Abstract
Graft-versus-host disease (GVHD) is the most common cause of nonrelapse mortality and morbidity after hematopoietic stem cell transplantation (HSCT). The well-documented involvement of heparanase in the process of inflammation and autoimmunity led us to investigate an association between HPSE gene single-nucleotide polymorphisms (SNPs) and the risk of GVHD. The present study indicates a highly significant correlation of HPSE gene SNPs rs4693608 and rs4364254 and their combination with the risk of developing acute GVHD. Moreover, the study revealed that discrepancy between recipient and donor in these SNPs may elevate significantly the risk of acute GVHD. This association was statistically significant when the recipients possessed genotype combinations dictating higher levels of heparanase compared with their human leukocyte antigen (HLA)–matched donors. In addition, HPSE gene SNPs disclosed a correlation with extensive chronic GVHD, nonrelapse mortality, and overall survival. Our study indicates involvement of heparanase in the development of acute and extensive chronic GVHD. Moreover, it suggests a possible mechanism for the aggressive behavior of T lymphocytes leading to GVHD when the recipients possess genotype combinations that dictate high levels of heparanase mRNA compared with their HLA-matched donors expressing low levels of heparanase.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is potentially effective curative therapy for a variety of malignant and nonmalignant hematologic diseases.1 The growing indications for HSCT and the improvement in supportive care and conditioning regimens led to an increased number of allogeneic transplantations.1 However, transplantation-related complications remain a major obstacle.2-4 Outcome of HSCT is affected by several variables, including disease and disease status, general condition, patient and donor age, and patient and donor human leukocyte antigen (HLA) matching.5,6 Disease relapse and transplantation-related complications, including graft-versus-host disease (GVHD), major infection, and organ toxicities such as interstitial pneumonitis and hepatic veno-occlusive disease (sinusoidal obstruction) are the major causes for transplantation failure.5,6 Long-term causes of morbidity include chronic GVHD and infection.7 Prediction and prevention of GVHD is thus one of the major tasks in trying to further improve transplantation outcome.
In addition to HLA matching, genetic diversity among patients and donors contributes to differences in individual responses to tissue injury, inflammation, and severity of acute and/or chronic GVHD.8,9 Previous studies have implicated polymorphisms in cytokine genes as factors affecting GVHD and survival.10-16 Heparanase is the predominant endoglycosidase responsible for heparan sulfate (HS) degradation and the associated release of HS-bound cytokines, chemokines, and bioactive angiogenic- and growth-promoting factors. Heparanase may therefore play an important role in the pathogenesis of GVHD.17-21
The human heparanase gene (HPSE) is located on chromosome 4 (4q21.3) and includes 12 exons that transcribe 1629-bp mRNA22,23 normally expressed in hematopoietic cells, platelets, cells of the immune system, mast cells, activated endothelial cells, keratinocytes, and cytotrophoblasts.20,21,24,25 The heparanase protein consists of 543 amino acids and is synthesized as a latent 65-kDa precursor whose processing involves proteolytic removal of a linker segment by cathepsin L26 and formation of a heterodimer consisting of 50- and 8-kDa subunits.26-28 Apart from its well-established involvement in cancer metastasis, heparanase has been shown to be involved in processes such as angiogenesis, inflammation, tissue repair and remodeling, and autoimmunity.20,21,25,28
We have recently determined genotype combinations of 2 single-nucleotide polymorphisms (SNPs; rs4693608 and rs4364254) in the HPSE gene that significantly correlate with low (LR), intermediate (MR), and high (HR) heparanase levels.29 This result led us to investigate the involvement of HPSE gene SNP combinations and heparanase in GVHD and HSCT outcome. The present study indicates a highly significant correlation between the 2 SNPs and their combinations and the risk of acute GVHD. Moreover, HPSE gene SNPs disclosed a significant correlation with extensive chronic GVHD, nonrelapse mortality, and overall survival (OS). Notably, our study revealed that discrepancy in HPSE gene SNP combinations between recipients and donors is a risk factor for developing acute GVHD. This association was statistically significant when the recipients possessed genotype combinations that dictate higher levels of heparanase mRNA compared with their HLA-matched donors.
Methods
Study populations
A total of 414 consecutive patients (240 men and 174 women) with hematologic malignancies and their HLA-matched donors were included in the study. Patients received transplants at the Bone Marrow Transplantation Department of the Chaim Sheba Medical Center over an 8-year period. All patients gave written informed consent, and the study was approved by the Institutional Review Board of the Chaim Sheba Medical Center. Patient and donor characteristics are presented in Table 1. A total of 245 patients received grafts from HLA-identical siblings and 169 patients received transplants from unrelated donors. A total of 405 patients received mobilized peripheral blood stem cells, while only 9 recipients received transplants of bone marrow grafts. The median age was 50 years (range, 17-75 years). HLA matching was performed for HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB using high-resolution molecular typing. Transplant conditioning regimens varied according to the type and status of the disease. Based on our previous studies correlating GVHD and pretransplantation regimens, we divided the conditioning regimens into 4 groups: (1) classical myeloablative, including Cy/TBI (high-dose cyclophosphamide and total body irradiation) and ivBuCy (high-dose intravenous busulfan and cyclophosphamide) regimens; (2) reduced-toxicity myeloablative conditioning consisting of fludarabine with high-dose busulfan or treosulfan; (3) reduced-intensity conditioning (RIC) containing fludarabine with reduced doses of busulfan; and (4) a fludarabine and melphalan regimen.30,31 Prophylaxis against GVHD consisted of cyclosporine A and a short course of methotrexate. GVHD prophylaxis was administered for 3 to 6 months and tapered afterward in patients with no active GVHD. G-CSF was administered from day 7 after transplantation until engraftment. A standard regimen of antibiotic and antimycotic prophylaxis was used to prevent bacterial and fungal infections. Acyclovir, sulfamethoxazole, and trimethoprim were administered for prevention of viral and Pneumocystis carinii infections, respectively.
Characteristics of transplant recipients and donors
| Variable/characteristic . | No. of cases (%) . |
|---|---|
| Age, y | |
| Median: 50 | |
| Range: 17-75 | |
| Sex | |
| Recipient | |
| Male | 240 (58.0) |
| Female | 174 (42.0) |
| Donor | |
| Male | 252 (60.9) |
| Female | 162 (39.1) |
| Recipient/donor | |
| Male/male | 151 (36.5) |
| Male/female | 90 (21.7) |
| Female/male | 100 (24.2) |
| Female/female | 73 (17.6) |
| Diagnosis | |
| AML | 178 (43.0) |
| ALL | 65 (15.7) |
| CML | 22 (5.3) |
| CLL | 15 (3.6) |
| MDS | 42 (10.1) |
| HL | 12 (2.9) |
| NHL | 43 (10.4) |
| MM | 25 (6.0) |
| MF | 5 (1.2) |
| AA | 4 (1.0) |
| PNH | 3 (0.7) |
| Donor | |
| Sibling | 245 (59.2) |
| mud/mm | 169 (40.8) |
| Conditioning regimen | |
| Classical myeloablative | 130 (31.4) |
| Reduced-intensity conditioning | 124 (29.9) |
| Reduced-toxicity myeloablative | 86 (17.9) |
| Fludarabine/melphalan | 74 (20.8) |
| Variable/characteristic . | No. of cases (%) . |
|---|---|
| Age, y | |
| Median: 50 | |
| Range: 17-75 | |
| Sex | |
| Recipient | |
| Male | 240 (58.0) |
| Female | 174 (42.0) |
| Donor | |
| Male | 252 (60.9) |
| Female | 162 (39.1) |
| Recipient/donor | |
| Male/male | 151 (36.5) |
| Male/female | 90 (21.7) |
| Female/male | 100 (24.2) |
| Female/female | 73 (17.6) |
| Diagnosis | |
| AML | 178 (43.0) |
| ALL | 65 (15.7) |
| CML | 22 (5.3) |
| CLL | 15 (3.6) |
| MDS | 42 (10.1) |
| HL | 12 (2.9) |
| NHL | 43 (10.4) |
| MM | 25 (6.0) |
| MF | 5 (1.2) |
| AA | 4 (1.0) |
| PNH | 3 (0.7) |
| Donor | |
| Sibling | 245 (59.2) |
| mud/mm | 169 (40.8) |
| Conditioning regimen | |
| Classical myeloablative | 130 (31.4) |
| Reduced-intensity conditioning | 124 (29.9) |
| Reduced-toxicity myeloablative | 86 (17.9) |
| Fludarabine/melphalan | 74 (20.8) |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; AA, aplastic anemia; classical myeloablative, Cy/TBI (high-dose cyclophosphamide and total body irradiation) and ivBuCy (high-dose intravenous busulfan and cyclophosphamide); CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MF, myelofibrosis; MM, multiple myeloma; mud/mm, matched unrelated or mismatched unrelated donor; NHL, non-Hodgkin lymphoma; PNH, paroxysmal nocturnal hemoglobinuria; reduced-intensity conditioning, fludarabine with reduced doses of busulfan; reduced-toxicity myeloablative, fludarabine with high-dose busulfan or treosulfan.
Evaluation of clinical outcome
The primary endpoints of analysis were incidence and severity of acute and chronic GVHD. Since the study included a heterogeneous group of patients, transplantation-related mortality (TRM), OS, and relapse incidence were considered secondary endpoints. HSCT data were collected prospectively during patient follow-up and recorded in an institutional database.
Acute GVHD was graded using the Glucksberg criteria.32 Statistical analysis was performed for grades II through IV and grades III and IV acute GVHD. Chronic GVHD was assessed using established criteria and graded as limited or extensive disease in patients who survived at least 100 days after HSCT.33
SNP genotyping
Genomic DNA was prepared from whole blood collected in disodium EDTA, applying the Puregene DNA isolation kit (Gentra). Genotyping of 2 HPSE gene SNPs rs4693608 and rs4364254 was carried out as described.34,35 Briefly, SNP rs4693608 was identified by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP)–based analysis, whereas SNP rs4364254 was detected by allele-specific amplification. The optimized PCR reaction mixture in a final volume of 15 μL consisted of Red Load TaqMaster (LAROVA GmbH), 10 to 20 pM of each forward and reverse primers, and 150 to 200 ng of the DNA template. All reactions were performed using a PTC-200 Peltier thermal cycler (MJ Research). The cycle conditions were 94°C for 4 minutes, followed by 30 cycles of 94°C for 15 seconds (denaturation), 54°C for 1 minute for SNP rs4693608 and 53°C for 45 seconds for SNP rs4364254, and 72°C for 1 minute (extension).34,35 The polymorphic alleles of the rs4693608 SNP were detected using HincII restriction endonuclease (Takara). Following digestion (2 hours at 37°C), 2 fragments of 83 bp and 41 bp were detected when the G allele was included. When the product remained resistant to HincII, the DNA included the A allele. The PCR products were separated by 2% to 3% agarose gel electrophoresis and visualized under ultraviolet light using ethidium bromide staining. Gels were photographed and analyzed by the EDAS 290 Electrophoresis Documentation and Analysis System (Kodak).34,35
Genotype combinations of rs4693608 and rs4364254 SNPs were signed as recently published.29 A total of 4 genotype combinations (GG-CC, GG-CT, GG-TT, and GA-CC) were included in group LR (low), 2 genotype combinations (GA-CT, GA-TT) were included in group MR (medium), and 2 additional combinations of genotypes (AA-TT, AA-CT) were included in group HR (high). Since the attribution of persons with AA-CC combination was unclear, these patients were excluded from the study. However, first evaluation of the AA-CC genotype in relation to certain groups was performed.
Statistical analysis
All major clinical outcome variables (acute GVHD, chronic GVHD, day 180 TRM, relapse incidence, and OS) were analyzed in relation to the rs4693608 and rs4364254 SNPs and their combinations according to the LR, MR, and HR groups of the recipients and donors, respectively. Genotype and allele frequencies of the SNPs were calculated by direct counting. Cumulative incidence was calculated for each of these parameters. Time to the clinical event (GVHD, TRM, relapse, and death) was measured from the date of HSCT. In the analysis of the cumulative incidence of GVHD, relapse was considered a competing risk.36 Relapse and TRM rates were estimated using cumulative incidence analysis and considered as competing risk. The probability of OS was analyzed using the Kaplan-Meier method.37 The effect of differences in patient and donor categorical variables on survival probabilities was studied with the log-rank test. Step-wise multivariate Cox regression models were adjusted for testing the independent prognostic relevance of HPSE gene SNPs. The limit for reverse selection procedures was 0.2. For the multivariate comparison, patient age, type of disease, patient and donor sex, type of donor, type of conditioning, and HPSE gene SNP combinations were considered. Analysis of discrepancy between recipient and donor was performed. As a first step, cumulative incidence analysis was performed for 9 possible variations of differences between recipients and donors. Then, all donor-recipient pairs were divided into 3 groups according to the risk of acute GVHD development (D1 for high risk of acute GVHD development, D2 for moderate risk, and D3 for low risk, respectively). Cumulative incidence analysis and the log-rank test were carried out. P values less than .05 were regarded as statistically significant. Calculations were performed using NCSS.
Results
Genotype and allele frequencies of HPSE gene rs4693608 and rs4364254 SNPs and their combinations in recipients, donors, and control populations
In the present study, patients with hematologic malignancies and their donors were assessed for a correlation between HPSE gene polymorphisms and HSCT outcomes. The genotype and allele frequencies of the SNPs are represented in Table 2. The SNP genotype frequencies did not deviate significantly from the Hardy-Weinberg distribution (data not shown). The genotype and allele frequencies of both related and unrelated donors were similar to those of the control group of healthy persons. The genotype and allele frequencies of the HSCT cohort were slightly higher in the HSCT group for SNP rs4693608 and the SNP combination rs4693608 and rs4364254, in comparison with the donor volunteers and the control group of healthy persons (Table 2), but these differences were not statistically significant. Disparity of HPSE gene SNPs between donors and recipients ranged from 35.1% to 38% and from 59.6% to 63.6% when the donors were sibling or unrelated, respectively (P < .001; Table 2).
Genotype and allele frequencies of HPSE gene SNPs rs4693608 and rs4364254 and their combinations in recipients, donors, and control healthy persons
| SNP/SNP combination . | Genotype/allele . | Recipients . | Total donors . | Related donors . | Disparity, % . | Unrelated donors . | Disparity, % . | χ2/P . | Controls . |
|---|---|---|---|---|---|---|---|---|---|
| rs4693608 | AA | 30.4% (126) | 23.9% (87) | 22.5% (48) | 38.0 | 25.8% (39) | 59.6 | 16.5/< .001 | 25.0% (27) |
| AG | 48.3% (200) | 54.9% (200) | 55.4% (118) | 54.3% (82) | 57.41% (62) | ||||
| GG | 21.3% (88) | 21.2% (77) | 22.1% (47) | 19.9% (30) | 17.6% (19) | ||||
| A | 54.6% (452) | 51.4% (374) | 50.2% (214) | 53.0% (160) | 53.7% (116) | ||||
| G | 45.4% (376) | 48.6% (354) | 49.8% (212) | 47.0% (142) | 46.3% (100) | ||||
| rs4364254 | TT | 44.7% (185) | 41.8% (152) | 39.4% (84) | 35.1 | 45.0% (68) | 62.9 | 27.4/< .001 | 45.4% (49) |
| TC | 40.3% (167) | 45.3% (165) | 46.0% (98) | 44.4% (67) | 46.3% (50) | ||||
| CC | 15.0% (62) | 12.9% (47) | 14.6% (31) | 10.6% (16) | 8.3% (9) | ||||
| T | 64.9% (537) | 64.4% (469) | 62.4% (266) | 67.2% (203) | 68.5% (148) | ||||
| C | 35.1% (291) | 35.6% (259) | 37.6% (160) | 32.8% (99) | 31.5% (68) | ||||
| rs4693608 and rs4364254: | AA-TT, AA-TC | 30.4% (126) | 23.9% (87) | 22.5% (48) | 37.1 | 25.8% (39) | 63.6 | 24.8/< .001 | 25.0% (27) |
| HR | AG-TC, AG-TT | 42.5% (176) | 48.1% (175) | 48.4% (103) | 47.7% (72) | 52.8% (57) | |||
| MR LR | GG-CC, GG-TC, GG-TT, AG-CC | 27.1% (112) | 28.0% (102) | 29.1% (62) | 26.5% (40) | 22.2% (24) |
| SNP/SNP combination . | Genotype/allele . | Recipients . | Total donors . | Related donors . | Disparity, % . | Unrelated donors . | Disparity, % . | χ2/P . | Controls . |
|---|---|---|---|---|---|---|---|---|---|
| rs4693608 | AA | 30.4% (126) | 23.9% (87) | 22.5% (48) | 38.0 | 25.8% (39) | 59.6 | 16.5/< .001 | 25.0% (27) |
| AG | 48.3% (200) | 54.9% (200) | 55.4% (118) | 54.3% (82) | 57.41% (62) | ||||
| GG | 21.3% (88) | 21.2% (77) | 22.1% (47) | 19.9% (30) | 17.6% (19) | ||||
| A | 54.6% (452) | 51.4% (374) | 50.2% (214) | 53.0% (160) | 53.7% (116) | ||||
| G | 45.4% (376) | 48.6% (354) | 49.8% (212) | 47.0% (142) | 46.3% (100) | ||||
| rs4364254 | TT | 44.7% (185) | 41.8% (152) | 39.4% (84) | 35.1 | 45.0% (68) | 62.9 | 27.4/< .001 | 45.4% (49) |
| TC | 40.3% (167) | 45.3% (165) | 46.0% (98) | 44.4% (67) | 46.3% (50) | ||||
| CC | 15.0% (62) | 12.9% (47) | 14.6% (31) | 10.6% (16) | 8.3% (9) | ||||
| T | 64.9% (537) | 64.4% (469) | 62.4% (266) | 67.2% (203) | 68.5% (148) | ||||
| C | 35.1% (291) | 35.6% (259) | 37.6% (160) | 32.8% (99) | 31.5% (68) | ||||
| rs4693608 and rs4364254: | AA-TT, AA-TC | 30.4% (126) | 23.9% (87) | 22.5% (48) | 37.1 | 25.8% (39) | 63.6 | 24.8/< .001 | 25.0% (27) |
| HR | AG-TC, AG-TT | 42.5% (176) | 48.1% (175) | 48.4% (103) | 47.7% (72) | 52.8% (57) | |||
| MR LR | GG-CC, GG-TC, GG-TT, AG-CC | 27.1% (112) | 28.0% (102) | 29.1% (62) | 26.5% (40) | 22.2% (24) |
Acute GVHD
A total of 149 patients developed acute GVHD. An additional 61 patients relapsed during the first 100 days after transplantation and were calculated as a competing risk. A total of 20 (13.4%) patients developed grade I acute GVHD, 56 (37.6%) developed grade II acute GVHD, 30 (20.1%) patients developed grade III acute GVHD, and 43 patients (28.9%) developed grade IV acute GVHD.
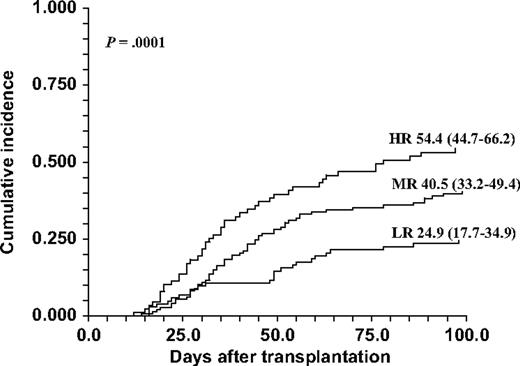
Univariate analysis of cumulative incidence of clinically significant (II-IV and III-IV) acute GVHD in association with rs4693608 and rs4364254 SNPs and SNP combinations is presented in Table 3. The cumulative rate of acute GVHD incidence (grades II-IV) for recipients carrying genotype AA was 52.7% (95% confidence interval [CI] 43.3-64.1); for recipients possessing genotype AG, 38.5% (95% CI 31.7-46.8); and for possessors of genotype GG, 24.3% (95% CI 16.2-36.3) at day 100 after HSCT (P = .001; Table 3). Cumulative incidence of acute GVHD (grades II-IV) for possessors of genotype CC was notably low (26.9% [95% CI 17.2-42.2]) compared with the cumulative incidence for patients carrying genotypes TC and TT (41.3% [95% CI 33.7-50.6] and 41.9% [95% CI 34.5-50.8], respectively). The most statistically significant association between HPSE gene SNPs and acute GVHD was obtained for rs4693608 and rs4364254 SNP genotype combinations. The cumulative rate of acute GVHD incidence (grades II-IV) for recipients of group HR was 54.4% (95% CI 44.7-66.2); for recipients of group MR, 40.5% (95% CI 33.2-49.4); and for recipients of group LR, 24.9% (95% CI 17.7-34.9) at day 100 after HSCT (P < .001; Table 3). These highly significant differences are depicted in Figure 1, further emphasizing the higher incidence of GVHD in group HR. The estimated incidence of severe acute GVHD (grades III-IV) was 42.7% (95% CI 32.8-55.5) in group HR, 21.8% (95% CI 15.6-30.6) in group MR, and 13.7% (95% CI 8.2-22.7) in group LR. The highly significant χ2 of 23.3 and P value less than .001 indicate that genotype combination HR is associated with increased risk of acute GVHD, especially severe acute GVHD, while genotype combination LR is correlated with a low risk of this complication. Donor genotypes or genotype combinations did not correlate with the development of acute GVHD (Table 3).
Univariate analysis of cumulative incidence of clinically significant (II-IV and III-IV) acute GVHD in association with HPSE gene SNPs and SNP combinations
| SNP/SNP combination . | Genotype/ genotype combination . | Cumulative incidence, % . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recipient . | Donor . | ||||||||||||
| II-IV . | 95% CI . | χ2/P . | III-IV . | 95% CI . | χ2/P . | II-IV . | 95% CI . | χ2/P . | III-IV . | 95% CI . | χ2/P . | ||
| rs4693608 | AA | 52.7 | 43.3-64.1 | 13.7/.001 | 40.9 | 31.4-53.3 | 18.7/< .001 | 35.6 | 26.1-48.5 | 4.8/.09 | 20.7 | 12.9-33.2 | .5/.78 |
| AG | 38.5 | 31.7-46.8 | 20.4 | 14.7-28.3 | 45.5 | 38.4-54.0 | 26.2 | 19.8-34.8 | |||||
| GG | 24.3 | 16.2-36.3 | 14.0 | 7.9-25.0 | 28.8 | 19.3-43.0 | 24.5 | 15.5-38.7 | |||||
| rs4364254 | TT | 41.9 | 34.5-50.8 | 4.4/.11 | 26.5 | 19.8-35.5 | 4.6/.098 | 41.0 | 33.3-50.6 | .34/.84 | 25.8 | 18.8-35.5 | .39/.82 |
| TC | 41.3 | 33.7-50.6 | 26.8 | 20.0-36.0 | 38.9 | 31.2-78.3 | 23.9 | 17.3-33.0 | |||||
| CC | 26.9 | 17.2-42.2 | 12.8 | 6.1-27.3 | 38.1 | 25.2-57.6 | 22.3 | 11.5-43.1 | |||||
| rs4693608 and rs4364254 | |||||||||||||
| HR | AA-TT, AA-TC | 54.4 | 44.7-66.2 | 18.6/< .001 | 42.7 | 32.8-55.5 | 23.3/< .001 | 36.1 | 26.5-49.1 | 1.3/.53 | 21.1 | 13.1-33.7 | .43/.81 |
| MR | AG-TC, AG-TT | 40.5 | 33.2-49.4 | 21.8 | 15.6-30.6 | 43.3 | 35.8-52.2 | 24.8 | 18.3-33.7 | ||||
| LR | GG-CC, GG-TC, GG-TT, AG-CC | 24.9 | 17.7-34.9 | 13.7 | 8.2-22.7 | 36.4 | 27.0-48.9 | 26.6 | 18.0-39.2 | ||||
| SNP/SNP combination . | Genotype/ genotype combination . | Cumulative incidence, % . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recipient . | Donor . | ||||||||||||
| II-IV . | 95% CI . | χ2/P . | III-IV . | 95% CI . | χ2/P . | II-IV . | 95% CI . | χ2/P . | III-IV . | 95% CI . | χ2/P . | ||
| rs4693608 | AA | 52.7 | 43.3-64.1 | 13.7/.001 | 40.9 | 31.4-53.3 | 18.7/< .001 | 35.6 | 26.1-48.5 | 4.8/.09 | 20.7 | 12.9-33.2 | .5/.78 |
| AG | 38.5 | 31.7-46.8 | 20.4 | 14.7-28.3 | 45.5 | 38.4-54.0 | 26.2 | 19.8-34.8 | |||||
| GG | 24.3 | 16.2-36.3 | 14.0 | 7.9-25.0 | 28.8 | 19.3-43.0 | 24.5 | 15.5-38.7 | |||||
| rs4364254 | TT | 41.9 | 34.5-50.8 | 4.4/.11 | 26.5 | 19.8-35.5 | 4.6/.098 | 41.0 | 33.3-50.6 | .34/.84 | 25.8 | 18.8-35.5 | .39/.82 |
| TC | 41.3 | 33.7-50.6 | 26.8 | 20.0-36.0 | 38.9 | 31.2-78.3 | 23.9 | 17.3-33.0 | |||||
| CC | 26.9 | 17.2-42.2 | 12.8 | 6.1-27.3 | 38.1 | 25.2-57.6 | 22.3 | 11.5-43.1 | |||||
| rs4693608 and rs4364254 | |||||||||||||
| HR | AA-TT, AA-TC | 54.4 | 44.7-66.2 | 18.6/< .001 | 42.7 | 32.8-55.5 | 23.3/< .001 | 36.1 | 26.5-49.1 | 1.3/.53 | 21.1 | 13.1-33.7 | .43/.81 |
| MR | AG-TC, AG-TT | 40.5 | 33.2-49.4 | 21.8 | 15.6-30.6 | 43.3 | 35.8-52.2 | 24.8 | 18.3-33.7 | ||||
| LR | GG-CC, GG-TC, GG-TT, AG-CC | 24.9 | 17.7-34.9 | 13.7 | 8.2-22.7 | 36.4 | 27.0-48.9 | 26.6 | 18.0-39.2 | ||||
Significant deviations (P < .05) are marked in bold.
Cumulative incidence of acute GVHD grades II through IV after HSCT according to the recipient HPSE gene SNPs rs4693608 and rs4364254 combinations. AA-TT and AA-TC genotype combinations were included in group HR; AG-TC and AG-TT genotype combinations were included in group MR; and GG-CC, GG-TC, GG-TT, and AG-CC genotype combinations were included in group LR. The incidence was higher in group HR compared with the MR and LR groups (P < .001).
Cumulative incidence of acute GVHD grades II through IV after HSCT according to the recipient HPSE gene SNPs rs4693608 and rs4364254 combinations. AA-TT and AA-TC genotype combinations were included in group HR; AG-TC and AG-TT genotype combinations were included in group MR; and GG-CC, GG-TC, GG-TT, and AG-CC genotype combinations were included in group LR. The incidence was higher in group HR compared with the MR and LR groups (P < .001).
First evaluation of AA-CC genotype relation to certain groups was done. Only 1 of 6 recipients with the AA-CC genotype developed acute GVHD grade II, whereas in the 4 patients possessing the same genotype, acute GVHD was not observed, and one of them developed early relapse. Presumably, this genotype may be included in the LR group.
Chronic GVHD
A total of 225 persons were evaluable for chronic GVHD analysis. A total of 137 patients received grafts from siblings, and 88 recipients received transplants of unrelated grafts. A total of 103 patients had limited chronic GVHD (54% CI [47.4-61.6]), 67 of whom received transplants from related donors and 36 received grafts from unrelated donors. The cumulative incidence of limited chronic GVHD was 49.4% (95% CI 41.7-58.6) and 41.8% (95% CI 32.5-53.7) for related and unrelated transplants, respectively. A total of 38 patients had extensive chronic GVHD; 25 of them received grafts from siblings and 13 received grafts from unrelated donors. Thus, the cumulative incidence of extensive chronic GVHD was 18.3% (95% CI 12.8-26.1) and 15% (95% CI 9.1-24.8) for recipients of related and unrelated transplants, respectively. HPSE gene SNP analysis did not disclose any significant correlation with limited chronic GVHD. Analysis of cumulative rate of extensive chronic GVHD revealed a significantly higher incidence (30.9% [95% CI 20.8-45.9]) for recipients of group HR as compared with recipients of group MR (14.3% [95% CI 8.8-23.3]) and recipients of group LR (9.9% [95% CI 4.9-20.0]; P < .001; Table 4). Thus, patient HPSE gene SNPs revealed a strong association with extensive chronic GVHD. Analysis of donor genotypes did not reveal a significant correlation to chronic GVHD.
Univariate analysis of cumulative incidence of HSCT outcome in association with HPSE gene SNP combinations
| Outcome . | SNP combinations . | Cumulative incidence, % . | 95% CI for cumulative incidence . | P . |
|---|---|---|---|---|
| Chronic GVHD limited | Recipient | .69 | ||
| HR | 34.6 | 24.0-49.7 | ||
| MR | 49.3 | 40.3-60.3 | ||
| LR | 51.6 | 41.1-64.9 | ||
| Donor | .16 | |||
| HR | 35.7 | 24.9-51.2 | ||
| MR | 44.6 | 35.9-55.5 | ||
| LR | 59.4 | 46.7-75.4 | ||
| Chronic GVHD extensive | Recipient | < .001 | ||
| HR | 30.9 | 20.8-45.9 | ||
| MR | 14.3 | 8.8-23.3 | ||
| LR | 9.9 | 4.9-20.0 | ||
| Donor | .37 | |||
| HR | 24.1 | 15.0-38.8 | ||
| MR | 16.8 | 10.9-26.0 | ||
| LR | 12.5 | 5.9-26.5 | ||
| TRM | Recipient | .01 | ||
| HR | 30.0 | 22.8-39.4 | ||
| MR | 17.5 | 12.7-24.1 | ||
| LR | 19.0 | 13.0-27.6 | ||
| Donor | .33 | |||
| HR | 15.7 | 9.5-25.8 | ||
| MR | 21.2 | 16.0-28.2 | ||
| LR | 23.8 | 16.8-33.7 | ||
| Relapse | Recipient | .93 | ||
| HR | 40.2 | 32.0-50.6 | ||
| MR | 50.1 | 42.9-58.4 | ||
| LR | 46.3 | 37.5-57.0 | ||
| Donor | .38 | |||
| HR | 45.2 | 34.3-56.8 | ||
| MR | 50.7 | 43.6-58.9 | ||
| LR | 42.8 | 33.9-54.1 |
| Outcome . | SNP combinations . | Cumulative incidence, % . | 95% CI for cumulative incidence . | P . |
|---|---|---|---|---|
| Chronic GVHD limited | Recipient | .69 | ||
| HR | 34.6 | 24.0-49.7 | ||
| MR | 49.3 | 40.3-60.3 | ||
| LR | 51.6 | 41.1-64.9 | ||
| Donor | .16 | |||
| HR | 35.7 | 24.9-51.2 | ||
| MR | 44.6 | 35.9-55.5 | ||
| LR | 59.4 | 46.7-75.4 | ||
| Chronic GVHD extensive | Recipient | < .001 | ||
| HR | 30.9 | 20.8-45.9 | ||
| MR | 14.3 | 8.8-23.3 | ||
| LR | 9.9 | 4.9-20.0 | ||
| Donor | .37 | |||
| HR | 24.1 | 15.0-38.8 | ||
| MR | 16.8 | 10.9-26.0 | ||
| LR | 12.5 | 5.9-26.5 | ||
| TRM | Recipient | .01 | ||
| HR | 30.0 | 22.8-39.4 | ||
| MR | 17.5 | 12.7-24.1 | ||
| LR | 19.0 | 13.0-27.6 | ||
| Donor | .33 | |||
| HR | 15.7 | 9.5-25.8 | ||
| MR | 21.2 | 16.0-28.2 | ||
| LR | 23.8 | 16.8-33.7 | ||
| Relapse | Recipient | .93 | ||
| HR | 40.2 | 32.0-50.6 | ||
| MR | 50.1 | 42.9-58.4 | ||
| LR | 46.3 | 37.5-57.0 | ||
| Donor | .38 | |||
| HR | 45.2 | 34.3-56.8 | ||
| MR | 50.7 | 43.6-58.9 | ||
| LR | 42.8 | 33.9-54.1 |
TRM, relapse, and OS
A total of 89 patients died of transplantation-related complications within the first 180 days after transplantation. On day 180, the cumulative incidence of death was 30.0% (95% CI 22.8-39.4) in recipient group HR, 17.5% (95% CI 12.7-24.1) in recipient group MR, and 19.0% (95% CI 13.0-27.6) in recipient group LR (P = .01; Table 4). Thus, univariate analysis of day 180 TRM indicated an association of nonrelapse mortality with genotype combination HR (Table 4). Donor genotypes did not reveal a significant association with day-180 TRM.
A total of 181 patients developed hematologic relapse (46.4% CI 41.6-51.7). Median time for relapse was 133 days (range, 20-1404 days). Analysis of association of HPSE gene SNPs with relapse incidence did not disclose a significant correlation (Table 4).
A total of 246 patients died after transplantation, of whom 152 patients died from relapse and 94 from nonrelapse mortality. Kaplan-Meier analysis revealed a 3.2-year OS of 40%. Donor HPSE gene polymorphisms had no influence on the overall risk of death (data not shown). However, there was a trend to association between genotype combination HR with poor OS (P = .058). OS of patients possessing HR combination was 32.5% (range, 23.1%-40.8%), while OS of other recipients was 38.6% (range, 32.2%-44.4%).
HPSE gene SNP discrepancy between recipients and donors
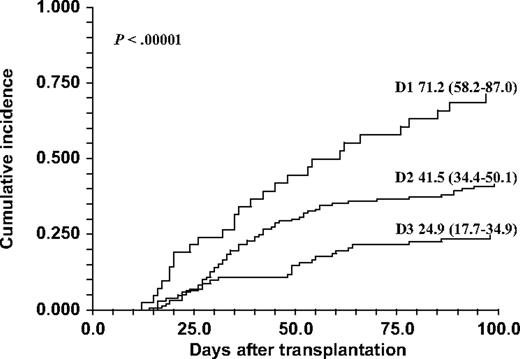
Complete analysis of differences between recipients and donors regarding HPSE gene SNPs (rs4693608 and rs4364254) was performed for 364 recipient-donor pairs for whom data were available. Cumulative incidence of clinically significant (grades II-IV and III-IV) acute GVHD was calculated for 9 possible variations of genotype combinations (HR-HR, HR-MR, HR-LR, MR-MR, MR-HR, MR-LR, LR-LR, LR-HR, and LR-MR). The analysis revealed highly significant differences among these genotype combinations (χ2 = 33.5, P < .001 for grades II-IV and χ2 = 40.4, P < .001 for grades III-IV; Table 5). Detailed examination of the results disclosed the following observations: (1) patient genotype combination LR exerted a protective effect against GVHD regardless of the donor genotype combinations; (2) the risk of acute GVHD in recipients with genotype HR was affected by the discrepancy with donor genotype; namely, HR recipients who received the transplant from donors with MR or LR genotypes had the highest rate of acute GVHD (HR-MR and HR-LR pairs); and (3) the remaining combinations, including the HR-HR pair, showed moderate risk of acute GVHD. Next, we divided all recipient-donor pairs to 3 groups according to potential risk for acute GVHD development. The first cohort, D1, contained pairs with high risk for developing acute GVHD (HR-MR and HR-LR pairs). The second group, D2, consisted of pairs with moderate risk of acute GVHD development (MR-MR, MR-HR, MR-LR and HR-HR pairs). The third group, D3, included 3 pairs with low risk of developing acute GVHD (LR-LR, LR-MR, and LR-HR pairs). Cumulative rate of acute GVHD incidence was 71.2% (95% CI 58.2-87.0) in the D1 group, 41.5% (95% CI 34.4-50.1) in the D2 group, and 24.9% (95% CI 17.7-34.9) in the D3 group (χ2 = 29.3, P < .001 for grades II-IV and χ2 = 34.1, P < .001 for grades III-IV; Table 6; Figure 2). Notably, the observed highly statistically significant P value indicates a strong association between HPSE gene SNP discrepancy in the D1 group and the risk of acute GVHD and even more so the risk of severe acute (grades III-IV) GVHD (Table 6).
Univariate analysis of cumulative incidence of clinically significant (II-IV and III-IV) acute GVHD in correlation with heparanase SNP discrepancy between recipients and donors
| Grade . | Recipient-donor pair . | Cumulative incidence, % . | 95% CI for cumulative incidence . | χ2/P . |
|---|---|---|---|---|
| II-IV | HR-HR | 40.2 | 26.4-61.3 | 33.5/< .001 |
| HR-MR | 74.1 | 59.4-92.5 | ||
| HR-LR | 63.6 | 40.7-99.5 | ||
| MR-MR | 38.2 | 29.0-50.5 | ||
| MR-LR | 52.6 | 34.4-80.6 | ||
| MR-HR | 45.2 | 28.5-71.6 | ||
| LR-LR | 23.3 | 13.9-39.0 | ||
| LR-MR | 29.7 | 17.6-49.9 | ||
| LR-HR | 17.9 | 6.4-50.2 | ||
| III-IV | HR-HR | 29.2 | 16.8-50.7 | 40.4/< .001 |
| HR-MR | 59.6 | 41.7-85.2 | ||
| HR-LR | 63.6 | 40.7-99.5 | ||
| MR-MR | 19.6 | 12.2-31.4 | ||
| MR-LR | 35.8 | 18.7-68.5 | ||
| MR-HR | 19.7 | 7.1-54.3 | ||
| LR-LR | 13.6 | 6.5-28.7 | ||
| LR-MR | 12.0 | 4.8-30.0 | ||
| LR-HR | 12.8 | 3.5-47.2 |
| Grade . | Recipient-donor pair . | Cumulative incidence, % . | 95% CI for cumulative incidence . | χ2/P . |
|---|---|---|---|---|
| II-IV | HR-HR | 40.2 | 26.4-61.3 | 33.5/< .001 |
| HR-MR | 74.1 | 59.4-92.5 | ||
| HR-LR | 63.6 | 40.7-99.5 | ||
| MR-MR | 38.2 | 29.0-50.5 | ||
| MR-LR | 52.6 | 34.4-80.6 | ||
| MR-HR | 45.2 | 28.5-71.6 | ||
| LR-LR | 23.3 | 13.9-39.0 | ||
| LR-MR | 29.7 | 17.6-49.9 | ||
| LR-HR | 17.9 | 6.4-50.2 | ||
| III-IV | HR-HR | 29.2 | 16.8-50.7 | 40.4/< .001 |
| HR-MR | 59.6 | 41.7-85.2 | ||
| HR-LR | 63.6 | 40.7-99.5 | ||
| MR-MR | 19.6 | 12.2-31.4 | ||
| MR-LR | 35.8 | 18.7-68.5 | ||
| MR-HR | 19.7 | 7.1-54.3 | ||
| LR-LR | 13.6 | 6.5-28.7 | ||
| LR-MR | 12.0 | 4.8-30.0 | ||
| LR-HR | 12.8 | 3.5-47.2 |
Significant deviations (P < .05) are marked in bold.
Univariate analysis of cumulative incidence of HSCT outcome in correlation with heparanase SNP discrepancy between recipients and donors
| Outcome . | Group of discrepancy . | Cumulative incidence, % . | 95% CI for cumulative incidence . | χ2/P . |
|---|---|---|---|---|
| Acute GVHD | ||||
| II-IV | D1 | 71.2 | 58.2-87.0 | 29.3/< .001 |
| D2 | 41.5 | 34.4-50.1 | ||
| D3 | 24.9 | 17.7-34.9 | ||
| III-IV | D1 | 61.4 | 46.5-81.1 | 34.1/< .001 |
| D2 | 23.3 | 17.2-31.7 | ||
| D3 | 13.7 | 8.2-22.7 | ||
| Chronic GVHD | ||||
| Limited | D1 | 32.0 | 18.1-56.7 | .87/.65 |
| D2 | 44.8 | 36.3-55.3 | ||
| D3 | 51.6 | 41.1-64.9 | ||
| Extensive | D1 | 32.0 | 18.1-56.7 | 7.9/.02 |
| D2 | 19.5 | 13.3-28.6 | ||
| D3 | 9.9 | 4.9-20.0 | ||
| TRM | D1 | 37.1 | 26.8-51.3 | 14.8/< .001 |
| D2 | 17.0 | 12.4-23.4 | ||
| D3 | 19.0 | 13.0-27.6 | ||
| Relapse | D1 | 39.6 | 28.9-54.1 | .3/.86 |
| D2 | 50.6 | 43.5-58.8 | ||
| D3 | 46.3 | 37.5-57.0 |
| Outcome . | Group of discrepancy . | Cumulative incidence, % . | 95% CI for cumulative incidence . | χ2/P . |
|---|---|---|---|---|
| Acute GVHD | ||||
| II-IV | D1 | 71.2 | 58.2-87.0 | 29.3/< .001 |
| D2 | 41.5 | 34.4-50.1 | ||
| D3 | 24.9 | 17.7-34.9 | ||
| III-IV | D1 | 61.4 | 46.5-81.1 | 34.1/< .001 |
| D2 | 23.3 | 17.2-31.7 | ||
| D3 | 13.7 | 8.2-22.7 | ||
| Chronic GVHD | ||||
| Limited | D1 | 32.0 | 18.1-56.7 | .87/.65 |
| D2 | 44.8 | 36.3-55.3 | ||
| D3 | 51.6 | 41.1-64.9 | ||
| Extensive | D1 | 32.0 | 18.1-56.7 | 7.9/.02 |
| D2 | 19.5 | 13.3-28.6 | ||
| D3 | 9.9 | 4.9-20.0 | ||
| TRM | D1 | 37.1 | 26.8-51.3 | 14.8/< .001 |
| D2 | 17.0 | 12.4-23.4 | ||
| D3 | 19.0 | 13.0-27.6 | ||
| Relapse | D1 | 39.6 | 28.9-54.1 | .3/.86 |
| D2 | 50.6 | 43.5-58.8 | ||
| D3 | 46.3 | 37.5-57.0 |
D1: HR-LR, HR-MR recipient-donor genotype combinations pairs. D2: MR-MR, MR-HR, MR-LR, HR-HR recipient-donor genotype combinations pairs. D3: LR-LR, LR-HR, LR-MR recipient-donor genotype combinations pairs. Significant deviations (P < .05) are marked in bold.
Cumulative incidence of acute GVHD grades II through IV after HSCT according to the discrepancy of HPSE gene SNPs rs4693608 and rs4364254 combinations between recipient and donor. The D1 group contains HR-MR and HR-LR recipient-donor genotype combination pairs; the D2 group contains MR-MR, MR-HR, MR-LR, and HR-HR recipient-donor pairs; and the D3 group contains LR-LR, LR-MR, and LR-HR recipient-donor pairs. The incidence was higher in group D1 compared with the D2 and D3 groups (P < .001).
Cumulative incidence of acute GVHD grades II through IV after HSCT according to the discrepancy of HPSE gene SNPs rs4693608 and rs4364254 combinations between recipient and donor. The D1 group contains HR-MR and HR-LR recipient-donor genotype combination pairs; the D2 group contains MR-MR, MR-HR, MR-LR, and HR-HR recipient-donor pairs; and the D3 group contains LR-LR, LR-MR, and LR-HR recipient-donor pairs. The incidence was higher in group D1 compared with the D2 and D3 groups (P < .001).
In addition, the effect of discrepancy between recipients and donors disclosed a correlation to extensive chronic GVHD (P = .02) and nonrelapse mortality (P < .001; Table 6). The Kaplan-Meier OS was poorer for the D1 group compared with the D2 and the D3 groups (P = .02).
Risk for acute GVHD according to donor type
Univariate analysis of the cumulative incidence of clinically significant (II-IV and III-IV) acute GVHD in association with HPSE gene SNP combinations and SNP disparity was performed in accordance to the type of donor (related vs unrelated; Table 7). The cumulative incidence of acute GVHD for recipients of group HR was higher when donors were unrelated (63.6% [95% CI 49.3-82.2]) compared with sibling donors (48.3% [95% CI 36.2-64.4]; P = .027 and P = .002 for related and unrelated donors, respectively; Table 7). The analysis revealed that the association between HPSE gene SNPs and risk of acute GVHD was higher in the group of unrelated donors compared with related donors (Table 7). However, examination of the discrepancy effect disclosed that the correlation level was the same for transplants from unrelated and related donors (P < .001 for both unrelated and related donors; Table 7), indicating that the discrepancy in HPSE gene SNPs between recipients and donors is more relevant for the risk of developing acute GVHD than the patients' genotype profile of HPSE gene SNPs.
Univariate analysis of cumulative incidence of clinically significant (II-IV and III-IV) acute GVHD in association with HPSE gene SNPs combination and their discrepancy in related versus unrelated transplants
| Grade . | Donor type . | SNPs combination/ group of discrepancy . | Cumulative incidence, % . | 95% CI for cumulative incidence . | χ2/P . |
|---|---|---|---|---|---|
| II-IV | Related | HR | 48.3 | 36.2-64.4 | 7.2/.027 |
| MR | 40.5 | 31.1-52.7 | |||
| LR | 25.8 | 16.9-39.4 | |||
| III-IV | HR | 35.7 | 24.4-52.4 | 8.9/.012 | |
| MR | 25.7 | 17.2-38.2 | |||
| LR | 13.7 | 7.2-26.2 | |||
| II-IV | Unrelated | HR | 63.6 | 49.3-82.2 | 12.7/.002 |
| MR | 40.6 | 29.9-54.9 | |||
| LR | 23.4 | 13.2-41.6 | |||
| III-IV | HR | 53.4 | 37.7-75.7 | 17.2/< .001 | |
| MR | 15.7 | 8.5-28.8 | |||
| LR | 13.7 | 6.0-31.3 | |||
| II-IV | Related | D1 | 70.4 | 51.7-95.9 | 14.4/< .001 |
| D2 | 40.5 | 31.6-52.0 | |||
| D3 | 25.8 | 16.9-39.4 | |||
| III-IV | D1 | 52.9 | 32.5-86.3 | 12.8/.002 | |
| D2 | 25.9 | 17.9-37.6 | |||
| D3 | 13.7 | 7.2-26.2 | |||
| II-IV | Unrelated | D1 | 72.5 | 56.0-93.9 | 14.1/< .001 |
| D2 | 43.0 | 32.3-57.3 | |||
| D3 | 23.4 | 13.2-41.6 | |||
| III-IV | D1 | 66.9 | 48.5-92.5 | 21.2/< .001 | |
| D2 | 18.7 | 10.9-32.1 | |||
| D3 | 13.7 | 6.0-31.3 |
| Grade . | Donor type . | SNPs combination/ group of discrepancy . | Cumulative incidence, % . | 95% CI for cumulative incidence . | χ2/P . |
|---|---|---|---|---|---|
| II-IV | Related | HR | 48.3 | 36.2-64.4 | 7.2/.027 |
| MR | 40.5 | 31.1-52.7 | |||
| LR | 25.8 | 16.9-39.4 | |||
| III-IV | HR | 35.7 | 24.4-52.4 | 8.9/.012 | |
| MR | 25.7 | 17.2-38.2 | |||
| LR | 13.7 | 7.2-26.2 | |||
| II-IV | Unrelated | HR | 63.6 | 49.3-82.2 | 12.7/.002 |
| MR | 40.6 | 29.9-54.9 | |||
| LR | 23.4 | 13.2-41.6 | |||
| III-IV | HR | 53.4 | 37.7-75.7 | 17.2/< .001 | |
| MR | 15.7 | 8.5-28.8 | |||
| LR | 13.7 | 6.0-31.3 | |||
| II-IV | Related | D1 | 70.4 | 51.7-95.9 | 14.4/< .001 |
| D2 | 40.5 | 31.6-52.0 | |||
| D3 | 25.8 | 16.9-39.4 | |||
| III-IV | D1 | 52.9 | 32.5-86.3 | 12.8/.002 | |
| D2 | 25.9 | 17.9-37.6 | |||
| D3 | 13.7 | 7.2-26.2 | |||
| II-IV | Unrelated | D1 | 72.5 | 56.0-93.9 | 14.1/< .001 |
| D2 | 43.0 | 32.3-57.3 | |||
| D3 | 23.4 | 13.2-41.6 | |||
| III-IV | D1 | 66.9 | 48.5-92.5 | 21.2/< .001 | |
| D2 | 18.7 | 10.9-32.1 | |||
| D3 | 13.7 | 6.0-31.3 |
D1: HR-LR, HR-MR recipient-donor genotype combinations pairs. D2: MR-MR, MR-HR, MR-LR, HR-HR recipient-donor genotype combinations pairs. D3: LR-LR, LR-HR, LR-MR recipient-donor genotype combinations pairs. Significant deviations (P < .05) are marked in bold.
Analysis of risk for acute GVHD in association with HPSE gene SNPs: relevance to conditioning regimen
Previous studies indicated that classical myeloablative and fludarabine and melphalan regimens were associated with increased risk of acute GVHD compared with RIC and reduced toxicity myeloablative conditioning regimens.30,31 Our estimations confirmed these results. Cumulative incidence of acute GVHD for myeloablative regimens was 47.2 (95% CI 38.6-57.8); for fludarabine/melphalan regimen, 53.9% (95% CI 42.0-69.1); for fludarabine/reduced-dose busulfan (RIC regimen), 28.0% (95% CI 20.2-38.9); and for fludarabine/high-dose busulfan and fludarabine/treosulfan regimens, 31.7% (95% CI 22.8-44.2; P = .001). Therefore, for further analysis, the fludarabine/melphalan regimen was combined with the myeloablative group (ie, myeloablative group), whereas the fludarabine/busulfan and fludarabine/treosulfan regimens were combined with the RIC group (reduced-toxicity group). Univariate analysis disclosed that the cumulative rate of acute GVHD incidence was higher in the myeloablative group compared with the reduced-toxicity group (Table 8). The level of association with recipient heparanase gene SNP combinations was highly significant in the group of patients receiving myeloablative conditioning regimen (χ2 = 12.5, P = .002 for grades II-IV and χ2 = 17.3, P < .001 for grades III-IV; Table 8). However, HPSE disparity enhanced the level of correlation in the reduced-toxicity group (χ2 = 14.8, P < .001 for grades II-IV and χ2 = 19.6, P < .001 for grades III-IV; Table 8). Thus, these observations indicate that the association of HPSE gene SNPs with the risk of acute GVHD was more prominent in patients receiving myeloablative conditioning regimens. However, HPSE disparity was a more important factor in the group of patients receiving reduced-toxicity conditioning.
Univariate analysis of cumulative incidence of clinically significant (II-IV and III-IV) acute GVHD in association with HPSE gene SNP combinations and their discrepancy according to the conditioning regimen
| Grade . | Conditioning regimen . | SNP combinations/ group of discrepancy . | Cumulative incidence, % . | 95% CI for cumulative incidence . | χ2/P . |
|---|---|---|---|---|---|
| II-IV | RIC, fludarabine/high-dose busulfan, fludarabine/treosulfan | HR | 36.5 | 24.0-55.5 | 6.5/.039 |
| MR | 34.8 | 25.4-47.9 | |||
| LR | 17.1 | 9.7-30.1 | |||
| III-IV | HR | 23.2 | 12.6-42.8 | 4.9/.08 | |
| MR | 16.2 | 9.1-28.7 | |||
| LR | 7.3 | 2.8-18.9 | |||
| II-IV | Myeloablative, fludarabine/melphalan | HR | 69.7 | 57.5-84.7 | 12.5/.002 |
| MR | 46.1 | 35.8-59.2 | |||
| LR | 34.7 | 23.1-52.3 | |||
| III-IV | HR | 59.5 | 45.9-77.3 | 17.3/< .001 | |
| MR | 27.7 | 18.3-41.7 | |||
| LR | 22.3 | 12.4-39.9 | |||
| II-IV | RIC, fludarabine/ high-dose busulfan, fludarabine/treosulfan | D1 | 58.8 | 39.5-87.6 | 14.8/< .001 |
| D2 | 35.1 | 25.7-47.9 | |||
| D3 | 17.1 | 9.7-30.1 | |||
| III-IV | D1 | 47.7 | 27.5-82.5 | 19.6/< .001 | |
| D2 | 14.4 | 7.8-26.5 | |||
| D3 | 7.3 | 2.8-18.9 | |||
| II-IV | Myeloablative, fludarabine/melphalan | D1 | 80.5 | 65.2-99.3 | 11.6/.003 |
| D2 | 47.4 | 37.6-59.8 | |||
| D3 | 34.7 | 23.1-52.3 | |||
| III-IV | D1 | 73.7 | 55.1-98.5 | 12.9/.002 | |
| D2 | 31.3 | 22.2-44.2 | |||
| D3 | 22.3 | 12.4-39.9 |
| Grade . | Conditioning regimen . | SNP combinations/ group of discrepancy . | Cumulative incidence, % . | 95% CI for cumulative incidence . | χ2/P . |
|---|---|---|---|---|---|
| II-IV | RIC, fludarabine/high-dose busulfan, fludarabine/treosulfan | HR | 36.5 | 24.0-55.5 | 6.5/.039 |
| MR | 34.8 | 25.4-47.9 | |||
| LR | 17.1 | 9.7-30.1 | |||
| III-IV | HR | 23.2 | 12.6-42.8 | 4.9/.08 | |
| MR | 16.2 | 9.1-28.7 | |||
| LR | 7.3 | 2.8-18.9 | |||
| II-IV | Myeloablative, fludarabine/melphalan | HR | 69.7 | 57.5-84.7 | 12.5/.002 |
| MR | 46.1 | 35.8-59.2 | |||
| LR | 34.7 | 23.1-52.3 | |||
| III-IV | HR | 59.5 | 45.9-77.3 | 17.3/< .001 | |
| MR | 27.7 | 18.3-41.7 | |||
| LR | 22.3 | 12.4-39.9 | |||
| II-IV | RIC, fludarabine/ high-dose busulfan, fludarabine/treosulfan | D1 | 58.8 | 39.5-87.6 | 14.8/< .001 |
| D2 | 35.1 | 25.7-47.9 | |||
| D3 | 17.1 | 9.7-30.1 | |||
| III-IV | D1 | 47.7 | 27.5-82.5 | 19.6/< .001 | |
| D2 | 14.4 | 7.8-26.5 | |||
| D3 | 7.3 | 2.8-18.9 | |||
| II-IV | Myeloablative, fludarabine/melphalan | D1 | 80.5 | 65.2-99.3 | 11.6/.003 |
| D2 | 47.4 | 37.6-59.8 | |||
| D3 | 34.7 | 23.1-52.3 | |||
| III-IV | D1 | 73.7 | 55.1-98.5 | 12.9/.002 | |
| D2 | 31.3 | 22.2-44.2 | |||
| D3 | 22.3 | 12.4-39.9 |
D1: HR-LR, HR-MR recipient-donor genotype combination pairs. D2: MR-MR, MR-HR, MR-LR, HR-HR recipient-donor genotype combination pairs. D3: LR-LR, LR-HR, LR-MR recipient-donor genotype combination pairs. Significant deviations (P < .05) are marked in bold.
RIC indicates reduced-intensity conditioning.
Multivariate analysis of risk factors associated with acute and extensive chronic GVHD, TRM, and OS
A multivariate analysis was performed to estimate clinical and genotypic risk factors for acute GVHD. The genotypic risk factors analyzed were HPSE gene rs4693608 and rs4364254 SNP combinations of recipients, and 3 risk groups for acute GVHD according to discrepancy between recipient and donor. The clinical risk factors were recipient age, sex of recipient and donor, type of donor (sibling or matched unrelated), type of disease, and conditioning. A P value less than .2 was a selected criterion for step-wise multivariate Cox regression model. Among the clinical risk factors 3 parameters—recipient age, sex, and type of conditioning—reached the limited criterion for a multivariate analysis of risk factors associated with acute GVHD. A multivariate analysis in this patient subset showed that recipient SNP combination hazard ratio (HR 2.7, [95% CI 1.6-4.4], P < .001) and myeloablative regimens (HR 1.9, [95% CI 1.3-2.8], P = .001) were the significant factors (Table 9 “In relation to recipient SNP combination”). A multivariate analysis of the effect of discrepancy between recipients and donors revealed that the D1 group (HR 3.9, [95%CI 2.2-6.7], P < .001) and the type of conditioning (myeloablative regimens; HR 1.7, [95% CI 1.1-2.5], P = .01) were the significant parameters (Table 9 “In relation to discrepancy effect”).
Summary of multivariate analysis between acute GVHD (II-IV) and clinical and genetic variables
| Variable . | n . | No. of events . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|---|
| In relation to recipient SNP combination | |||||
| Age, y | |||||
| 50 or younger | 210 | 73 | |||
| Older than 50 | 204 | 56 | .9 | .6-1.3 | .66 |
| Sex | |||||
| Male/male, female/female | 224 | 79 | |||
| Female/male | 100 | 27 | .6 | .4-1.0 | .07 |
| Male/female | 90 | 23 | .7 | .5-1.2 | .19 |
| Conditioning | |||||
| Myeloablative, fludarabine/ melphalan | 205 | 80 | 1.9 | 1.3-2.8 | .001 |
| RIC, fludarabine/ busulfan, fludarabine/treosulfan | 209 | 49 | |||
| Recipient SNP combination | |||||
| HR | 121 | 46 | 2.7 | 1.6-4.4 | < .001 |
| MR | 177 | 58 | 1.6 | 1.0-2.6 | .045 |
| LR | 116 | 25 | |||
| In relation to discrepancy effect | |||||
| Age, y | |||||
| 50 or younger | 185 | 64 | |||
| Older than 50 | 182 | 53 | 1.0 | .7-1.4 | .81 |
| Sex | |||||
| Male/male, female/female | 199 | 71 | |||
| Female/male | 88 | 24 | .7 | .4-1.1 | .14 |
| Male/female | 80 | 22 | .8 | .5-1.2 | .27 |
| Conditioning | |||||
| Myeloablative, fludarabine/ melphalan | 183 | 71 | 1.7 | 1.1-2.5 | .01 |
| RIC, fludarabine/ busulfan, fludarabine/treosulfan | 184 | 46 | |||
| Groups of discrepancy | |||||
| D1 | 63 | 28 | 3.9 | 2.2-6.7 | < .001 |
| D2 | 188 | 64 | 1.8 | 1.1-2.8 | .02 |
| D3 | 116 | 25 |
| Variable . | n . | No. of events . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|---|
| In relation to recipient SNP combination | |||||
| Age, y | |||||
| 50 or younger | 210 | 73 | |||
| Older than 50 | 204 | 56 | .9 | .6-1.3 | .66 |
| Sex | |||||
| Male/male, female/female | 224 | 79 | |||
| Female/male | 100 | 27 | .6 | .4-1.0 | .07 |
| Male/female | 90 | 23 | .7 | .5-1.2 | .19 |
| Conditioning | |||||
| Myeloablative, fludarabine/ melphalan | 205 | 80 | 1.9 | 1.3-2.8 | .001 |
| RIC, fludarabine/ busulfan, fludarabine/treosulfan | 209 | 49 | |||
| Recipient SNP combination | |||||
| HR | 121 | 46 | 2.7 | 1.6-4.4 | < .001 |
| MR | 177 | 58 | 1.6 | 1.0-2.6 | .045 |
| LR | 116 | 25 | |||
| In relation to discrepancy effect | |||||
| Age, y | |||||
| 50 or younger | 185 | 64 | |||
| Older than 50 | 182 | 53 | 1.0 | .7-1.4 | .81 |
| Sex | |||||
| Male/male, female/female | 199 | 71 | |||
| Female/male | 88 | 24 | .7 | .4-1.1 | .14 |
| Male/female | 80 | 22 | .8 | .5-1.2 | .27 |
| Conditioning | |||||
| Myeloablative, fludarabine/ melphalan | 183 | 71 | 1.7 | 1.1-2.5 | .01 |
| RIC, fludarabine/ busulfan, fludarabine/treosulfan | 184 | 46 | |||
| Groups of discrepancy | |||||
| D1 | 63 | 28 | 3.9 | 2.2-6.7 | < .001 |
| D2 | 188 | 64 | 1.8 | 1.1-2.8 | .02 |
| D3 | 116 | 25 |
Significant deviations (P < .05) are marked in bold.
As for extensive chronic GVHD, the clinical factors that reached the limited criterion .2 for a multivariate analysis of risk factors were recipient age, conditioning, and incidence of acute GVHD. A multivariate analysis disclosed that prior acute GVHD (HR 5.1, [95% CI 2.5-10.8], P < .001) and recipient SNP combination HR (HR 2.6, [95% CI 1.1-6.7], P = .039) were the significant factors (supplemental Table 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Analysis of the effect of HPSE disparity between recipients and donors showed that only history of acute GVHD was a significant parameter (HR 5.4, [95% CI 2.5-11.9], P < .001), while the D1 group revealed a trend to association (HR 2.6, [95% CI .9-7.7], P = .07; supplemental Table 1B).
Sex, type of conditioning, and incidence of acute GVHD reached the limitation criterion for a multivariate analysis of risk factors associated with TRM and OS. Donor type was an additional parameter associated with OS. In both analyses, acute GVHD incidence was the most significant parameter (HR 22.9, [95% CI 7.0-74.5], P < .001 for TRM, and HR 3.3, [95% CI 2.3-4.8], P < .001 for OS; supplemental Tables 2-3). Transplantation from women to men was also a significant parameter (HR 2.3, [95% CI 1.1-4.9], P = .03 for TRM, and HR 1.5, [95% CI 1.0-2.4], P = .05 for OS; supplemental Tables 2-3). Unrelated donor was an additional significant parameter among risk factors associated with OS (HR 1.4, [95% CI 1.0-2.0], P = .05; supplemental Table 3).
Thus, the multivariate analysis revealed a strong association of HPSE gene SNPs with the risk of acute GVHD and a statistically significant correlation with extensive chronic GVHD. The significant associations of heparanase gene SNPs with TRM and OS, observed by univariate analysis, appear secondary to the effect on acute GVHD incidence.
Discussion
We have demonstrated a highly significant association between 2 HPSE gene SNPs (rs4693608 and rs4364254) and their combinations and the risk of acute GVHD. The genotype combination HR, associated with increased heparanase mRNA expression,29 correlated with a high risk of acute GVHD. Conversely, the genotype combination LR, associated with decreased level of heparanase mRNA,29 correlated with a low risk of GVHD. Moreover, disparities between recipient and donor pairs in HPSE gene SNP combinations significantly increased the probability of developing acute GVHD after HSCT. The D1 group, in which this association was disclosed, included pairs where the recipient possessed the genotype combination HR, while the donors were carriers of genotype combinations associated with lower heparanase expression levels (ie, MR or LR groups, HR-MR, and HR-LR pairs). The D1 group showed a significantly higher risk of acute GVHD compared with the D2 and D3 groups. In subsequent analysis, we found an association between increased risk of extensive chronic GVHD and either the recipient genotype combination HR or the D1 group. Moreover, our study revealed a significant association between HPSE gene SNP combinations and both transplantation-related mortality (TRM) and OS after HSCT, an association that appears secondary to the effect on acute GVHD.
The pathophysiology of acute GVHD resembles inflammatory cellular responses involving primarily donor T cells, cytokines, and possibly other cell subsets of the immune system.2,3 Inflammation requires the translocation of leukocytes from the circulation into extravascular compartments. The extracellular microenvironment possesses myriad signals that cooperate in a dialogue with the immune cells, coordinating their behavior as they migrate into inflamed tissues. The cross-talk between immune cells and the surroundings, profoundly regulates their response to inflammatory insults.38,39 Extracellular matrix (ECM)–degrading enzymes released by activated inflammatory cells act on macromolecular constituents of this milieu and thereby enable the inflammatory cells to migrate and reach their target organs. The action of these enzymes creates not only a path for cells to follow, but also releases a variety of ECM-bound cytokines and degradation fragments.40 This rationale led us to investigate an association between HPSE gene SNPs, shown to correlate with heparanase expression29 and the risk of GVHD after HSCT.
During GVHD, mature donor lymphocytes, primarily alloreactive effector T cells, extravasate and migrate through the ECM toward the affected tissues expressing high levels of HLA-DR.2,41 These processes involve a vast array of cellular and molecular pathways that closely resemble inflammatory and autoimmune processes, leading to tissue destruction.41 T cells, the key cell type involved in GVHD, were shown to secrete heparanase from preformed pools immediately after activation,42 suggesting a critical role of the enzyme in the GVHD inflammatory process.42 T cells and other cells (ie, monocytes, dendritic cells, mast cells) participating in the GVHD inflammatory process secrete soluble mediators that bind to HS in the ECM. ECM-anchored mediators include cytokines and chemokines such as TNFα, IFNγ, IL-7, IL-2, RANTES and MIP-1β, as well as the growth factors (ie, bFGF, HGF, TGFβ),43 all shown to play a role in the pathogenesis of acute and chronic GVHD.1,2,41 Enzymatic cleavage of the HS by heparanase in the ECM releases these mediators and creates a chemoattractant gradient38 that activates and attracts cells toward the GVHD-affected tissues.
We have previously demonstrated a correlation between the heparanase SNPs currently shown to be associated with GVHD and heparanase expression levels in healthy persons.29 Therefore, our current findings may suggest that patients possessing genotype combination HR secrete high levels of heparanase compared with patients carrying the MR and LR genotype combinations, which release intermediate and low levels of heparanase, respectively. Levels of signals affected by heparanase may thus be higher in patients possessing the HR genotype and belonging to the D1 group compared with possessors of the MR and LR genotypes, comprising the D2 and D3 groups, respectively.
Furthermore, our study revealed that discrepancy of HPSE gene SNPs between recipients and donors was the most prominent factor for risk of acute GVHD, especially in the group receiving reduced-toxicity conditioning regimens. Hence, we speculate that heparanase-related differences in level of signals between recipients and donors may lead to activation and subsequent proliferation and differentiation of donor T cells. Since the strongest association was observed in the D1 group, it is conceivable that in the D1, as opposed to the D2 and D3 groups, the recipient signal threshold, affected by heparanase, is higher compared with that of their donors. This delicate balance may be further modulated by the recipient and donor inflammatory cytokine polymorphism,10-16 and may lead to hyperactivation of donor T cells and thereby elevate the risk of acute GVHD.
Apart from release of ECM-bound inflammatory mediators mediated by enzymatically active heparanase,44 heparanase and/or heparan sulfate degradation fragments may activate T cells, monocytes, and dendritic cells, resulting in synthesis and secretion of cytokines (ie, TNFα, IL-1) and matrix metalloproteinases (MMPs), known to be involved in GVHD.45-47 Some of these activities are exerted by latent and enzymatically inactive heparanase.45,46 Heparanase was also shown to be expressed by the vascular endothelium at the site of inflammation,48 resulting in vascular leakage that may further aggravate organ toxicity of the lung and liver in GVHD. Moreover, up-regulation of heparanase has been found in the colonic epithelium of inflammatory bowel disease (IBD),49 which in many aspects resembles gastrointestinal tract involvement in GVHD. Similarly, heparanase activity is dramatically elevated in sinovial fluid of patients with rheumatoid arthritis (RA),50 further pointing to its possible role in chronic GVHD, which is an autoimmune disorder occasionally associated with arthralgia and sinovitis.
In summary, our study demonstrates a strong correlation between the heparanase SNPs and the development of acute GVHD, suggesting an important role for heparanase in hyperactivation of donor T lymphocytes toward recipient tissues. This implies that inhibition of heparanase activity may serve as a potential new approach for the treatment of GVHD. In fact, we have recently demonstrated that heparanase gene silencing48,51 or newly developed heparanase-inhibiting compounds (ie, nonanticoagulant N-acetylated, glycol-split heparin)52 effectively inhibit DTH inflammation48 and tumorigenesis.52,53 Our findings may be applied to construct a clinically useful model that incorporates clinical and genetic information to predict the risk of GVHD and thereby improve patient outcome. It is conceptually attractive that a single genetic variant (ie, heparanase) might be used to assist in predicting and hopefully treating GVHD, which is still the main obstacle for successful allogeneic transplantation. This may be a first step toward constructing a risk “index” of clinical and genetic variables that will not only enable selecting an optimal unrelated donor out of multiple potential donors, but also identify recipients at highest risk of complications for whom alternative therapies should be considered.54 A necessary step for achieving this goal is to thoroughly investigate SNP-SNP interaction predicting susceptibility to acute GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Israel Science Foundation (grant 549/06); National Cancer Institute, National Institutes of Health (NIH; grant RO1-CA106456); and the DKFZ-MOST Cooperation Program in Cancer Research. I.V. is a Research Professor of the Israel Cancer Research Fund (ICRF).
National Institutes of Health
Authorship
Contribution: O.O. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; A.S. collected data and cowrote the manuscript; A.R. collected data; I.V. codirected the study and cowrote the manuscript; and A.N. directed the study and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arnon Nagler, Director Division of Hematology, BMT & CBB, Chaim Sheba Medical Center, Tel-Hashomer, Israel; e-mail: a.nagler@sheba.health.gov.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal