Abstract
T cells contribute to the pathophysiology of ischemic stroke by yet unknown mechanisms. Mice with transgenic T-cell receptors (TCRs) and mutations in costimulatory molecules were used to define the minimal immunologic requirements for T cell–mediated ischemic brain damage. Stroke was induced in recombination activating gene 1–deficient (RAG1−/−) mice devoid of T and B cells, RAG1−/− mice reconstituted with B cells or T cells, TCR-transgenic mice bearing 1 single CD8+ (2C/RAG2, OTI/RAG1 mice) or CD4+ (OTII/RAG1, 2D2/RAG1 mice) TCR, mice lacking accessory molecules of TCR stimulation (CD28−/−, PD1−/−, B7-H1−/− mice), or mice deficient in nonclassical T cells (natural killer T [NKT] and γδ T cells) by transient middle cerebral artery occlusion (tMCAO). Stroke outcome was assessed at day 1. RAG1−/− mice and RAG1−/− mice reconstituted with B cells developed significantly smaller brain infarctions compared with controls, but thrombus formation after FeCl3-induced vessel injury was unimpaired. In contrast, TCR-transgenic mice and mice lacking costimulatory TCR signals were fully susceptible to tMCAO similar to mice lacking NKT and γδ T cells. These findings were corroborated by adoptive transfer experiments. Our data demonstrate that T cells critically contribute to cerebral ischemia, but their detrimental effect neither depends on antigen recognition nor TCR costimulation or thrombus formation.
Introduction
Ischemic stroke induces a profound local inflammatory response involving various types of immune cells.1 Although previous research mainly focused on the role of neutrophils and monocytes, the contribution of T cells during brain infarction is less clear.2-4 T cells have been identified in the brain as early as 24 hours after ischemia.5,6 Antibodies directed against lymphocyte adhesion receptors blocked T-cell transmigration and reduced stroke volumes at day 1 after transient middle cerebral artery occlusion (tMCAO) in rats.7,8 Two seminal papers9,10 described that recombination activating gene 1 (RAG1)–deficient mice, which lack functional T and B cells, are protected from cerebral ischemia after 24 hours, and that this protection is lost upon reconstitution of RAG1−/− mice with T cells from wild-type littermates. Detailed analysis of lymphocyte subsets revealed that T cells, but not B cells, are detrimental during stroke, but the underlying molecular mechanisms are unknown.9
T cells encompass 2 major subgroups defined by the expression of the CD4 or CD8 molecule: CD4+ helper and CD8+ cytotoxic T cells. T cells are usually activated by specific antigens presented on the surface of antigen-presenting cells (APCs). Numerous cell types such as monocytes, dendritic cells, or endothelial cells can serve as professional or nonprofessional APCs. Antigen recognition and T-cell activation is a 2-step process involving cellular surface molecules on both T cells and APCs. Signal 1 originates from the engagement of the T-cell receptor (TCR) and its coreceptors (CD4 and CD8) to antigens bound to the major histocompatibility complex (MHC) expressed on APCs. The second signal is mediated by costimulatory factors (eg, interleukin-2 [IL-2] or the ligation of cell-surface molecules complementary to TCR binding).11 Costimulatory molecules are mandatory for the full activation of T cells and can determine their fate: proliferation or anergy with ensuing cell death. Although it is well established that both CD4+ and CD8+ T cells are detrimental in brain ischemia/reperfusion (I/R) injury,9,10 the minimal immunologic requirements for their activation and the underlying intercellular mechanisms are unknown.
Usually, T cells can recognize a plethora of different antigens due to somatic recombination of the TCR. In the present study, we took advantage of transgenic mice bearing identical TCRs on CD4+ or CD8+ T cells, respectively (OTI/RAG1 mice, 2C/RAG2 mice, OTII/RAG1 mice, and 2D2/RAG1 mice).12-15 These genetically altered T cells can bind only 1 predefined antigen (eg, ovalbumin) and are unable to trigger a specific immune response against other antigens. Thus, they provide the unique opportunity to analyze whether the detrimental effect of T-cell subsets in stroke depends on antigen recognition (adaptive immunity) or antigen-nonspecific cellular interactions. To further analyze the role of accessory signals of TCR stimulation during stroke development, mice lacking essential costimulatory molecules such as CD28,16 programmed death 1 (PD1),17 or B7 homolog 1 (B7-H1)18 were used. In these mutants the full activation of T cells is repressed.
Another potential mechanism by which T cells could contribute to the pathophysiology of ischemic stroke is propagation of thrombus formation.19 To test whether T-cell deficiency generally alters the ability to form stable thrombi in mice, 2 well-established thrombus formation models were used: collagen-coated flow chambers allow comparison of platelet adhesion and aggregation in RAG1−/− or wild-type mice under in vitro conditions.20 To induce intravascular thrombus formation in vivo, FeCl3 was applied to the surface of mesenterial arteries.21 FeCl3 causes endothelial damage and subsequent exposure of the subendothelial matrix to the bloodstream, leading to rapidly evolving thrombi. In both models, thrombus formation can be efficiently inhibited by anti-GPIb and anti-GVI antibodies. Importantly, the same antibodies also protect mice from ischemic stroke after tMCAO, suggesting that similar mechanisms of thrombus formation are operative in vitro and in vivo.22
We here provide evidence that T cells exert antigen-independent detrimental effects during experimental stroke in mice and are dispensable for thrombus formation.
Methods
Animals
Animal experiments were approved by the Bezirksregierung of Unterfranken and conducted according to recent expert recommendations for research in basic stroke studies.23
A total of 219 mice were used in this study. Transgenic mice were backcrossed for at least 10 generations to the C57Bl/6 background except for OTI/RAG1 mice, which were backcrossed for at least 10 generations to the 129/SJ background. RAG1−/− mice completely lack mature T cells and B cells.24 The following TCR-transgenic mice (on RAG1−/− or RAG2−/− background) were used: MHC class I–restricted OTI/RAG1 mice specific for ovalbumin257-264,12 MHC class I–restricted high-affinity 2C/RAG2 mice specific for the peptide sequence SIYRYYGL,13 MHC class II–restricted OTII/RAG1 mice specific for ovalbumin323-339,14 and MHC class II–restricted 2D2/RAG1 mice specific for MOG35-55.15 For analyzing the significance of TCR costimulation during experimental stroke, CD28−/− mice (The Jackson Laboratory; stock no. 002666),16 PD1−/− mice17 (kindly provided by T. Honjo, Department of Medical Chemistry, Graduate School of Medicine, Kyoto University, Kyoto, Japan), and B7-H1−/− mice (generated by L. Chen, Department of Dermatology, Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD)18 were used. To assess the contribution of specialized lymphocyte subsets placed in between the adaptive and innate immune system for early stroke development, mice devoid of natural killer T (NKT) cells (CD1d−/−; kindly provided by M. Lutz, University of Wuerzburg)25 and γδ T cells (The Jackson Laboratory; stock no. 002120)26 were included. Commercial C57Bl/6 mice (Harlan Winkelmann) always served as wild-type (WT) controls.
A synopsis of the different transgenic mouse strains used in this study is provided in Table 1.
Immunologic characteristics of the different transgenic mouse strains
| Strain . | Specifity . | Deficiency . | Genetic background . |
|---|---|---|---|
| 2C/RAG2 | TCR-transgenic mouse, specificity for SIYRYYG, a neoantigen, MHC I–restricted | Mature T and B cells other than specific TCR | C57Bl/6 (> 10 generations) |
| OTI/RAG1 | TCR-transgenic mouse, specificity for a peptide from ovalbumin, SIINFEKL, MHC I–restricted | Mature T and B cells other than specific TCR | 129/SJ (> 10 generations) |
| OTII/RAG1 | TCR-transgenic mouse, specificity for a peptide from ovalbumin, SIINFEKL, MHC II–restricted | Mature T and B cells other than specific TCR | C57Bl/6 (> 10 generations) |
| 2D2/RAG1 | TCR-transgenic mouse, specificity for a peptide from myelin-oligodendrocyte protein (MOG35–55), MHC II–restricted | Mature T and B cells other than specific TCR | C57Bl/6 (> 10 generations) |
| CD28−/− | — | Costimulatory TCR CD28 | C57Bl/6 (> 10 generations) |
| B7-H1−/− | — | Coinhibitory TCR ligand B7-H1 (CD274) | C57Bl/6 (> 10 generations) |
| PD1−/− | — | Coinhibitory TCR PD1 | C57Bl/6 (> 10 generations) |
| CD1d−/− | — | NKT cells | C57Bl/6 (> 10 generations) |
| γΔ−/− | — | γΔ T cells | C57Bl/6 (> 10 generations) |
| Strain . | Specifity . | Deficiency . | Genetic background . |
|---|---|---|---|
| 2C/RAG2 | TCR-transgenic mouse, specificity for SIYRYYG, a neoantigen, MHC I–restricted | Mature T and B cells other than specific TCR | C57Bl/6 (> 10 generations) |
| OTI/RAG1 | TCR-transgenic mouse, specificity for a peptide from ovalbumin, SIINFEKL, MHC I–restricted | Mature T and B cells other than specific TCR | 129/SJ (> 10 generations) |
| OTII/RAG1 | TCR-transgenic mouse, specificity for a peptide from ovalbumin, SIINFEKL, MHC II–restricted | Mature T and B cells other than specific TCR | C57Bl/6 (> 10 generations) |
| 2D2/RAG1 | TCR-transgenic mouse, specificity for a peptide from myelin-oligodendrocyte protein (MOG35–55), MHC II–restricted | Mature T and B cells other than specific TCR | C57Bl/6 (> 10 generations) |
| CD28−/− | — | Costimulatory TCR CD28 | C57Bl/6 (> 10 generations) |
| B7-H1−/− | — | Coinhibitory TCR ligand B7-H1 (CD274) | C57Bl/6 (> 10 generations) |
| PD1−/− | — | Coinhibitory TCR PD1 | C57Bl/6 (> 10 generations) |
| CD1d−/− | — | NKT cells | C57Bl/6 (> 10 generations) |
| γΔ−/− | — | γΔ T cells | C57Bl/6 (> 10 generations) |
— indicates not applicable.
Induction of cerebral ischemia
Focal cerebral ischemia was induced in 6- to 8-week-old mice by 60 minutes of tMCAO as described.21,22,27 Briefly, mice were anesthetized with 2.5% isoflurane (Abbott) in a 70% N2O/30% O2 mixture. After a midline skin incision in the neck, the proximal common carotid artery and the external carotid artery were ligated, and a standardized silicon rubber-coated 6.0 nylon monofilament (6021910; Doccol Corp) was inserted and advanced via the right internal carotid artery to occlude the origin of the right MCA. Operators (P.K., T.S., M.A., and C.K.) were unaware of the genotype, and operation time per animal did not exceed 15 minutes. The intraluminal suture was left in situ for 60 minutes. Animals were then reanesthetized, and the occluding monofilament was withdrawn to allow reperfusion.
For reconstitution of RAG1−/− mice with B cells, B cells were isolated from spleen-cell suspensions of WT C57Bl/6 mice in RPMI medium containing 1% l-glutamine, 1% fetal calf serum (FCS), and 1% penicillin/streptomycin. After removal of red blood cells with ammonium chloride, B cells were enriched using CD19 microbeads (Miltenyi Biotec). B cells were resuspended to 107 cells per 100 μL in phosphate-buffered saline (PBS) and subsequently injected intravenously into RAG1−/− mice. After 24 hours, blood from reconstituted mice was stained for B220, CD4, and CD8 (BD) and analyzed on a FACScalibur (BD; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After that, B cell–reconstituted RAG1−/− mice were immediately subjected to tMCAO (n = 10). For reconstitution of RAG1−/− mice with CD3+ T cells, immune cells were isolated from spleen and lymph node cell suspensions of WT C57Bl/6 mice in RPMI medium containing 1% l-glutamine, 1% FCS, and 1% penicillin/streptomycin. After removal of red blood cells with ammonium chloride, T cells were enriched using the Pan T Cell Isolation Kit (Miltenyi Biotec), resuspended to 107 cells per 100 μL in PBS and subsequently injected intravenously into RAG1−/− mice. T cell–reconstituted RAG1−/− mice were subjected to tMCAO 24 hours after injection (n = 5). In addition, RAG1−/− mice were reconstituted with immune cells from OTI/RAG1 mice, 2D2/RAG1 mice, CD28−/− mice, and PD1−/− mice. For that, single-cell suspensions were generated by mashing the spleens and lymph nodes through a 70-μm strainer and lysing red blood cells with ammonium chloride. After that, 1.5 × 107 immune cells resuspended in 100 μL PBS were injected intravenously into RAG1−/− mice that were subjected to tMCAO 24 hours afterward (n = 5/group).
Assessment of functional outcome
Neurologic function was assessed by 3 independent and blinded investigators (M.A., P.K., and T.S.). At 24 hours after tMCAO, the modified Bederson score28 was used to determine global neurologic function according to the following scoring system: 0, no deficit; 1, forelimb flexion; 2, decreased resistance to lateral push; 3, unidirectional circling; 4, longitudinal spinning; and 5, no movement. Motor function and coordination were evaluated by the grip test.29 For this test, the mouse was placed midway on a string between 2 supports and rated as follows: 0, falls off; 1, hangs onto string by 1 or both forepaws; 2, same as for 1, and attempts to climb onto string; 3, hangs onto string by 1 or both forepaws plus 1 or both hindpaws; 4, hangs onto string by fore- and hindpaws plus tail wrapped around string; and 5, escape (to the supports).
Determination of infarct size
Animals were killed 24 hours after tMCAO. Brains were quickly removed and cut in three 2-mm-thick coronal sections using a mouse brain slice matrix (Harvard Apparatus). The slices were stained for 20 minutes at 37°C with 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich) in PBS to visualize the infarctions.30 Planimetric measurements (ImageJ software; National Institutes of Health) were performed blinded to the groups and edema-corrected infarct volumes were quantified as described.21,22,27
Laser-Doppler flowmetry
Laser-Doppler flowmetry (Moor Instruments) was used to monitor regional cerebral blood flow (rCBF) in the MCA territory (6 mm lateral and 2 mm posterior from bregma) of WT mice, RAG1−/− mice, CD1d−/− mice, PD1−/− mice, OTI/RAG1 mice, OTII/RAG1 mice, CD28−/− mice, B7-H1−/− mice, and 2D2/RAG1 mice (n = 4/group).31 After advancing the thread, the decrease in rCBF was similar in all groups (P > .05), indicating on the one hand sufficient occlusion of the MCA and on the other hand excluding preformed differences in rCBF between the genotypes (supplemental Figure 2A). At 10 minutes after reperfusion, rCBF was reconstituted to more than 70% of baseline levels and again did not significantly differ between WT and transgenic mice (P > .05).
Assessment of the cerebral vasculature
For assessment of the cerebral vasculature, WT and RAG1−/− mice (n = 4/group) were deeply anesthetized with CO2 and transcardially perfused with 4% paraformaldehyde (PFA), followed by 3 mL of black ink diluted in 4% PFA (1:5 vol/vol). Brains were carefully removed, fixed in4% PFA overnight at 4°C, and the Circle of Willis and major arteries were examined under a microscope (supplemental Figure 2B). A complete Circle of Willis was identified in all animals studied, and the distribution of the MCA trunk and branch appeared to be anatomically identical among the genotypes. To further quantitatively examine the vascular structures, the development of the posterior communicating arteries (PComAs), which can affect brain sensitivity to ischemia,32 was examined. The mean score of PComAs in both hemispheres showed no significant differences between WT and RAG1−/− mutants (3.3 ± 0.6 in WT mice vs 3.8 ± 0.8 in RAG1−/− mice; P > .05).
Platelet adhesion under flow conditions
Rectangular coverslips (24 × 60 mm) were coated with 0.2 mg/mL fibrillar type I collagen (Nycomed) for 1 hour at 37°C and blocked with 1% bovine serum albumin (BSA). Heparinized whole blood was perfused as previously described.20 Image analysis was performed offline using Metavue software (Visitron). Thrombus formation was expressed as the mean percentage of total area covered by thrombi.
Intravital microscopy of thrombus formation in FeCl3-injured mesenteric arterioles
RAG1−/− mice or WT controls were anesthetized, and the mesentery was exteriorized through a midline abdominal incision. Arterioles measuring 35 to 60 μm in diameter were visualized at 10-fold magnification with an inverted microscope (Axiovert 200; Carl Zeiss Inc) equipped with a 100-W HBO fluorescent lamp source and a camera (CoolSNAP-EZ; Visitron). Digital images were recorded and analyzed offline using Metavue software. Injury was induced by topical application of a 3-mm2 filter paper saturated with 20% FeCl3 for 10 seconds. Adhesion and aggregation of fluorescently labeled platelets (DyLight 488–conjugated anti-GPIX Ig derivative) in arterioles was monitored for 40 minutes or until complete occlusion occurred (blood flow stopped for more than 1 minute).
Statistical analysis
Data are expressed as means plus or minus SD. For statistical analysis, the PrismGraph 4.0 software package was used (GraphPad Software). Infarct volumes and neurologic scores were tested for Gaussian distribution with the D'Agostino and Pearson omnibus normality test and then analyzed by Bonferroni-corrected 1-way analysis of variance (ANOVA). Data from clotting assays were compared using the 2-tailed Student t test. P values less than .05 were considered statistically significant. Detailed power and type-II (beta) error calculations on infarct volumes are provided in the supplemental Methods.
Results
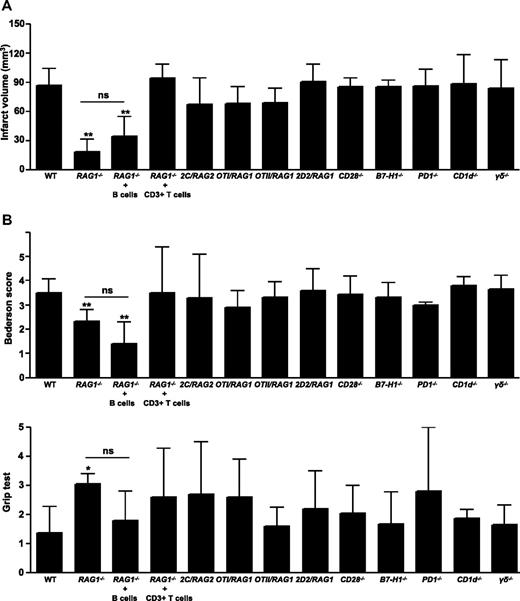
To confirm that T cells play a major role in the pathophysiology of acute ischemic stroke,9,10 RAG1−/− mice, which completely lack mature T cells and B cells, were subjected to 60 minutes of tMCAO. At 24 hours after reperfusion, the infarct volumes in RAG1−/− mice were significantly reduced to approximately 30% of the infarct volumes in WT mice (18.6 ± 12.5 mm3 vs 67.9 ± 16.7 mm3; P < .001), which is in line with previous reports (Figure 1A; supplemental Figure 3).9,10 The reduction in infarct size was functionally relevant, as the Bederson score (2.3 ± 0.5 vs 3.5 ± 0.6; P < .001) assessing global neurologic function and the grip test (3.1 ± 0.4 vs 1.4 ± 0.9; P < .05) that specifically measures motor function were significantly better in RAG1−/− mice (Figure 1B). Reconstitution of RAG1−/− mice with B cells did not significantly affect stroke protection (Figure 1A-B; supplemental Figure 3), indicating that B cells are probably less important for early stroke evolution. The pathophysiologic relevance of T cells was further underlined in RAG1−/− mice reconstituted with CD3+ T cells, because these animals were again fully susceptible to tMCAO and developed infarctions and neurologic deficits similar to WT controls (Figure 1A-B; supplemental Figure 3). This confirms previous reports.9,10
Infarct volumes and functional outcomes 24 hours after focal cerebral ischemia in WT mice, RAG1−/− mice lacking T cells and B cells, RAG1−/− mice reconstituted with B cells or CD3+ T cells, TCR-transgenic mice bearing only a single CD8+ (2C/RAG2, OTI/RAG1) or CD4+ (OTII/RAG1, 2D2/RAG1) TCR clone of defined specificity, mice lacking essential counterreceptors of TCR costimulation (CD28−/−, B7-H1−/−, and PD1−/−), CD1d−/− (devoid of NKT cells), and γδ T cell–deficient mice. (A) Brain infarct volumes from the different animals groups indicated 24 hours after tMCAO as measured by planimetry (n = 10 per group, except for RAG1−/− mice reconstituted with CD3+ T cells: n = 5). Note that RAG1−/− mice developed significantly smaller strokes compared with wild-type (WT) controls, while no significant differences (ns) in infarct volumes were observed between RAG1−/− mice and RAG1−/− mice reconstituted with B cells. In contrast, reconstitution of RAG1−/− mice with CD3+ T cells rescued the phenotype and infarct volumes, and functional deficits returned to WT levels. (B) Top panel shows neurologic Bederson score and bottom panel shows grip test from the different groups indicated as assessed at day 1 after tMCAO (n = 10 per group, except for RAG1−/− mice reconstituted with CD3+ T cells: n = 5). *P < .05 and **P < .001, Bonferroni-corrected 1-way ANOVA compared with WT controls. Error bars represent SD.
Infarct volumes and functional outcomes 24 hours after focal cerebral ischemia in WT mice, RAG1−/− mice lacking T cells and B cells, RAG1−/− mice reconstituted with B cells or CD3+ T cells, TCR-transgenic mice bearing only a single CD8+ (2C/RAG2, OTI/RAG1) or CD4+ (OTII/RAG1, 2D2/RAG1) TCR clone of defined specificity, mice lacking essential counterreceptors of TCR costimulation (CD28−/−, B7-H1−/−, and PD1−/−), CD1d−/− (devoid of NKT cells), and γδ T cell–deficient mice. (A) Brain infarct volumes from the different animals groups indicated 24 hours after tMCAO as measured by planimetry (n = 10 per group, except for RAG1−/− mice reconstituted with CD3+ T cells: n = 5). Note that RAG1−/− mice developed significantly smaller strokes compared with wild-type (WT) controls, while no significant differences (ns) in infarct volumes were observed between RAG1−/− mice and RAG1−/− mice reconstituted with B cells. In contrast, reconstitution of RAG1−/− mice with CD3+ T cells rescued the phenotype and infarct volumes, and functional deficits returned to WT levels. (B) Top panel shows neurologic Bederson score and bottom panel shows grip test from the different groups indicated as assessed at day 1 after tMCAO (n = 10 per group, except for RAG1−/− mice reconstituted with CD3+ T cells: n = 5). *P < .05 and **P < .001, Bonferroni-corrected 1-way ANOVA compared with WT controls. Error bars represent SD.
To further analyze whether T cells contribute to ischemic brain damage via adaptive immune mechanisms (eg, antigen recognition and engagement), mice transgenically overexpressing either 1 single MHC class I–restricted (OTI/RAG1, 2C/RAG2) or MHC class II–restricted (OTII/RAG1, 2D2/RAG1) TCR of known antigen specificity also underwent tMCAO. In contrast to RAG1−/− mice, TCR-transgenic mice recognizing only 1 defined antigen were fully susceptible to focal brain ischemia (Figure 1A-B; supplemental Figure 3). This indicates that T cell–driven adaptive immune mechanisms (eg, engagement of the TCR with MHC class I orII molecules on APCs) are not required to exert their detrimental effect during the acute phase of ischemic stroke. We next investigated a possible contribution of essential counterreceptors of TCR costimulation in experimental stroke. In line with the observation that TCR engagement is dispensable for stroke development, infarctions and neurologic deficits in mice devoid of CD28, B7-H1, and PD1 were comparable with WT animals 24 hours after tMCAO (Figure 1A-B; supplemental Figure 3). This demonstrates that accessory TCR signaling via the PD1-B7-H1 or CD28-B7 pathway is also not essential for early stroke progression. Hence, the formation of an immunologic synapse is not required for the detrimental effects of T cells for early stroke development.
RAG1−/− mice are also devoid of unconventional lymphocyte subpopulations in between the adaptive and innate immune system such as NKT cells or γδ T cells (so-called “nonclassical” T-cell populations). These populations carry a TCR, but are considered to act rather on the innate than the adaptive arm of the immune system. To address their functional contribution to early stroke development, CD1d−/− and γδ T cell–deficient mice underwent tMCAO. Again, no differences in infarct volumes and functional deficits were observed compared with WT controls (Figure 1A-B; supplemental Figure 3), indicating that these populations probably do not confer injury.
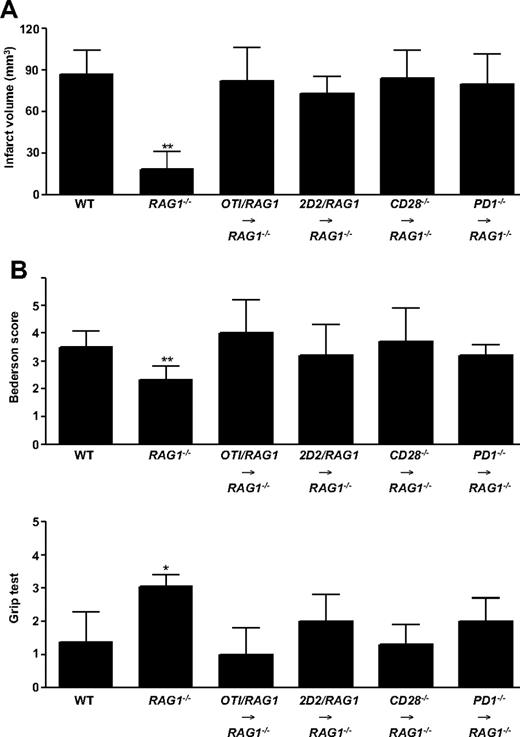
To further substantiate our hypothesis that the early detrimental T-cell effects in ischemic stroke neither depend on adaptive immune mechanisms nor costimulatory pathways, RAG1−/− mice were reconstituted with splenocytes from OTI/RAG1 mice, 2D2/RAG1 mice, CD28−/− mice, or PD1−/− mice and subjected to 60 minutes of tMCAO afterward. Splenocyte reconstitution reversed the stroke-protective effect observed in RAG1−/− mice. Infarct volumes and functional outcomes in the reconstituted RAG1−/− mice did not significantly differ from WT controls at day 1 (Figure 2A-B; supplemental Figure 4). This further supports the notion that a single TCR is sufficient to induce the T cell–dependent detrimental effect in the tMCAO model and that the costimulatory molecules CD28 and PD1 are dispensable as already shown in the corresponding knockout mice (see Figure 1).
Infarct volumes and functional outcomes 24 hours after focal cerebral ischemia in WT mice and RAG1−/− mice lacking T cells and B cells, as well as RAG1−/− mice reconstituted with splenocytes from OTI/RAG1 mice, 2D2/RAG1 mice, CD28−/− mice, or PD1−/− mice. (A) Brain infarct volumes from the different animals groups indicated 24 hours after tMCAO as measured by planimetry (n = 5-10 per group). Note that the protection from stroke in RAG1−/− mice was overcome by the adoptive transfer of splenocytes. (B) Top panel shows neurologic Bederson score and bottom panel shows grip test from the different groups indicated as assessed at day 1 after tMCAO (n = 5-10 per group). *P < .05 and **P < .001, Bonferroni-corrected 1-way ANOVA compared with WT controls. Error bars represent SD.
Infarct volumes and functional outcomes 24 hours after focal cerebral ischemia in WT mice and RAG1−/− mice lacking T cells and B cells, as well as RAG1−/− mice reconstituted with splenocytes from OTI/RAG1 mice, 2D2/RAG1 mice, CD28−/− mice, or PD1−/− mice. (A) Brain infarct volumes from the different animals groups indicated 24 hours after tMCAO as measured by planimetry (n = 5-10 per group). Note that the protection from stroke in RAG1−/− mice was overcome by the adoptive transfer of splenocytes. (B) Top panel shows neurologic Bederson score and bottom panel shows grip test from the different groups indicated as assessed at day 1 after tMCAO (n = 5-10 per group). *P < .05 and **P < .001, Bonferroni-corrected 1-way ANOVA compared with WT controls. Error bars represent SD.
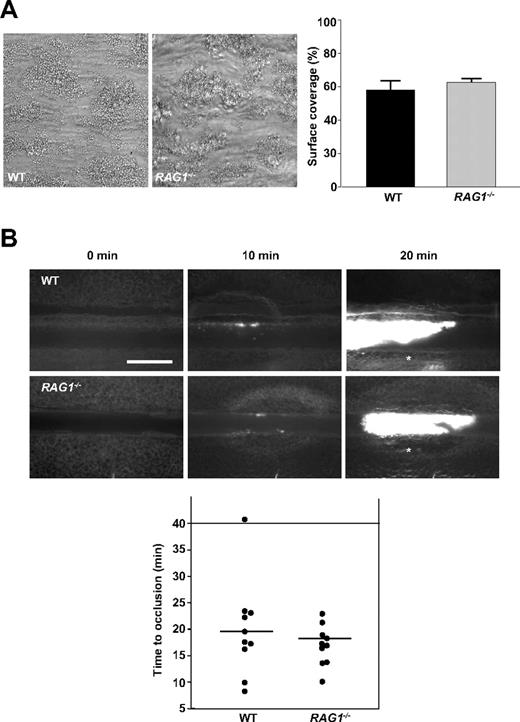
To investigate alternative mechanisms that could account for the profound resistance of RAG1−/− mice against ischemic stroke, we analyzed their ability to form stable thrombi. This was done in 2 standardized in vitro and in vivo thrombus formation models: on collagen-coated surfaces in a whole-blood perfusion system under high shear conditions (1000 s−1),20 platelets from both WT and RAG1−/− mice adhered to collagen fibers and formed aggregates within 2 minutes that consistently grew into large thrombi (Figure 3A). By the end of the perfusion period, the surface area covered by platelets did not significantly differ between the groups (58.2% ± 5.1% vs 62.7% ± 1.8%; P > .05). We also studied the effects of RAG1 deficiency on occlusive thrombus formation in vivo by fluorescence microscopy after FeCl3-induced injury on mesenteric arterioles. In WT mice, formation of small platelet aggregates was observed approximately 10 minutes after injury, with progression to complete vessel occlusion in 9 of 10 mice within 30 minutes (mean occlusion time = 18.1 minutes ± 5.5 minutes; Figure 3B). Again, no significant differences were observed in damaged arterioles of RAG1−/− mice (mean occlusion time = 17.5 minutes ± 3.8 minutes; P > .05; Figure 3B). These findings indicate that T cells are dispensable for stable clot formation. Thus, it is unlikely that the protective effect of T-cell deficiency in stroke is due to impaired thrombus stability.
Thrombus formation in RAG1−/− mice is not affected in vitro and in vivo. (A) Platelets in whole blood from RAG1−/− mice form stable thrombi when perfused over a collagen-coated surface at a shear rate of 1000 s−1. Left panel shows representative phase-contrast images (40×/0.25 NA air objective). Right panel shows mean surface coverage by thrombi in RAG1−/− mice and WT controls (n = 5 per group). P > .05, 2-tailed Student t test compared with WT controls. (B) In vivo analysis of thrombus formation in RAG1−/− mice and WT controls (n = 10 per group). Mesenteric arterioles were treated with FeCl3 and adhesion, and thrombus formation of fluorescently labeled platelets were monitored by in vivo fluorescence microscopy. Representative images (top) and the time to vessel occlusion (bottom) are shown. Each symbol represents 1 animal, and horizontal bars depict means. The asterisk (top) indicates occlusion of the vessels. Scale bar equals 25 μm. P > .05, 2-tailed Student t test compared with WT control.
Thrombus formation in RAG1−/− mice is not affected in vitro and in vivo. (A) Platelets in whole blood from RAG1−/− mice form stable thrombi when perfused over a collagen-coated surface at a shear rate of 1000 s−1. Left panel shows representative phase-contrast images (40×/0.25 NA air objective). Right panel shows mean surface coverage by thrombi in RAG1−/− mice and WT controls (n = 5 per group). P > .05, 2-tailed Student t test compared with WT controls. (B) In vivo analysis of thrombus formation in RAG1−/− mice and WT controls (n = 10 per group). Mesenteric arterioles were treated with FeCl3 and adhesion, and thrombus formation of fluorescently labeled platelets were monitored by in vivo fluorescence microscopy. Representative images (top) and the time to vessel occlusion (bottom) are shown. Each symbol represents 1 animal, and horizontal bars depict means. The asterisk (top) indicates occlusion of the vessels. Scale bar equals 25 μm. P > .05, 2-tailed Student t test compared with WT control.
Discussion
The main finding of this study is that the early detrimental effects of T cells during acute ischemic stroke are not related to adaptive immune mechanisms (ie, antigen recognition or costimulatory pathways). In addition, the fact that thrombus formation in RAG1−/− mice was unimpaired in vitro and in vivo indicates that T cells probably also do not critically contribute to intravascular thrombosis during focal cerebral ischemia.
Ischemic stroke induces a profound local inflammatory response. Within hours, various types of immune cells transmigrate over the activated endothelium to invade the damaged brain in a timed fashion.1 Whereas previous studies mostly focused on neutrophils and monocytes,2-4 the role of lymphocytes, especially T cells in ischemic stroke, has long been neglected, although T cells are localized in close vicinity to blood vessels in the infarct boundary as early as 24 hours after experimental focal cerebral ischemia in rodents.5,6 Antibodies directed against the adhesion receptor α4 integrin blocked T-cell trafficking into the postischemic brain and reduced stroke volume at day 1 after tMCAO in rats.7,8 Importantly, certain T-cell subsets such as regulatory T cells might also have beneficial effects on tissue regeneration and repair at the subacute phase of ischemic stroke.33 The supposed pathophysiologic relevance of T cells during early stages of infarct development was recently confirmed using transgenic mouse strains that lack functional T cells (ie, RAG1−/− mice9,10 and severe combined immunodeficient [SCID] mice).34 Although these mice are also devoid of B cells, B cells are probably less important for mediating ischemic brain damage. B-cell deficiency alone did not alter infarct dynamics,9 and transferring B cells into RAG1−/− mice as shown in our study did not neutralize the neuroprotective effect of immune deficiency. Importantly, RAG1−/− mice supplemented with CD3+ T cells were fully susceptible to ischemic brain damage, proving that T cells are instrumental in early stroke development. In line with our findings in brain infarction, T cells are also involved in the pathogenesis of ischemia/reperfusion injury in other vascular beds, including the intestine, liver, and kidney.35-37
Under normal conditions, the intact blood-brain barrier (BBB) limits access of immune cells and humoral factors to the central nervous system (CNS), rendering the brain an “immune-privileged organ.” However, up-regulation of cell adhesion molecules and transient BBB disruption after stroke allows lymphocytes to enter the brain and brain antigens to enter the peripheral circulation. Recently, it has been shown that this encounter of normally sequestered brain antigens by the peripheral immune system is principally sufficient to induce specific (auto)immune responses. Mononuclear cells (MNCs) from the brains of rats treated with lipopolysaccharide (LPS) and subjected to tMCAO afterward were sensitized to myelin basic protein (MBP) after 1 month.38 Moreover, 6 hours and 22 hours after tMCAO, spleen cells from stroke-injured mice secreted significantly enhanced levels of proinflammatory cytokines such as tumor necrosis factor–α (TNF-α) or interferonγ (IFN-γ) upon restimulation.39 Interestingly, (auto)antibodies to brain antigens (eg, MBP, neurofilaments, and the N-methyl-D-aspertate receptor) have been described after human stroke.40-42 However, although specific immune responses seem to occur in the course of brain infarction, their functional relevance for stroke development, especially during the initial phase, has been unknown so far. Here, we demonstrate that the presence of 1 transgenic MHC class I–restricted (CD8+) or MHC class II–restricted (CD4+) TCR of predefined antigen specificity is sufficient to fully reconstitute susceptibility to experimental stroke, indicating that the ability of CD4+ or CD8+ T cells to recognize (auto)antigens is no major determinant of stroke development. Similarly, the absence of costimulatory molecules necessary to fully activate T cells had no effect on early poststroke outcome. These findings exclude a major role of T cell–driven adaptive immune responses or the formation of an immunologic synapse at least during the early stages of brain infarction.
Several “unconventional” lymphocyte subpopulations exist that are placed at the intersection between the innate and adaptive immune system and could be of pathophysiologic relevance in ischemic stroke. These cells are also characterized by the expression of a TCR, but their function is rather related to the innate arm of the immune system. NKT cells share properties of both T cells and NK cells. They express a limited TCR repertoire, recognize glycolipids presented by CD1d molecules rather than peptide-MHC complexes, and produce large quantities of cytokines upon activation.43 The other subpopulation, γδ T cells, possess a distinct TCR on their surface composed of 1 γ-chain and 1 δ-chain (instead of α- and β-TCR chains).44 Like NKT cells, γδ T cells are peculiar in that they do not seem to require antigen processing and MHC presentation of peptide epitopes but also have a prominent role in recognition of lipid antigens. Interestingly, a critical role of IL-17–producing γδ T cells during the late stage of infarct development was recently postulated.10 Our experiments using the corresponding knockout mice suggest that CD1d-restricted NKT cells and γδ T cells are probably of minor importance during early infarct evolution, but these findings await further confirmation by adoptive transfer experiments.
Platelet-derived thrombus formation is one major pathomechanism that drives ischemic brain damage after tMCAO in mice.45 Activated T cells can interact with aggregated platelets (eg, via the CD40/CD40L pathway), thereby propagating intravascular thrombosis.2 We therefore hypothesized that T cell–deficient RAG1−/− mice are protected from ischemic stroke due to their reduced ability to form vessel-occlusive thrombi. However, the extent and time course of thrombus formation in RAG1−/− mice was neither altered in a whole-blood in vitro perfusion system under high shear flow conditions, nor in vivo after chemical injury of mesenteric arterioles. In our previous studies, blockade of GPIb and GPVI receptors as well as FXII prevented thrombus formation to the same extent in these models after tMCAO, indicating that similar mechanisms are operative.22,27
Because protection from ischemic stroke in T cell–deficient mice did not depend on adaptive immune pathways, and thrombus formation was unimpaired in RAG1−/− mice, other mechanisms must account for the detrimental effect of T cells in stroke. The brain endothelium becomes rapidly activated during cerebral ischemia.46 As a consequence, various cell adhesion receptors such as lymphocyte function antigen-1 (LFA-1), macrophage antigen-1 (Mac-1), and very late antigen-4 (VLA-4) are up-regulated.46,47 It has been shown that T cells can engage these receptors, leading to microvascular dysfunction, impaired capillary reperfusion, and worse outcome after experimental stroke, an observation referred to as “no reflow phenomenon.”46-48 In line with these findings, microvascular integrity was highly preserved in RAG1−/− mice after tMCAO.9 Other possible mechanisms that might mediate stroke resistance in the absence of T ells include reduced production of proinflammatory cytokines such as IFNγ and TNFα and less apoptosis, as well as altered T cell–microglia interactions.1,2,49
Our study confirms that T cells play a detrimental pathophysiologic role in the early stage of ischemic stroke. However, T cell–driven adaptive immune pathways (ie, the formation of an immunologic synapse) are not involved herein. Whether this is also true at later stages of ischemic stroke (eg, during the recovery phase) needs to be further addressed. T-cell deficiency in RAG1−/− mice also had no influence on clot formation; thus, the mechanisms by which T cells aggravate early I/R injury in the brain remain elusive.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Melanie Glaser and Theresa Moritz for excellent technical assistance and Prof Rudolf Martini and Dr Antje Kroner-Milsch, Wuerzburg, for providing the RAG1−/− mice.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 688, A1, B1 to G.S. and B.N.; A13 to C.K.; and SFB 581, A8 to H.W.), the Interdisziplinäres Zentrum für Klinische Forschung, University of Wuerzburg, Germany (IZKF, Z-4/87 to C.K. and B.N.), the Rudolf Virchow Center, Wuerzburg, Germany, and TEVA Deutschland GmbH, Germany.
Authorship
Contribution: C.K. conceived and funded the project, supervised the stroke experiments, and drafted the manuscript; P.K., T.S., and M.A. performed the stroke experiments, collected the functional scores, and analyzed the data; I.H. performed the clotting experiments; N.S. and A.D. guided the isolation of immune cell subpopulations and planned the reconstitution experiments; and B.N., H.W., and G.S. conceived and funded the project and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for N.S., A.D., and H.W. is Department of Neurology–Inflammatory Disorders of the Nervous System and Neurooncology, University of Muenster, Muenster, Germany.
Correspondence: Christoph Kleinschnitz, Department of Neurology, Julius-Maximilians-University of Wuerzburg, Josef-Schneider Strasse 11, D-97080 Wuerzburg, Germany; e-mail: christoph.kleinschnitz@mail.uni-wuerzburg.de.
References
Author notes
C.K., H.W., and G.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal