Abstract
Interleukin-17A (IL-17A) and IL-17F are 2 of several cytokines produced by T helper 17 cells (Th17), which are able to indirectly induce the recruitment of neutrophils. Recently, human Th17 cells have been phenotypically characterized and shown to express discrete chemokine receptors, including CCR2 and CCR6. Herein, we show that highly purified neutrophils cultured with interferon-γ plus lipopolysaccharide produce the CCL2 and CCL20 chemokines, the known ligands of CCR2 and CCR6, respectively. Accordingly, supernatants from activated neutrophils induced chemotaxis of Th17 cells, which was greatly suppressed by anti-CCL20 and anti-CCL2 antibodies. We also discovered that activated Th17 cells could directly chemoattract neutrophils via the release of biologically active CXCL8. Consistent with this reciprocal recruitment, neutrophils and Th17 cells were found in gut tissue from Crohn disease and synovial fluid from rheumatoid arthritis patients. Finally, we report that, although human Th17 cells can directly interact with freshly isolated or preactivated neutrophils via granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-α, and interferon-γ release, these latter cells cannot be activated by IL-17A and IL-17F, because of their lack of IL-17RC expression. Collectively, our results reveal a novel chemokine-dependent reciprocal cross-talk between neutrophils and Th17 cells, which may represent a useful target for the treatment of chronic inflammatory diseases.
Introduction
Th17 cells, named for their ability to produce interleukin-17A (IL-17A; also known as IL-17), are a novel subset of CD4+ effector T helper (Th) cells that have been recently described. Th17 cells also produce IL-17F, as well as other cytokines and chemokines, including IL-21, IL-22, IL-26, and CCL20 (reviewed in Ouyang et al1 and Tesmer et al2 ). IL-17A and IL-17F belong to a family of cytokines that includes IL-17B, IL-17C, IL-17D, IL-25 (originally known as IL-17E), and the heterodimer IL-17A/F. IL-17A and IL-17F have a high degree of sequence similarity and share many biologic properties, IL-17A being more potent than IL-17F.1-3 The biologic activities of IL-17A and IL-17F are dependent on their binding to a multimeric receptor complex, composed of at least 2 IL-17RA subunits and 1 IL-17RC subunit (the precise stoichiometry being still unknown).3 This, in turn, recruits a cytoplasmic adaptor protein, Act1, which is essential for intracellular signal transduction.4
Recent characterization of human Th17 cells has shown their selective expression of IL-23R, CCR6, CD161, and the transcription factor RORγt.5-7 In addition, Th17 cells share tissue trafficking and homeostatic chemokine receptors, such as CCR2, CCR4, CXCR4, CXCR5, and CXCR6, with other T-cell subsets.7-9 Th17 cells appear to be essential for the pathogenesis of chronic inflammatory diseases, such as psoriasis, Crohn disease (CD), and allergic asthma.10 Interestingly, a preferential CCL20-mediated recruitment of CCR6-expressing Th17 cells into the inflamed joints of rheumatoid arthritis (RA) patients has also been described.11
The activity of IL-17A is classically defined by its ability to induce the expression of a variety of proinflammatory mediators, such as IL-1, IL-6, tumor necrosis factor-α (TNF-α), CXCL8, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) by epithelial, endothelial, and other stromal cells, which ultimately leads to the recruitment and activation of neutrophils.1 Neutrophils are phagocytic cells of the innate immune system that act as the first line of defense against invading pathogens. On activation with different mediators, neutrophils synthesize numerous proteins involved in their effector response, including a variety of chemokines potentially implicated in the recruitment of distinct leukocyte subpopulations.12-14 However, whether neutrophils respond directly to IL-17A and IL-17F remains controversial. Although it has been shown that IL-17A is unable to affect the rate of spontaneous neutrophil apoptosis by itself,15,16 IL-17A seems able to attenuate the antiapoptotic effects of GM-CSF,16 as well as increase pneumococcal killing when present at extremely high concentrations (> 1 μg/mL).17 Based on these premises, the aim of our study was to define whether highly purified populations of human neutrophils are able to directly chemoattract Th17 cells. We also evaluated whether human Th17 cells can directly attract neutrophils and/or modulate their effector functions via IL-17A, IL-17F, or other cytokines.
Herein, we report a direct reciprocal recruitment between activated neutrophils and Th17 cells that is mediated by endogenous chemokines. We also show that both IL-17A and IL-17F are unable to directly modulate neutrophil responses, probably because of their lack of the IL-17RC subunit of the IL-17R complex. However, we show that activated Th17 cells are able to modulate neutrophil responses in an IL-17–independent manner, precisely via the release of GM-CSF, TNF-α, and interferon-γ (IFN-γ).
Methods
Cell purification and culture
Neutrophils were enriched (> 99.7% purity) from Ficoll-Paque–isolated granulocytes (obtained either from peripheral blood of healthy donors or synovial fluid [SF] from RA patients) by positively removing all contaminating cells with mAbs against CD3, CD56, CD19, CD36, CD49d, and Gly-A, using a custom-made EasySep kit (StemCell Technologies).18 Th clones were generated as previously reported.5 Peripheral CD4+ T-cell populations enriched for IL-17+ cells (up to 50%) were obtained by the use of immunomagnetic cell sorting as already described.19 CD4+ T cells were purified from peripheral blood and SF of RA patients by a specific EasySep kit (StemCell Technologies). CD4+ CCR6+ T cells were purified by positive selection of the negatively purified CD4+ T cells labeled with FITC-CCR6 Abs (R&D Systems) using MACS anti-FITC Microbeads (Miltenyi Biotec). Monocytes were isolated (> 95% purity) from peripheral blood mononuclear cells of healthy donors by negative selection using a specific EasySep kit (StemCell Technologies). Neutrophils were suspended in RPMI 1640 medium, supplemented with 10% low endotoxin fetal bovine serum (< 0.5 EU/mL; BioWhittaker-Lonza), usually treated with 100 U/mL IFN-γ (R&D Systems) and/or 100 ng/mL Ultra Pure Escherichia coli lipopolysaccharide (LPS; 0111:B4 strain, from InvivoGen), and/or 5 ng/mL TNF-α (PeproTech), 100 ng/mL IL-17A (purchased from R&D Systems and PeproTech), or IL-17F (R&D Systems), 10 ng/mL GM-CSF (PeproTech), 100nM formyl-methionyl-leucyl-phenylalanine (fMLF; Sigma-Aldrich), and then plated in 6- or 24-well tissue culture plates (Nunc) at 37°C, 5% CO2 atmosphere. Th clones were stimulated for up to 72 hours with anti-CD3 and anti-CD28 mAbs (5 μg/mL, BD Biosciences) or phorbol myristate acetate/ionomycin (Sigma-Aldrich), as previously reported.5 In selected experiments, neutrophils were cultured with supernatants from anti-CD3/anti-CD28-stimulated Th17 clones, preincubated as indicated for 30 minutes with 10 μg/mL Abs neutralizing IL-17 (R&D Systems), TNF-α (B154.2 hybridoma), IFN-γ (B133.3 hybridoma), IL-6, and GM-CSF (PeproTech), or isotype-related Abs (BioLegend). At the indicated times, cell-free supernatants were harvested and stored at −20°C, whereas pelleted cells were subsequently used for flow cytometry, extracted for total RNA or prepared for Western blot analyses. RA (n = 10) and CD (n = 3) patients were enrolled at first diagnosis after providing informed consent in accordance with the ethical standards of the Regional Committee (Tuscany, Italy) on Human Experimentation and the Declaration of Helsinki. All reagents used were of the highest available grade and were dissolved in pyrogen-free water for clinical use. All procedures and experiments in the study were in accordance with the ethical standards of and approved by the Institutional Review Board of the University of Verona and the Regional Committee on Human Experimentation.
Chemotaxis
Chemotaxis was assessed using 96-transwell Boyden chambers of 3- and 5-μm pore size (Corning Costar) for neutrophils and T cells, respectively. Cell suspensions (2 × 106/mL) were added to the top chambers, whereas leukocyte-derived supernatants, preincubated or not for 30 minutes with Abs neutralizing CCL20 (5 μg/mL, R&D Systems), CCL2, CXCL10, and CXCL8 (5 μg/mL, PeproTech) or their isotype-related antibodies (5 μg/mL, BioLegend), were added to the bottom wells. After a 1- or 2-hour incubation for neutrophils and Th clones, respectively, migrated cells were counted with CyQuant cell proliferation assay kit (Invitrogen SRL).
Statistical analysis
The unpaired t test or 1-way analysis of variance with Dunnett multiple comparison test was used for statistical significance evaluation. Data are expressed as mean plus or minus SEM, unless otherwise specified. A P value of less than .05 was considered significant.
All other procedures, performed according to standard methodologies and described in previous publications, are detailed in full and available in supplemental Methods and figures (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
Highly purified human neutrophils produce chemokines that chemoattract Th17 and Th1 cells
Preliminary studies that were performed using human neutrophils isolated by magnetic negative selection yielded a highly purified (> 99.7%) cell population that was devoid of any lymphocytes (CD3+CD16−CCR3−), monocytes, or eosinophils (CD16low CCR3+) that often contaminate Ficoll-Paque isolated granulocytes (supplemental Figure 1A). Such experiments not only confirmed previous observations that neutrophils release maximal levels of CCL20, CCL2, and CXCL10 in response to IFN-γ plus LPS (γL),12-14 but also revealed that they do not produce classic Th2-type recruiting chemokines, including CCL17 or CCL22 (supplemental Figure 1B).
Because CCL2 and CCL20 are potentially involved in the recruitment of Th17 cells,10-12 we evaluated whether supernatants harvested from activated neutrophils could attract Th17 cells in vitro. For this purpose, we initially used peripheral CD4+ T-cell populations enriched for IL-17A–producing cells (Th17),19 but also containing IFN-γ (Th1)– and IL-4 (Th2)–producing cells (Figure 1A). These experiments revealed that supernatants from γL-stimulated neutrophils exhibit a significant chemotactic effect on IL-17A+–enriched cells, whereas the supernatants from IFN-γ– or LPS-stimulated neutrophils only induce a minimal chemotactic effect, comparable with that exerted by unconditioned neutrophil supernatants (Figure 1B). Accordingly, higher percentages of IL-17A– and IFN-γ–, but not IL-4–, producing cells were found to migrate toward γL-conditioned supernatants compared with resting supernatants (Figure 1C). In addition, the T cells recruited by γL-conditioned neutrophil supernatants expressed higher levels of IL-23R and IL-17A mRNAs than T cells migrating in response to supernatants from resting neutrophils, supporting the hypothesis that activated neutrophils possess the ability to induce Th17 cell chemotaxis (Figure 1D).
Activated neutrophils can recruit Th17 and Th1 cells via chemokine release. (A) IL-17A+–enriched cells were evaluated by flow cytometry for intracellular IL-17A, IFN-γ, and IL-4 expression (to determine the percentages of positive cells). (B) Chemotaxis of IL-17A+–enriched cells toward supernatants (supnts) from IFN-γ–, LPS-, and IFN-γ plus LPS–conditioned neutrophils (n = 4). (C) IL-17A+-enriched cells, migrated in response to supernatants from resting (■) and IFN-γ plus LPS (γL)–activated (□) neutrophils (n = 4), were assessed for cytokine production by flow cytometry, on polyclonal stimulation. Bars represent the percentage of cells producing the indicated cytokine (mean ± SEM). (D) Real-time RT-PCR gene expression analysis performed with IL-17A+–enriched cells that have migrated toward supernatants from resting and γL-activated neutrophils. Results are expressed as mean normalized expression (MNE) units ± SE. One representative of 3 independent experiments with similar results is shown. (E-F) Chemotaxis of Th17, Th1, and (where indicated) Th2 clones toward: supernatants from γL-conditioned neutrophils (n ≥ 4; E); γL-conditioned supernatants, preincubated as indicated with αCCL2, αCCL20, and αCXCL10 neutralizing Abs (n = 4; F). *P < .05. ND indicates not done.
Activated neutrophils can recruit Th17 and Th1 cells via chemokine release. (A) IL-17A+–enriched cells were evaluated by flow cytometry for intracellular IL-17A, IFN-γ, and IL-4 expression (to determine the percentages of positive cells). (B) Chemotaxis of IL-17A+–enriched cells toward supernatants (supnts) from IFN-γ–, LPS-, and IFN-γ plus LPS–conditioned neutrophils (n = 4). (C) IL-17A+-enriched cells, migrated in response to supernatants from resting (■) and IFN-γ plus LPS (γL)–activated (□) neutrophils (n = 4), were assessed for cytokine production by flow cytometry, on polyclonal stimulation. Bars represent the percentage of cells producing the indicated cytokine (mean ± SEM). (D) Real-time RT-PCR gene expression analysis performed with IL-17A+–enriched cells that have migrated toward supernatants from resting and γL-activated neutrophils. Results are expressed as mean normalized expression (MNE) units ± SE. One representative of 3 independent experiments with similar results is shown. (E-F) Chemotaxis of Th17, Th1, and (where indicated) Th2 clones toward: supernatants from γL-conditioned neutrophils (n ≥ 4; E); γL-conditioned supernatants, preincubated as indicated with αCCL2, αCCL20, and αCXCL10 neutralizing Abs (n = 4; F). *P < .05. ND indicates not done.
To unequivocally prove that Th17 cells effectively migrate in response to neutrophil-conditioned supernatants, we performed chemotaxis assays using human Th17, Th1, and Th2 clones generated and characterized as previously described5,6 (supplemental Figure 2A). As in the case of IL-17A+–enriched CD4+ T cells, supernatants from resting neutrophils induced a weak but detectable chemotaxis of all 3 clone types. In contrast, supernatants from γL-stimulated neutrophils significantly augmented the chemotaxis of Th17 and Th1, but not Th2, clones (Figure 1E), consistent with the inability of neutrophils to produce CCL17 and CCL22 (supplemental Figure 1B). To identify whether CCL20, CCL2, or CXCL10, all present in γL-conditioned supernatants, was responsible for the increased Th17 and Th1 cell chemotaxis, we preincubated γL-conditioned supernatants with specific neutralizing (or isotype-related) Abs. This allowed us to identify CCL20 and CCL2 as the chemokines mainly responsible for inducing chemotaxis in Th17 clones (Figure 1F). The same approach identified CXCL10 and CCL2 as the chemokines responsible for recruitment of Th1 clones by supernatants harvested from γL-stimulated neutrophils (Figure 1F). In this latter case, Abs neutralizing CCL20 were not used because rCCL20 (up to 100 ng/mL) was unable to induce the recruitment of Th1 cells (data not shown). All isotype control Abs were ineffective in chemotaxis assays (data not shown). Notably, the patterns of chemokine receptors expressed by our Th clones (supplemental Figure 2B) not only correlated with their chemotactic responses (Figure 1F), but also matched data previously described for Th cell subsets derived from adult peripheral blood and tonsils.8 Indeed, Th17 and Th1 clones stained strongly positive for CCR6/CCR2 and CXCR3/CCR2, respectively, whereas Th2 clones were CCR6-, CCR2-, and CXCR3-negative (supplemental Figure 2B). In addition, the majority of Th17 cells expressed both CCR2 and CCR6, whereas Th1 cells were positive only for CCR2 (supplemental Figure 2C). Taken together, our data demonstrate that optimally activated neutrophils release chemokines that can either selectively or redundantly chemoattract distinct Th cell subpopulations: CCL20 and CXCL10 selectively chemoattract Th17 and Th1 cells, respectively, whereas CCL2 recruits both Th17 and Th1 cells.
Activated Th17 cells can directly recruit human neutrophils via endogenous CXCL8
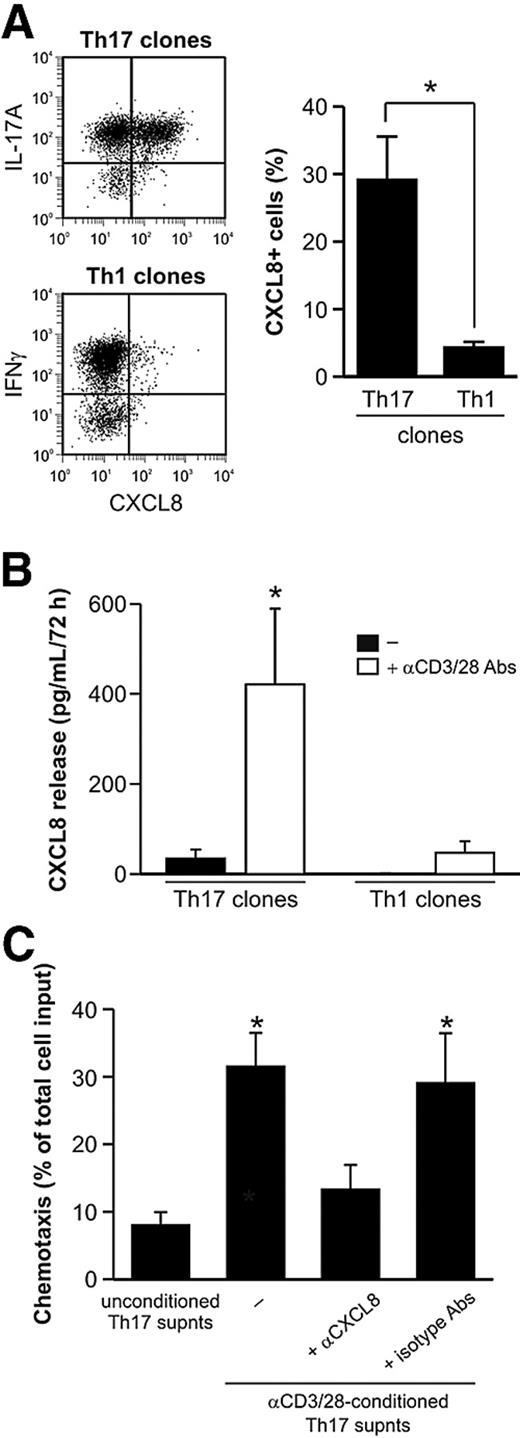
Whereas Th17 cells are well known to indirectly recruit and activate neutrophils via an IL-17A– and IL-17F–mediated induction of CXCL8, CXCL1, and G-CSF by epithelial and other stromal cells,20 it is currently unclear whether they can directly attract neutrophils. Because it was recently shown that CCR6+ T cells from lesional skin of acute generalized exanthematous pustulosis patients can produce CXCL8,21 we examined whether Th17 cells are able to express and secrete functionally active CXCL8. This indeed appeared to be the case, as revealed by the capacity of Th17 but not Th1 clones to both produce and release CXCL8 on activation (Figure 2A-B), Furthermore, we performed chemotaxis assays, which demonstrated that supernatants from anti-CD3/CD28–activated Th17 clones significantly increased the chemotactic response of neutrophils (Figure 2C) compared with their basal chemotaxis toward unconditioned T cell–derived supernatants. Finally, neutrophil migration induced by supernatants derived from activated Th17 clones was greatly suppressed by specific CXCL8-neutralizing, but not isotype-matched, Abs (Figure 2C), indicating that the chemotactic effect of Th17 cell supernatants on neutrophils can be mainly attributed to CXCL8. Collectively, these data demonstrate that human Th17 cells, once activated, promote a direct CXCL8-dependent chemotactic effect on neutrophils.
Activated Th17 clones can recruit neutrophils through CXCL8 release. (A) CXCL8 expression in phorbol myristate acetate/ionomycin-activated Th17 and Th1 clones, as evaluated by representative intracellular staining (left panel) and shown as the mean (± SE) values of percentage of positive cells (Th17: n = 9; Th1: n = 5, right panel). (B) CXCL8 concentrations in supernatants harvested from resting or αCD3/αCD28-stimulated Th17 and Th1 clones (n = 4). (C) Chemotaxis of neutrophils toward supernatants harvested from αCD3/αCD28-stimulated Th17 clones, in the presence or absence of αCXCL8, or related isotype, Abs (n = 4). *P < .05.
Activated Th17 clones can recruit neutrophils through CXCL8 release. (A) CXCL8 expression in phorbol myristate acetate/ionomycin-activated Th17 and Th1 clones, as evaluated by representative intracellular staining (left panel) and shown as the mean (± SE) values of percentage of positive cells (Th17: n = 9; Th1: n = 5, right panel). (B) CXCL8 concentrations in supernatants harvested from resting or αCD3/αCD28-stimulated Th17 and Th1 clones (n = 4). (C) Chemotaxis of neutrophils toward supernatants harvested from αCD3/αCD28-stimulated Th17 clones, in the presence or absence of αCXCL8, or related isotype, Abs (n = 4). *P < .05.
Neutrophils and Th17 cells colocalize in inflamed tissues from CD and RA patients
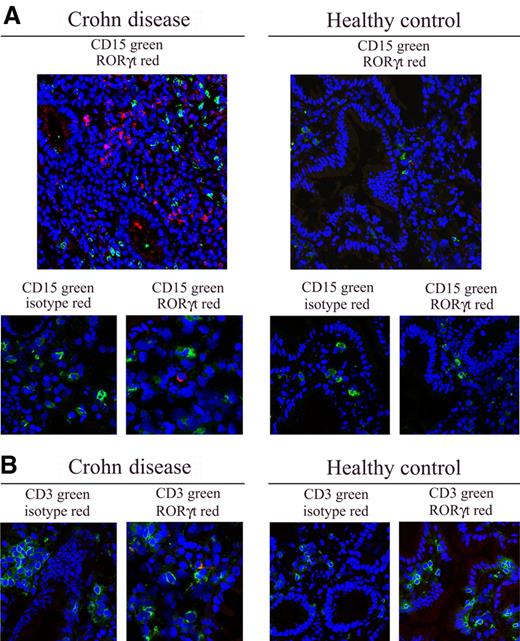
Subsequently, we verified whether human neutrophils and Th17 cells colocalize in inflamed tissues isolated from selected inflammatory diseases in which Th17 cells have been shown to infiltrate and play a pathogenic role, namely CD and RA.2,11 In CD, confocal microscopy analysis performed on gut specimens revealed a remarkable infiltration of CD15+ neutrophils and RORγt+ cells (Figure 3A), or CD161+ cells (not shown), that colocalize in the same areas. By contrast, few to no CD15+ neutrophils or RORγt+ Th17 cells were detectable in gut specimens from healthy subjects (Figure 3A). To confirm that RORγt-expressing cells were T lymphocytes, we performed double staining with CD3 and RORγt antibodies. As shown in Figure 3B, RORγt+ cells coexpressed the CD3 molecule in CD patients, but not in healthy donors. No staining was obtained with isotype control Abs (Figure 3), confirming the specificity of the different antibodies used.
Neutrophils and Th17 cells colocalize in CD. (A) Confocal microscopy analysis was performed on gut tissue section of CD patients and healthy donors for CD15+ (green) and RORγt+ (red) cells. Nuclei were counterstained with TO-PRO-3 (blue). Cropped images of CD15, RORγt, and related isotype Abs (A bottom panels), as well as of CD3, RORγt, and related isotype Abs (B) are also reported (original magnification ×400, pixel 1024 × 1024). Representative images from 3 patients and 3 healthy donors. Images were acquired with a 40×/1.3 NA oil objective (corresponding to a 400× magnification). Single confocal images of cells were obtained at nuclear equatorial level by using the 1 Airy unit formula for the adjustment of the pinhole diameter, corresponding to an optical slice of 0.7 mm (for FITC emission images). Images were acquired and analyzed using the LSM 5 software (Carl Zeiss). The slides were mounted with Vectashield mounting medium (Vector Laboratories).
Neutrophils and Th17 cells colocalize in CD. (A) Confocal microscopy analysis was performed on gut tissue section of CD patients and healthy donors for CD15+ (green) and RORγt+ (red) cells. Nuclei were counterstained with TO-PRO-3 (blue). Cropped images of CD15, RORγt, and related isotype Abs (A bottom panels), as well as of CD3, RORγt, and related isotype Abs (B) are also reported (original magnification ×400, pixel 1024 × 1024). Representative images from 3 patients and 3 healthy donors. Images were acquired with a 40×/1.3 NA oil objective (corresponding to a 400× magnification). Single confocal images of cells were obtained at nuclear equatorial level by using the 1 Airy unit formula for the adjustment of the pinhole diameter, corresponding to an optical slice of 0.7 mm (for FITC emission images). Images were acquired and analyzed using the LSM 5 software (Carl Zeiss). The slides were mounted with Vectashield mounting medium (Vector Laboratories).
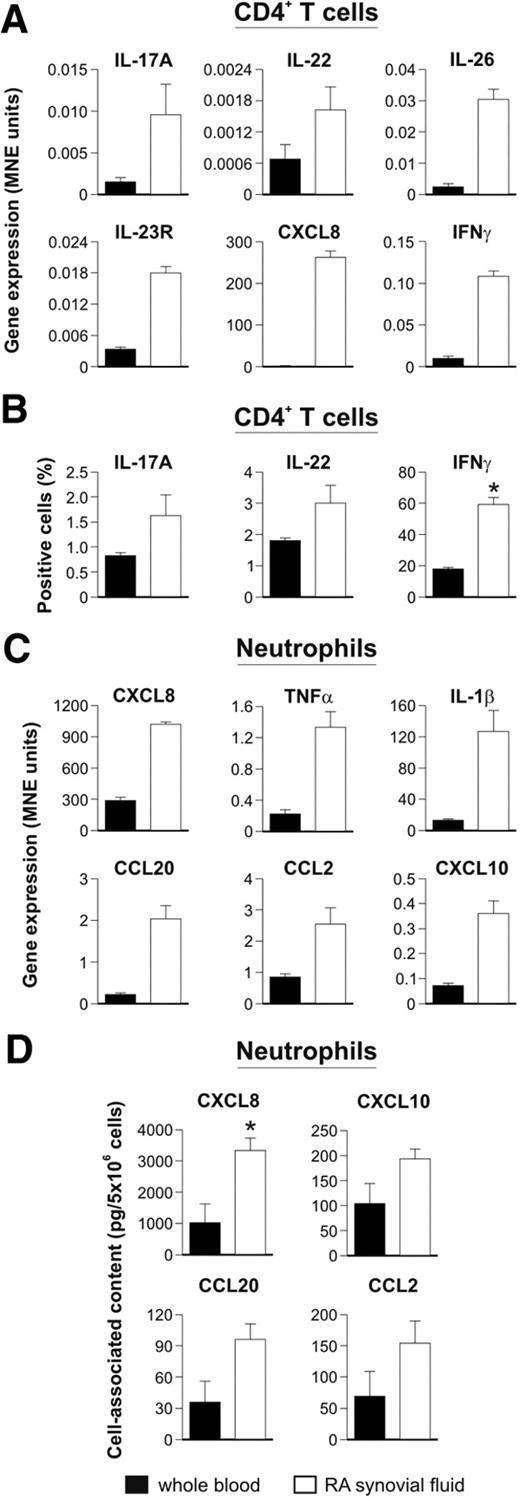
In the case of RA, we purified and molecularly analyzed the various leukocyte types present in SF of patients, to ascertain whether neutrophils and Th17 cells could be identified in the same samples. Magnetically purified CD4+ T cells from RA SF were found to display a typical Th17-“cytokine signature,” as determined by their increased expression of IL-17A, IL-23R, IL-22, IL-26 (Figure 4A), and IL-17F (data not shown) mRNAs, as well as CD161 by flow cytometry (data not shown), compared with that observed in magnetically purified CD4+ T cells from the peripheral blood. In addition, the expression of IFN-γ mRNA was higher in CD4+ T cells from RA SF (Figure 4A). In addition, CD4+ T cells from peripheral blood and SF lymphocytes were evaluated for their ability to produce cytokines. As reported in Figure 4B, the frequencies of IL-17A–, IL-22–, and IFN-γ–producing cells among CD4+ T cells in SF were higher than those observed in the peripheral blood of the same patients, suggesting a direct involvement of these cells in RA-associated inflammation. These results are in agreement with previous observations showing an increased abundance of Th17 cells in RA SF as opposed to the blood of both RA patients and healthy subjects.22 Interestingly, RA CD4+ T cells were also found to express high levels of CXCL8 mRNA (Figure 4A), which, in agreement with the results shown in Figure 2A and B, might reflect a local Th17 cell activation. However, the increased expression of Th17-related genes in RA SF CD4+ T cells could also derive from a selective recruitment and, in turn, enrichment of Th17 cells. In line with the latter hypothesis, high yields of CCL20 (5.48 ± 3.11 ng/mL, n = 5), CCL2 (149.67 ± 77.24 ng/mL), and CXCL10 (0.87 ± 0.57 ng/mL) as well as IL-17A (0.64 ± 0.45 ng/mL) and CXCL8 (0.61 ± 0.09 ng/mL) were detected in all RA SF analyzed.
Neutrophils and Th17 cells colocalize in RA. Real-time RT-PCR gene expression analysis (A,C), and protein expression profile (B,D), performed in CD4+ T cells (A-B) and neutrophils (C-D), magnetically purified from the peripheral blood and SF of RA patients. (A,C) Results are expressed as mean normalized expression (MNE) units ± SE of representative samples (n = 3). (B) The mean value of the percentage (± SE) of CD4+ T lymphocytes from peripheral blood or SF of RA patients producing the indicated cytokine on polyclonal stimulation are shown (n = 7). (D) Mean values (± SEM) of cell-associated CXCL8, CXCL10, CCL20, and CCL2 measured by ELISA in lysates of peripheral blood and SF RA neutrophils (n = 3). *P < .05.
Neutrophils and Th17 cells colocalize in RA. Real-time RT-PCR gene expression analysis (A,C), and protein expression profile (B,D), performed in CD4+ T cells (A-B) and neutrophils (C-D), magnetically purified from the peripheral blood and SF of RA patients. (A,C) Results are expressed as mean normalized expression (MNE) units ± SE of representative samples (n = 3). (B) The mean value of the percentage (± SE) of CD4+ T lymphocytes from peripheral blood or SF of RA patients producing the indicated cytokine on polyclonal stimulation are shown (n = 7). (D) Mean values (± SEM) of cell-associated CXCL8, CXCL10, CCL20, and CCL2 measured by ELISA in lysates of peripheral blood and SF RA neutrophils (n = 3). *P < .05.
In the same samples, neutrophils purified by magnetic negative selection were found to express elevated amounts of CXCL8, TNF-α, and IL-1β transcripts (Figure 4C) as well as intracellular CXCL8 (Figure 4D), consistent with these cells being in an activated state.23 Remarkably, RA SF neutrophils also displayed high amounts of CCL2 and, as previously described, CCL2024 and CXCL1025 mRNAs (Figure 4C), along with a cell-associated CCL2, CCL20, and CXCL10 content higher than that of peripheral neutrophils (Figure 4D). In such a context, endogenous TLR4 ligands, in addition to LPS, could cooperate with IFN-γ for the production of those chemokines. These data suggest that neutrophils could participate in the recruitment of not only pathogenic Th17, but also Th1,26 cells into RA joints. Notably, we also found that CD4+ T cells and neutrophils isolated from the SF of a patient with septic arthritis resulting from Streptococcus agalactiae displayed an RA-like gene expression pattern (data not shown), suggesting that neutrophils and Th17 cells colocalize in other types of inflammatory arthritis.
IL-17A and IL-17F are unable to modulate neutrophil responses
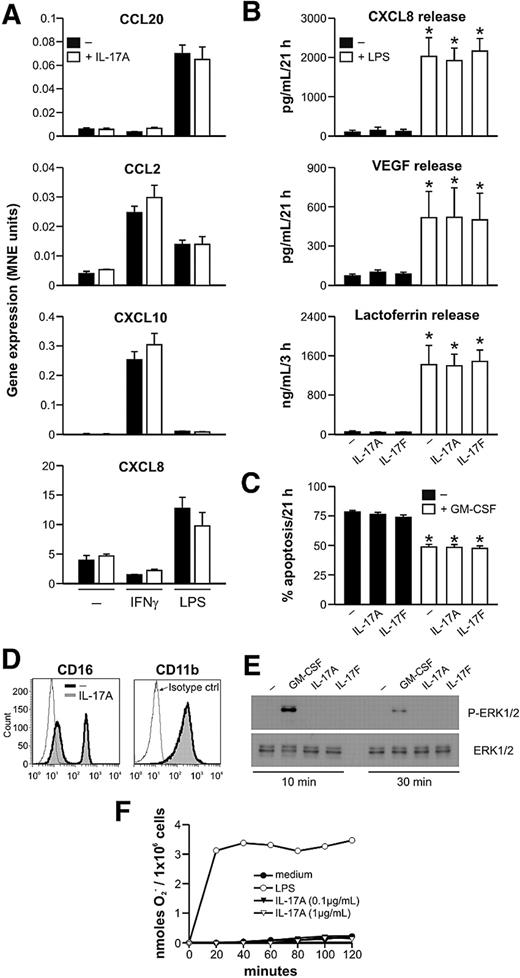
In further experiments, we investigated whether IL-17A and IL-17F, either by themselves or in combination with other agonists, could influence neutrophil responses. IL-17A and IL-17F are known to activate IL-6, CXCL8, and G-CSF production by fibroblasts and epithelial cells. These cytokines enhance chemokine gene expression and induce the secretion of vascular endothelial growth factor (VEGF) in various cell types, such as synoviocytes.1,3 In addition, IL-17A and IL-17F have been reported to markedly synergize with several cytokines, including IL-1β, TNF-α, and IFN-γ, and to additively cooperate with TLR ligands, such as LPS, in inducing cytokine and chemokine production.1,3 As shown in Figure 5A, we observed that IL-17F (data not shown) and IL-17A (from 2 commercial sources and at doses ranging from 0.01 to 100 ng/mL) have no direct effect in human neutrophils on CCL20, CCL2, CXCL10, and CXCL8 gene expression, nor were they able to modulate chemokine gene expression after IFN-γ or LPS stimulation. We further observed that IL-17A and IL-17F neither influenced the release of CXCL8, VEGF, and lactoferrin in response to LPS (Figure 5B) nor affected the rate of spontaneous neutrophil apoptosis15,16 (Figure 5C). In contrast with other results,16 IL-17A and IL-17F were also unable to antagonize the protective effects of GM-CSF on neutrophil survival (Figure 5C). We did not detect any IL-17A– or IL-17F–dependent modulation of CD16 Fcγ receptor, CD11b integrin, or CD66b adhesion molecule expression (Figure 5D; and data not shown). Similarly, although it has been described in other cell types,3 we did not see any IL-17A– or IL-17F–dependent effect on the phosphorylation state of MAPK ERK1/2, (Figure 5E), a signaling pathway implicated in IL-17-induced mRNA stabilization. In addition, a neutrophil preincubation with 0.1 to 1 μg/mL IL-17A for 2 hours failed to prime an fMLF-induced respiratory burst (Figure 5F). Finally, addition of 0.1 to 1 μg/mL IL-17A to neutrophils preincubated with IFN-γ plus LPS or IFN-γ plus TNF-α for 21 hours neither activated MAPK ERK1/2 phosphorylation (supplemental Figure 3A) nor influenced the extracellular yields of CXCL8, CXCL10, and CCL2 (supplemental Figure 3B) or affected the rate of apoptosis (supplemental Figure 3C) measured after an additional 24 hours of incubation. Similarly, the prosurvival effect of GM-CSF, observed on its addition to neutrophils preincubated for 21 hours in medium, was also not influenced by the presence of IL-17A or IL-17F (data not shown).
Human neutrophils are unresponsive to IL-17A and IL-17F. (A-D) Neutrophils were cultured with IL-17A or IL-17F for up to 21 hours, in the presence or absence of IFN-γ, LPS, or GM-CSF as indicated. (A) Neutrophils were harvested after 3 hours for gene expression analysis by real-time RT-PCR. Results are expressed as MNE units ± SE. (B-D) Neutrophils were incubated for 3 and 21 hours before measuring: (B) CXCL8, VEGF, and lactoferrin supernatant levels (n = 3); (C) their degree of apoptosis (as assessed by annexin V/propidium iodide staining; n = 4); (D) CD16 and CD11b surface expression. (E) Neutrophils were incubated with IL-17A, IL-17F, or GM-CSF and then evaluated for phospho-ERK1/2 (P-ERK1/2) analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (F) Neutrophils were incubated with fMLF in the presence of IL-17A or LPS, and superoxide anion (O2−) generation was measured for up to 2 hours. (A,D-F) One representative of at least 3 independent experiments with similar results. *P < .05.
Human neutrophils are unresponsive to IL-17A and IL-17F. (A-D) Neutrophils were cultured with IL-17A or IL-17F for up to 21 hours, in the presence or absence of IFN-γ, LPS, or GM-CSF as indicated. (A) Neutrophils were harvested after 3 hours for gene expression analysis by real-time RT-PCR. Results are expressed as MNE units ± SE. (B-D) Neutrophils were incubated for 3 and 21 hours before measuring: (B) CXCL8, VEGF, and lactoferrin supernatant levels (n = 3); (C) their degree of apoptosis (as assessed by annexin V/propidium iodide staining; n = 4); (D) CD16 and CD11b surface expression. (E) Neutrophils were incubated with IL-17A, IL-17F, or GM-CSF and then evaluated for phospho-ERK1/2 (P-ERK1/2) analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (F) Neutrophils were incubated with fMLF in the presence of IL-17A or LPS, and superoxide anion (O2−) generation was measured for up to 2 hours. (A,D-F) One representative of at least 3 independent experiments with similar results. *P < .05.
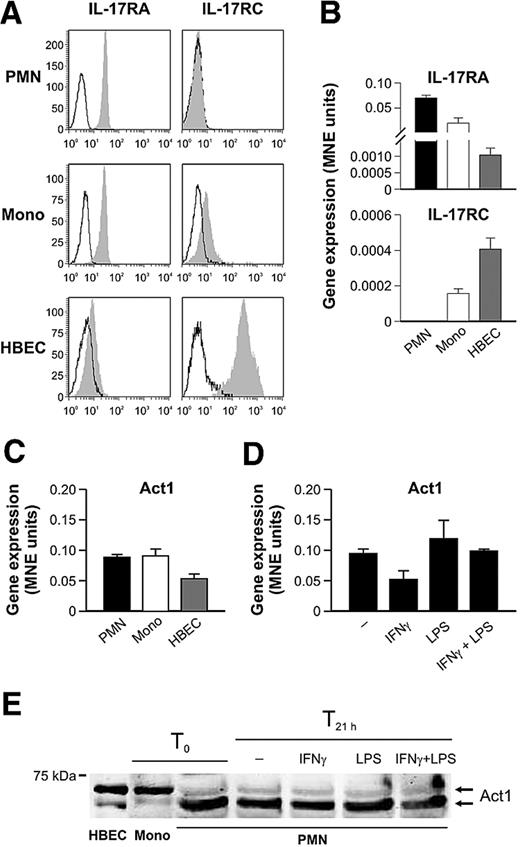
Human neutrophils do not express IL-17RC
Because the previous results argue for a nonfunctional IL-17/IL-17R signaling pathway in neutrophils and because the biologic activities of IL-17A and IL-17F rely on the formation of a multimeric receptor composed of at least the IL-17RA and IL-17RC subunits,3 we next examined whether highly purified human neutrophils express these IL-17R components. By both flow cytometry and real-time RT-PCR analyses, not only could we confirm previous observations16 that freshly isolated neutrophils constitutively express the IL-17RA chain, but we also uncovered that they do not display any IL-17RC, either at the protein or the mRNA level, in contrast to autologous monocytes (Figure 6A-B). Moreover, we were not able to detect surface IL-17RC either in in vivo activated neutrophils, such as in those harvested from RA SF (supplemental Figure 4A), or in in vitro neutrophils treated for up to 21 hours with IFN-γ and/or LPS (supplemental Figure 4B), RA SF, or GM-CSF (data not shown). This suggests that IL-17RC gene is not susceptible to any modulation. Interestingly, we noticed a slight down-regulation of surface IL-17RA in LPS and IFN-γ plus LPS-activated neutrophils (supplemental Figure 4B), which was not caused by the binding of endogenously produced IL-17A (supplemental Figure 4C), a phenomenon recently proposed by Gaffen27 to limit signaling by receptor-mediated IL-17 internalization.
Human neutrophils express IL-17RA but not IL-17RC. Freshly isolated neutrophils (PMN), monocytes (Mono), and HBECs were evaluated for IL-17RA and IL-17RC surface (A) and mRNA (B) expression by flow cytometry (empty histogram: isotype control Abs; filled histogram: specific Abs) and real-time RT-PCR, respectively. (C-D) Act1 mRNA expression was evaluated in freshly isolated neutrophils, monocytes, and HBECs (C) as well as in neutrophils incubated for 21 hours with IFN-γ and/or LPS (D). (E) Act1 protein expression was evaluated by Western blot analysis of whole-cell extracts prepared from HBECs and freshly prepared monocytes (T0), as well as of cytoplasmic cavitates prepared from neutrophils, freshly isolated (T0) or incubated for 21 hours with IFN-γ and/or LPS (T21 h). One representative of at least 3 independent experiments with similar results is shown in each panel.
Human neutrophils express IL-17RA but not IL-17RC. Freshly isolated neutrophils (PMN), monocytes (Mono), and HBECs were evaluated for IL-17RA and IL-17RC surface (A) and mRNA (B) expression by flow cytometry (empty histogram: isotype control Abs; filled histogram: specific Abs) and real-time RT-PCR, respectively. (C-D) Act1 mRNA expression was evaluated in freshly isolated neutrophils, monocytes, and HBECs (C) as well as in neutrophils incubated for 21 hours with IFN-γ and/or LPS (D). (E) Act1 protein expression was evaluated by Western blot analysis of whole-cell extracts prepared from HBECs and freshly prepared monocytes (T0), as well as of cytoplasmic cavitates prepared from neutrophils, freshly isolated (T0) or incubated for 21 hours with IFN-γ and/or LPS (T21 h). One representative of at least 3 independent experiments with similar results is shown in each panel.
Act1 is an essential component of the IL-17–dependent signaling pathway that ultimately drives the induction of cytokine and chemokine gene expression.28 For this reason, we established that, in human neutrophils, Act1 is not only expressed at the mRNA (Figure 6C-D) and protein (Figure 6E) levels in amounts substantially similar to those present in autologous monocytes, but its expression does not significantly change on neutrophil activation for up to 21 hours with IFN-γ and/or LPS (Figure 6D-E), GM-CSF, or fMLF (not shown). The human bronchial epithelial cell (HBEC) line is fully responsive to both IL-17A and IL-17F in terms of IL-6, CXCL8, and G-CSF gene expression induction and CXCL8 production and release (data not shown). Used as a positive control, these cells expressed not only the IL-17RA and IL-17RC subunits (Figure 6A-B), but also substantial levels of Act1 mRNA and protein (Figure 6C,E). Interestingly, whereas Act1 was immunodetected as a doublet in all 3 cell types under examination, corroborating previous observations,28-30 the expression ratio between the upper and lower Act1 band was reversed in neutrophils compared with monocytes and HBEC cells (Figure 6E). Together, our results prove that human neutrophils, whether freshly isolated or cytokine-primed, are unresponsive to IL-17A and IL-17F, probably because they lack the IL-17RC subunit.
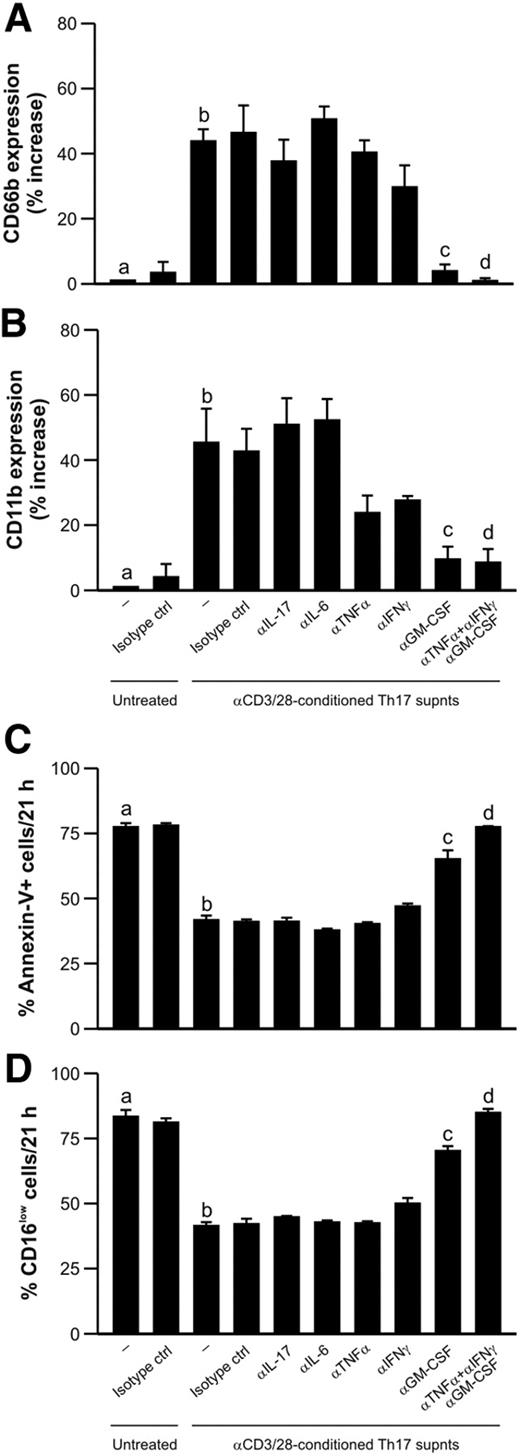
Activated Th17 clones modulate neutrophil responses through an IL-17–independent mechanism
In light of the inability of IL-17A and IL-17F to act on neutrophils, we investigated whether supernatants from anti-CD3/CD28-activated Th17 clones were able to activate neutrophils, and, if so, we hoped to identify the responsible factor(s). We first established that conditioned supernatants from Th17 cells were very potent in positively modulating neutrophil expression of CD66b (Figure 7A) and CD11b (Figure 7B), 2 activation markers implicated in neutrophil adhesion that are rapidly mobilized from granules to the cell surface on stimulation.31,32 In addition, Th17 cell-derived supernatants were found to reduce neutrophil apoptosis (Figure 7C-D), which was assessed by both annexin V binding and CD16 surface expression. Because Th17-derived supernatants contained TNF-α (46.7 ± 7.0 pg/mL, n = 4), IL-6 (13.1 ± 0.1 pg/mL), and GM-CSF (39.1 ± 27.1 pg/mL) in addition to IL-17A (16.2 ± 3.3 ng/mL) and IFN-γ (detectable, however, only by intracellular staining; supplemental Figure 2A), we preincubated with specific neutralizing antibodies directed against IL-17A, TNF-α, GM-CSF, IL-6, and IFN-γ, either individually or in combination, before their addition to neutrophils. By doing so, we confirmed that IL-17A is unable to modulate neutrophil responses, similar to IL-6 (Figure 7). In contrast, we identified GM-CSF as the Th17-derived cytokine that more potently acts on neutrophils, based on the capacity of an anti–GM-CSF neutralizing antibody to significantly reduce the increased CD66b and CD11b expression (Figure 7A-B) and the delay in apoptosis (Figure 7C-D). Minor effects could also be attributed to TNF-α and IFN-γ, as their specific neutralizing antibodies reduced the effects of Th17-conditioned supernatants on CD11b expression (Figure 7B). Remarkably, the simultaneous use of neutralizing antibodies against TNF-α, IFN-γ, and GM-CSF completely suppressed the effects of Th17-conditioned supernatants on the neutrophil responses under investigation (Figure 7). This further demonstrates that Th17 cells can modulate neutrophil functions through an IL-17–independent mechanism, most probably through the release of GM-CSF, TNF-α, and IFN-γ. We confirmed this in further experiments by demonstrating that the prosurvival effect exerted by conditioned supernatants from activated Th17 cells on preactivated neutrophils was not influenced by neutralizing antibodies directed against IL-17A, once again consistent with the absence of IL-17RC in neutrophils (supplemental Figure 5).
Activated Th17 clones can modulate human neutrophil responses through an IL-17–independent mechanism. Neutrophils were left untreated or cultured for 21 hours with supernatants from αCD3/αCD28-stimulated Th17 clones, in the presence or absence of neutralizing Abs against IL-17, IL-6, TNF-α, IFN-γ, GM-CSF, or TNF-α, IFN-γ, and GM-CSF in combination (or appropriate isotype control Abs), before evaluating their surface expression of CD66b (A) and CD11b (B) and their degree of apoptosis, as assessed by annexin V/PI staining (C) and by CD16 surface expression (D; n = 4). (A-B) Data are expressed as percentage of increase over the untreated condition. b vs a, P < .05; c vs b, P < .05; d vs b, P < .05.
Activated Th17 clones can modulate human neutrophil responses through an IL-17–independent mechanism. Neutrophils were left untreated or cultured for 21 hours with supernatants from αCD3/αCD28-stimulated Th17 clones, in the presence or absence of neutralizing Abs against IL-17, IL-6, TNF-α, IFN-γ, GM-CSF, or TNF-α, IFN-γ, and GM-CSF in combination (or appropriate isotype control Abs), before evaluating their surface expression of CD66b (A) and CD11b (B) and their degree of apoptosis, as assessed by annexin V/PI staining (C) and by CD16 surface expression (D; n = 4). (A-B) Data are expressed as percentage of increase over the untreated condition. b vs a, P < .05; c vs b, P < .05; d vs b, P < .05.
Discussion
In this study, we have revealed that activated neutrophils and Th17 cells participate in direct cross-talk. One important feature of this interaction is that the 2 cell types can trigger, on activation, a reciprocal recruitment via the release of endogenous chemokines. In this context, we also report that activated neutrophils promote direct chemotaxis of Th1 cells. We found that supernatants harvested from highly purified neutrophils, after stimulation with IFN-γ plus LPS (γL), can exhibit a remarkable chemotactic effect on peripheral IL-17A+-enriched CD4+ T-cell populations. The subsequent use of discrete Th clones and specific neutralizing antibodies allowed us to identify CCL20 and CCL2 as the chemokines responsible for this chemotactic activity. By the same approach, we also identified CXCL10 and CCL2 as the chemokines mediating the recruitment of Th1 clones by neutrophils, consistent with previous studies showing that supernatants from γL-stimulated neutrophils induce migration and rapid integrin-dependent adhesion of CXCR3-expressing lymphocytes.14 In contrast, we found that Th2 clones were not recruited by neutrophils, which was in agreement with the inability of γL-stimulated neutrophils to produce CCL17 and CCL22 (current study; and Andrew et al33 ). These data differ from a recent paper demonstrating that blood neutrophils from cystic fibrosis patients can secrete CCL17.34 However, although the experimental settings34 are different from ours, the involvement of potential contaminating cells able to produce CCL17 and CCL22, such as eosinophils,35 was not adequately excluded.34 In any case, the capacity of neutrophils to chemoattract Th17 cells via CCL2 and CCL20 is in line with the role of these 2 agonists to elicit migratory responses in murine Th17-polarized cells,36 as well as with the importance of the CCR6/CCL20 axis in directing the migration of Th17 cells in inflammatory disease.11,37,38 Importantly, and in addition to the fact that neutrophils preferentially participate into the recruitment of Th17 and Th1 subpopulations, various studies suggest that neutrophils could also contribute to the cytokine balance governing Th host responses during bacterial infections. For instance, neutrophils could play a role in Borellia burgdorferi NapA-driven Th17 cell inflammation in Lyme arthritis through the release of IL-23, an essential survival factor for Th17 cells.39 Moreover, the early recruitment of neutrophils was shown to contribute to Th1 polarization in a murine model of Legionella pneumophila pneumonia40 and in the development of resistance to Trypanosoma cruzi in BALB/c mice by altering the expression of Th1/Th2 cytokines in favor of a Th1 response.41 Overall, it seems that neutrophils preferentially recruit and modulate the functions of Th17 and Th1 cells, as opposed to Th2 cells.
In this work, we also show that, on activation, human Th17 cells can directly chemoattract neutrophils through the production and release of CXCL8. This may represent a more efficient mechanism for Th17 cells to rapidly recruit and interact with neutrophils, as opposed to the indirect actions of Th17-derived IL-17A and IL-17F, which function via induction of chemokine secretion by epithelial and endothelial cells.20 Although the ability of Th17 cells to produce CXCL8 has never been formally reported, our findings are in agreement with the recent description of a minor subpopulation of CD4+ T cells capable of producing CXCL8 in the peripheral blood of healthy donors.21 These cells were also shown to produce TNF-α, but not IFN-γ, IL-4, IL-5, IL-10, and IL-13,21 which, curiously, are all features of human Th17 cells. Our in vitro observations lead us to speculate that the neutrophil/Th17 interaction might create a pathogenic proinflammatory loop that ultimately amplifies the local accumulation of neutrophils and Th17 cells during inflammatory disease. Accordingly, we show that neutrophils and Th17 cells colocalize in gut tissue from CD and synovial fluid from RA patients. Other examples of colocalized neutrophils and Th17 cells have been shown, for instance, in asthma,42 gastric Helicobacter pylori infection,43 an experimental model for inflammatory bowel disease44 and in a model of experimental autoimmune encephalitis induced by the adoptive transfer of Th17-polarized myelin-reactive T cells.45 Our identification of neutrophil-derived CCL20 and CCL2, neutrophil-derived CXCL10, and Th17 cell–derived CXCL8 as the mediators involved in the recruitment of Th17 cells, Th1 cells, and neutrophils, respectively, reinforces the notion that targeting chemokines may be a useful treatment for chronic inflammatory and autoimmune diseases.46
In this paper, we also report that Th17 cells, although potentially promoting a prompt neutrophil recruitment via CXCL8, do not affect any neutrophil effector functions via a direct action of IL-17A or IL-17F. This includes previously reported effects such as the attenuation of the antiapoptotic effects by GM-CSF.16 Although such discrepancies might derive from contaminating cells in the neutrophil preparations obtained by standard purification methods,16,17 we offer a solid molecular basis to explain the neutrophil unresponsiveness to 17A or IL-17F: namely, an impaired expression of IL-17RC that is not inducible on neutrophil activation, despite the presence of IL-17RA and the cytoplasmic adaptor protein Act1. Interestingly, Act1 displayed an expression pattern different to that detected in autologous monocytes or HBECs, which are both responsive to both IL-17A and IL-17F. Whether or not this atypical Act1 expression in neutrophils is related to their lack of responsiveness to IL-17 remains to be determined. Regardless, neutrophils resemble human T cells, which also express only IL-17RA, and therefore do not up-regulate IL-17 target genes in response to IL-17A.47 In addition, our data also match the behavior of murine neutrophils, in which the IL-17/IL-17R signaling pathway does not seem to play a significant role, as IL-17RAKO neutrophils appear to have no intrinsic functional defects.48 Furthermore, several differences exist between the human and murine IL-17 receptors and cytokines. In human, IL-17RC binds IL-17A and IL-17F with similar affinities, whereas IL-17RA binds IL-17A with a 1000-fold affinity higher than IL-17F. In mouse, IL-17RA binds both IL-17A and IL-17F, whereas only IL-17F binds to IL-17RC, implying that mouse cells expressing only IL-17RA should respond to IL-17A.49 Accordingly, a recent study showed that murine CD4+ T cells, which express IL-17RA and Act1, but not IL-17RC, secrete CCL2, CCL3, GM-CSF, IL-1β, and IL-9 in response to IL-17A, but not IL-17F.50 Finally, it was recently shown that murine IL-17A not only directly induces neutrophil chemotaxis in vitro (a finding that we never observed using human neutrophils and IL-17A; M.P., A.M., and M.A.C., unpublished observations, February 2009), but is also able to direct the migration of neutrophils into the peritoneal cavity.51 Therefore, the relative contributions of IL-17A and IL-17F in inflammation are different in mice and humans, and this should be considered when testing IL-17 family antagonists in mouse models of human disease.
Further observations that, in our opinion, consolidate the biologic relevance of a neutrophil-Th17 cell network, include the capacity of activated Th17 cells to directly modulate several responses in both freshly isolated and preactivated neutrophils. These include cell survival and antigen expression, which are affected through IL-17–independent mechanisms. According to our experiments, there is a clear role for Th17-derived TNF-α, IFN-γ, and especially GM-CSF, which are all cytokines known to act as classic neutrophil-activating factors.52 Therefore, the importance and the role of Th17-derived cytokines different apart from IL-17A or IL-17F should not be underestimated when considering the downstream effects of Th17 cells.
In conclusion, the findings of this work extend our knowledge of the ability of neutrophils to communicate with other cells of the innate and adaptive immune system, as has been previously shown for dendritic cells, CD8+ T cells, and B cells.12,53-55 The fact that neutrophils may also directly interact with discrete Th cell subpopulations might have important implications in our understanding of the pathogenic mechanisms involved in chronic inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gabriella Bergamini and Paola Melotti for providing and maintaining the human 16HBE14o-bronchial epithelial cell line and Ingrid Saba and Ariel C. Bulua for critical reading.
This work was supported by Ministero dell'Istruzione, dell'Università e della Ricerca, University of Verona (Joint Project grant), Fondazione Cariverona, Associazione Italiana per la Ricerca sul Cancro (5839), Ente Cassa di Risparmio (Florence, Italy), EU Projects SENS-IT-IV (FP6-LSBH-CT-2006-018861), and INNOCHEM (FP6-LSHB-CT-2005-518167). M.P. holds a Canadian Institutes of Health Research fellowship and C.C. holds an Associazione Italiana per la Ricerca sul Cancro fellowship.
Authorship
Contribution: M.P., L.M., A.M., E.L., N.T., and C.C. performed experiments; M.P., L.M., and A.M. analyzed results and made the figures; M.P., L.C., C.L., F.A., and M.A.C. designed the research; and M.P., F.A., S.R., and M.A.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco A. Cassatella, Department of Pathology, Section of General Pathology, Strada Le Grazie 8, 37134 Verona, Italy; e-mail: marco.cassatella@univr.it; and Francesco Annunziato, Department of Internal Medicine, Immunology and Cellular Therapies Unit, Viale Pieraccini 6, I-50134 Firenze, Italy; e-mail: f.annunziato@dmi.unifi.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal