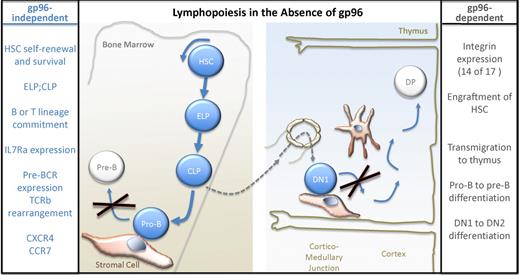
Initially identified as a glucose-regulated protein, gp96 (also referred to as grp94 or tumor rejection antigen 1) is an abundant endoplasmic reticulum protein with multiple biologic functions.2 As a chaperone molecule during protein folding, gp96 is up-regulated during cell stress and plays a key role in unfolded protein responses (UPR). When released into the extracellular milieu after necrosis, soluble gp96 can function as an adjuvant for antitumor immune responses and can activate macrophages through Toll-like receptor 2 (TLR2). In addition, gp96 can bind short peptides and elicit immune responses. Staron and colleagues use an inducible cre-lox gp96KO mouse to overcome embryonic lethality and reveal new roles for gp96 in mediating the pre-B to pro-B and DN1-DN2 transitions during lymphopoiesis (see figure).
Staron and colleagues demonstrate that loss of gp96 leads to block in early B- and T-cell development (large X) and decreased transmigration of lymphoid precursors to the thymus (----). HSC indicates hematopoietic stem cell; ELP, early lymphocyte progenitor; and CLP, common lymphocyte progenitor.
Staron and colleagues demonstrate that loss of gp96 leads to block in early B- and T-cell development (large X) and decreased transmigration of lymphoid precursors to the thymus (----). HSC indicates hematopoietic stem cell; ELP, early lymphocyte progenitor; and CLP, common lymphocyte progenitor.
As a chaperone protein, gp96's nonredundant role during lymphopoiesis could be caused by aberrant expression of its client proteins. An essential role of gp96 as a chaperone for TLRs and integrins is conserved in drosophila3 and appears to be dependent on gp96 binding of ATP.4 Elegant work by Randow and Seed showed that gp96 deficiency resulted in the inability of TLR4 to traffic to the cell surface,4 and a previous study by Liu and Li demonstrated that mature B cells lacking gp96 were unable to express cell-surface α4 and β2 integrins.5 The loss of these integrins rendered cells unable to traffic to lymph nodes, peritoneal cavity, or splenic marginal zone even though they expressed appropriate chemokine receptors.
Cell adhesion and migration are integrin-mediated functions that are also essential for many steps during lymphopoiesis. The earlier Liu and Li study used a CD19 cre-lox to assess gp96's role in the late pro-B- to mature B-cell stages. In the current study, Staron and colleagues extend these studies using ERT2-Cre mice crossed to floxed gp96 mice, which reveal a striking loss of all α integrins and most β integrins in hematopoietic cells. The loss of gp96 and its partners arrests B-cell development at the pro-B-cell stage, transmigration to the thymus, and DN1 to DN2 differentiation (see figure). One might hypothesize that gp96 client integrins are especially critical for transmigration steps such as the egress of T-cell precursors from bone marrow to thymus because there are fewer thymic progenitors identified in these mice. Both early B-cell and early T-cell development require cell-to-cell contact with specialized stromal cells for differentiation.6,7 gp96-lacking precursors were unable to progress through these crucial steps, likely implicating integrins in the tightly choreographed lympho-stromal dance.
Integrins signal both outside-in and inside-out and such cross-talk appears to be critical for the function of hematopoietic stem cells (HSCs), because gp96KO HSCs were able to self-renew but could not facilitate engraftment on their own. Chimeric transplants were required for engraftment of gp96KO HSCs, suggesting that wild-type, gp96-, and integrin-expressing HSCs were necessary to signal host stromal cells to support lymphopoiesis. Myelopoiesis appears to proceed normally without gp96; however, loss of gp96 does cause thrombocytopenia, likely reflecting the critical role of integrins in platelet production and/or function. It is not entirely clear whether the defects in lymphopoiesis observed are entirely caused by the lack of integrin expression, although similar pro-B and DN1 developmental arrest is seen in α4 integrin knockout mice. gp96 has been identified at the cell surface of immature thymocytes8 and thus it remains possible that surface gp96 has other functional roles than just as chaperone.
Is gp96 a specialized immune chaperone? Perhaps yes, as other chaperones can mediate the UPR, but gp96 is nonredundant for B- and T-cell development, especially at the stages requiring transmigration. In contrast, gp96-specific loss in Purkinje cells of the cerebellum does not effect function, whereas lack of the related chaperone grp78 leads to accelerated cerebellar degeneration.9 Such findings challenge a passive view of chaperones as a generic protective mechanism. Instead, these studies shed light on chaperones as subspecialists who carefully choose their dance card partners and are critical to specific lineages or tissues.
In summary, blocks in T- and B-cell development at stages that require interaction with stromal cells reveal a previously unappreciated role for gp96 as a specialized chaperone, nonredundant for immune development, and highlight what is likely to be essential roles for integrins in the specialized microenvironments of primary lymphoid organs.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal