Wiskott-Aldrich syndrome is caused by mutations in the WASP gene, which affects the function of most hematopoietic cells and leads to immunodeficiency.1 In this issue of Blood, Morales-Tirado and colleagues show that T helper cells lacking WASp have a selective deficiency in their memory response.2
The Wiskott-Aldrich syndrome protein (WASp) is a crucial regulator of actin polymerization. Its gene is located on the X chromosome and when mutated, it leads to thrombocytopenia, immunodeficiency, and eczema. WASp is expressed in hematopoietic cells and in its absence, migration, adhesion, activation, and antigen uptake malfunction. It has previously been shown that WASp plays a role in T-cell activation, being important for the regulation of the so-called immunologic synapse.3 This membrane structure, crucial for activation of T cells, is formed between T cells and antigen-presenting cells and is composed of clusters of T-cell receptor, adhesion molecules, and signaling molecules. It has also been suggested that WASp can regulate transcriptional activation in T cells independent of its role in actin polymerization.4
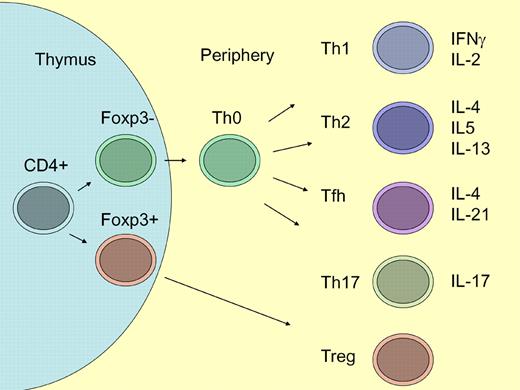
CD4+ T helper cells can be divided into subpopulations, depending on expression of transcription factors and/or cytokines (see figure). Th1 cells secrete IL-2 and IFNγ, which are particularly important for cell-mediated responses, whereas Th2 cells secrete IL-4, IL-5, and IL-13, which play important roles in humoral immunity and atopic allergy. There are also more newly discovered CD4+ cell populations such as Th17 cells, T follicular T helper cells, and Treg cells.5,6
Differentiation of CD4+ T cells. In the thymus, a subpopulation of CD4+ cells become positive for the transcription factor Foxp3 and develop into regulatory T (Treg) cells.6 CD4+Foxp3− cells leave the thymus and give rise to Th0 cells. Depending on the type of antigen-dependent signals, Th0 cells develop into Th1, Th2, follicular T helper (Tfh), or Th17 cells.5
Differentiation of CD4+ T cells. In the thymus, a subpopulation of CD4+ cells become positive for the transcription factor Foxp3 and develop into regulatory T (Treg) cells.6 CD4+Foxp3− cells leave the thymus and give rise to Th0 cells. Depending on the type of antigen-dependent signals, Th0 cells develop into Th1, Th2, follicular T helper (Tfh), or Th17 cells.5
In this issue of Blood, Morales-Tirado et al show that cytokine secretion from activated primed T helper cells is affected in the absence of WASp.2 They demonstrate that absence of WASp leads to lower IFNγ and IL-4 secretion in primed, in vitro–activated Th1 and Th2 cells, respectively. Secretion of IFNγ can be rescued by signals from antigen-presenting cells, whereas secretion of IL-4 cannot. Levels of other Th2 cytokines, such as IL-5, IL-10, and IL-13, were also lower in the absence of WASp. Early events in Th2 differentiation are not affected by the absence of WASp and there is no measurable defect in transcriptional activation of the il4 gene in the memory response. Thus, there is an uncoupling of IL-4 protein production and transcription in Th2 effector function. Morales-Tirado et al give evidence that this effect could be due to a deficiency in activation of the signaling molecule ERK, which might regulate translation or protein stability.
In their experiment, Morales-Tirado et al went on to analyze T helper cell function in parasite responses in vivo, studying WASp-deficient T cells in a WASp-sufficient environment. They found that T cells mediated protective immunity and parasite clearance in a Th1 response, but were unable to support a Th2 response to the nematode Nippostrongylus brasiliensis. Thus, there was a significant delay in parasite clearance, which seemed to be due to lower IL-4 and IL-13 levels as well as reduced recruitment of innate effectors to infected tissue.
The experiments performed by Morales-Tirado et al are puzzling in view of the elevated IgE levels in WAS patients,7 considering that IgE production is dependent on IL-4. If Th2 cells are deficient in IL-4 secretion, where does IL-4 come from? Morales-Tirado et al show that basophils and the minor T-cell subpopulation γδ T cells can produce IL-4 in a manner independent of WASp. They propose that these cells are responsible for IL-4 production in WAS patients.
The paper by Morales-Tirado et al puts forward new aspects of WASp regulation in T cells. Their findings are interesting but do not always agree with previous data. More studies are needed to fully understand why there is reduced cytokine production in WASp-deficient T helper cells. Whereas Morales-Tirado et al measured steady-state levels of cytokine protein or mRNA, it would be interesting to determine rates of synthesis or degradation. Furthermore, it will be very important to confirm their findings using T cells from WAS patients. The paper by Morales-Tirado et al gives further insights into the immunodeficiency in WAS patients and puts additional focus on the T cells. This could be helpful in finding better treatments for the disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal