ chain are indicated in panels A and B.
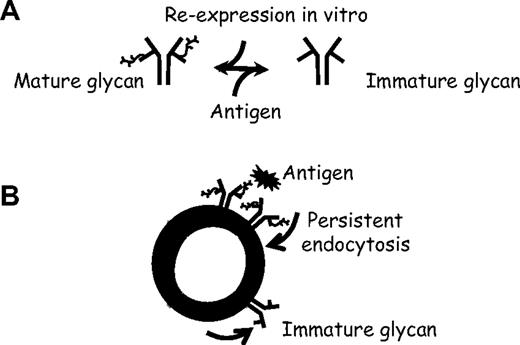
In summary, after ligation of the sIgM, normal B cells undergo conversion of the fully N-glycosylated form of surface μ chain to the immature form. The finding that CLL cells already express this form on isolation from blood indicates that this process, which all B cells appear to be capable of, has been operating in vivo. The changes in glycoforms occurring during perturbation of the sIgM are outlined in Figure 7A, which shows oppositional drives on the BCR sIgM. Antigen drives expression of immature glycan, whereas incubation in vitro in the absence of antigen allows restoration of mature glycan. The cellular context is shown in Figure 7B, indicating that binding of antigen to sIgM (mimicked by treatment with anti-μ) of both normal B cells and CLL cells induces antigen-specific endocytosis. This process, which may be persistent in vivo, apparently acts as a potential stimulus for glycoform modulation to an immature form.
Reversible antigen-driven modulation of sIgM in B cells. (A) Oppositional changes in μ chain glycoforms mediated either by antigen (toward immature form) or by re-expression in vitro (toward mature form). (B) Hypothetical model of the effect of antigen on sIgM of B cells in vivo, suggesting that persistent exposure to antigen, followed by endocytosis, drives expression of immature glycan.
Reversible antigen-driven modulation of sIgM in B cells. (A) Oppositional changes in μ chain glycoforms mediated either by antigen (toward immature form) or by re-expression in vitro (toward mature form). (B) Hypothetical model of the effect of antigen on sIgM of B cells in vivo, suggesting that persistent exposure to antigen, followed by endocytosis, drives expression of immature glycan.
Discussion
The BCR has a critical influence on the fate of both normal B cells and of the majority of B-cell malignancies.1,33 Not only does it mediate tonic survival signals,34 but binding and presentation of antigen leads to proliferation and differentiation, processes which depend on interaction with CD4+ T cells.4 The fate of antigen-specific B cells appears to be determined by integration of multiple signals from antigen and from the microenvironment.35 B-cell malignancies opportunistically retain the ability to exploit these signals, and the sIg is the key molecule involved. For CLL, this is underlined by the fact that the 2 clinical subsets are distinguished by the nature of the sIg.
It is now clear that the sIgM of many cases of CLL cells is interacting with antigen in vivo. The consequence of this is down-regulation of expression, apparently more marked in M-CLL, which is associated with a reduced ability to respond to ligation of sIgM in vitro.11 This anergic status can be partly mimicked in mouse models using transgenic B cells directed against a single antigen (HEL) and crossed with an HEL-expressing strain.36 The setting there is of continuous confrontation of the B cells with constitutively expressed antigen, and the surviving B cells are anergic.26 The effects of this on the totality of B-cell IgM were to slow biosythesis and to convert it to the “immature” form normally located in the ER.
Immaturity is defined as having highly mannosylated glycans, which are susceptible to removal by EndoH. All glycoproteins, including IgM μ chains, generally acquire mature glycans during transit through the cell via the Golgi stacks by addition of further sugar chains. Complex glycans are the rule for surface or secreted glycoproteins.29 This is the case for μ chain, where the majority of the 5 N-glycosylation sites in serum IgM carry complex glycans.27 A very small proportion have mannosylated sites, and these are relatively inaccessible.27 In the double-transgenic tolerizing mouse model, B-cell anergy was associated with an increased level of immature forms, leading to the conclusion that the sIgM was trapped in the ER.26 A similar conclusion was reached from a study of CLL cells, which paralleled the mouse model in showing increased total levels of immature μ chains compared with normal B cells.24,25 However, restriction of the analysis to total cellular IgM did not allow discrimination between events at the cell surface and in the intracellular sites.
The present data support the finding of a relative increase in immature sIgM in CLL cells, compared with normal B cells. However, it is now possible to separate sIgM from intracellular IgM, and this reveals that the immature form is not trapped in the ER, but transits to the cell surface. The surface immature form was found at variable levels in 34 patients with CLL studied, but clearly dominates in U-CLL. Reversibility to mature form by incubation in vitro is consistent with an influence of BCR engagement in vivo. This was supported by the observation that normal blood B cells of both naive and memory subsets generate the surface immature form after ligation of sIgM in vitro. These are likely to include the precursors at least of U-CLL.37 Although we ensured that only viable cells were being studied, we did consider if the minor levels of apoptosis detected could contribute to glycan modification. However, the fact that re-expression or endocytosis in vitro, both procedures which stress normal or CLL B cells, are modified in opposite directions, argues against any contribution from apoptotic mechanisms.
Transient induction of mannosylated surface μ chains appears to be a result of engagement of sIgM in normal and malignant B cells and raises the question of whether this is simply a consequence of endocytosis and re-expression of sIgM, or if there might be a function for this form. An interesting parallel with other B-cell malignancies suggests that expression of mannosylated Ig may be important, but that it is arrived at by different strategies. In follicular lymphoma, N-glycosylation sites are introduced into the Ig variable regions during somatic mutation and, in contrast to normal B cells, they are present in 80% to 100% of patients, highly indicative of positive selection.38,39 Analysis of the glycans revealed that they are of the immature mannosylated form.40 Because the Ig variable regions of CLL do not acquire these sites, even though M-CLL undergoes somatic mutation, it was concluded that this constitutive expression was a feature of germinal center lymphomas, perhaps conferring continuing interaction with local lectin-bearing stromal cells. However, it now appears that CLL cells can express mannosylated sIgM transiently, and that this is also a feature of antigen-activated normal B cells. This raises the question of whether transient expression and interaction with lectin-bearing cells of innate immunity in the bone marrow or lymph node sites may contribute to the growth and/or survival of CLL cells.
A possible pathway of events is illustrated in Figure 7. Tumor cells are likely to engage antigen in the tissues where microenvironmental support is available. Endocytosis and conversion of sIgM to a mannosylated glycoform may confer an advantageous interaction with mannose-binding lectins in the site. Intriguingly, both glycoforms of sIgM can mediate signaling as measured by overall tyrosine phosphorylation. It will be important to compare the level of phosphorylation of defined intermediates of the signaling pathway, including p72Syk; this is in progress. Escape from antigen apparently reverses glycoform modulation, as seen in the blood and in vitro, where restoration of fully glycosylated sIgM occurs.
Although numbers of patients analyzed for levels of mannosylated sIgM are relatively low, there appears to be an intriguing relative increase in U-CLL (P = .022). At present, the analysis is technically demanding and will have to be streamlined to look for other correlations. Previously, we and others have reported that the sIgM of patients with U-CLL tends to signal more commonly in vitro than in patients with M-CLL.10,11 The trend was not evident in this relatively small cohort, where we specifically excluded patients expressing very low levels of sIgM. It appears that glycoform conversion is more extensive in U-CLL and raises the question of how this links to variable [Ca2+]i signaling, especially as both glycoforms are capable of mediating signals. The likely explanation is that glycoform conversion arises during endocytosis and antigen presentation. The connection between sIg-mediated signaling and endocytosis in B cells has long been debated.41,42 However, there is evidence for separation of the 2 pathways from the finding that knocking out intracellular mediators such as Vav1/3 isoforms does not affect signaling but inhibits endocytosis.43 It appears that U-CLL and M-CLL mimic their normal B-cell counterparts in dealing with antigen differently, and downstream effects including microenvironmental interactions could affect tumor cell behavior.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs A. S. Duncombe, A. Jacob, H. McCarthy, B. Kennedy, and H. Grech for providing clinical material.
This work was supported by Tenovus UK, Tenovus Solentside, the Experimental Cancer Medicine Center (ECMC) network, and the Kay Kendall Leukemia Fund.
Authorship
Contribution: S.K., K.N.P., G.P., and F.K.S. participated in designing the research; S.K., C.I.M., V.C., and I.W. performed research; S.K., K.N.P., G.P., and F.K.S. collected data; S.K., K.N.P., G.P., V.C., and F.K.S. analyzed and interpreted data; S.K. performed statistical analysis; and S.K., K.N.P., G.P., and F.K.S. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Freda Stevenson, Molecular Immunology Group, Cancer Sciences Division, Southampton University Hospitals Trust, Southampton General Hospital, Tremona Rd, Southampton SO16 6YD, United Kingdom; e-mail: fs@soton.ac.uk.