Abstract
Phenotypic markers associated with human hematopoietic stem cells (HSCs) were developed and validated using uncultured cells. Because phenotype and function can be dissociated during culture, better markers to prospectively track and isolate HSCs in ex vivo cultures could be instrumental in advancing HSC-based therapies. Using an expansion system previously shown to increase hematopoietic progenitors and SCID-repopulating cells (SRCs), we demonstrated that the rhodamine-low phenotype was lost, whereas AC133 expression was retained throughout culture. Furthermore, the AC133+CD38− subpopulation was significantly enriched in long-term culture-initiating cells (LTC-IC) and SRCs after culture. Preculture and postculture analysis of total nucleated cell and LTC-IC number, and limiting dilution analysis in NOD/SCID mice, showed a 43-fold expansion of the AC133+CD38− subpopulation that corresponded to a 7.3-fold and 4.4-fold expansion of LTC-ICs and SRCs in this subpopulation, respectively. Thus, AC133+CD38− is an improved marker that tracks and enriches for LTC-IC and SRC in ex vivo cultures.
Introduction
The CD34+CD38− phenotype has been predominantly used to enrich hematopoietic stem cells (HSCs) from uncultured cells.1 However, during culture, this phenotype does not accurately reflect HSC activity because lineage-negative (Lin−)CD34+CD38+ cells lose CD38 expression during culture, acquiring the CD34+CD38− phenotype without gaining SCID-repopulating cell (SRC) function.2 Hence, we examined whether other methods to purify HSCs from uncultured hematopoietic cells could be applied to enrich HSCs in culture. Using a culture technology capable of definitively expanding human HSCs greater than 3-fold,3 we specifically examined AC133, CD38, and rhodamine expression during culture. These markers have been extensively used to characterize uncultured hematopoietic progenitors and HSCs; however, their effectiveness in identifying these cells after culture has not been determined.
The rhodamine-low (Rholo) phenotype, which results from effluxing of the Rhodamine123 dye by the P-glycoprotein efflux pump,4 enriches long-term culture-initiating cells (LTC-ICs) from primary hematopoietic tissues.5-7 AC133 is a pentaspan glycoprotein expressed on hematopoietic progenitors,8 although the function of AC133 is not understood. Uncultured bone marrow AC133+CD34hi cells are enriched in primitive progenitors and are capable of engrafting secondary recipients.9 AC133 has also been used to isolate neural stem cells,10 endothelial progenitor cells,11 and brain and colon cancer stem cells.12,13 The absence of CD38 expression in conjunction with either the RholoCD34+ or AC133+ phenotype on uncultured human hematopoietic cells significantly enriches both normal and leukemic HSCs.14,15 Herein we show that the AC133+, but not the Rholo phenotype, is a better marker of cultured progenitors and the AC133+CD38− phenotype tracks with and significantly enriches for LTC-IC and SRC function in HSC expansion cultures. Therefore, the AC133+CD38− phenotype serves as an improved marker to accurately track and isolate primitive progenitors and HSCs in ex vivo cultures.
Methods
Umbilical cord blood (UCB) samples were collected according to ethics board–approved procedures at Mount Sinai (Toronto, ON) and Joseph Brant Memorial (Burlington, ON) hospitals. Lin− cells were isolated using StemSep and then cultured in StemSpan media (StemCell Technologies), 100 ng/mL stem cell factor (SCF) and 100 ng/mL FMS-like tyrosine kinase 3, and 50 ng/mL thrombopoietin (R&D Systems) with a Lin− reselection and media exchange at day 4 as previously described.3 Cells were stained with Rhodamine123 (Kodak), AC133-phycoerythrin (AC133; Miltenyi Biotec), CD38-fluorescein isothiocyanate (T16; Coulter), and CD34-allophycocyanin (581; Coulter) according to the manufacturer's protocols. Cells were analyzed on the FACSCanto flow cytometer (BD Biosciences) or sorted on the MoFlo cytometer (Dako North America).
Colony-forming unit cells (CFU-C) were cultured in methylcellulose (4434; StemCell Technologies). LTC-ICs were maintained on M210B4 cells for 5 weeks with half media (H5100; StemCell Technologies) exchange weekly and then harvested and plated in methylcellulose.
Sublethally irradiated (3.6 Gy) NOD/SCID mice were transplanted with cultured cells via tail vein injection. Mice were killed 7 to 8 weeks after transplantation. Bone marrow was harvested from femurs, stained with antibodies to human CD45(J.33), HLA-ABC(B9.12.1), CD19(J4.119), or CD33(D3HL60.251; Coulter), and then analyzed on the LSRII flow cytometer (BD Biosciences).
Results and discussion
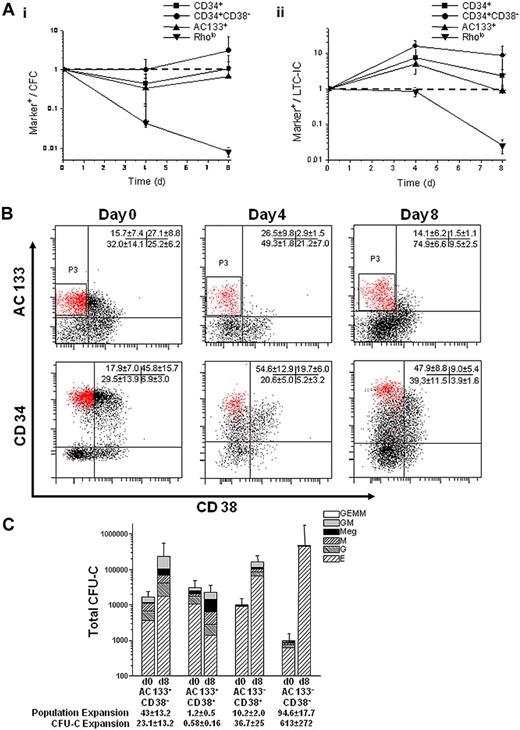
To assess whether AC133 or Rholo expression could effectively monitor progenitor function in ex vivo cultures, we cultured umbilical cord blood Lin− cells using periodic reselection, as previously described.16 At days 0, 4, and 8, cells were plated in CFU-C and LTC-IC assays and analyzed for AC133+, Rholo, CD34+, and CD34+CD38− expression by flow cytometry. We plotted marker expression over CFU-C or LTC-IC output with time, theorizing that the expression of an appropriate marker would correlate with progenitor function. The Rholo phenotype was lost by day 8; however, AC133 expression was retained throughout culture and appeared to correlate with LTC-IC output, especially compared with the more commonly used CD34+CD38− or CD34+ phenotypes (Figure 1A).
AC133 expression correlates with progenitor function, and the AC133+CD38− phenotype marks a CD34hiCD38− subpopulation. (A) The expression of AC133+, Rholo, CD34+, and CD34+CD38− markers was quantitatively evaluated for their progenitor function in culture by the following relationship: percentage of marker+ cells/number of CFU-C or LTC-ICs. Correlation between marker expression and (Ai) CFU-C or (Aii) LTC-IC function was tracked over 8 days in culture and normalized to 1 based on day 0 values. (CFU-Cs per 500 cells plated on days 0, 4, and 8 were 53.5 ± 44, 159 ± 32, and 60.5 ± 25.2, respectively. LTC-ICs per 2000 cells plated at day 0, 4, and 8 were 39 ± 12, 9 ± 1.4, and 16.3 ± 10.2, respectively.) An ideal marker would closely correlate with progenitor function resulting in a constant relative frequency during culture (dashed line). (B) Representative flow cytometry plots comparing the expression of AC133 and CD38 with CD34 and CD38 on UCB Lin− cells cultured for 8 days (n = 9 independent experiments). Cells were stained with either AC133 and CD38 or CD34 and CD38. AC133+CD38- cells (P3) mark a CD34hiCD38− subpopulation that enriches the CD34+CD38− population 2.5-fold (P < .001). (C) UCB Lin− cells were sorted into 4 populations (AC133+CD38−, AC133+CD38+, AC133−CD38+, and AC133−CD38−) before and after culture and then plated in CFU-C clonogenic assays (n = 5). The AC133+CD38− subpopulation contained more CFU-GM after culture than the AC133+CD38+ (P = .005), AC133−CD38+ (P = .02), and AC133−CD38− (P = .1) subpopulations and more CFU-mix after culture than the AC133+CD38+ (P = .05), AC133−CD38+ (P = .06), and AC133−CD38− (P = .01) subpopulations.
AC133 expression correlates with progenitor function, and the AC133+CD38− phenotype marks a CD34hiCD38− subpopulation. (A) The expression of AC133+, Rholo, CD34+, and CD34+CD38− markers was quantitatively evaluated for their progenitor function in culture by the following relationship: percentage of marker+ cells/number of CFU-C or LTC-ICs. Correlation between marker expression and (Ai) CFU-C or (Aii) LTC-IC function was tracked over 8 days in culture and normalized to 1 based on day 0 values. (CFU-Cs per 500 cells plated on days 0, 4, and 8 were 53.5 ± 44, 159 ± 32, and 60.5 ± 25.2, respectively. LTC-ICs per 2000 cells plated at day 0, 4, and 8 were 39 ± 12, 9 ± 1.4, and 16.3 ± 10.2, respectively.) An ideal marker would closely correlate with progenitor function resulting in a constant relative frequency during culture (dashed line). (B) Representative flow cytometry plots comparing the expression of AC133 and CD38 with CD34 and CD38 on UCB Lin− cells cultured for 8 days (n = 9 independent experiments). Cells were stained with either AC133 and CD38 or CD34 and CD38. AC133+CD38- cells (P3) mark a CD34hiCD38− subpopulation that enriches the CD34+CD38− population 2.5-fold (P < .001). (C) UCB Lin− cells were sorted into 4 populations (AC133+CD38−, AC133+CD38+, AC133−CD38+, and AC133−CD38−) before and after culture and then plated in CFU-C clonogenic assays (n = 5). The AC133+CD38− subpopulation contained more CFU-GM after culture than the AC133+CD38+ (P = .005), AC133−CD38+ (P = .02), and AC133−CD38− (P = .1) subpopulations and more CFU-mix after culture than the AC133+CD38+ (P = .05), AC133−CD38+ (P = .06), and AC133−CD38− (P = .01) subpopulations.
We next used CD38 to further characterize the AC133+ population. At day 0, AC133+CD38− and CD34+CD38− expression was very similar. However, as the culture progressed, AC133+CD38− expression remained relatively constant compared with the percentage of CD34+CD38− cells, which increased 2.7-fold, and the AC133+CD38− phenotype marked a CD34hiCD38− subpopulation (Figure 1B). Although the relative percentage of AC133+CD38− cells remained constant throughout the 8-day culture, this subpopulation expanded 43-fold (± 13.2-fold). To determine whether the expansion of AC133+CD38− cells corresponded with progenitor function and expansion during culture, uncultured day 0 Lin− and day 8 bioreactor-expanded cells were sorted into 4 populations (AC133+CD38−, AC133+CD38+, AC133−CD38+, and AC133−CD38−) and plated in CFU-C and LTC-IC assays. The expansion of AC133+CD38− cells resulted in a 23.1-fold (± 13.2-fold) expansion of CFU-C in this subpopulation, which contained more CFU-GM (P ≤ .1) and CFU-mix (P ≤ .06) after culture than the other subpopulations. The expansion of the AC133−CD38+ and AC133−CD38− subpopulations (10.2 ± 1.9-fold and 94.6 ± 17.7-fold, respectively) also resulted an expansion of CFU-C in each subpopulation (36.7 ± 25-fold and 613 ± 272-fold, respectively). However, BFU-E were the predominant progenitor type in these subpopulations (Figure 1C; supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
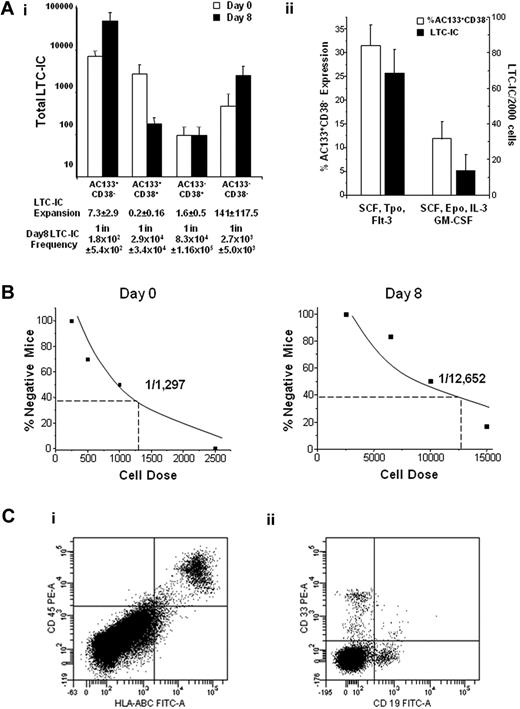
The expansion of the AC133+CD38− and AC133−CD38− populations also corresponded with an expansion of LTC-ICs during culture. However, the majority of LTC-ICs were contained in the AC133+CD38− population (Figure 2Ai; P ≤ .009), and the frequency of LTC-IC was significantly higher in this subpopulation than the other subpopulations (P < .05). We further demonstrated that the AC133+CD38− phenotype reflects LTC-IC function by culturing AC133+CD38− cells in either supportive or differentiation-inducing cultures. AC133+CD38− expression decreased in differentiation cultures, which also paralleled a decrease in LTC-IC activity of 68.4 (± 13.5) versus 13.6 (± 9.4; P < .01) LTC-ICs in supportive and differentiation cultures, respectively (Figure 2Aii).
The AC133+CD38− phenotype corresponds with LTC-IC and SRC function and expansion. UCB Lin− cells were sorted into 4 populations (AC133+CD38−, AC133+CD38+, AC133−CD38+, and AC133−CD38−) before and after culture and then plated in LTC-IC clonogenic assays (n ≥ 3) or transplanted into NOD/SCID mice. (Ai) The AC133+CD38− population contained significantly more LTC-ICs than the AC133+CD38+ (P = .002), AC133−CD38+ (P = .002), and AC133−CD38− (P = .009) populations. (Aii) AC133+CD38− cells were placed in either supportive (SCF, FMS-like tyrosine kinase 3 ligand, thrombopoietin) or differentiation (SCF, erythropoietin, interleukin-3, and GM-CSF) cultures. AC133+CD38− expression was 31.5% ± 4.4% versus 11.9% ± 3.7% (P = .04), and LTC-IC frequency was 68.4 plus or minus 13.5 and 13.6 ± 9.4 per 2000 cells (P < .01) in the supportive and differentiation cultures, respectively. (B) Cultured cells were sorted and transplanted intravenously into NOD/SCID mice, and analyzed 7 to 8 weeks after transplantation. Because the majority of SRC activity was present in the AC133+CD38− population, limiting dilution analysis in NOD/SCID mice (n = 52) was performed with day 0 AC133+CD38− uncultured cells and day 8 AC133+CD38− cultured cells. (C) Mice engrafted with AC133+CD38− cells were secondarily transplanted into NOD/SCID mice and analyzed for the presence of human (Ci) CD45+HLA-ABC+ and (Cii) CD19+ and CD33+ cells 8 weeks after transplantation (n = 2). Statistics were calculated using the Mann-Whitney U test.
The AC133+CD38− phenotype corresponds with LTC-IC and SRC function and expansion. UCB Lin− cells were sorted into 4 populations (AC133+CD38−, AC133+CD38+, AC133−CD38+, and AC133−CD38−) before and after culture and then plated in LTC-IC clonogenic assays (n ≥ 3) or transplanted into NOD/SCID mice. (Ai) The AC133+CD38− population contained significantly more LTC-ICs than the AC133+CD38+ (P = .002), AC133−CD38+ (P = .002), and AC133−CD38− (P = .009) populations. (Aii) AC133+CD38− cells were placed in either supportive (SCF, FMS-like tyrosine kinase 3 ligand, thrombopoietin) or differentiation (SCF, erythropoietin, interleukin-3, and GM-CSF) cultures. AC133+CD38− expression was 31.5% ± 4.4% versus 11.9% ± 3.7% (P = .04), and LTC-IC frequency was 68.4 plus or minus 13.5 and 13.6 ± 9.4 per 2000 cells (P < .01) in the supportive and differentiation cultures, respectively. (B) Cultured cells were sorted and transplanted intravenously into NOD/SCID mice, and analyzed 7 to 8 weeks after transplantation. Because the majority of SRC activity was present in the AC133+CD38− population, limiting dilution analysis in NOD/SCID mice (n = 52) was performed with day 0 AC133+CD38− uncultured cells and day 8 AC133+CD38− cultured cells. (C) Mice engrafted with AC133+CD38− cells were secondarily transplanted into NOD/SCID mice and analyzed for the presence of human (Ci) CD45+HLA-ABC+ and (Cii) CD19+ and CD33+ cells 8 weeks after transplantation (n = 2). Statistics were calculated using the Mann-Whitney U test.
To assess SRC activity, cells from the 4 subpopulations were transplanted into NOD/SCID mice. Engraftment of human cells was observed in 7 of 8 mice transplanted with either 5 × 104 or 1 × 105 bioreactor-derived AC133+CD38− cells. In contrast, 14 mice were transplanted with 5.5 × 104 to 5.1 × 106 cultured AC133−CD38− cells, but only 2 mice transplanted with 1.77 × 106 and 5.1 × 106 AC133−CD38− cells engrafted, and none of the mice transplanted with the entire sorted subpopulation of AC133+CD38+ (n = 7) or AC133−CD38+ (n = 10) cultured cells was engrafted. Thus, although there is a very low frequency of SRC present in the AC133−CD38− population as with uncultured CD34−CD38− cells,17 SRC activity is predominantly contained in the cultured AC133+CD38− subpopulation (supplemental Table 1).
Limiting dilution analysis was then performed to determine whether the AC133+CD38− phenotype corresponds with SRC expansion. The frequency of SRC in the uncultured and cultured AC133+CD38− population was 1 in 1297 (± 422) and 1 in 294 (± 87), respectively (after the 1 in 12 652 frequency is corrected for the 43-fold expansion of this subpopulation after culture, Figure 2B; supplemental Table 1). The data thus indicated a 4.4-fold SRC expansion after culture. We previously reported SRC expansions of 3.3-fold to 5-fold in our bioprocess3,16 ; therefore, the cultured AC133+CD38− cells contain the majority of the bioreactor-generated SRCs, and this phenotype corresponds well with SRC expansion. Moreover, bone marrow from primary recipients engrafted secondary recipients with both CD19+ and CD33+ human cells, thus indicating self-renewal potential of cultured AC133+CD38− marked SRCs (Figure 2C). Together, these data demonstrate that the AC133+CD38− phenotype reflects LTC-IC and SRC function and expansion in this particular bioprocess and therefore may be used to significantly enrich these cells in culture.
Markers capable of quantifying and enriching HSCs after culture are poorly developed. New markers and strategies are needed to facilitate high-throughput screens, prospectively predict the success of engraftment after transplantation of ex vivo manipulated HSCs, and enable real-time cell fate tracking studies in vitro. This study demonstrates that the AC133+CD38− phenotype is an improved strategy to enrich for and track primitive progenitors and HSC during ex vivo culture.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sherry Zhao at the Flow Cytometry Facility at the Hospital for Sick Children Toronto for her expertise and help with cell sorting, John Dick and Monica Doedens for providing us with some NOD/SCID mice, Nobuko Yamanaka for her invaluable technical support, and Geoff Clarke, Kelly Purpura, William Stanford, and members of the Zandstra laboratory for their helpful discussions.
This work was supported by funding from the Canadian Institutes of Health Research (MOP-57885) and Natural Sciences and Engineering Research Council/Collaborative Research and Development (PJ 335195-05; P.W.Z.). P.W.Z. is a Canada Research Chair in Stem Cell Bioengineering.
Authorship
Contribution: C.Y.I. designed and performed the experiments, analyzed the data, and wrote and edited the paper; D.C.K. designed and performed the experiments and analyzed the data; G.J.M. assisted with data analysis; M.Y. provided technical support; I.R. provided the NOD/SCID mice and assisted with the transplantation studies; and P.W.Z. designed the experiments and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter W. Zandstra, University of Toronto, Donnelly Bldg CCBR, RM 1116, 160 College St, Toronto, ON, Canada M5S 3E1; e-mail: peter.zandstra@utoronto.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal