Abstract
B-cell chronic lymphocytic leukemia (CLL) is the most common human leukemia. 13q14 deletions are most common chromosomal alterations in CLL. We previously reported that miR-15/16 is a target of 13q14 deletions and plays a tumor suppressor role by targeting BCL2. Because DLEU7 is located near miR-15/16 and is also positioned within a minimal deleted region, we investigated whether DLEU7 could also play a tumor suppressor role. Recent studies of transgenic mouse models demonstrated the importance of the nuclear factor-κB (NF-κB) pathway in CLL. To examine the possible role of DLEU7 in CLL, we investigated the effect of DLEU7 expression on NF-κB and nuclear factor of activated T cells (NFAT) activity. We found that DLEU7 functions as a potent NF-κB and NFAT inhibitor by physically interacting and inhibiting TACI and BCMA, members of the tumor necrosis factor (TNF) receptor family involved in B-CLL. In addition, DLEU7 expression in A549 lung cancer cells resulted in a decrease in S phase and increased apoptosis. The results suggest that loss of DLEU7 may cooperate with the loss of miR-15/16 in the pathogenesis of CLL.
Introduction
Chronic lymphocytic leukemia (CLL) lymphocytes have mature appearance and the B220+CD5+ phenotype.1,2 Several chromosomal aberrations occur frequently in CLL cases, including 13q deletions (∼ 50%), 11q23 deletions (18%), trisomy 12 (12%), and 17p deletions (7%).3 The 13q14 deletion is the most common B-CLL aberration and is seen by cytogenetics in approximately half of the cases.3 13q14 is seen predominantly in the indolent form of CLL and is associated with low levels of ZAP70 expression and mutated variable region genes of immunoglobulins.4 Analysis of an approximately 30-kb deletion at 13q14.3 and chromosomal breakpoint mapping of translocation t(2:13)(q32;q14) led to the discovery of 2 physically linked microRNAs, miR-15a and miR-16-1, as targets of these deletions.5 Furthermore, both, miR-15a and miR-16-1 were reduced in expression in most CLL cases,5 and further studies indicated that miR-15a/miR-16-1 negatively regulate Bcl2 expression.6 These findings indicated that down-regulation of miR-15/16 and subsequent Bcl2 up-regulation contribute to CLL pathogenesis.5 A high-resolution map of 13q14 deletions using 171 CLL samples was recently reported.7 These data indicated that the minimal deleted region, in addition to miR-15/16, also contains the DLEU7 gene (Figure 1A).7 DLEU7 was previously identified as a candidate tumor suppressor gene at 13q14.8 It encodes a 221–amino acid protein with no homology to known proteins. No mutations in DLEU7 were found in 45 CLL samples, although the DLEU7 promoter was methylated in 61% of CLL.8 Since DLEU7 is the only protein-coding gene located within the minimal deleted region at 13q14, we investigated whether DLEU7 can function as a tumor suppressor.
Methods
CLL samples and real-time PCR experiments
A total of 25 CLL samples were obtained after informed consent from patients diagnosed with CLL from the CLL Research Consortium. Research was performed with the approval of the Institutional Review Board of Ohio State University. Briefly, blood was obtained from patients with CLL, and lymphocytes were isolated through Ficoll/Hypaque gradient centrifugation (Amersham Biosciences) and processed for RNA extraction by using the standard TRIzol method (Invitrogen). Real-time polymerase chain reaction (PCR) experiments were carried out using Hs004155700_m1 (DLEU7), 000389 (miR-15a), and 000391 (miR-16-1) assays for real-time PCR (Applied Biosystems) according to the manufacturer's protocol. GAPDH was used as a control.
DNA constructs
Expression constructs, containing full-length human DLEU7, TNFRSF13B (TACI), TNFRSF17 (BCMA), TNFRSF5 (CD40), and TRAF6 were purchased from OriGene. Expression constructs, containing Myc-tagged open reading frames (ORFs) of human TACI and BCMA, named TACI-myc and BCMA-myc, respectively, were also purchased from OriGene. Full-length human DLEU7 ORF with an HA tag was cloned into a pCMV5 vector9 to obtain the pCMV5-hDLEU7-HA construct. The GLTX5-HA construct was created by cloning GLTX5 ORF into the pCMV-HA vector (BD Biosciences). To obtain expression constructs encoding GFP-hDLEU7 and FHIT-GFP fusion proteins hDLEU7 and FHIT ORFs were cloned into a pEGFP-N1 vector from Clontech.
Dual-luciferase Reporter Assay System and Renilla luciferase reporter vector pRL-TK were purchased from Promega. The NF-κB reporter construct pNF-κB-Luc was purchased from Stratagene. The NFAT reporterconstruct pNFAT-Luc was purchased from Affymetrix. The construct encoding NFATC1 (NFAT2) under the cytomegalovirus (CMV) promoter, pCMV-NFAT, was purchased from OriGene.
Cell culture, transfection, Western blotting, and immunoprecipitation experiments
HEK 293, NIH-3T3, A549, K562, SUPT11, CA46 and Daudi cells were grown in RPMI 1640 medium with 10% fetal bovine serum (FBS) and 25 μg/mL gentamicin at 37°C in a humidified atmosphere of 5% CO2 in air. Human CD19+ B cells were purchased from Lonza. FuGene 6 transfection reagent and protease inhibitor cocktail tablets were obtained from Roche. Transfections, except luciferase assay experiments; cell lysate preparations and Western blot analysis were carried out as described previously.10 Immunoblots were developed using Pierce ECL Western Blotting substrate or SuperSignal West Femto Maximum Sensitivity Substrate from Thermo Scientific. Human recombinant TNF-α was purchased from Calbiochem. Antibodies used for Western 6blots and immunoprecipitation experiments were: anti-Dleu7, anti-Myc, anti-Myc–HRP, anti-GFP, anti-GFP–HRP (Santa Cruz Biotechnology), anti-HA (Covance), and anti-HA–HRP (Roche).
Immunofluorescence
NIH-3T3 cells were grown on Human Fibronectin Cellware 2-Well CultureSlides (BD Biosciences). Immunofluorescence experiments were carried out as previously described using a Zeiss LCM 510 confocal microscope, a 63×/1.2 W water objective, and MBF ImageJ software.11 Secondary antibodies used for immunofluorescence were as follows: goat anti–mouse Alexa Fluor 546 (red) and goat anti–rabbit Alexa Fluor 488 (green), all purchased from Invitrogen.
Cell-cycle and apoptosis analysis
Recombinant adenovirus expressing human DLEU7 and GFP (Ad5-DLEU7) was obtained using the BD Adeno-X Expression System 1 (BD Biosciences) according to the manufacturer's protocol. Packaged recombinant adenovirus particles were harvested by lysing transfected HEK 293 cells. Consequent cell-cycle and apoptosis experiments were performed in the A549 lung cancer cell line, which was infected with Ad5-control or Ad5-DLEU7 virus. Flow cytometry experiments were carried out on BD LSR II flow cytometer, controlled by BD FACSDiva software (BD Biosciences). Cell-cycle experiments were carried out using the Click-It EdU Flow Cytometry Assay Kit from Invitrogen according to the manufacturer's protocol. Apoptotic cells were detected using APC annexin V from BD Biosciences and the MitoProbe DiIC1 (5) Assay Kit for Flow Cytometry from Molecular Probes (Invitrogen).
Luciferase assay
HEK 293 cells were transfected with the indicated constructs. Firefly and renilla luciferase activities were assayed with the dual luciferase assay system (Promega) and firefly luciferase activity was normalized to renilla luciferase activity, as suggested by the manufacturer. All experiments were carried out in triplicate and repeated 3 times with consistent results.
Results
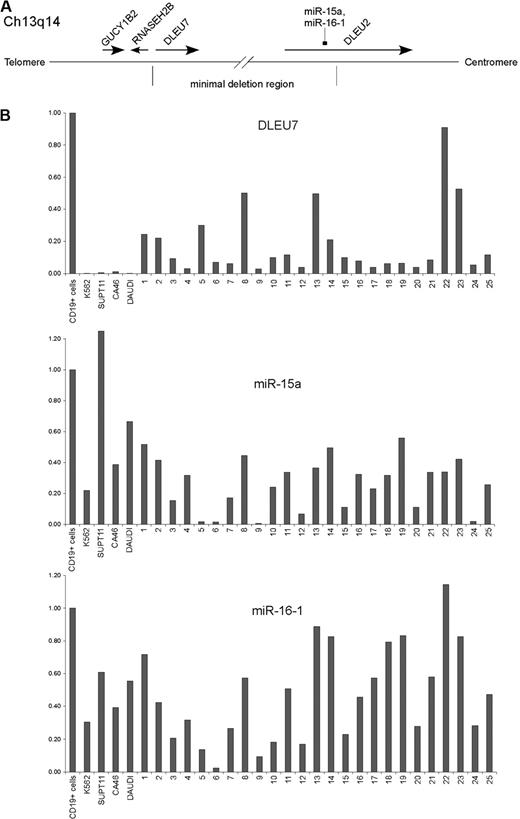
We first examined the expression of DLEU7, miR-15a and miR-16-1 in B-CLL samples. Figure 1B shows real-time reverse transcriptase (RT)–PCR results in normal CD19+ B cells, 4 leukemia cell lines (K562, SUPT11, CA46, and Daudi) and 25 CLL samples (1-25). All 25 B-CLL samples showed decreased DLEU7 expression, while 13 of 25 samples showed decreases of 10-fold or more (Figure 1B). miR-15a expression was decreased in all B-CLL samples compared with normal CD19+ B cells, and 23 of 25 samples showed decreases of 2-fold or more. Similarly, miR-16-1 expression was decreased in 24 of 25 samples (Figure 1B). These results are consistent with previously reported observations that DLEU7 essentially is not expressed in CLL,8 and that miR-15/16 expression is down-regulated in CLL.5
DLEU7 at 13q14. (A) Schematic representation of minimal deleted region at 13q14 in CLL (adapted from Ouillette et al7 Fig 2 ). (B) Expression of DLEU7, miR-15a, and miR-16-1 in B-CLL samples. Relative expression levels of DLEU7, miR-15a, and miR-16-1 in 25 B-CLL samples, 4 lymphoid cell lines, and in normal CD19+ cells were assayed by real-time PCR. Expression levels of DLEU7, miR-15a, and miR-16-1 in CD19+ cells was set at 1. Expression levels of DLEU7, miR-15a, and miR-16-1 were normalized to the expression of GAPDH.
DLEU7 at 13q14. (A) Schematic representation of minimal deleted region at 13q14 in CLL (adapted from Ouillette et al7 Fig 2 ). (B) Expression of DLEU7, miR-15a, and miR-16-1 in B-CLL samples. Relative expression levels of DLEU7, miR-15a, and miR-16-1 in 25 B-CLL samples, 4 lymphoid cell lines, and in normal CD19+ cells were assayed by real-time PCR. Expression levels of DLEU7, miR-15a, and miR-16-1 in CD19+ cells was set at 1. Expression levels of DLEU7, miR-15a, and miR-16-1 were normalized to the expression of GAPDH.
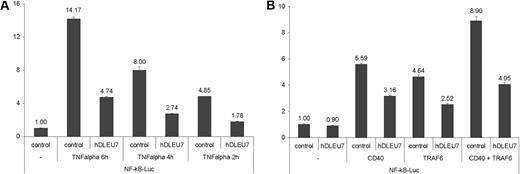
We proceeded to determine whether DLEU7 could play a tumor suppressor role in CLL. Recent studies of transgenic mouse models demonstrated the importance of the NF-κB pathway in CLL (reviewed in Pekarsky et al12 ). For example, transgenic expression of a proliferation-inducing TNF ligand (APRIL), a member of the tumor necrosis factor (TNF) superfamily involved in NF-κB activation, resulted in significant expansions of B220+CD5+ cells.13 Recently, we reported that Tcl1 activation caused B-CLL by activation of the NF-κB pathway and inhibition of AP-1.14 Since recent functional studies and studies of animal models suggested a role for the NF-κB pathway in the pathogenesis of CLL,12 we examined the possibility that Dleu7 might function as an inhibitor of NF-κB. NF-κB can be routinely activated in mammalian cells by TNF-α stimulation.15 To determine whether Dleu7 expression affects the transactivating activity of NF-κB, we used a commercial NF-κB reporter construct, pNF-κB-Luc, expressing luciferase under the control of an NF-κB–responsive element. NIH-3T3 cells were transfected with the constructs indicated in Figure 2A, and the NF-κB reporter was activated by treatment with TNF-α for 2, 4, or 6 hours. Figure 2A shows that Dleu7 expression inhibits NF-κB activity approximately 2.5- to 3-fold in each of 3 time points. NF-κB activity could also be activated by overexpression of various molecules involved in TNF–NF-κB pathway.16,17 To confirm that Dleu7 can function as an inhibitor of NF-κB, we activated NF-κB activity in 293 cells by TRAF5, TRAF6, and CD40 and investigated whether Dleu7 inhibits NF-κB in these settings. HEK 293 cells were transfected with the constructs indicated in Figure 2B. Figure 2B shows that Dleu7 significantly inhibits NF-κB activity induced by overexpression of TRAF6, CD40, and both TRAF6 and CD40. No such effect was observed when NF-κB was activated by TRAF5 (data not shown).
Dleu7 inhibits NF-κB–dependent transcription. (A) NIH-3T3 cells were cotransfected with 250 ng of pNF-κB-Luc reporter and 50 ng of pRL-TK Renilla reporter constructs. In addition, 1.5 mg of CMV5-empty vector or 1.5 mg of CMV5-hDLEU7-HA was used. In addition, cells were treated with 50 ng/mL TNF-α for 6, 4, or 2 hours before lysis where indicated. Firefly and Renilla luciferase activities were assayed with the Dual-Luciferase Assay System from Promega, and firefly luciferase activity was normalized to Renilla luciferase activity. The normalized promoter activity of pNF-κB-Luc in NIH-3T3 cells transfected with CMV5-empty vector without TNF-α treatment was set as 1. Experiments were repeated 3 times in triplicate. Columns indicate the mean; bars, SD. (B) HEK 293 cells were cotransfected with 50 ng of pNF-κB-Luc reporter and 50 ng of pRL-TK Renilla reporter constructs. In addition, 1.5 mg of CMV5-empty vector or 1.5 mg of CMV5-hDLEU7-HA was used. In addition, 100 ng of CMV6-CD40, CMV6-TRAF6, or a combination of both were used where indicated. Firefly and Renilla luciferase activities were assayed with the Dual-Luciferase Assay System from Promega, and firefly luciferase activity was normalized to Renilla luciferase activity. The normalized promoter activity of pNF-κB-Luc in HEK 293 cells transfected with CMV5-empty vector was set as 1. Experiments were repeated 3 times in triplicate. Columns indicate the mean; bars, SD.
Dleu7 inhibits NF-κB–dependent transcription. (A) NIH-3T3 cells were cotransfected with 250 ng of pNF-κB-Luc reporter and 50 ng of pRL-TK Renilla reporter constructs. In addition, 1.5 mg of CMV5-empty vector or 1.5 mg of CMV5-hDLEU7-HA was used. In addition, cells were treated with 50 ng/mL TNF-α for 6, 4, or 2 hours before lysis where indicated. Firefly and Renilla luciferase activities were assayed with the Dual-Luciferase Assay System from Promega, and firefly luciferase activity was normalized to Renilla luciferase activity. The normalized promoter activity of pNF-κB-Luc in NIH-3T3 cells transfected with CMV5-empty vector without TNF-α treatment was set as 1. Experiments were repeated 3 times in triplicate. Columns indicate the mean; bars, SD. (B) HEK 293 cells were cotransfected with 50 ng of pNF-κB-Luc reporter and 50 ng of pRL-TK Renilla reporter constructs. In addition, 1.5 mg of CMV5-empty vector or 1.5 mg of CMV5-hDLEU7-HA was used. In addition, 100 ng of CMV6-CD40, CMV6-TRAF6, or a combination of both were used where indicated. Firefly and Renilla luciferase activities were assayed with the Dual-Luciferase Assay System from Promega, and firefly luciferase activity was normalized to Renilla luciferase activity. The normalized promoter activity of pNF-κB-Luc in HEK 293 cells transfected with CMV5-empty vector was set as 1. Experiments were repeated 3 times in triplicate. Columns indicate the mean; bars, SD.
A previous study described a transgenic mouse line specifically expressing APRIL (a proliferation-inducing ligand) in lymphoid cells.13 An assessment of 9- to 12-month-old transgenics by flow cytometric analysis revealed significant expansions of B220+CD5+ cells in mesenteric lymph nodes and Peyer patches.13 The incidence and severity of these alterations increased over time, suggesting progressive expansion of mature CD5+ B cells, a disease similar to human CLL. APRIL and its close relative BAFF are 2 recent members of the TNF superfamily, expressing almost exclusively in hematopoietic cells.18 Both BAFF and APRIL show elevated expression levels in patients with various B-cell malignancies, including diffuse large-cell lymphoma, mantle cell lymphoma, and CLL.18 APRIL binds with high affinity to 2 receptors, BCMA (B-cell maturation antigen) and TACI.18 BCMA is detected in mature B and T cells, whereas TACI is mostly expressed in activated T cells and subpopulations of B cells.18 BCMA and TACI are members of the TNF receptor superfamily.18 They interact with various TRAFs and stimulate the NF-κB pathway.18 All these data suggest that NF-κB activation through TACI and BCMA is important in the pathogenesis of CLL. Because we found that Dleu7 functions as an inhibitor of NF-κB activation by the TNF pathway, we investigated whether Dleu7 expression has an effect on NF-κB activation by TACI and BCMA.
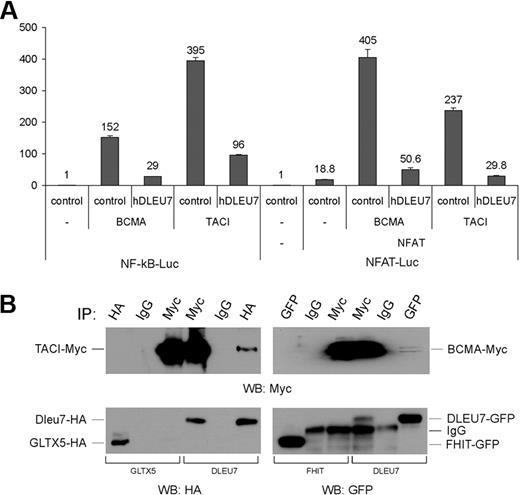
To test this hypothesis, we carried out experiments similar to that described in Figure 2B. HEK 293 cells were transfected with constructs indicated on Figure 3A (left). BCMA expression resulted in 152-fold NF-κB activation, which was inhibited by Dleu7 more than 5-fold (Figure 3A left). Similarly, Figure 3A (left) shows that Dleu7 expression inhibited NF-κB activation by TACI more than 4-fold. Thus, these data clearly indicate that Dleu7 functions as an NF-κB inhibitor in a pathway critical for the pathogenesis of CLL.
Interaction of Dleu7 with members of the TNFR superfamily, BCMA and TACI, interferes with NF-κB and NFAT trans-activation. (A) HEK293 cells were cotransfected with 50 ng of pNF-κB-Luc reporter or 250 ng of pNFAT-Luc reporter, respectively, and 50 ng of pRL-TK Renilla reporter constructs. In addition, 1.5 μg of CMV5-empty vector or 1.5 μg of CMV5-hDLEU7-HA was used. In addition, 250 ng of CMV6-BCMA and 25 ng of CMV6-TACI (in the case of NF-κB-Luc) and 5 ng of CMV-NFAT, 100 ng of CMV6-BCMA and 5 ng of CMV6-TACI (in the case of NFAT-Luc) were used where indicated. Firefly and Renilla luciferase activities were assayed with the Dual-Luciferase Assay System from Promega, and firefly luciferase activity was normalized to Renilla luciferase activity. The normalized promoter activity of pNF-κB-Luc and pNFAT-Luc in HEK 293 cells transfected with CMV5-empty vector was set as 1, respectively. Experiments were repeated 3 times in triplicate. Columns indicate the mean; bars, SD. (B) Left panels show HEK 293 cells cotransfected with GLTX5-HA (negative control) and TACI-Myc, or hDLEU7-HA and TACI-Myc constructs. After lysis, immunoprecipitations were carried out using anti-HA antibody, mouse immunoglobulin G, or anti-Myc antibody. Western blotting was carried out as indicated. Right panels show HEK 293 cells cotransfected with BCMA-myc and FHIT-GFP (negative control), or BCMA-Myc and hDLEU7-GFP constructs. After lysis, immunoprecipitations were carried out using anti-GFP antibody, mouse immunoglobulin G, or anti-Myc antibody. Western blotting was carried out as indicated.
Interaction of Dleu7 with members of the TNFR superfamily, BCMA and TACI, interferes with NF-κB and NFAT trans-activation. (A) HEK293 cells were cotransfected with 50 ng of pNF-κB-Luc reporter or 250 ng of pNFAT-Luc reporter, respectively, and 50 ng of pRL-TK Renilla reporter constructs. In addition, 1.5 μg of CMV5-empty vector or 1.5 μg of CMV5-hDLEU7-HA was used. In addition, 250 ng of CMV6-BCMA and 25 ng of CMV6-TACI (in the case of NF-κB-Luc) and 5 ng of CMV-NFAT, 100 ng of CMV6-BCMA and 5 ng of CMV6-TACI (in the case of NFAT-Luc) were used where indicated. Firefly and Renilla luciferase activities were assayed with the Dual-Luciferase Assay System from Promega, and firefly luciferase activity was normalized to Renilla luciferase activity. The normalized promoter activity of pNF-κB-Luc and pNFAT-Luc in HEK 293 cells transfected with CMV5-empty vector was set as 1, respectively. Experiments were repeated 3 times in triplicate. Columns indicate the mean; bars, SD. (B) Left panels show HEK 293 cells cotransfected with GLTX5-HA (negative control) and TACI-Myc, or hDLEU7-HA and TACI-Myc constructs. After lysis, immunoprecipitations were carried out using anti-HA antibody, mouse immunoglobulin G, or anti-Myc antibody. Western blotting was carried out as indicated. Right panels show HEK 293 cells cotransfected with BCMA-myc and FHIT-GFP (negative control), or BCMA-Myc and hDLEU7-GFP constructs. After lysis, immunoprecipitations were carried out using anti-GFP antibody, mouse immunoglobulin G, or anti-Myc antibody. Western blotting was carried out as indicated.
NFAT can be activated by TACI and BCMA.19 Previous reports showed that nuclear NFAT is a hallmark of unstimulated CLL cells, and that NFAT is necessary for CD5 expression in B cells.20,21 Since Dleu7 inhibits NF-κB activation by TACI and BCMA, we investigated whether Dleu7 can inhibit the ability of TACI and BCMA to induce NFAT transactivating function. To determine whether Dleu7 expression affects the transactivating activity of NFAT, we used a commercial NFAT reporter construct, pNFAT-Luc, expressing luciferase under the control of an NFAT-responsive element. HEK 293 cells were transfected with constructs indicated in Figure 3A (right). NFAT alone activated the reporter approximately 18-fold, whereas expression of BCMA increased this activation to approximately 400-fold. Dleu7 expression inhibited this activation approximately 8-fold. Similar inhibition (∼ 8-fold) was observed when we used TACI to induce NFAT-dependent activation (Figure 3A right). Thus, we conclude that Dleu7 functions as NFAT inhibitor. Because TACI and BCMA activate NFAT by a different mechanism from that used for NF-κB activation,19 it is reasonable to speculate that Dleu7 functions as a TACI and BCMA inhibitor by direct association with TACI and BCMA. To test this hypothesis, we carried out coimmunoprecipitation experiments. HEK 293 cells were cotransfected with TACI-myc and DLEU7-HA, or GLTX5-HA (as a negative control). Figure 3B (left) shows that TACI was coimmunoprecipitated with Dleu7 in both directions, while no positive coimmunoprecipitates were detected between GLTX5 (used as a negative control) and TACI. Similarly, HEK 293 cells were cotransfected with BCMA-myc and DLEU7-GFP, or FHIT-GFP (as a negative control). Figure 3B (right) shows that BCMA was coimmunoprecipitated with Dleu7 in both directions, while no positive coimmunoprecipitates were detected between FHIT (used as a negative control) and BCMA. These results indicate that Dleu7 functions as a TACI and BCMA inhibitor by direct association with these molecules.
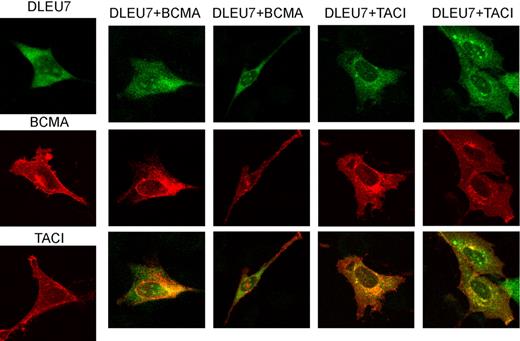
To determine intracellular localization of Dleu7 and its complexes with TACI and BCMA, we carried out immunofluorescence experiments. NIH-3T3 cells were used since B-CLL cell lines practically do not exist, and NIH-3T3 cells are commonly used for intracellular localization studies because of their morphology.10 NIH-3T3 cells were transfected with the indicated constructs. Figure 4 shows that Dleu7 alone mostly localized in the cytoplasm with weaker nuclear staining. TACI and BCMA also show cytoplasmic localization and significant presence at the plasma membrane. Figure 4 (bottom) shows that Dleu7-TACI and Dleu7-BCMA complexes (yellow) localized mostly in the cytoplasm; some colocalization is also evident at the plasma membrane. These data serve as additional evidence that Dleu7 inhibits TACI and BCMA function by direct association.
Intercellular localization of Dleu7, BCMA, and TACI. NIH-3T3 cells were transfected with DLEU7-HA, BCMA-Myc, and TACI-Myc constructs. After 16 hours, cells were fixed, permeabilized, and immunostained with rat anti-HA and mouse anti–c-Myc antibodies. Secondary goat anti–rat Alexa Fluor 488 and goat anti-mouse Alexa Fluor 546 antibodies were used to visualize intercellular localization of BCMA (red), TACI (red), and Dleu7 (green). Colocalization of BCMA and Dleu7 or TACI and Dleu7 is shown in yellow.
Intercellular localization of Dleu7, BCMA, and TACI. NIH-3T3 cells were transfected with DLEU7-HA, BCMA-Myc, and TACI-Myc constructs. After 16 hours, cells were fixed, permeabilized, and immunostained with rat anti-HA and mouse anti–c-Myc antibodies. Secondary goat anti–rat Alexa Fluor 488 and goat anti-mouse Alexa Fluor 546 antibodies were used to visualize intercellular localization of BCMA (red), TACI (red), and Dleu7 (green). Colocalization of BCMA and Dleu7 or TACI and Dleu7 is shown in yellow.
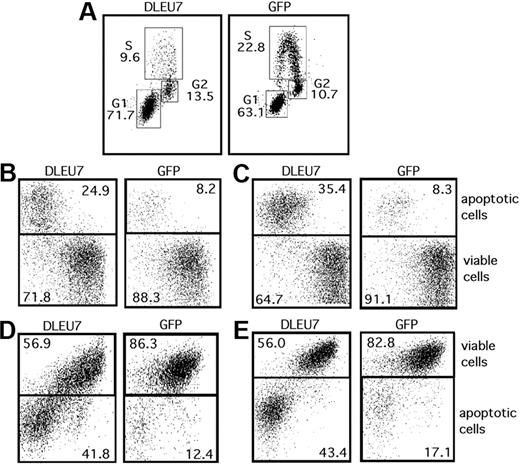
Since we proved that Dleu7 inhibits NF-κB– and NFAT-dependent transcription and inhibits TACI and BCMA function by direct association, it is reasonable to speculate that Dleu7's actions will result in increased cell death. Because there are very few CLL cell lines, we used A549 lung cancer cells extensively used previously in apoptotic studies.22 Apoptosis in these cells can be manipulated by exogenous gene expression as well as treatments with proapoptotic drugs.22 To show that this Dleu7 expression causes apoptosis, we used adenovirus expressing Dleu7 and GFP (Ad-DLEU7) and, as a control, adenovirus expressing GFP only (Ad-GFP). We first examined whether Dleu7 expression results in changes in the cell cycle. A total of 5 × 104 cells/well were infected with 80 multiplicity of infection (MOI) Ad-Dleu7 or Ad-GFP. After 36 hours, cell cycle was measured using an EdU assay kit. This method is superior to the older propidium iodide method because it achieves clear separation of the S phase (Figure 5). Figure 5A shows that Dleu7 expression results in a more than 2-fold decrease of cells in the S phase (9.6% vs 22.8%). At the same time, G1 and G2 populations were increased (71.7% vs 63.1% and 13.5% vs 10.7%, respectively). To determine whether Dleu7 expression has any effect on apoptosis, we used 2 different methods: incorporation of annexin V, in which apoptotic cells are stained and labeled with annexin V (Figure 5B-C); and DiIc1(5) assay, in which live cells are stained, but apoptotic cells remained unstained (Figure 5D-E). A total of 2.5 × 104 cells/well were infected with 350 MOI of Ad-Dleu7 or Ad-GFP. Apoptosis was measured 96 hours after infection (Figure 5B,D) and 120 hours after infection (Figure 5C,E). Figure 5B and C shows that Dleu7 expression resulted in more than 3-fold induction in apoptosis as measured by annexin V incorporation (24.9% vs 8.2% after 96 hours, and 35.4% vs 8.3% after 120 hours). Similar results were obtained using DiIc1(5) assay (41.8% vs 12.4% after 96 hours, and 43.4% vs 17.1% after 120 hours; Figure 5D-E). These data clearly indicate that Dleu7 expression results in increased apoptosis in A549 cells.
Dleu7 expression results in cell death. (A) A549 cells were infected with 80 MOI of Ad5-DLEU7 or Ad5-control virus; 2 days later, cells were harvested and used in the Click-iT EdU Flow Cytometry Assay. Plot data for cells labeled with Click-iT EdU Pacific Blue fluorescence (y-axis) and cells labeled with Click-iT CellCycle 488-Red (7-ADD) fluorescence (x-axis). A total of 3 subgroups, representing the percentage of cells in S phase, G1 phase, and G2 phase of cell cycle, are shown for cells infected with Ad5-DLEU7 and Ad5-control, respectively. (B-C) A549 cells were infected with 350 MOI of Ad5-DLEU7 or Ad5-control virus; 4 days (B) or 5 days (C) later, cells were harvested and treated with APC annexin V conjugate, which binds apoptotic cells. Panels show plot data for cells labeled with APC annexin V fluorescence (y-axis) and GFP green fluorescence for infected cells (x-axis). A total of 2 subgroups, representing the percentage of apoptotic cells and viable cells, are shown. (D-E) A549 cells were infected with 350 MOI of Ad5-DLEU7 or Ad5-control virus; 4 days (D) or 5 days (E) later, cells were harvested and treated with MitoProbe DiIC1(5) dye, which accumulates in mitochondria with active membrane potentials. DiIC1(5) stain intensity decreases in apoptotic cells. Panels show plot data for cells labeled with MitoProbe DiIC1(5) fluorescence (y-axis) and GFP green fluorescence for infected cells (x-axis). A total of 2 subgroups, representing the percentage of apoptotic cells and viable cells, are shown.
Dleu7 expression results in cell death. (A) A549 cells were infected with 80 MOI of Ad5-DLEU7 or Ad5-control virus; 2 days later, cells were harvested and used in the Click-iT EdU Flow Cytometry Assay. Plot data for cells labeled with Click-iT EdU Pacific Blue fluorescence (y-axis) and cells labeled with Click-iT CellCycle 488-Red (7-ADD) fluorescence (x-axis). A total of 3 subgroups, representing the percentage of cells in S phase, G1 phase, and G2 phase of cell cycle, are shown for cells infected with Ad5-DLEU7 and Ad5-control, respectively. (B-C) A549 cells were infected with 350 MOI of Ad5-DLEU7 or Ad5-control virus; 4 days (B) or 5 days (C) later, cells were harvested and treated with APC annexin V conjugate, which binds apoptotic cells. Panels show plot data for cells labeled with APC annexin V fluorescence (y-axis) and GFP green fluorescence for infected cells (x-axis). A total of 2 subgroups, representing the percentage of apoptotic cells and viable cells, are shown. (D-E) A549 cells were infected with 350 MOI of Ad5-DLEU7 or Ad5-control virus; 4 days (D) or 5 days (E) later, cells were harvested and treated with MitoProbe DiIC1(5) dye, which accumulates in mitochondria with active membrane potentials. DiIC1(5) stain intensity decreases in apoptotic cells. Panels show plot data for cells labeled with MitoProbe DiIC1(5) fluorescence (y-axis) and GFP green fluorescence for infected cells (x-axis). A total of 2 subgroups, representing the percentage of apoptotic cells and viable cells, are shown.
Discussion
The 13q14 deletion is the most common CLL aberration and is observed by cytogenetics in approximately half of the cases and by loss of heterozygosity (LOH) in approximately 70% of cases. Previous reports demonstrated that the miR-15a/miR-16-1 cluster is located within the deleted region and showed a reduction in expression of miR-15a/miR-16-1 in most CLL cases.5 A subsequent report demonstrated that miR-15/16 targets Bcl2 expression, contributing to the pathogenesis of CLL.6 A recent report described a high-resolution map of 13q14 and showed that DLEU7 is the only protein-coding gene located within the minimal deleted region at 13q14.7 This prompted us to investigate whether DLEU7 can function as a tumor suppressor. Our results clearly show that Dleu7 functions as a potent inhibitor of NF-κB signaling by inhibiting TACI and BCMA, critical transducers of proliferative signals in B cells. All 3 known mouse models of CLL use abnormal activation of the NF-κB pathway. Eμ-TCL1 mice abnormally express Tcl1 in mouse B cells, and Tcl1 functons as an activator of NF-κB–dependent transcription.14,23 The APRIL transgenic mouse model induces NF-κB signaling through the TNF pathway using TACI and BCMA as transducers.13 Finally, the BCL2/TRAF2DN mouse model of B-CLL also uses NF-κB because TRAFs are important transducers of NF-κB signaling.24 The importance of our results is greatly enhanced by the fact that both APRIL and BCL2/TRAF2DN CLL mouse models use the same pathway: transduction NF-κB–activating signals through TACI and BCMA.
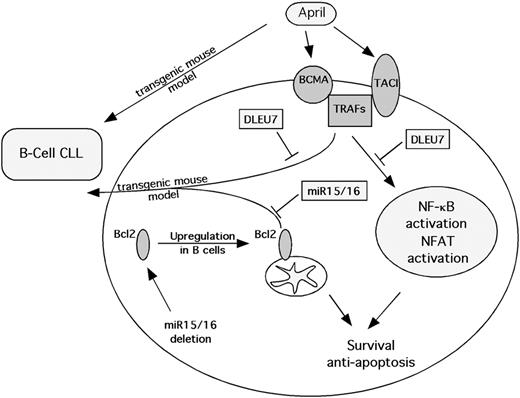
The BCL2/TRAF2DN transgenic mouse model represents a very interesting parallel and a confirmation of our findings. Bcl2 transgenic mice were produced by using a construct which mimics t(14;18) translocation, juxtaposing the BCL2 oncogene with the immunoglobulin heavy-chain locus at 14q32 observed in human follicular lymphomas. These mice did not develop any tumor phenotype until very late in life and did not show an increase in the B220+CD5+ cell population, but showed polyclonal expansions of B cells and prolonged B-cell survival in vitro.25 TRAF2 transgenics overexpressed the TRAF2 mutant, lacking the N-terminal RING and zinc finger domains located at the N-terminal of the molecule (TRAF2DN), which mimics TRAF1.26 These mice developed splenomegaly, lymphadenopathy, and an increased number of B cells, but failed to develop any frank hematologic malignancy and did not show an increase in CD5+ B cells. In contrast, TRAF2DN/Bcl2 double-transgenic mice over time developed severe splenomegaly, and most animals developed B-cell leukemias of a B220+CD5+ phenotype similar to CLL, with a B-cell blood count as high as 40 times that of normal, and died prematurely at the age of 6 to 14 months.24 Our results suggest that 13q14 contains 2 cooperating tumor suppressors, miR-15/16 and DLEU7. Inactivation of miR-15/16 causes a constitutive increase of Bcl2 expression, and DLEU7 inactivation causes induction of TNF signaling through TRAFs. These 2 events cause the CLL phenotype closely resembling that of TRAF2DN/Bcl2 transgenic mouse model. Figure 6 shows a schematic representation of the cooperation of Dleu7 and miR-15/16 in the pathogenesis of CLL. Deregulated expression of APRIL resulting in activation of NF-κB and NFAT causes CLL in mice. Normally, Dleu7 inhibits this activation by direct association with TACI and BCMA. Inactivation of DLEU7 leads to the loss of this inhibition, increase in survival, and inhibition of apoptosis. As mentioned, TRAF2 and Bcl2 cooperate in the development of CLL. Normally, miR-15/16 inhibits Bcl2 expression, and Dleu7 inhibits TRAFs by association with TACI and BCMA. Deletions at 13q14 result in the loss of miR-15/16 and DLEU7, 2 tumor suppressors. miR-15/16 deletions cause Bcl2 overexpression, and inactivation of DLEU7 causes induction of TNF signaling through TRAFs. Cooperation of these 2 events results in the pathogenesis of CLL.
Schematic representation of Dleu7 tumor-suppressor function in B cells.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The research was supported by an American Cancer Society Research Scholar grant (Y.P.) and National Institutes of Health grant PO1-CA81534 of the CLL Research Consortium (L.R., T.K., and C.M.C.).
National Institutes of Health
Authorship
Contribution: A.P., A.E., N.N., U.S., and H.A. performed research; L.R. and T.K. provided vital reagents; and Y.P. and C.M.C. analyzed data and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yuri Pekarsky, Comprehensive Cancer Center, Ohio State University, 460 W 12th Ave, BRT Rm 1090, Columbus, OH 43210; e-mail: pekarsky.yuri@osumc.edu.
References
Author notes
A.P. and A.E. contributed equally to the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal