Abstract
Certain malignant B cells rely on B-cell receptor (BCR)–mediated survival signals. Spleen tyrosine kinase (Syk) initiates and amplifies the BCR signal. In in vivo analyses of B-cell lymphoma cell lines and primary tumors, Syk inhibition induces apoptosis. These data prompted a phase 1/2 clinical trial of fostamatinib disodium, the first clinically available oral Syk inhibitor, in patients with recurrent B-cell non-Hodgkin lymphoma (B-NHL). Dose-limiting toxicity in the phase 1 portion was neutropenia, diarrhea, and thrombocytopenia, and 200 mg twice daily was chosen for phase 2 testing. Sixty-eight patients with recurrent B-NHL were then enrolled in 3 cohorts: (1) diffuse large B-cell lymphoma (DLBCL), (2) follicular lymphoma (FL), and (3) other NHL, including mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), mucosa-associated lymphoid tissue lymphoma, lymphoplasmacytic lymphomas, and small lymphocytic leukemia/chronic lymphocytic leukemia (SLL/CLL). Common toxicities included diarrhea, fatigue, cytopenias, hypertension, and nausea. Objective response rates were 22% (5 of 23) for DLBCL, 10% (2 of 21) for FL, 55% (6 of 11) for SLL/CLL, and 11% (1/9) for MCL. Median progression-free survival was 4.2 months. Disrupting BCR-induced signaling by inhibiting Syk represents a novel and active therapeutic approach for NHL and SLL/CLL. This trial was registered at www.clinicaltrials.gov as #NCT00446095.
Introduction
Despite intensive efforts to develop improved therapy in the past decade, mantle cell lymphomas (MCL) and the indolent non-Hodgkin lymphoma (NHL), including small lymphocytic leukemia/chronic lymphocytic leukemia (SLL/CLL), remain essentially incurable. In addition, despite the inclusion of rituximab in standard chemotherapy regimens, almost one-half of patients with clinical high-risk diffuse large B-cell lymphoma (DLBCL) are not cured.1 More effective and tolerable therapeutic agents are needed to improve the outcome for these patients.
Most B-cell malignancies express the B-cell receptor (BCR).2 BCR signaling induces receptor oligomerization, immunoglobulin (Ig) α and β phosphorylation and the recruitment and activation of the spleen tyrosine kinase (Syk). Thereafter, Syk initiates downstream events and amplifies the original BCR signal.3,4 In addition to ligand-induced BCR signaling, there are critical “tonic” (nonligand dependent) BCR maintenance or survival signals that occur in the absence of receptor engagement.4,5 The role of tonic BCR survival signals was first shown in murine models in which the inducible loss of murine BCR expression or Ig α function led to the death of mature B cells.6,7 Although the molecular mechanisms regulating tonic BCR signaling remain to be defined, several studies highlight the critical role of Syk.8,9
Studies that used small interfering RNA to inhibit BCR expression have shown that constitutive signaling by BCR is critical for the survival and proliferation of both murine and human B-cell lymphomas.10 The primary outcome of BCR signaling in these cells appeared to be the activation of Syk.11 Activation of Syk leads to several downstream events that promote cell survival, including activation of phosphatidylinositol 3-kinase and Akt,12 and the phosphorylation of multiple proliferation proteins, including RAS, PLC-γ, and MAPK kinases.
A subset of DLBCLs exhibits coordinate overexpression of components of the BCR signaling cascade and tonic BCR-dependent survival signals.8,13,14 In such primary DLBCLs and DLBCL cell lines, targeted inhibition of Syk abrogates BCR signaling and induces apoptosis. Additional evidence that Syk is a rational therapeutic target for DLBCL comes from a murine model of MYC overexpression in which BCR signaling drives lymphomagenesis; a model that was used to determine the role of Syk in the maintenance of aggressive B-cell lymphoma. This model integrates both tonic signaling and the signaling associated with antigen binding, both of which are mediated by Syk.15 Tumor cells deficient in Syk failed to expand in vivo, and the inhibition of Syk led to tumor regression in vivo.16
Evidence that BCR and Syk are valid targets for the therapy of indolent B-cell NHL comes from the comparison of the single-cell signaling profiles of follicular lymphoma (FL) cells to those of nonmalignant B cells within individual patient biopsies.17 BCR-mediated signaling through Syk occurred more rapidly, to a greater degree, and for a longer duration in FL cells compared with the tumor-infiltrating nonmalignant B cells. This suggests that enhanced signaling through BCR may contribute to the malignant phenotype of FL. Similarly, in a MCL line, Syk was found to be amplified at the DNA level and overexpressed at both the RNA and protein levels. After treatment with piceatannol (a Syk inhibitor), cell proliferation arrest and apoptosis were observed and were correlated to the degree of Syk overexpression.18
Taken together, these data provide rationale for inhibiting the BCR, and its downstream target Syk, as a therapeutic approach for a variety of B-cell NHLs.19
R406 is a potent and selective inhibitor of Syk.20 Fostamatinib disodium (R788; FosD) is a prodrug of R406 available in an oral formulation and is also under clinical development for the treatment of rheumatoid arthritis21 and immune thrombocytopenic purpura.22 Pharmacokinetic and pharmacodynamic data from several human clinical studies have shown that twice daily administration is sufficient to achieve a significant reduction in Syk activity, with no adverse effects on innate immunity or hemostasis.20 Herein, we present the results of the first phase 1/2 trial of FosD in the treatment of patients with relapsed and refractory B-cell NHL and CLL.
Methods
Patients
This study was registered at www.clinicaltrials.gov (NCT00446095). Patients with relapsed or refractory B-cell lymphoid malignancies were enrolled, and treatment was initiated between February 2007 and January 2008. Written informed consent was obtained in accordance with the Declaration of Helsinki, applicable federal regulations, and requirements of local institutional review boards, and all participating institutions approved this study. Eligibility criteria included relapsed/refractory B-cell malignancy (DLBCL, FL, MCL, mucosa-associated lymphoid tissue lymphoma, marginal zone lymphoma [MZL], and SLL/CLL), age of 18 years or older, measurable disease, Karnofsky performance status of at least 60, absolute neutrophil count (ANC) of at least 1.5 × 109/L (1500/μL), hemoglobin level of at least 90.0 g/L (9.0 g/dL), platelet count of at least 75.0 × 109/L (75 000/μL), aspartate aminotransferase and alanine aminotransferase less than 3 times upper limit of normal (up to 5 times upper limit of normal if tumor involvement), bilirubin level no greater than 25.7 μmol/L (1.5 mg/dL), creatinine level no greater than 176 800 μmol/L (2.0 mg/dL), no systemic steroids greater than the equivalent of 10 mg/d prednisone, no CYP3A4 inducer/inhibitor within 1 week before treatment, and no significant gastrointestinal disease, difficulty swallowing, or malabsorption.
Study design
This was a phase 1/2 study. Phase 1 consisted of 2 cohorts of 6 patients each who were sequentially assigned to receive 1 of 2 dose levels of FosD (provided by Rigel Pharmaceuticals) 200 mg by mouth twice daily or 250 mg by mouth twice daily. These doses were chosen on the basis of previous experience in rheumatoid arthritis.23 In the event of unacceptable toxicity in the first cohort, a dose deescalation was planned to be 150 mg by mouth twice daily. After the completion of 4 weeks of FosD administration in the first cohort, dose-limiting toxicities and adverse events were examined by a Data Safety Monitoring Board (DSMB), which determined it safe to accrue to the second dose cohort. On the completion of 4 weeks of treatment for the second cohort of patients, the DSMB reviewed the aggregate data from the 2 cohorts and recommended the dose level for the phase 2 portion of the study. Patients in the phase 1 portion could continue on therapy until unacceptable toxicity or disease progression occurred. Phase 1 dose-limiting toxicities were defined as absolute neutrophil count (ANC) of grade 3 or 4 plus fever; ANC of less than 0.5 × 109/L (500/μL) for 5 days or longer; platelet count less than 25 × 109/L (25 000/μL); grade 3 or 4 nausea, vomiting, diarrhea if persistent despite optimal antiemetic or antidiarrheal therapy; grade 3 elevation of transaminases if persistent for longer than 7 days; any other grade 3 or 4 toxicity except alopecia; need for delay or modification of dose.
In phase 2, patients were enrolled into 1 of 3 groups according to histology (DLBCL, FL, and other, which included MZL, MCL, SLL/CLL, and lymphoplasmacytic lymphoma). Patients in each group received FosD by mouth twice daily until unacceptable toxicity or progression. Common Toxicity Criteria grade 3 or 4 toxicity, including febrile neutropenia, ANC less than 0.5 × 109/L (500/μL) for 5 days, nausea or vomiting persistent despite optimal therapy, increase in alanine aminotransferase or aspartate aminotransferase persisting for longer than 7 days prompted interruption of the study drug. A dose reduction of 50 mg by mouth twice daily was instituted when the study drug was resumed. A reduction in dose to less than 100 mg by mouth twice daily was permitted only after discussion with the sponsor.
Supportive care
Patients were not permitted to receive any other antineoplastic therapy or immunosuppressive agents while on study. Use of growth factor was allowed per institution-specific guidelines. All other necessary supportive care was encouraged.
Response assessment
Initial tumor response, assessed per the Revised Response Criteria for Lymphoma,24 was determined after 8 weeks (56 days) of treatment; subsequent response assessments were performed at 12-week intervals. Patients with SLL/CLL were assessed according to lymphoma response criteria only.
Pharmacokinetic/pharmacodynamic evaluations
Samples were obtained for pharmacokinetic (PK) analysis on days 1, 8, 29, and 57 and every 12 weeks thereafter. Samples were drawn before the dose and at 1, 2, and 4 hours after the dose on days 1, 8, and 29. Additional samples were obtained on day 57 and at 12-week intervals thereafter. Pharmacodynamic assessments included analyses of normal circulating CD14+ cells as a measure of FosD activity, because CD14+ cell counts were inversely correlated with FosD in previous studies.25
Statistical methods
Sample size in phase 1 was based on a standard phase 1 design. The sample size for phase 2, allowed the exclusion of a response rate of 25% if no responses were seen within a treatment group.
The intent to treat population included all patients receiving any amount of the study drug. All efficacy and safety analyses were based on the intent to treat population. Best response was used for all response analyses. The number and percentage of patients achieving a response (complete response [CR] plus partial response [PR]) was calculated for each treatment group and overall. The 95% confidence intervals (95% CIs) for response were calculated by using the exact method. The number and percentage of patients achieving clinical benefit (defined as responding patients and those with stable disease) were also calculated. Progression-free survival (PFS) was determined by the Kaplan-Meier method.
Results
Patients
Phase 1 component.
Thirteen patients were enrolled in 2 cohorts; 6 in cohort 1 (200 mg twice daily) and 7 in cohort 2 (250 mg twice daily; details in Table 1). The median age in the phase 1 component was 74 years (range, 52-91 years). Histologies included DLBCL (3 patients), MCL (3 patients), FL (5 patients), and SLL/CLL (2 patients). Most patients had received extensive prior therapy (median, 4 prior regimens; range, 1-6 prior regimens), including prior autologous stem cell transplantation (ASCT; n = 2) and prior radioimmunotherapy (n = 4).
Demographics and disease history by phase/cohort/group for phases 1 and 2
| . | Phase 1 . | Phase 2 . | |||||
|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 6) . | Cohort 2 (n = 7) . | Total (n = 13) . | Group 1 (n = 23) . | Group 2 (n = 21) . | Group 3 (n = 24) . | Total (n = 68) . | |
| Median age, y (range) | 78.5 (70-91) | 61.0 (52-80) | 72.0 (52-91) | 63.0 (41-87) | 59.0 (41-74) | 62 (51-77) | 61.5 (41-87) |
| Male (%) | 33.3 | 28.6 | 30.8 | 78.3 | 61.9 | 70.8 | 70.6 |
| Race (%) | |||||||
| White | 100.0 | 71.4 | 84.6 | 87.0 | 90.5 | 87.5 | 88.2 |
| Black | 0 | 14.3 | 7.7 | 8.7 | 0 | 12.5 | 7.4 |
| Asian | 0 | 14.3 | 7.7 | 0 | 9.5 | 0 | 2.9 |
| Lymphoma type, n (%) | |||||||
| DLBCL* | 2 (33.3) | 2 (28.6) | 4 (30.8) | 23 (100.0) | 0 | 0 | 23 (33.8) |
| FL | 2 (33.3) | 2 (28.6) | 4 (30.8) | 0 | 21 (100.0) | 0 | 21 (30.9) |
| MC | 2 (33.3) | 1 (14.3) | 3 (23.1) | 0 | 0 | 9 (37.5) | 9 (13.2) |
| CLL/SLL | 0 | 2 (28.6) | 2 (15.4) | 0 | 0 | 11 (45.8) | 11 (16.2) |
| MZL | 0 | 0 | 0 | 0 | 0 | 2 (8.3) | 2 (2.9) |
| MALT lymphoma | 0 | 0 | 0 | 0 | 0 | 1 (4.2) | 1 (1.5) |
| Other | 0 | 0 | 0 | 0 | 0 | 1 (4.2) | 1 (1.5) |
| Median months since diagnosis (range) | 69.0 (26-135) | 39.0 (17-207) | 63.0 (17-207) | 31.0 (5-216) | 78.0 (17-184) | 50.5 (7-137) | 49.0 (5-216) |
| Prior therapy | |||||||
| Median number (range) | 4.0 (1-7) | 4.0 (1-5) | 4.0 (1-7) | 4.0 (2-10) | 4.0 (1-11) | 2.5 (1-7) | 4.0 (1-11) |
| Prior rituximab, n | 6 | 7 | 13 | 22 | 21 | 22 | 65 |
| Rituximab within 6 mo, n | 1 | 2 | 3 | 9 | 4 | 4 | 17 |
| Prior autologous transplantation, n (%) | 0 | 2 (28.6) | 2 (15.4) | 12 (52.2) | 5 (23.8) | 2 (8.3) | 19 (27.9) |
| Prior radioimmunotherapy, n | 2 | 2 | 4 | 2 | 9 | 5 | 16 |
| Multiagent chemotherapy, n | 6 | 7 | 13 | 23 | 19 | 22 | 64 |
| Fludarabine-containing regimen, n | 1 | 3 | 4 | 1 | 7 | 7 | 15 |
| . | Phase 1 . | Phase 2 . | |||||
|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 6) . | Cohort 2 (n = 7) . | Total (n = 13) . | Group 1 (n = 23) . | Group 2 (n = 21) . | Group 3 (n = 24) . | Total (n = 68) . | |
| Median age, y (range) | 78.5 (70-91) | 61.0 (52-80) | 72.0 (52-91) | 63.0 (41-87) | 59.0 (41-74) | 62 (51-77) | 61.5 (41-87) |
| Male (%) | 33.3 | 28.6 | 30.8 | 78.3 | 61.9 | 70.8 | 70.6 |
| Race (%) | |||||||
| White | 100.0 | 71.4 | 84.6 | 87.0 | 90.5 | 87.5 | 88.2 |
| Black | 0 | 14.3 | 7.7 | 8.7 | 0 | 12.5 | 7.4 |
| Asian | 0 | 14.3 | 7.7 | 0 | 9.5 | 0 | 2.9 |
| Lymphoma type, n (%) | |||||||
| DLBCL* | 2 (33.3) | 2 (28.6) | 4 (30.8) | 23 (100.0) | 0 | 0 | 23 (33.8) |
| FL | 2 (33.3) | 2 (28.6) | 4 (30.8) | 0 | 21 (100.0) | 0 | 21 (30.9) |
| MC | 2 (33.3) | 1 (14.3) | 3 (23.1) | 0 | 0 | 9 (37.5) | 9 (13.2) |
| CLL/SLL | 0 | 2 (28.6) | 2 (15.4) | 0 | 0 | 11 (45.8) | 11 (16.2) |
| MZL | 0 | 0 | 0 | 0 | 0 | 2 (8.3) | 2 (2.9) |
| MALT lymphoma | 0 | 0 | 0 | 0 | 0 | 1 (4.2) | 1 (1.5) |
| Other | 0 | 0 | 0 | 0 | 0 | 1 (4.2) | 1 (1.5) |
| Median months since diagnosis (range) | 69.0 (26-135) | 39.0 (17-207) | 63.0 (17-207) | 31.0 (5-216) | 78.0 (17-184) | 50.5 (7-137) | 49.0 (5-216) |
| Prior therapy | |||||||
| Median number (range) | 4.0 (1-7) | 4.0 (1-5) | 4.0 (1-7) | 4.0 (2-10) | 4.0 (1-11) | 2.5 (1-7) | 4.0 (1-11) |
| Prior rituximab, n | 6 | 7 | 13 | 22 | 21 | 22 | 65 |
| Rituximab within 6 mo, n | 1 | 2 | 3 | 9 | 4 | 4 | 17 |
| Prior autologous transplantation, n (%) | 0 | 2 (28.6) | 2 (15.4) | 12 (52.2) | 5 (23.8) | 2 (8.3) | 19 (27.9) |
| Prior radioimmunotherapy, n | 2 | 2 | 4 | 2 | 9 | 5 | 16 |
| Multiagent chemotherapy, n | 6 | 7 | 13 | 23 | 19 | 22 | 64 |
| Fludarabine-containing regimen, n | 1 | 3 | 4 | 1 | 7 | 7 | 15 |
MALT indicates mucosa-associated lymphoid tissue.
Nine patients had transformed DLBCL: 1 in cohort 1 of phase 1, 2 in cohort 2 of phase 1, and 6 in group 1 of phase 2.
Phase 2 component.
After review by the DSMB, the dose of 200 mg twice daily was chosen for phase 2 testing. Patients (median age, 63 years; range, 41-87 years) were enrolled in 3 cohorts; 23 with DLBCL (group 1: 17 de novo DLBCL, 6 transformed LBCL), 21 with FL (group 2), and 24 with other lymphomas (group 3), including 11 with SLL/CLL, 9 with MCL, 3 with MZL, and 1 with lymphoplasmacytic lymphoma. The median number of prior therapies was 4, 4, and 2 in groups 1, 2, and 3, respectively. There was no difference in the median number of prior therapies for patients with transformed LBCL compared with those with de novo DLBCL. In the DLBCL cohort, 12 patients (9 de novo and 3 transformed LBCL) had undergone prior ASCT. In the FL cohort, 9 patients had received prior radioimmunotherapy, and 5 had received prior ASCT. In the other cohort (group 3), prior therapies included aggressive fludarabine-containing combinations and the fractionated cyclophosphamide, vincristine, Adriamycin, and dexamethasone/methotrexate/cytarabine regimen for MCL. Approximately 33% of patients with DLBCL and 11% of patients with FL had an International Prognostic Index or FL-International Prognostic Index score indicative of poor prognosis at diagnosis. Additional details can be found in Table 1.
Adverse events
Adverse events in phase 1 included neutropenia, anemia, thrombocytopenia, fatigue/asthenia, and diarrhea. Severe events were infrequent; most events were grade 1 or 2 in severity; see details in Table 2. One patient with CLL in phase 1 was withdrawn because of leukocytosis after treatment with FosD. On the basis of the observation of dose-dependent toxicities, with an increased rate of adverse events at the 250-mg twice daily level, the DSMB determined that 200 mg twice daily would be the appropriate dose level for the phase 2 trial. The dose-limiting toxicity was felt to be a constellation of diarrhea, neutropenia, and thrombocytopenia.
Phase 1–related adverse events by grade
| Adverse event . | Cohort 1 (n = 6) . | Cohort 2 (n = 7) . | Total (n = 13) . | |||
|---|---|---|---|---|---|---|
| % . | Grade 3/4, % . | % . | Grade3/4, % . | % . | Grade 3/4, % . | |
| Diarrhea | 83 | 17 | 71 | 0 | 77 | 8 |
| Fatigue | 33 | 0 | 43 | 14 | 39 | 8 |
| Leukopenia | 50 | 33 | 14 | 14 | 31 | 23 |
| Hypertension | 0 | 0 | 57 | 0 | 31 | 0 |
| Neutropenia | 33 | 33 | 14 | 14 | 23 | 23 |
| Anemia | 50 | 50 | 0 | 0 | 23 | 23 |
| Asthenia | 17 | 17 | 14 | 0 | 15 | 8 |
| Nausea | 0 | 0 | 29 | 0 | 15 | 0 |
| Dyspepsia | 0 | 0 | 29 | 0 | 15 | 0 |
| Thrombocytopenia | 17 | 0 | 14 | 0 | 15 | 0 |
| Headache | 0 | 0 | 29 | 0 | 15 | 0 |
| Adverse event . | Cohort 1 (n = 6) . | Cohort 2 (n = 7) . | Total (n = 13) . | |||
|---|---|---|---|---|---|---|
| % . | Grade 3/4, % . | % . | Grade3/4, % . | % . | Grade 3/4, % . | |
| Diarrhea | 83 | 17 | 71 | 0 | 77 | 8 |
| Fatigue | 33 | 0 | 43 | 14 | 39 | 8 |
| Leukopenia | 50 | 33 | 14 | 14 | 31 | 23 |
| Hypertension | 0 | 0 | 57 | 0 | 31 | 0 |
| Neutropenia | 33 | 33 | 14 | 14 | 23 | 23 |
| Anemia | 50 | 50 | 0 | 0 | 23 | 23 |
| Asthenia | 17 | 17 | 14 | 0 | 15 | 8 |
| Nausea | 0 | 0 | 29 | 0 | 15 | 0 |
| Dyspepsia | 0 | 0 | 29 | 0 | 15 | 0 |
| Thrombocytopenia | 17 | 0 | 14 | 0 | 15 | 0 |
| Headache | 0 | 0 | 29 | 0 | 15 | 0 |
Common related adverse events for the phase 2 study are detailed in Table 3; those occurring in greater than 20% of patients in phase 2 included diarrhea, fatigue, cytopenias, hypertension, and nausea. With the exception of hematologic toxicities, most of these adverse events were grade 1 and grade 2 according to the Common Toxicity Criteria for Adverse Events Version 3. Febrile neutropenia occurred in 8% of patients. There were a total of 9 instances when infection occurred in the setting of neutropenia. These infections included a viral process (n = 1), pneumonia/pneumonitis (n = 2), dental abscess/tooth infection (n = 2), upper respiratory infection (n = 2), vaginal yeast infection (n = 1), and possible colitis (n = 1). No grade 5 events were thought to be related to therapy; however, 2 patients died while on study because of sepsis (n = 1) and pneumonia after influenza A infection (n = 1). Serious adverse events related to therapy included 5 episodes of febrile neutropenia and 1 episode each of pancytopenia, abdominal pain, diarrhea, renal failure, and superior vena cava occlusion. Renal failure occurred in a patient with baseline renal insufficiency; there was no evidence of tumor lysis, and no clear cause for the renal failure was determined. Only 2 patients were withdrawn from the phase 2 portion of the study for adverse events. One patient developed influenza A, but there were no reported opportunistic infections considered related to the FosD therapy.
Most common phase 2–related adverse events
| Adverse event . | Group 1 (n = 23) . | Group 2 (n = 21) . | Group 3 (n = 24) . | Total (n = 68) . | ||||
|---|---|---|---|---|---|---|---|---|
| % . | Grade 3/4, % . | % . | Grade 3/4, % . | % . | Grade 3/4, % . | % . | Grade 3/4, % . | |
| Fatigue | 26 | 0 | 48 | 0 | 50 | 0 | 41 | 0 |
| Diarrhea | 22 | 0 | 48 | 0 | 54 | 0 | 41 | 0 |
| Neutropenia | 22 | 13 | 33 | 14 | 38 | 25 | 31 | 18 |
| Anemia | 17 | 4 | 29 | 5 | 33 | 13 | 27 | 7 |
| Thrombocytopenia | 26 | 4 | 19 | 5 | 25 | 0 | 24 | 3 |
| Hypertension | 0 | 0 | 43 | 14 | 29 | 4 | 24 | 6 |
| Nausea | 4 | 0 | 43 | 0 | 17 | 4 | 21 | 2 |
| AST level increased | 22 | 4 | 19 | 5 | 13 | 4 | 18 | 4 |
| Headache | 4 | 0 | 33 | 0 | 13 | 0 | 16 | 0 |
| Leukopenia | 4 | 0 | 24 | 14 | 8 | 8 | 12 | 7 |
| Dizziness | 0 | 0 | 19 | 0 | 13 | 0 | 10 | 0 |
| Frequent bowel movements | 0 | 0 | 14 | 0 | 17 | 0 | 10 | 0 |
| Dysgeusia | 9 | 0 | 19 | 0 | 4 | 0 | 10 | 0 |
| ALT level increased | 9 | 4 | 14 | 5 | 8 | 4 | 10 | 4 |
| Dyspnea | 4 | 0 | 14 | 0 | 13 | 0 | 10 | 0 |
| Pyrexia | 13 | 0 | 5 | 0 | 8 | 0 | 9 | 0 |
| Blood alkaline phosphatase level increased | 9 | 0 | 14 | 5 | 4 | 0 | 9 | 2 |
| Febrile neutropenia | 4 | 4 | 5 | 0 | 13 | 8 | 7 | 4 |
| Upper abdominal pain | 0 | 0 | 5 | 0 | 17 | 4 | 7 | 2 |
| Dyspepsia | 4 | 0 | 14 | 0 | 4 | 0 | 7 | 0 |
| Increased appetite | 4 | 0 | 10 | 0 | 8 | 0 | 7 | 0 |
| Vomiting | 0 | 0 | 10 | 0 | 8 | 4 | 6 | 2 |
| Flatulence | 0 | 0 | 14 | 0 | 4 | 0 | 6 | 0 |
| Cough | 9 | 0 | 5 | 0 | 4 | 0 | 6 | 0 |
| Blood bilirubin level increased | 4 | 0 | 5 | 0 | 0 | 0 | 6 | 0 |
| Adverse event . | Group 1 (n = 23) . | Group 2 (n = 21) . | Group 3 (n = 24) . | Total (n = 68) . | ||||
|---|---|---|---|---|---|---|---|---|
| % . | Grade 3/4, % . | % . | Grade 3/4, % . | % . | Grade 3/4, % . | % . | Grade 3/4, % . | |
| Fatigue | 26 | 0 | 48 | 0 | 50 | 0 | 41 | 0 |
| Diarrhea | 22 | 0 | 48 | 0 | 54 | 0 | 41 | 0 |
| Neutropenia | 22 | 13 | 33 | 14 | 38 | 25 | 31 | 18 |
| Anemia | 17 | 4 | 29 | 5 | 33 | 13 | 27 | 7 |
| Thrombocytopenia | 26 | 4 | 19 | 5 | 25 | 0 | 24 | 3 |
| Hypertension | 0 | 0 | 43 | 14 | 29 | 4 | 24 | 6 |
| Nausea | 4 | 0 | 43 | 0 | 17 | 4 | 21 | 2 |
| AST level increased | 22 | 4 | 19 | 5 | 13 | 4 | 18 | 4 |
| Headache | 4 | 0 | 33 | 0 | 13 | 0 | 16 | 0 |
| Leukopenia | 4 | 0 | 24 | 14 | 8 | 8 | 12 | 7 |
| Dizziness | 0 | 0 | 19 | 0 | 13 | 0 | 10 | 0 |
| Frequent bowel movements | 0 | 0 | 14 | 0 | 17 | 0 | 10 | 0 |
| Dysgeusia | 9 | 0 | 19 | 0 | 4 | 0 | 10 | 0 |
| ALT level increased | 9 | 4 | 14 | 5 | 8 | 4 | 10 | 4 |
| Dyspnea | 4 | 0 | 14 | 0 | 13 | 0 | 10 | 0 |
| Pyrexia | 13 | 0 | 5 | 0 | 8 | 0 | 9 | 0 |
| Blood alkaline phosphatase level increased | 9 | 0 | 14 | 5 | 4 | 0 | 9 | 2 |
| Febrile neutropenia | 4 | 4 | 5 | 0 | 13 | 8 | 7 | 4 |
| Upper abdominal pain | 0 | 0 | 5 | 0 | 17 | 4 | 7 | 2 |
| Dyspepsia | 4 | 0 | 14 | 0 | 4 | 0 | 7 | 0 |
| Increased appetite | 4 | 0 | 10 | 0 | 8 | 0 | 7 | 0 |
| Vomiting | 0 | 0 | 10 | 0 | 8 | 4 | 6 | 2 |
| Flatulence | 0 | 0 | 14 | 0 | 4 | 0 | 6 | 0 |
| Cough | 9 | 0 | 5 | 0 | 4 | 0 | 6 | 0 |
| Blood bilirubin level increased | 4 | 0 | 5 | 0 | 0 | 0 | 6 | 0 |
Eight patients in phase 2 required a dose adjustment (to 150 mg twice daily) per protocol; 3 for neutropenia, 2 for hypertension, and 1 each for elevated results of liver function tests, fever, and anemia. Two patients required 2 dose reductions (to 150 mg daily). Seventeen patients (25%) had dose interruptions during the course of therapy. These interruptions lasted a median of 20 days (range, 1-57 days).
Nineteen patients (6 with a prior history of hypertension) were reported to have new onset or worsening hypertension in the phase 2 portion of the study, including 6 with grade 3 hypertension (defined as requiring drug therapy or additional therapy if previously treated). Hypertension most commonly occurred within 1 month of initiation of FosD. Of the 13 patients who required a new antihypertensive regimen, the majority (n = 8) required a single oral medication to establish blood pressure control. Hypertension resolved quickly after discontinuation of FosD.
Interestingly, all patients with SLL/CLL initially had increases in circulating lymphocyte count observed during the first 29 days of therapy; in 9 of the 13 patients with SLL/CLL (phase 1 and phase 2) this increase exceeded 50% of baseline circulating lymphocytes. The first patient in phase 1 was withdrawn for this reason. In several subsequent patients, circulating lymphocytes peaked at day 15 or 29 and fell slowly during the remainder of the time on therapy.
Clinical response and patient outcome
In the phase 1 portion of the trial, all patients in cohort 1 had stable disease after treatment with FosD with a median duration of 5.3 months (range, 1.8-10.4 months). In cohort 2 of the phase 1 study, one patient with FL had a PR with a response duration of 13.3 months. Two additional patients (with FL and SLL/CLL) had stable disease, with a duration of 4.8 and 13.7 months, respectively.
Responses for the phase 2 portion of the trial are summarized in Table 4. One patient with de novo DLBCL had a CR, 4 patients (including 1 with transformed LBCL) had a PR, and 4 additional patients (2 with transformed LBCL) had stable disease; the overall response rate (CR + PR) in the DLBCL cohort was 22% (95% CI, 7.5%-43.7%), and the median PFS was 2.7 months (95% CI, 1.3-4.5 months). Two patients with FL had a best response of PR to therapy (overall response rate [ORR], 10%; 95% CI, 1.2%-30%), and 11 additional patients had stable disease after therapy; the median PFS was 6.4 months (95% CI, 2.0-8.3 months). Six of 11 patients with SLL/CLL had a PR to therapy (ORR, 55%; 95% CI, 23%-83%) assessed according to lymphoma response criteria26 ; 2 additional patients had stable disease after therapy. The median PFS for patients with SLL/CLL was 6.4 months (95% CI, 2.2-7.1 months). Of the 6 responding patients with CLL, Zap-70 was positive (n = 3), negative (n = 1), and unknown (n = 2); cytogenetic abnormalitiesincluded trisomy 12 (n = 1), del13 (n = 1), normal (n = 1), complex (n = 1), and unknown (n = 2). One patient with MCL had a PR (ORR, 11%; 95% CI, 0.3%-48%); 4 additional patients with MCL had stable disease after therapy.
Phase 2 overall response and PFS by group/indication
| Response . | Group 1, DLBCL . | Group 2, FL . | Group 3 . | |||
|---|---|---|---|---|---|---|
| De novo (n = 17) . | Transformed (n = 6) . | (n = 21) . | CLL/SLL (n = 11) . | MCL (n = 9) . | Other (n = 4) . | |
| CR, n | 1 | 0 | 0 | 0 | 0 | 0 |
| PR, n | 3 | 1 | 2 | 6 | 1 | 0 |
| SD, n | 2 | 2 | 11 | 2 | 4 | 1 |
| PD, n | 8 | 3 | 7 | 3 | 4 | 2 |
| Not evaluable, n | 3 | 0 | 1 | 0 | 0 | 1 |
| ORR (CR + PR), n (%) | 4 (23.5%) | 1 (16.7%) | 2 (9.5%) | 6 (54.5%) | 1 (11.1%) | 0 |
| CR + PR + SD, n (%) | 6 (35.3%) | 3 (50.0%) | 13 (61.9%) | 8 (72.7%) | 5 (55.6%) | 1 (25.0%) |
| PFS, mo (95% CI) | —* | —* | 4.6 (2.0-8.3) | 6.4 (2.2-7.1) | 3.8 (1.9-4.6) | 1.9 (1.8-N/A) |
| Response . | Group 1, DLBCL . | Group 2, FL . | Group 3 . | |||
|---|---|---|---|---|---|---|
| De novo (n = 17) . | Transformed (n = 6) . | (n = 21) . | CLL/SLL (n = 11) . | MCL (n = 9) . | Other (n = 4) . | |
| CR, n | 1 | 0 | 0 | 0 | 0 | 0 |
| PR, n | 3 | 1 | 2 | 6 | 1 | 0 |
| SD, n | 2 | 2 | 11 | 2 | 4 | 1 |
| PD, n | 8 | 3 | 7 | 3 | 4 | 2 |
| Not evaluable, n | 3 | 0 | 1 | 0 | 0 | 1 |
| ORR (CR + PR), n (%) | 4 (23.5%) | 1 (16.7%) | 2 (9.5%) | 6 (54.5%) | 1 (11.1%) | 0 |
| CR + PR + SD, n (%) | 6 (35.3%) | 3 (50.0%) | 13 (61.9%) | 8 (72.7%) | 5 (55.6%) | 1 (25.0%) |
| PFS, mo (95% CI) | —* | —* | 4.6 (2.0-8.3) | 6.4 (2.2-7.1) | 3.8 (1.9-4.6) | 1.9 (1.8-N/A) |
SD indicates stable disease; PD, progressive disease; and —, not applicable.
DLBCL combined was 2.7 (1.3-4.5).
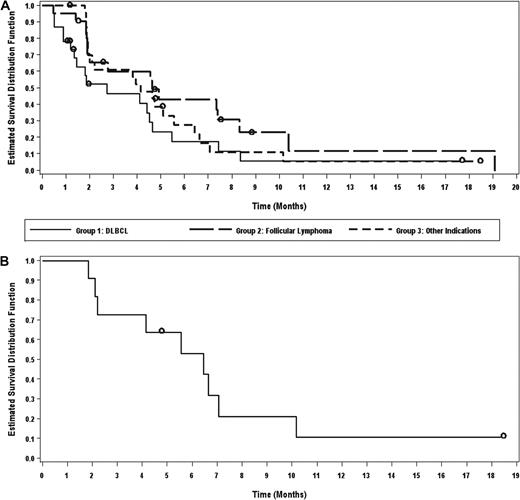
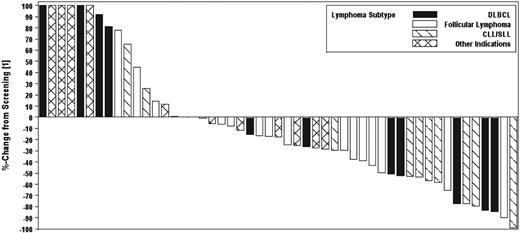
For all patients in phase 2, the median time to response was 57 days because responses generally occurred at the time of first response evaluation. The median duration of response was 4.3 months (range, 1-13 months). The median PFS for all patients in phase 2 was 4.1 months (95% CI, 2.1-5.6 months). The median duration of therapy was 75 days (range, 3-511 days). PFS by cohort is shown in Figure 1A. PFS for patients with SLL/CLL is shown in Figure 1B. In addition, a waterfall plot of best responses by histology is shown in Figure 2.
Progression-free survival. PFS by cohort (A) and of patients with SLL/CLL (B).
Waterfall plot of best responses in evaluable patients observed by cohort in the phase 2 study. Six subjects had greater than 100% increase in size of lesions; these values are listed at 100% for display purposes. Seventeen patients are not included because of early termination of drug because of toxicity or disease progression and no formal postscreening assessment.
Waterfall plot of best responses in evaluable patients observed by cohort in the phase 2 study. Six subjects had greater than 100% increase in size of lesions; these values are listed at 100% for display purposes. Seventeen patients are not included because of early termination of drug because of toxicity or disease progression and no formal postscreening assessment.
As of July 1, 2009, 2 patients remain on study treatment, 1 with transformed LBCL and 1 with SLL/CLL. Nine deaths have been reported, 8 from complications of progressive lymphoma and 1 from influenza pneumonia.
PK studies
On day 1 of FosD administration in the phase 1 study, the maximum plasma concentrations were 668 plus or minus 258 ng/mL and 1020 plus or minus 781 ng/mL, and the estimates of the area under the curve at 0 to 4 hour were 1800 plus or minus 602 ng × h/mL and 2590 plus or minus 1900 ng × h/mL, at 200 mg and 250 mg twice daily, respectively. Plasma concentrations increased approximately 2-fold with continued administration, but beyond day 29 there was no apparent change in FosD concentrations over time. For comparison to in vitro data with R406, 1800 ng/mL is roughly 3.6mM. No correlation between PK and clinical outcome was detected.
Pharmacodynamic studies
In previous human studies involving FosD, a correlation was observed between FosD dose/R406 plasma levels and number of CD14+ circulating cells in peripheral blood.25 We therefore evaluated this pharmacodynamic marker before and after therapy in all patients with the use of conventional flow cytometric evaluation. The median percentage of decrease of CD14+ cells on day 29 of therapy compared with baseline was 35%. There was no correlation between the magnitude of decrease in CD14+ cells after therapy with FosD and tumor response or development of cytopenias or hypertension (P = NS). Moreover, there was no apparent correlation between changes in CD14+ counts and R406 area under the curve, suggesting this may not be an optimal pharmacodynamic marker in patients with lymphoma and CLL.
Discussion
In this first phase 1/2 clinical trial of the specific Syk inhibitor FosD, we have demonstrated both safety and efficacy in the treatment of B-cell NHLs and SLL/CLL. Despite a heavily pretreated relapsed and refractory patient population, responses were observed in DLBCL, FL, MCL, and SLL/CLL. Common toxicities observed in our study included diarrhea, fatigue, and cytopenias, similar to toxicities observed in rheumatoid arthritis studies that used lower doses of FosD.21 Although neutropenia was frequently observed, febrile neutropenia was uncommon despite the heavily pretreated nature of the patient population. We observed mild-to-moderate hypertension in 22% of patients, generally occurring within the first month of initiation of therapy, and typically managed with the addition of a single oral agent. The onset of hypertension did not appear to correlate with a prior history of hypertension. The mechanism of this observed hypertension is not known, but it has been reported in rheumatoid arthritis trials with FosD and may be related to off-target inhibition of vascular endothelial growth factor receptor.21 FosD has also been reported to inhibit FLT-320 and Ret kinase27 ; however, it is unlikely these targets contributed to toxicity or lymphoma responses. Although grade 3 neutropenia was observed in a subset of patients, there were few severe infections and no opportunistic infections, even with extended treatment (longer than 1 year in some patients). These findings are consistent with earlier murine safety studies in which the reduction in lymphocytes and bone marrow cellularity after treatment with FosD had no adverse effects on the humoral immune response.28
More than 20% of the patients in our series with multiply relapsed/refractory DLBCL responded to therapy with FosD. In vitro evidence of DLBCL sensitivity to Syk inhibition, manifested by a dose-dependent reduction in basal proliferation, has been shown in a variety of cell lines treated with piceatannol (a less specific Syk inhibitor).10 Off-target effects are always possible, but these data support the notion that Syk inhibition is responsible for the clinical outcomes observed in our study. Chen et al8 recently published a comprehensive preclinical evaluation of R406 in DLBCL. In their study, DLBCL cell lines and primary tumors with an intact BCR signaling pathway were highly sensitive to R406-mediated apoptosis. Responses to R406 were detected in primary DLBCLs with evidence of both tonic and induced BCR signaling. Of interest, R406-sensitive, BCR-dependent DLBCLs were independently identified as BCR-type tumors by transcriptional profiling.8,13 In the preclinical evaluation of R406, only cell lines and primary tumors with absent surface IgG or IgM or lower levels of cell-surface IgG had ineffective BCR signaling. We were unable to determine IgG expression in DLBCLs from the current trial because of the lack of fresh tissue. Future trials in DLBCL that limit enrollment to patients expressing cell-surface IgM or high levels of cell-surface IgG are under development.
Although the response rates were lower in MCL and FL, prolonged stable disease was observed in several patients with FL. The mechanism by which Syk inhibition leads to clinical responses in these diseases may be different from that in DLBCL. It is not clear whether tonic signaling through BCR is required to maintain survival in subgroups of FL and MCL. However, in a study that used piceatannol or small interfering RNA plasmids to inhibit Syk in vitro, potent inhibition of mammalian target of rapamycin (mTOR) activity was shown in both FL and MCL cell lines.29 Syk therefore appears to also play a central role in mTOR activation; this is of particular relevance because targeting of mTOR signaling with temsirolimus has resulted in significant therapeutic activity in indolent lymphoma and MCL.30
The highest response rate in our trial, by conventional lymphoma response criteria,26 was observed in patients with SLL/CLL. Tonic signaling through BCR has been identified in a subset of patients with CLL, particularly in CLL with unmutated IgH.31,32 Mutation analysis was not performed in our patients; however, clinical responses were observed in patients with SLL/CLL who did and did not express Zap-70, a robust surrogate marker for IgH mutation status.33 This suggests that patients with both mutated and unmutated IgH responded to treatment with FosD in our trial. Additional rational for inhibiting Syk as therapy for SLL/CLL comes from a recent analysis of spontaneous regression in patients with SLL/CLL, which determined Syk was overexpressed and that functional BCR signaling was present in this subset of cases compared with normal B lymphocytes.34
In a laboratory model of CLL, induction of the chemoattractants CCL3 and CCL4 was abrogated by the inhibition of Syk, suggesting that cells within the CLL microenvironment induce these chemokines through BCR activation.35 Quiroga et al36 have found that BCR can trigger increased CLL cell migration toward the chemokines CXCL12 and CXCL13. Moreover, in their laboratory study, BCR activation also enhanced CLL cell migration beneath marrow stromal cells. Migration of CLL cells could be inhibited by R406, suggesting that the BCR-induced CLL survival signals in the tissue microenvironments can be disrupted by Syk inhibition. This laboratory finding is of particular interest, given our observation that several patients with CLL had rapid nodal responses in the setting of a transient worsening of lymphocytosis. We speculate that the inhibition of Syk in these patients may have disrupted the nodal microenvironment and increased the trafficking of CLL cells out of nodal tissues. Little is known about the equilibrium between the circulating state and the lymph node resident state in CLL, but nodal disease appears to be more resistant to other novel therapeutic agents.37 Additional studies are underway to identify how Syk inhibition leads to responses in CLL, including a detailed evaluation of the malignant microenvironment in nodal tissue.
The future development of FosD is likely to include rational combinations. Rituximab, an anti-CD20 monoclonal antibody with several mechanisms of action,38 confers a survival benefit in both indolent and aggressive lymphomas when combined with chemotherapy39,40 and improves PFS in CLL.41 The cytotoxic activity of another monoclonal antibody, gemtuzumab ozogamicin, in acute myelogenous leukemia correlates negatively with the expression of Syk,42 suggesting the possibility that the antibody-dependent cellular cytotoxicity activity of rituximab could be affected negatively by FosD. In vitro and in vivo studies are ongoing to determine whether FosD alters the response to rituximab. Other rational agents to combine with FosD may include mTOR inhibitors, because mTOR is a potential downstream target of Syk. In addition, there is evidence that a ubiquitin/proteasome-dependent mechanism contributes to Syk regulation,43 and proteasome inhibitors that have significant activity in NHL44 may be synergistic with FosD. Finally, because cAMP negatively regulates Syk, and inhibition of PDE4B expression significantly improved the efficacy of Syk inhibitors in several murine models of DLBCL, approaches that inhibit both Syk and PDE4B are under investigation.45
The inhibition of a critical B-cell lymphoma survival pathway with FosD results in tumor cell death and clinical responses in a significant proportion of heavily pretreated patients with SLL/CLL and DLBCL. The most common toxicities are hematologic and reversible; no B cell–specific toxicities were observed. Additional clinical trials are planned to identify lymphomas dependent on the BCR pathway and to further analyze this promising rational targeted therapy for B-cell lymphomas and leukemia.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in part in oral form at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 7, 2008.
The publication costs of this Article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Rigel Pharmaceuticals. J.W.F. is a Clinical Scholar of the Leukemia & Lymphoma Society and is supported in part by the University of Rochester Specialized Program of Research Excellence (SPORE) in Lymphoma (CA-130805).
National Institutes of Health
Authorship
Contribution: J.W.F., J.P.L., A.M.L., J.M.V., R.L., and M.A.S. designed the research; J.W.F., J. Sharman, J.S.-C., P.B.J., S.D.V., A.L., J.P.L., L.D.C., R.S., S.A.G., J. Sweetenham, and J.M.V. performed the research; J.W.F., J. Sharman, J.S.-C., M.P.S., A.M.L., R.L., and M.A.S. analyzed data; and J.W.F., A.L., R.L., and M.A.S. wrote the paper.
Conflict-of-interest disclosure: A.M.L. and M.P.S. are employees at Rigel. J.W.F., J. Sweetenham, P.B.J., J.M.V., S.D.V., R.S., J.P.L., L.D.C., S.A.G., and R.L. have received research support for this trial from Rigel. The remaining authors declare no competing financial interests.
Correspondence: Jonathan W. Friedberg, James P. Wilmot Cancer Center, University of Rochester, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: jonathan_friedberg@urmc.rochester.edu.