In this issue of Blood, Loges and colleagues have identified a novel tumor-promoting mechanism, whereby tumors educate tumor-associated macrophages to produce high levels of the mitogen Gas6, leading to tumor growth and metastasis.1 Surprisingly, this newly identified protumoral activity of tumor-associated macrophages is restricted to the selective induction of cancer cell proliferation, without interfering with cancer cell survival, tumor-associated inflammation, and angiogenesis.
Gas6 is a member of the vitamin K–dependent ligand family, homologous to the blood coagulation protein S.2 These molecules bind the family of receptor tyrosine kinases (TAMRs)—that includes Tyro-3, Axl, and Mer—and control various cellular functions, including macrophage clearance of apoptotic cells and natural killer cell differentiation.3 Gas6 may also play a role as a key factor in cell survival4 and platelet aggregation.3,5
Genetic events, including gene amplification, mutations, and altered protein expression, promote the oncogenic potential of TAMRs and have been found in various human cancers.3 TAMRs display a certain degree of promiscuity and robustness, in that TAMR ligands share affinity for the entire family of receptor. Within this scenario, Gas6 has prominent affinity for Axl, which has transforming properties.3 Contrasting evidence exists as to the prognostic significance of Gas6/Axl in cancer patients. Whereas increased Gas6/Axl interaction predicts poor prognosis in patients with glioblastoma and ovarian carcinoma,6 an improved prognosis was observed in patients with renal cell carcinoma (RCC),6 demonstrating the complexity of the system.
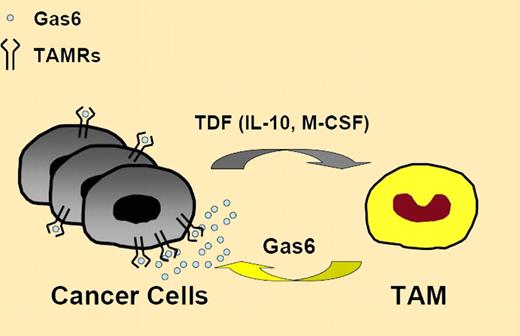
Loges et al provide new evidence on the regulation and significance of Gas6/Axl activity in cancer. Within the tumor microenvironment, tumor-associated macrophages acquire the capacity to express high levels of Gas6, suggesting that unidentified tumor-derived factor(s) contributes to their protumoral education.7 Among the possible candidates, IL-10 and M-CSF were able to induce Gas6 up-regulation in vitro. Interestingly, these factors promote M2 polarization of macrophages7 and help shape the protumoral M2 phenotype (see figure) of tumor-associated macrophages.7,8 It remains to be determined whether Gas6 represents a prototypical M2 marker and whether expression of high Gas6 levels is a feature of other tumor-associated myeloid cells, including myeloid-derived suppressor cells.9
Tumor-derived factors (TDF), likely including IL-10 and M-CSF, educate TAM for Gas6 production to fuel cancer cell proliferation. TAMRs indicates TAM family of receptor tyrosine kinases; TAM, tumor-associated macrophages; M-CSF, macrophage colony-stimulating factor; and IL-10, interleukin-10.
Tumor-derived factors (TDF), likely including IL-10 and M-CSF, educate TAM for Gas6 production to fuel cancer cell proliferation. TAMRs indicates TAM family of receptor tyrosine kinases; TAM, tumor-associated macrophages; M-CSF, macrophage colony-stimulating factor; and IL-10, interleukin-10.
Using different ectopic and orthotopic syngeneic tumor models, Loges et al demonstrate that inhibition of Gas6 does not influence accumulation of CD45+ leukocytes, tumor-associated fibroblasts, angiogenesis, and coagulation, but it is limited to the selective inhibition of cancer cell proliferation. Further, the observed reduction in metastasis formation was secondary to reduced primary tumor growth. This scenario is rather surprising, as TAMRs are largely expressed by stromal components, including endothelial cells and leukocytes.3
The complex regulation of the Gas6 actions is highlighted by reports on its functional interplay with central regulators of inflammation and cell metabolism. Whereas activation of Gas6/Mer-dependent PI3K/akt pathway was reported to influence NF-κB activation,3,10 alteration of the Von Hippel–Lindau tumor suppressor gene in clear cell renal cell carcinoma patients (ccRCC), a condition promoting a pseudohypoxic response and the consequent activation of the hypoxia-inducible factor-1 (HIF-1),11 results in enhanced expression of Axl.6 As tumor-associated macrophages accumulate in the hypoxic regions of a tumor12 and activation of PI3K/Akt leads to HIF-1 activity,13 it is tempting to speculate a possible link between the hypoxia/HIF-1 and Gas6/Axl pathways.
The contrasting information on the prognostic significance of Gas6/Axl in cancer, along with the multiple mechanisms involved in its regulation, suggest that the complexity of its biology is also contest- and tissue-specific. Although development of TAMRs antagonists may have therapeutic limitations, due to the possible induction of autoimmune disorders (eg, Lupus-like syndrome),3 the cancer-restricted action of Gas6, along with its inertness on stroma cells, suggests that its therapeutic inhibition may well integrate strategies targeting cancer-related inflammation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal