Although the 3 isoforms of Akt regulate cell growth, proliferation, and survival in a wide variety of cell types, their role in B-cell development is unknown. We assessed B-cell maturation in the bone marrow (BM) and periphery in chimeras established with fetal liver progenitors lacking Akt1 and/or Akt2. We found that the generation of marginal zone (MZ) and B1 B cells, 2 key sources of antibacterial antibodies, was highly dependent on the combined expression of Akt1 and Akt2. In contrast, Akt1/2 deficiency did not negatively affect the generation of transitional or mature follicular B cells in the periphery or their precursors in the BM. However, Akt1/2-deficient follicular B cells exhibited a profound survival defect when forced to compete against wild-type B cells in vivo. Altogether, these studies show that Akt signaling plays a key role in peripheral B-cell maturation and survival.
Introduction
In adults, mature B cells derive from a series of precursors in the bone marrow (BM) and periphery. B-lineage committed pro-B cells in the BM undergo V-DJ recombination at the immunoglobulin (Ig) heavy chain locus, and cells possessing functional heavy chains are selected via the pre-B cell receptor (pre-BCR) to generate pre-B cells.1 The majority of Ig light chain rearrangements occur in pre-B cells, and cells with productive light chain rearrangements yield immature B-cell receptor–positive (BCR+) B cells.2 Newly formed BCR+ cells in the BM either die or mature further after entering peripheral lymphoid tissues such as the spleen.3 One outcome of peripheral B-cell maturation is the selection of recently formed “transitional” B cells into functionally distinct B-cell subpopulations. Whereas the bulk of surviving transitional B cells yield follicular B cells, so named because of their enrichment in B cell–rich follicles in the spleen and lymph nodes, small numbers of transitional B cells differentiate into marginal zone (MZ) or B1 B cells, the main sources of antibody to T cell–independent bacterial pathogens.4,5
The Akt family of serine/threonine kinases is expressed in 3 distinctly coded isoforms termed Akt-1, Akt-2, and Akt-3.6 All 3 proteins share similar functions and structures,7 and are known to enhance cellular metabolism and positively regulate cell survival and proliferation by activating numerous downstream biochemical pathways.8 Activation of Akt requires phosphatidylinositol-3 kinase (PI-3K), a heterodimeric lipid kinase recruited to the plasma membrane upon ligation of a variety of surface receptors.9 In B cells, PI-3K and Akt are activated upon ligation of a BCR coreceptor complex consisting of CD19, CD81, or CD21.10,–12 Interestingly, mice lacking CD19 or the catalytic subunit of PI-3K fail to develop MZ or B1 B cells,13,–15 suggesting that the CD19/PI-3K pathway optimizes BCR-mediated peripheral B-cell maturation and selection. Consistent with this model, aggregation of the BCR leads to CD19-dependent phosphorylation and activation of Akt.11 However, the CD19/PI-3K pathway activates several additional signaling pathways with important roles in BCR-mediated B-cell activation. Therefore, whether the Akt pathway plays an essential role in peripheral B-cell maturation and selection remains unclear.
Recent observations show that Akt activity is required for early stages of T-cell development in the thymus. Specifically, thymocyte progenitors lacking Akt1 and Akt2 were unable to effectively transit the DN3 to double-positive transition,16,17 which is characterized by a robust proliferative burst mediated by the pre-T cell receptor.18 Because pro-B cells with functional IgH rearrangements undergo analogous pre-BCR–mediated proliferative and differentiative events to generate pre-B cells,1 Akt signaling may also promote progression through the pro- to pre-B cell transition in the BM. To address these issues, we assessed the generation of B-lineage precursors and mature B-cell subsets in the BM and periphery of chimeric mice established with various degrees of deficiency in Akt1 and/or Akt2. Our data illustrate that although Akt1/2-deficient pro-B cells readily generate downstream precursors, mature B-cell survival and selection of cells into the MZ and B1 subpopulations are substantially compromised in the absence of Akt1 and Akt2.
Methods
Mice
Chimeras
As recently described,16 fetal liver cells from B6.Ly5SJL or B6-backcrossed (Ly5B6+) embryos lacking 1 or 2 alleles of Akt1 and/or Akt2 were harvested at days 14.5 to 16.5 postcoitus, and cultured overnight in α-minimum essential medium with 20% fetal bovine serum, 2.2 g/L sodium bicarbonate, 2mM glutamine, penstrep, 10 ng/mL interleukin-6 (IL-6), 20 ng/mL IL-3, and 100 μg/mL stem cell factor. The resulting cells were genotyped by polymerase chain reaction (PCR), and 2.5 × 105 cells injected intravenously into irradiated (2 doses of 550 rads 4 hours apart) B6.Ly5SJL females. For double chimeras, B6.Ly5SJL progenitors were mixed 50:50 with Akt-deficient cells before injection into irradiated B6.Ly5SJL females. Hosts were maintained on water containing a sulfamethoxazole suspension (400 mg of sulfamethoxazole and 80 mg of trimethoprim per 500 mL of water) for 2 days before and at least 2 weeks after irradiation.
Flow cytometry
For flow cytometric analyses, red blood cell–depleted cell suspensions from BM, spleen, or peritoneal cavity were prepared and stained with optimal dilutions of directly conjugated antibodies in fluorescence-activated cell sorting (FACS) buffer (1× phosphate-buffered saline, 5% bovine serum albumin, 1mM ethylenediaminetetraacetic acid) before analysis using a 14-color LSR II flow cytometer (BD Biosciences) equipped with 4 lasers for excitation of ultraviolet-, violet-, blue-, and red-excited dyes. Cells (1-2 × 106) were first incubated on ice for 15 minutes with FcR Block (2.4G2; Pharmingen), and then stained with optimal dilutions of antibodies in a 96-well round-bottom plates in a final volume of 50 μL. Antibodies used included fluorescein–, phycoerythrin (PE)–, PE–cyanin 5.5 (Cy5.5)–, PE-Cy7–, allophycocyanin (APC)–, APC-Cy5.5–, APC-Cy7–, APC-Alexa750–, or biotin (BI)–conjugated versions of monoclonal antibodies to the following cell surface antigens: B220 (RA3-6B2), CD43 (S7), CD23 (B3B4), CD21/35 (7G6), IgM (331), CD5 (53-7.3), Ly5B6 (104), Ly5SJL (A20), CD11b (M1/70), Gr-1 (8C5), F4/80 (BM8) Ter-119, CD3 (2C11), CD127/IL-7Rα (A7R34), CD135/Flt3 (A2F10), CD117/c-kit (2B8), C1qR/AA4 (AA4.1), Sca-1/Ly6 A/E (E13-161.7), and CD19 (6D5). Polyclonal fluorescein-conjugated Fab goat–anti-IgM antibodies (Jackson ImmunoResearch) were also used. BI-conjugated antibodies were revealed with streptavidin coupled to PE-TexasRed (Caltag). All directly conjugated antibodies were purchased from eBiosciences except for APC-Cy5.5 CD19 (Caltag), APC-Cy7 B220 (Pharmingen), and AA4, CD21/35, and TER-119, which were purified and conjugated by standard methods in our laboratory. Nonviable cells were excluded from all analyses by staining with the ultraviolet-excited DNA dye DAPI (4,6 diamidino-2-phenylindole). All flow cytometric data were analyzed by uploading files into FlowJo 8.8 (TreeStar Inc). Cell sorting was performed on FACSAria 3 lasers (Becton Dickinson) for excitation of violet-, blue-, and red-excited dyes.
In vitro cultures
Follicular splenic B cells were isolated by positive selection using BI-CD23 antibodies and streptavidin microbeads with (magnetic-activated cell sorting) LS columns (Miltenyi Biotec) according to the manufacturer's instructions. To assess cell division, cells were labeled with 5μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) for 2 minutes in phosphate-buffered saline. Subsequently, 2 × 104 CD23+ cells per well were cultured in a 96-well plate in RPMI 1640 with 10% fetal bovine serum, 2mM l-glutamine, 0.1mM nonessential amino acids, 1× OPI, 55μM β-ME, and 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES). Cells were stimulated with F(ab′)2 goat anti–mouse IgM (Jackson ImmunoResearch) or lipopolysaccharide (LPS; Sigma-Aldrich). Cells were cultured for 3 days before harvesting and assessing CFSE dilution on a FACSCanto (Becton Dickinson).
Quantitative RT-PCR
Cells from at least 3 C57BL/6 mice were pooled and 1 × 105 cells from each respective population sorted on a FACSAria. Tissue samples from liver and brain were not sorted but isolated in bulk. QIAGEN RNeasy Mini Kits were used to isolate RNA, and RNA was reverse transcribed using first-strand cDNA synthesis kit (GE Healthcare). Quantitative reverse-transcription–PCR (qRT-PCR) was performed using prevalidated Taqman primer/probes set for Akt1, Akt2, and Akt3 with 18s used as an endogenous control (Applied Biosystems). Each primer/probe set efficiently amplified control cDNA samples. Data were normalized to respective Akt isoform expression in follicular B cells (Figure 6A). In addition, Δ–threshold cycle (Ct) values were calculated by subtracting the average Ct for the 18s primer/probe for each population from the respective Akt isoform (Figure 6B). For Figure 6C, Akt1−/−Akt2−/− B-cell populations were sorted and Akt3 transcript levels compared with the corresponding population from wild-type mice.
Statistical analysis
Results
Early B-cell development without Akt1 and Akt2
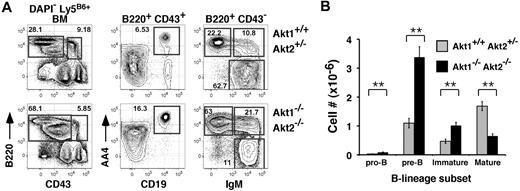
Mice lacking Akt1 or Akt2 alone exhibited no significant differences in BM or splenic B-cell development compared with littermate controls (not shown). To assess the role of Akt1 and Akt2 in B-cell development, we transferred fetal liver cells carrying null mutations for Akt1 and/or Akt2 into lethally irradiated Ly5 congenic hosts, and allowed 12 to 14 weeks for full reconstitution. Use of the fetal liver chimeras was required because of the neonatal lethal phenotype of Akt1−/−Akt2−/− (Akt1/2 DKO) mice.16 Although combined expression of Akt1 and Akt2 is required for thymopoiesis beyond the pre-TCR checkpoint, in the BM Akt1/2 DKO progenitors readily generated pre-BCR-dependent pre-B cells as well as downstream immature B cells (Figure 1A). Indeed, if anything, frequencies of Akt1/2 DKO donor-derived pro- (B220low CD43+ AA4+ CD19+), pre- (B220low CD43− AA4+ CD19+ sIgM−), and immature (B220low CD43− AA4+ CD19+ sIgM+) BM B cells were elevated. Numbers of pro-, pre-, and immature B cells was increased approximately 3-, 4-, and 2-fold, respectively, compared with chimeras reconstituted with Akt1+/+Akt2+/− progenitors (Figure 1B). In contrast, frequencies and cell numbers of mature recirculating BM B cells derived from Akt1/2 DKO progenitors were decreased significantly compared with Akt1+/+Akt2+/− progenitor controls (Figure 1A-B). We conclude that the combined expression of Akt1 and Akt2 is not required to generate pre-B and immature B cells.
Akt-deficient progenitors generate marrow B-lineage precursors. (A) Representative flow cytometric analysis of BM cells from chimeras established with Akt1+/+Akt2+/− (top) or Akt1−/−Akt2−/− (bottom) progenitors 12 to 14 weeks previously. The left-most plots are gated on viable donor-derived (DAPI− Ly5B6+) cells. Numbers in plots indicate the frequency of events in the indicated gate as a function of the indicated parent population. Pro-B, CD43+ B220+ CD19+ AA4+; pre-B, CD43low B220+ IgM− AA4+; immature B, CD43− B220+ IgM+ AA4+; mature B, CD43− B220+ IgM+ AA4−. (B) Absolute cell numbers for respective BM populations were calculated using the gates shown in panel A. Error bars indicate the SEM for each group; n = 5. Data are representative of 2 separate experiments. *P ≤ .01.
Akt-deficient progenitors generate marrow B-lineage precursors. (A) Representative flow cytometric analysis of BM cells from chimeras established with Akt1+/+Akt2+/− (top) or Akt1−/−Akt2−/− (bottom) progenitors 12 to 14 weeks previously. The left-most plots are gated on viable donor-derived (DAPI− Ly5B6+) cells. Numbers in plots indicate the frequency of events in the indicated gate as a function of the indicated parent population. Pro-B, CD43+ B220+ CD19+ AA4+; pre-B, CD43low B220+ IgM− AA4+; immature B, CD43− B220+ IgM+ AA4+; mature B, CD43− B220+ IgM+ AA4−. (B) Absolute cell numbers for respective BM populations were calculated using the gates shown in panel A. Error bars indicate the SEM for each group; n = 5. Data are representative of 2 separate experiments. *P ≤ .01.
Akt1-2 deficient precursors fail to generate MZ B cells
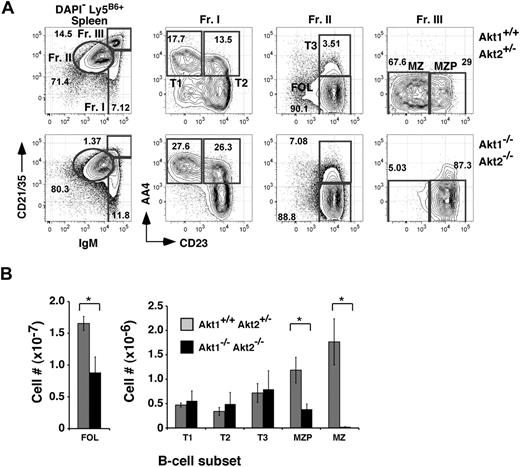
Because numbers of Akt1/2 DKO mature B cells in the BM were decreased, we assessed the peripheral B-cell compartments in Akt1/2 DKO reconstituted animals. In chimeras established with Akt1/2 DKO progenitors, the donor-derived follicular B-cell pool was decreased only 2-fold compared with chimeras generated with Akt1+/+Akt2+/− progenitors. Furthermore, frequencies of AA4+ transitional B-cell subpopulations, defined by differential expression levels for CD23 and IgM (termed T1, T2, and T3),21 were largely unaffected. In contrast, the development of MZ B cells was highly dependent on Akt, as we were unable to detect donor-derived CD21highIgMhighCD23low MZ B cells in chimeras established with Akt1/2 DKO progenitors (Figure 2). Numbers of splenic MZ B-cell precursors (MZPs), considered immediate precursors for MZ B cells,22 were decreased 3-fold compared with control chimeras (Figure 2B). Analyses of mice lacking either Akt1 or Akt2 exhibited a normal MZ B-cell population, suggesting that Akt1 and Akt2 perform overlapping functions required for MZ B-cell development. Altogether, these data show that MZ B-cell development is critically dependent on the combined expression of Akt1 and Akt2.
MZ B-cell development is highly dependent on Akt1/2. (A) Representative analysis of splenocytes from chimeras established with Akt1+/+Akt2+/− (top) or Akt1−/−Akt2−/− (bottom) progenitors 12 to 14 weeks previously. Viable donor-derived cells were gated as in Figure 1. Numbers in plots show the frequency of events as a function of the indicated parent gate. (B) Absolute cell numbers for respective splenic B-cell subsets were calculated using the gates shown in panel A. Error bars indicate the SEM for each group; n = 5. Data are representative of 2 separate experiments. *P ≤ .05.
MZ B-cell development is highly dependent on Akt1/2. (A) Representative analysis of splenocytes from chimeras established with Akt1+/+Akt2+/− (top) or Akt1−/−Akt2−/− (bottom) progenitors 12 to 14 weeks previously. Viable donor-derived cells were gated as in Figure 1. Numbers in plots show the frequency of events as a function of the indicated parent gate. (B) Absolute cell numbers for respective splenic B-cell subsets were calculated using the gates shown in panel A. Error bars indicate the SEM for each group; n = 5. Data are representative of 2 separate experiments. *P ≤ .05.
Defective B1 B-cell development in Akt1/2-deficient chimeras
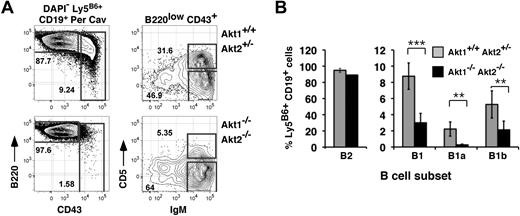
Given that mice lacking CD19 or the catalytic subunit of PI-3K, p110δ, fail to generate MZ and B1 B cells,13,–15 we also analyzed frequencies of donor-derived B1 B cells in the peritoneal cavity. As shown (Figure 3), whereas animals reconstituted with Akt1+/+Akt2+/− progenitors generated abundant numbers of CD19+ CD43+ B1 peritoneal cavity B cells, the donor-derived B1 B-cell pool was detectable but significantly diminished in chimeras established with Akt1/2 DKO progenitors. Furthermore, frequencies of CD5+ B1a cells were severely decreased, whereas CD5− CD43+ B1b cells were reduced only 2-fold. We conclude that the generation of B1a B cells is also highly dependent on the Akt pathway.
Requirement for Akt1/2 for B1 B-cell development. (A) Representative analysis of peritoneal cavity lymphocytes from chimeras established with Akt1+/+Akt2+/− (top) or Akt1−/−Akt2−/− (bottom) progenitors 12 to 14 weeks previously. The leftmost plots are gated on viable donor-derived B cells (DAPI− Ly5B6+ CD19+). Numbers in plots show the frequency of events as a function of the indicated parent gate. (B) Average frequencies for the indicated subsets were calculated using the gates shown in panel A. B2, CD19+ B220+ CD43−; B1, CD19+ B220+ CD43+; B1a, CD19+ B220+ CD43+ CD5+; B1b, CD19+ B220+ CD43+ CD5−. Error bars indicate the SEM for each group; n = 5. Data are representative of 2 separate experiments. **P ≤ .01; ***P ≤ .005.
Requirement for Akt1/2 for B1 B-cell development. (A) Representative analysis of peritoneal cavity lymphocytes from chimeras established with Akt1+/+Akt2+/− (top) or Akt1−/−Akt2−/− (bottom) progenitors 12 to 14 weeks previously. The leftmost plots are gated on viable donor-derived B cells (DAPI− Ly5B6+ CD19+). Numbers in plots show the frequency of events as a function of the indicated parent gate. (B) Average frequencies for the indicated subsets were calculated using the gates shown in panel A. B2, CD19+ B220+ CD43−; B1, CD19+ B220+ CD43+; B1a, CD19+ B220+ CD43+ CD5+; B1b, CD19+ B220+ CD43+ CD5−. Error bars indicate the SEM for each group; n = 5. Data are representative of 2 separate experiments. **P ≤ .01; ***P ≤ .005.
Diminished proliferation to BCR aggregation without Akt1/2
Previous reports suggest that both B1a and MZ B-cell development require optimal selection via the BCR. In addition, past studies suggest that one common consequence of BCR aggregation is activation of Akt isoforms.23 We therefore assessed BCR-mediated activation and proliferation in Akt1/2-deficient B cells. We isolated and stimulated CFSE-labeled CD23+ splenic B cells from Akt1/2 DKO or control reconstituted chimeras with increasing concentrations of anti-IgM antibodies. After 3 days, Akt1/2-deficient B cells exhibited decreases in the number of cell divisions induced by IgM aggregation and markedly decreased survival as assessed by uptake of the DNA dye 7-amino-actinomycin D (7AAD; Figure 4A), resulting in significant decreases in numbers of viable B cells (Figure 4B). Furthermore, although the number of cell divisions occurring in response to the TLR-4 agonist lipopolysaccharide (LPS) was largely unchanged between Akt1/2 DKO and Akt1+/+Akt2+/− B cells, overall cell recoveries for Akt1/2 DKO follicular B cells were significantly reduced, likely due to increased cell death (Figure 4). These data suggest that, whereas cellular survival in vitro is diminished without Akt1 and Akt2 regardless of culture conditions, optimal BCR-mediated B-cell proliferation requires Akt1 and Akt2.
Akt1/2-deficient B cells exhibit a defective BCR-mediated proliferative response. (A) CFSE-labeled CD23+ follicular B cells were left unstimulated (top plots) or stimulated with 50 mg/mL anti-IgM antibodies (middle) or 1 μg/mL LPS (bottom) for 3 days, stained with the viability dye 7AAD, and analyzed by flow cytometry. The right-most overlay histograms were gated on viable (7AAD−) cells using the gates indicated in the corresponding plots. Black line indicates Akt1−/−Akt2−/−; gray filled curves, Akt1+/+Akt2+/−. (B) Mean numbers of viable Akt1−/−Akt2−/− or Akt1+/+Akt2+/− B cells recovered from triplicate cultures stimulated with the indicated concentrations of anti-IgM antibodies or LPS were calculated by flow cytometry using the 7AAD− gates shown in panel A. Error bars indicate SEMs from 4 animals per group. Data are representative of 2 separate experiments. ***P ≤ .005.
Akt1/2-deficient B cells exhibit a defective BCR-mediated proliferative response. (A) CFSE-labeled CD23+ follicular B cells were left unstimulated (top plots) or stimulated with 50 mg/mL anti-IgM antibodies (middle) or 1 μg/mL LPS (bottom) for 3 days, stained with the viability dye 7AAD, and analyzed by flow cytometry. The right-most overlay histograms were gated on viable (7AAD−) cells using the gates indicated in the corresponding plots. Black line indicates Akt1−/−Akt2−/−; gray filled curves, Akt1+/+Akt2+/−. (B) Mean numbers of viable Akt1−/−Akt2−/− or Akt1+/+Akt2+/− B cells recovered from triplicate cultures stimulated with the indicated concentrations of anti-IgM antibodies or LPS were calculated by flow cytometry using the 7AAD− gates shown in panel A. Error bars indicate SEMs from 4 animals per group. Data are representative of 2 separate experiments. ***P ≤ .005.
The Akt pathway promotes competitive fitness of mature B cells in vivo
Past work illustrates that mature B cells lacking CD19 or the tec tyrosine kinase Btk are unable to compete against wild-type (WT) B cells in vivo.24,–26 This feature correlates with the inability of mature B cells from these mutants to undergo cell division upon BCR aggregation in vitro. Given the suboptimal survival of Akt1/2 DKO B cells in response to BCR cross-linking, we reasoned that Akt1/2 DKO B cells might also survive poorly when forced to compete against WT B cells. To test this possibility, we generated double chimeras in which equal numbers of C57BL/6 (Ly5B6) backcrossed Akt1/2 DKO or control Akt1+/+Akt2+/− fetal liver progenitors were mixed with wild-type progenitors from congenic B6.Ly5SJL mice before transplantation into irradiated B6.Ly5SJL adults. Four months later, recipients were assessed for the representation of Akt1+/+Akt2+/− or Akt1/2 DKO-derived (both Ly5B6+) versus B6.Ly5SJL-derived (Ly5SJL+) B-lineage cells including BM pro- and pre-B cells, immature (AA4+ IgM+) B cells in the BM and spleen, and mature splenic follicular, MZP, and MZ B cells. To control for engraftment of progenitors from each donor, we also assessed the relative contribution of C57BL/6 versus B6.Ly5SJL progenitors to lineage− c-Kit+ Sca1+ (LSK) BM progenitor pool, which contains hematopoietic stem cells and early multipotent progenitors.
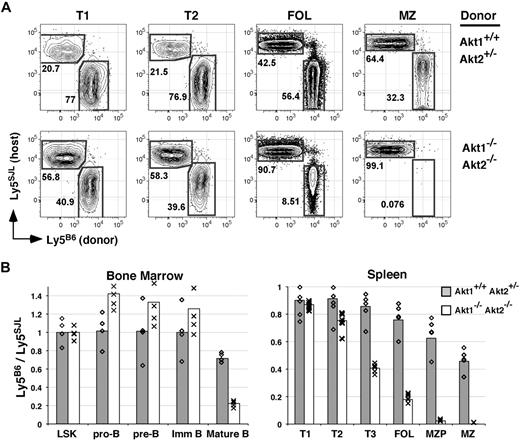
In the BM, Akt1/2-deficient cells were highly competitive with WT cells within both the LSK pool and within all B-lineage precursor populations including immature IgM+ B cells, as the degree of chimerism for each of these populations derived from Akt1/2 DKO progenitors paralleled that observed for Akt1+/+Akt2+/− progenitors (Figure 5B). In sharp contrast, all mature B-cell populations including follicular and MZP B cells in the spleen and mature BM B cells were dramatically underrepresented in Akt1/2 DKO + WT compared with Akt1+/+Akt2+/− + WT double chimeras (Figure 5A-B). In addition, whereas Akt1/2 DKO and Akt1+/+Akt2+/− cells were equally competitive within the least mature (T1) splenic transitional subset, we noted a partial loss of Akt1/2 DKO B cells within the more mature T2 transitional subset. Furthermore, in additional double chimeras, Akt1−/− or Akt2−/− mature B cells displayed a substantial albeit incomplete inability to compete against WT cells (not shown). Altogether, these data suggest that the Akt pathway initiates cellular processes required for competitive survival as immature B cells transit from the T2 population into mature B-cell pools.
Akt1/2-deficient B cells compete poorly with wild-type B cells. (A) Double chimeras were analyzed at 16 weeks after transplantation. (A) Representation of the indicated B-cell subset derived from WT (Ly5SJL+) or Akt1+/+Akt2+/− (top) or Akt1−/−Akt2−/− (bottom) progenitors. Each subset was gated as shown in Figure 2. (B) The average ratio of cells in the indicated subpopulation derived from Ly5B6+ and Ly5SJL+ progenitors in double chimeras established with Akt1+/+Akt2+/− or Akt1−/−Akt2−/− progenitors was calculated using gates shown in Figures 1 and 2 with 4 or 5 animals per group. Individual mice are indicated by ◇ and ×. LSK indicates Lineage− c-Kit+ Sca-1+. Data are representative of 2 separate experiments.
Akt1/2-deficient B cells compete poorly with wild-type B cells. (A) Double chimeras were analyzed at 16 weeks after transplantation. (A) Representation of the indicated B-cell subset derived from WT (Ly5SJL+) or Akt1+/+Akt2+/− (top) or Akt1−/−Akt2−/− (bottom) progenitors. Each subset was gated as shown in Figure 2. (B) The average ratio of cells in the indicated subpopulation derived from Ly5B6+ and Ly5SJL+ progenitors in double chimeras established with Akt1+/+Akt2+/− or Akt1−/−Akt2−/− progenitors was calculated using gates shown in Figures 1 and 2 with 4 or 5 animals per group. Individual mice are indicated by ◇ and ×. LSK indicates Lineage− c-Kit+ Sca-1+. Data are representative of 2 separate experiments.
Akt isoform expression
Because pre-B cell frequencies were increased and MZ, MZP, and B1 B-cell frequencies decreased upon deletion of Akt1 and Akt2, we next sought to better define the expression of each Akt isoform throughout the B-cell lineage. Our initial analyses sought to address expression of each isoform in each immature and mature B-cell subpopulation. As shown in Figure 6A, Akt1 and Akt3 transcripts were present at 3- to 6-fold higher levels for wild-type pro- and immature B cells in the BM relative to follicular B cells in the spleen. Although Akt3 transcripts were expressed at higher levels in immature relative to follicular B cells, the expression of Akt3 was still lower than brain, a tissue shown previously to express high levels of Akt3.27 Interestingly, Akt1 and Akt3 expression appeared to be down-regulated as pro-B cells give rise to pre-B cells, up-regulated in immature B cells, and again down-regulated as immature B cells give rise to mature B cells. In addition, relative to follicular B cells, transcript abundance for Akt1 and Akt3 was decreased further in MZ B cells and their immediate precursors (MZPs) in the spleen, and in both peritoneal cavity B1 subpopulations. These observations contrast with the expression pattern for Akt2, which did not vary substantially among any of the B-lineage subsets examined (Figure 6A) and was expressed at lower overall levels compared with Akt1 and Akt3.
Expression of Akt isoforms in B-lineage cells. (A) Relative Akt isoform expression in sorted BM, splenic, and peritoneal B-cell populations measured by qRT-PCR. Data are expressed relative to wild-type follicular B cells. Brain and liver cDNA serve as positive controls for Akt3 and Akt2 mRNA, respectively. (B) Δ-Ct values for Akt1, Akt2, and Akt3 relative to endogenous control 18s, within each of the indicated B-cell populations as in panel A. (C) Akt3 transcript abundance in sorted B-cell populations from Akt1/2 DKO chimeras relative to the corresponding wild-type population. Error bars represent relative quantity maximum and minimum for each sample (A,C) or SD of Δ-Ct for each sample (B).
Expression of Akt isoforms in B-lineage cells. (A) Relative Akt isoform expression in sorted BM, splenic, and peritoneal B-cell populations measured by qRT-PCR. Data are expressed relative to wild-type follicular B cells. Brain and liver cDNA serve as positive controls for Akt3 and Akt2 mRNA, respectively. (B) Δ-Ct values for Akt1, Akt2, and Akt3 relative to endogenous control 18s, within each of the indicated B-cell populations as in panel A. (C) Akt3 transcript abundance in sorted B-cell populations from Akt1/2 DKO chimeras relative to the corresponding wild-type population. Error bars represent relative quantity maximum and minimum for each sample (A,C) or SD of Δ-Ct for each sample (B).
Given that past studies have shown that Akt3 is expressed in unfractionated splenic B cells,16 and pre-B cell development in wild-type and Akt1/2 DKO precursors may be highly dependent on Akt3, we sought to further quantify overall expression of each Akt isoform for each B-lineage population relative to 18s RNA. To this end, for each B-lineage population we divided the mean signal (Ct) for each Akt isoform by the mean Ct for 18s RNA, thus yielding a Δ-Ct for each Akt isoform within each population. As shown in Figure 6B, whereas Akt1 and Akt3 are expressed at similar levels among BM and peripheral B-cell subsets, Akt2 is expressed at substantially lower levels (resulting in a significantly higher Δ-Ct) in every population. In contrast, samples from mouse liver, a source of abundant Akt2 transcripts,28 demonstrated significantly lower Δ-Ct values.
Finally, to address whether Akt3 expression increases in B-lineage cells such as pre-B cells lacking Akt1 and Akt2, we compared Akt3 transcript abundance in wild-type and Akt1/2 DKO B-lineage cells. As shown in Figure 6C, Akt3 expression was only modestly increased (1.6-fold) in Akt1/2 DKO pre-B cells compared with their wild-type counterparts. Interestingly, whereas Akt3 transcript expression was unchanged in most other B-lineage cells, Akt3 transcripts were increased 3-fold in Akt1/2 DKO MZPs compared with wild-type MZPs (Figure 6C). Thus, although the absence of Akt1 and Akt2 in MZPs appears to result in increased mRNA levels for Akt3, this increase is insufficient to rescue MZ B-cell development. Altogether, these data show that although Akt3 mRNA is expressed at substantial levels in developing and mature B cells, Akt3 expression alone is unable to drive MZ and B1 B-cell development and follicular B-cell competitive fitness.
Discussion
Unraveling the biochemical processes underlying B-cell maturation and selection is essential for understanding both normal and neoplastic differentiation within the B-cell lineage. Past work describing discrete B-lineage precursors and functionally unique BCR+ subpopulations has provided a framework for understanding immune system defects in the context of normal B-cell development, and has raised questions about how newly formed B cells are selected to mature into the follicular, MZ, or B1 compartments. In this study, we identify a role for the Akt serine/threonine kinase family in this process.
Given that Akt activity promotes a variety of essential biochemical processes,6 it was noteworthy that Akt1/2 deficiency affected the development of peripheral B-cell subpopulations without resulting in the loss of B-lineage precursors in the BM. Indeed, numbers of pre-B and immature B cells in the BM were slightly elevated when generated from Akt1/2 DKO progenitors (Figure 1). In contrast, the generation of MZ B cells was highly dependent on the Akt pathway, as we were unable to detect even small numbers of these cells after reconstitution with Akt1/2 DKO progenitors (Figure 2). Frequencies of B1a B cells were also substantially reduced (Figure 3). Therefore our data show that MZ and B1 B cells are especially dependent on Akt1 and Akt2.
The BLyS/BAFF cytokine family also plays an essential role in peripheral B-cell survival,29 and recent evidence suggests that BCR-mediated signals may enhance responsiveness of B cells to BLyS by regulating cross talk between the canonical and noncanonical nuclear factor κB (NF-κB) pathways.30 Thus, one possibility is that BCR-mediated Akt signaling increases sensitivity of B cells to BLyS. Alternatively, BR-3, a main prosurvival BLyS receptor expressed by follicular B cells, may enhance B-cell longevity by activating the PI-3K/Akt pathway directly. The latter possibility is supported by data showing that PI-3K inhibitors prevent BLyS-induced Akt phosphorylation and survival of cultured B cells.31 Although both models could explain the failure of follicular B cells to thrive in mixed chimeras, 2 lines of reasoning suggest that the failure of Akt1/2-deficient progenitors to generate B1a and MZ B cells cannot be explained by diminished BLyS responsiveness alone. First, maintenance of B1 cells does not require BR-3.32 Second, although MZ B cells are not detected in BR-3 mutant mice, the MZ B-cell pool is restored through retroviral expression of Bcl-xL of BR-3 mutant progenitors.33 However, we were unable to restore MZ B-cell production from Akt1/2-deficient precursors through enforced Bcl-xL expression (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Therefore, we suggest that Akt plays additional MZ and B1 B cell–specific functions beyond promoting their survival via the BCR and BLyS pathways.
Surprisingly, we were unable to restore the MZ B-cell pool by introducing retrovirus for the highly antiapoptotic Bcl-2 family member Bcl-xL into Akt1/2-deficient progenitors (supplemental Figure 1). This result contrasts with past experiments showing that MZ B-cell development in CD19−/− mice is restored through ectopic expression of Bcl-2, the founding antiapoptotic member of the Bcl-2 family.24 Thus, although Akt activation is clearly downstream of the CD19/PI-3K pathway in B cells, it is tempting to speculate that Akt proteins play additional roles in peripheral B cells beyond cellular survival and that additional receptor-ligand interactions may activate the Akt pathway in B cells. With regard to complementation strategies involving enforced expression of Bcl-2 family members, in our view our retroviral system is a highly effective approach for testing whether loss of a specific cell population reflects increased apoptosis, as Bcl-xL prevents apoptosis rather potently when its expression is enforced in B-lineage cells,34,–36 and in this retroviral system expression of virus-encoded proteins is not subject to lineage and stage-specific regulatory mechanisms. Indeed, although we failed to rescue the Akt1/2-related MZ B-cell defect through enforced Bcl-xL expression, this approach led to robust increases in numbers of mature recirculating Akt1/2-deficient B cells in the BM (supplemental Figure 1).
Although numbers of Akt1/2 DKO follicular B cells declined only 2-fold relative to wild-type cells in separate animals, Akt1/2-deficient follicular B cells exhibited a clear inability to survive when forced to compete with WT cells in the same animals (Figure 5). Previous work has highlighted the importance of constitutive or “tonic” BCR signaling to maintain peripheral B-cell numbers. Indeed, induced ablation of surface BCR expression or Ig-α, a key component of the BCR signaling complex, leads to rapid loss mature naive B cells.37,38 Interestingly, although follicular B cells lacking CD19 or Btk fail to thrive when forced to compete against WT cells, progenitors lacking CD19, Btk, or Akt1/2 all generate near normal numbers of follicular B cells without competition from WT B cells.24,–26 Why is the prosurvival role for CD19, Btk, and Akt1/2 observed only in vivo through such competition experiments? Assuming that activation of Akt1/2 and Btk in vivo is always CD19 dependent, one possibility is that tonic BCR signaling activates both CD19-dependent and CD19-independent pathways that combine to focus on common prosurvival effector mechanisms.
One argument against the notion of BCR-dependent but CD19-independent prosurvival mechanisms in mature B cells comes from a very recent report by Srinivasan et al showing that enforced expression of a constitutively active form of the catalytic PI-3K subunit P110α promotes peripheral B-cell survival after ablation of the BCR.39 This work illustrates the critical role played by the CD19/PI-3K pathway in peripheral B-cell maturation and, although not establishing a role for Akt proteins in peripheral B-cell homeostasis directly, is consistent with our data on the role of the Akt pathway in these processes. Notably, Srinivasan et al also showed that activating the canonical NF-κB pathway is insufficient to rescue mature B cells upon BCR deletion.39 Therefore, although Akt-mediated NF-κB activity may regulate key events within the pre-B cell pool, it may play a less than prominent role in peripheral B-cell survival as controlled by the proposed BCR/CD19–PI-3K–Akt pathway. Alternatively, given that the Akt pathway is also activated by BR-3 signaling and canonical NF-κB activity allows for BLyS-independent B-cell survival,31,40,41 induced NF-κB activity may restore follicular B-cell survival and/or B1 and MZ B-cell development in the context of Akt deficiency. Further experiments are needed to address these fundamental questions.
Recent data from Derudder et al indicate that NF-κB deficiency leads to increased frequencies of pre-B cells without affecting Ig κ rearrangement.42 Given that Akt signaling regulates NF-κB activity in other contexts, the increased numbers of pre-B cells produced by Akt1/2 DKO progenitors may reflect decreased NF-κB activity in pro- and/or pre-B cells. Thus, experiments testing whether enforced NF-κB activity decreases numbers of pre-B cells and increases numbers of MZ and B1 B cells in the context of Akt deficiency might provide important information concerning the mechanism of Akt-dependent development and selection within B-lineage precursors in the marrow.
Given that Akt regulates several biochemical processes associated with general cellular metabolism, one must speculate whether MZ and B1 B cells possess increased or perhaps unique metabolic requirements. However whether cells found in different B-cell subsets possess unique metabolic requirements has not been addressed. An alternative possibility is that Akt regulates the activity of specific transcription factors that are required for MZ B-cell development. This viewpoint is supported by data showing that enforced Akt activity antagonizes Rag1 and Rag2 expression mediated by the Foxo1 transcription factor.43 Indeed, heightened Foxo1-driven RAG1/2 expression due to decreased Akt activity may explain the observed increase in frequencies in pre-B cells generated from Akt1/2 DKO progenitors (Figure 1). Likewise, Akt1/2 deficiency may deregulate the expression of certain transcription factors required for the MZ B-cell fate. Clearly, additional studies will be required to better understand the role of each Akt isoform in B-cell development and selection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Michael Cancro and Radhika Goenka for helpful discussions and Drs Lakshmi Srinivasan and Klaus Rajewsky for sharing unpublished results. We also gratefully acknowledge the expert technical support in flow cytometry provided by Ryan Wychowanec and all staff members of the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource.
This work was supported by National Institutes of Health grants F31-AI056671 (M.M.J.), RO1-AI063345 (J.R.), R01-DK56886 (M.J.B.), PO1-CA093615 (G.K.), R37-GM053526 (G.K.), and RO1-AG546776 (D.A.), and a Career Development Award from the Leukemia & Lymphoma Society (D.A.).
National Institutes of Health
Authorship
Contribution: M.C. performed research and wrote the paper; M.M.J., M.T., D.L.N., and J.R. performed research; M.J.B. and G.K. provided key reagents and analyzed data; and D.A. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Allman, University of Pennsylvania, 36th & Hamilton Walk, 230 John Morgan Bldg, Philadelphia, PA 19104-6082; e-mail: dallman@mail.med.upenn.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal