Abstract
Enabling engraftment of allogeneic T cell–depleted bone marrow (TDBM) under reduced-intensity conditioning represents a major challenge in bone marrow transplantation (BMT). Anti–third-party cytotoxic T lymphocytes (CTLs) were previously shown to be endowed with marked ability to delete host antidonor T cells in vitro, but were found to be less effective in vivo. This could result from diminished lymph node (LN) homing caused by the prolonged activation, which induces a CD44+CD62L− effector phenotype, and thereby prevents effective colocalization with, and neutralization of, alloreactive host T cells (HTCs). In the present study, LN homing, determined by imaging, was enhanced upon culture conditions that favor the acquisition of CD44+CD62L+ central memory cell (Tcm) phenotype by anti–third-party CD8+ cells. These Tcm-like cells displayed strong proliferation and prolonged persistence in BM transplant recipients. Importantly, adoptively transferred HTCs bearing a transgenic T-cell receptor (TCR) with antidonor specificity were efficiently deleted only by donor-type Tcms. All these attributes were found to be associated with improved efficacy in overcoming T cell–mediated rejection of TDBM, thereby enabling high survival rate and long-term donor chimerism, without causing graft-versus-host disease. In conclusion, anti–third-party Tcms, which home to recipient LNs and effectively delete antidonor T cells, could provide an effective and novel tool for overcoming rejection of BM allografts.
Introduction
Graft-versus-host disease (GVHD) remains a major obstacle in bone marrow transplantation (BMT). BMT without GVHD can be achieved in severe combined immunodeficient patients by using T cell–depleted BM (TDBM) transplant or by using purified hematopoietic stem cells.1 However, in leukemia patients, the benefit of GVHD prevention was offset by increased rejection rate of the TDBM transplant,2,3 mediated by radiotherapy- and chemotherapy-resistant host-derived T cells (HTCs).4,5 This barrier could be overcome by combining supralethal conditioning with functional inactivation of HTCs by “megadose” of purified CD34 stem cells.6-9 Nevertheless, this strategy is hampered by considerable toxicity of the conditioning agents10-12 and by increased risk of opportunistic infections due to slow immune reconstitution. Therefore, developing new strategies to achieve engraftment of TDBM transplant after reduced-intensity conditioning (RIC), which spares a substantial level of host immunity, is warranted.
The finding that cells within the CD34 fraction are endowed with potent veto activity13-15 has provided new insights into the mechanism by which CD34 megadose transplants can overcome the residual immunity in leukemia patients, after lethal conditioning. Veto activity was defined in 1980 by Miller16 as the capacity to specifically suppress cytotoxic T lymphocyte precursor cells (CTLps), directed against antigens (Ags) of the veto cells themselves, but not against third-party Ags. Therefore, using veto cells as specific immunosuppressants in the transplantation setting, eliminating only host CTLps directed against the donor Ags, while sparing clones directed against pathogens or tumor cells, is attractive. Because the number of purified human CD34 cells that can be harvested is limited, and insufficient for achieving engraftment under RIC, the availability of other regulatory cell types is crucial.17 Activated effector CD8+ T cells (CTLs) were shown to possess the strongest veto activity.18 However, their use is limited because of their marked GVH reactivity. We have previously described one approach to generate CTLs with highly reduced GVH reactivity by stimulation against third-party stimulators in the absence of exogenous cytokines.19 This approach was based on the observation that only activated CTLps are capable of surviving the cytokine deprivation in the primary culture, and that these anti–third-party clones expand throughout the culture. We have previously shown that fully allogeneic anti–third-party CTLs can support engraftment of allogeneic TDBM transplant without causing GVHD.19 Nevertheless, the in vivo activity of anti–third-party CTLs was markedly diminished compared with that exhibited in vitro, requiring the administration of large numbers of CTLs in conjunction with the immunosuppressive drug rapamycin. The reduced in vivo efficacy could be explained by prolonged ex vivo culture of the CTLs, which likely induces an effector phenotype associated with a migration pattern different from that displayed by naive HTCs.20,21 Thus, it is possible that anti–third-party CTLs fail to colocalize with the HTCs, preventing the veto CTLs from deleting antidonor HTCs at the lymph nodes (LNs). In turn, these antidonor cells are activated, lose their sensitivity to veto cell–induced apoptosis,22 and egress from the LNs in high numbers.
Activated CD8+ T cells were shown to differentiate in vitro under the influence of interleukin-15 (IL-15) into cells with a central memory–like phenotype.23-25 Central memory T cells (Tcms) express the LN homing receptors CD62L and CCR726 and, like naive T cells, localize to T-cell areas of all secondary lymphoid organs.27 Consistent with their memory state, Tcms respond faster and more vigorously than naive T cells when re-encountering cognate Ag.24,28 CD8+ Tcms have been shown to possess enhanced ability, compared with effector or effector-memory (Tem) CD8+ cells, to confer host protection against pathogens and tumors.23,28,29 Similarly to “natural” Tcms, in vitro–generated Tcm-like cells are incapable of immediate cytotoxicity and display the typical Tcm migratory pattern.27 Thus, our working hypothesis was that if it were possible to generate, ex vivo, anti–third-party CD8+ T cells bearing a Tcm phenotype, such cells might be more effective in tolerance induction.
In the present study, we evaluated this hypothesis. Initially, we verified by imaging that anti–third-party CTLs fail to colocalize with HTCs during the first few days after BMT. We then developed culture conditions that favor the acquisition of Tcm phenotype by the anti–third-party CD8+ cells, and demonstrated that such cells exhibit enhanced LN homing as well as superior proliferation and persistence in vivo. Most importantly, we demonstrated that these anti–third-party Tcms specifically delete in vivo host antidonor T cells. When tested in a stringent mouse model for T-cell–mediated BM allograft rejection, these attributes were found to be associated with a robust potential to overcome BM allograft rejection, thus attaining long-term chimerism sustainable for more than a year.
Methods
Animals
Female 6- to 12-week-old BALB/c, CB6, FVB, C57BL/6, C3H/Hej, (C3H × C57BL/6)F1, and BALB/c-NUDE mice were obtained from Harlan Laboratories. A breeding pair of transgenic (Tg) 2c mice expressing a T-cell receptor (TCR) with specificity for H-2Ld was kindly provided by Janko Nikolic-Zugic (Sloan-Kettering). Progeny of these Tg, (C3H × BALB/c)F1, and C57BL/6-NUDE mice were bred at the Weizmann Institute Animal Center. All mice were kept in small cages (5 animals in each cage) and fed sterile food and acid water. The Weizmann Institute of Science Institutional Animal Care and Use Committee approved these studies.
Flow cytometric analysis
Fluorescence-activated cell sorting (FACS) analysis was performed using a modified Becton Dickinson FACScan. Cells were stained with labeled antibodies specific for CD8α–phycoerythrin (PE)/fluorescein isothiocyanate (FITC)/allophycocyanin (APC), CD3-PE/FITC/APC, CD62L-PE/FITC/APC, CD44-PE/FITC/APC, H2Kb-PE/FITC, H2Dd-PE/FITC, H2Kk-PE/FITC (BD Pharmingen), 1B2 biotinylated, and streptavidin-APC (Jackson ImmunoResearch Laboratories). Annexin V and 7-amino-actinomycin D (7AAD) staining were done according to the manufacturer's instructions (BD Pharmingen).
Preparation of host nonreactive anti–third-party cells
Anti–third-party CTLs were prepared as previously described.19 Briefly, splenocytes of the donor mice were cultured against irradiated third-party splenocytes for 6 days under cytokine deprivation. Subsequently, CD8+ cells were positively selected using Magnetic Particles (BD Pharmingen). The isolated cells were restimulated with irradiated third-party splenocytes, and recombinant human IL-2 (rhIL-2, 40 U/mL; Eurocetus) was added every second day. Anti–third-party Tcms were grown similarly, but the cells underwent only 60 hours of cytokine deprivation; subsequently, CD8+ cells were positively selected and cultured in an Ag-free environment. rhIL-15 (20 ng/mL; R&D Systems) was added every second day. To attain a purified population at the end of the culture (day 16), the Tcms were positively selected for CD62L (magnetic-activated cell sorting [MACS]).
T cell–mediated BM allograft rejection and GVHD model
Host mice (12-13 weeks of age) were exposed to a single dose of lethal total body irradiation (TBI) at the indicated levels on day −2 (C3H/HeJ mice and the more radiosensitive BALB/c mice were conditioned with 10 Gy and 8 Gy of TBI, respectively). On day −1, the mice intravenously received 1.25 × 104 HTCs (CD4+CD8+ positively selected [MACS]). On day 0, 3 × 106 allogeneic NUDE BM cells were transplanted in conjunction with the evaluated veto cells. In the GVHD model, C3H mice were supralethally irradiated (11 Gy) on day −2, and on day 0 received a transplant of 5 × 106 allogeneic NUDE BM cells with or without the cells evaluated for GVHD activity. The mice were monitored for survival and weight loss.
In vivo imaging
Considering that emitted light absorption is relatively low in white mice compared with dark mice, in vivo imaging experiments were performed in BALB/c recipients. Before transplantation, target cells were incubated in 10 mL of phosphate-buffered saline containing 0.5% ethanol and 1.5μg/mL 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DIR, a near-infrared lipophilic carbocyanine dye; Invitrogen). After 60 minutes of incubation at room temperature, cells were washed twice. At the specified time after transplantation, the mice were monitored by the optical whole-body imaging system (IVIS 100; Xenogen) coupled with a Pixelfly QE (PCO) charge-coupled device camera. Image processing was performed using Living Image 2.5 software (Xenogen).
In vitro assay for veto activity in the 2c TCR Tg mouse model
The in vitro assay for veto activity was done as previously described.30 Briefly, 2c splenocytes were stimulated with irradiated BALB/c splenocytes in the presence of the evaluated veto cells at the concentrations specified. Cultures were incubated for 72 hours in 96-well plates. The deletion of the 2c cells was monitored by FACS analysis measuring the level of surviving (7AAD−) CD8+ 2c cells, specifically stained by the 1B2 antibody. The inhibitory activity was calculated by the following formula: (1 − number of 1B2 CD8 cells in the assessed well/number of 1B2 CD8 cells in the control well) × 100.
In vivo assay for veto activity in the 2c TCR Tg mouse model
Lethally irradiated (10 Gy) C57BL/6 mice received 1 × 105 purified CD8+ 2c cells (MACS) and 5 × 105 irradiated (20 Gy) BALB/c splenocytes. The following day, mice received a transplant of 1 × 106 C57BL/6-NUDE BM cells and 5 × 106 Tcms. Recipients were killed 8 days after transplantation, their spleens were harvested, and the deletion of the 2c T cells was monitored by FACS as described in the previous section for the in vitro assay.
Statistical analysis
The analysis of survival data was performed using Kaplan-Meier curves (log-rank test). Comparison of means was conducted using the Student t test.
Results
Anti–third-party CTLs fail to colocalize with HTCs in the lymph nodes
Host nonreactive anti–third-party CTLs (termed CTLs), generated by prolonged activation against third-party stimulators, likely develop into effector CTLs,24,29 which do not home to the LNs,20,21,27 and therefore might fail to colocalize with rejecting HTCs in BM transplant recipients. Considering that several factors including lymphopenia,31 rejection process,32 and irradiation33,34 could all affect the migration pattern and persistence of adoptively transferred cells, we initially tested the homing pattern of the CTLs in a stringent T cell–mediated graft rejection model, in which the efficacy of the CTLs was previously evaluated.19 In this stringent model, lethally irradiated mice receive allogeneic TDBM in the presence or absence of a fixed number of HTCs that induce rejection that results in fatal anemia. To track the migration pattern of the different infused T cells, the cells were labeled with DIR dye and monitored by in vivo imaging (IVIS).
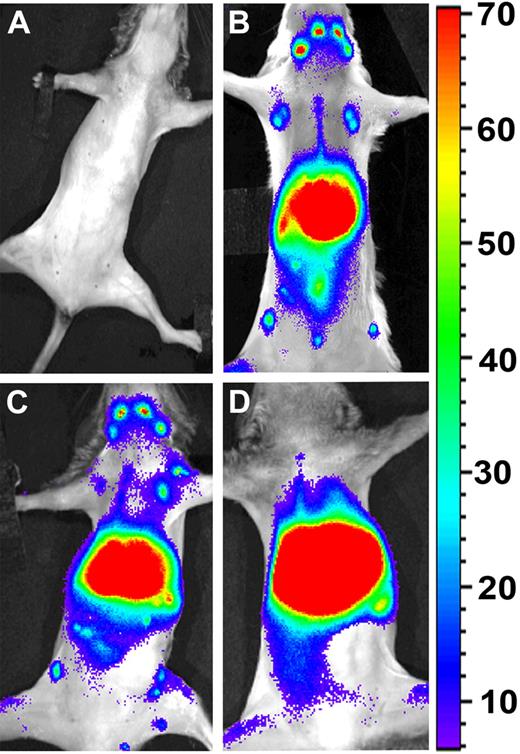
As shown in Figure 1, 36 hours after the infusion of the labeled cells, syngeneic (HTCs) and allogeneic naive T cells exhibited marked homing to the LNs (Figure 1B and C, respectively). In contrast, allogeneic CTLs were found mainly in the liver and lungs, but were clearly excluded from the LNs (Figure 1D). Failure of the CTLs to colocalize with HTCs at the critical time point when the latter cells undergo stimulation at the LNs against donor Ags, might suggest a reasonable explanation for their low efficiency in overcoming graft rejection in vivo. The ability of allogeneic naive T cells to colocalize with HTCs implies that allogeneic T cells, exhibiting the appropriate phenotype, can home to the LNs in the context of the special milieu of the graft rejection model.
Anti–third-party CTLs are excluded from the LNs, and do not colocalize with HTCs in the early time points after BM transplantation. BALB/c mice were conditioned with 8 Gy of lethal TBI and infused with different cell mixtures as follows: (A) C57BL/6-NUDE BM (4 × 106). (B) C57BL/6-NUDE BM + HTCs (1 × 104) + DIR-labeled syngeneic naive HTCs (7 × 106). (C) C57BL/6-NUDE BM + HTCs (1 × 104) + DIR-labeled naive allogeneic CB6-derived CD8+ T cells (7 × 106). (D) C57BL/6-NUDE BM + HTCs (1 × 104) + DIR-labeled allogeneic CB6-derived anti–third-party CTLs (7 × 106). Images were taken 36 hours after transplantation using IVIS. A representative mouse (of 6 mice in 2 independent experiments) is shown for each group.
Anti–third-party CTLs are excluded from the LNs, and do not colocalize with HTCs in the early time points after BM transplantation. BALB/c mice were conditioned with 8 Gy of lethal TBI and infused with different cell mixtures as follows: (A) C57BL/6-NUDE BM (4 × 106). (B) C57BL/6-NUDE BM + HTCs (1 × 104) + DIR-labeled syngeneic naive HTCs (7 × 106). (C) C57BL/6-NUDE BM + HTCs (1 × 104) + DIR-labeled naive allogeneic CB6-derived CD8+ T cells (7 × 106). (D) C57BL/6-NUDE BM + HTCs (1 × 104) + DIR-labeled allogeneic CB6-derived anti–third-party CTLs (7 × 106). Images were taken 36 hours after transplantation using IVIS. A representative mouse (of 6 mice in 2 independent experiments) is shown for each group.
Induction of anti–third-party Tcms in vitro
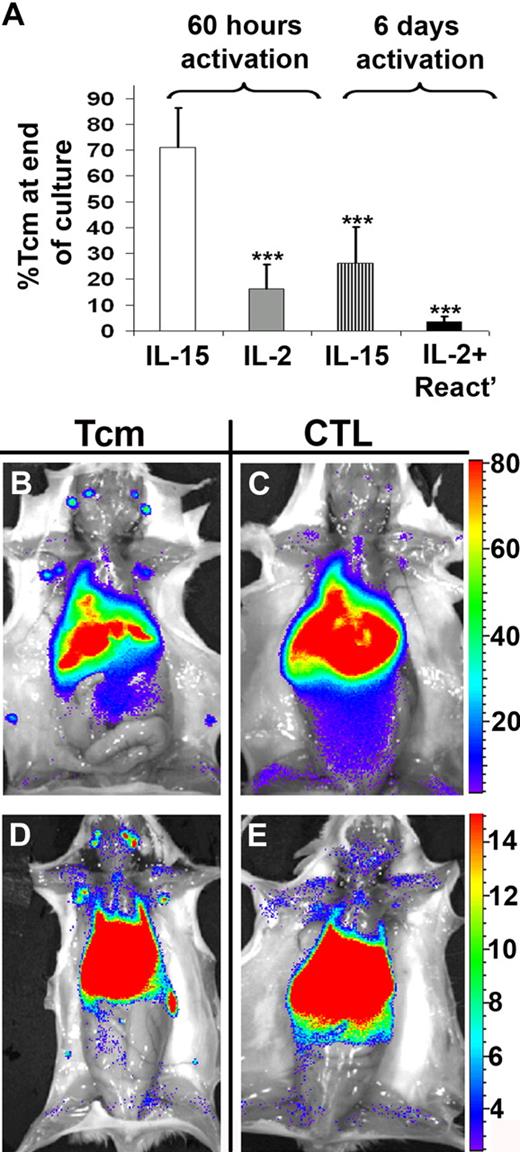
To address the poor LN homing exhibited by CTLs, we tested the potential of IL-15 to induce a Tcm phenotype in anti–third-party CD8+ T cells. Considering that depletion of GVH reactivity requires cytokine deprivation before expansion in the presence of the tested cytokines, we initially attempted to use the 6-day cytokine deprivation (used to generate the CTLs) before addition of IL-15. However, this procedure led to irreversible induction of effector phenotype. In contrast, short-term cytokine deprivation afforded marked induction of Tcm phenotype. Thus, as shown in Figure 2A, when examined on day 15 of culture, a short 60-hour stimulation followed by culture with IL-15 in the absence of Ag yielded a 71% CD44+CD62L+ Tcm phenotype, compared with only 26% found after 6 days of cytokine deprivation. Moreover, addition of various combinations and concentrations of cytokines that were previously shown to be involved in the development and maintenance of Tcms (including IL-15, IL-2, and IL-7)24,25,27,35 failed to induce significant Tcm phenotype after the long, 6-day stimulation culture, and IL-15 by itself was much less effective even when tested after an intermediate, 4-day cytokine deprivation period (data not shown). Our data, which indicate that CD8+ T cells undergoing a long activation protocol cannot be effectively induced to acquire a Tcm phenotype, are in line with previous studies indicating a limited time window in which Ag-activated T cells can be driven by IL-15 to a Tcm phenotype.24,25 Trying to replace IL-15 with IL-2 after the short, 60-hour stimulation was also associated with significantly reduced levels of CD44+CD62L+ expression (16%, Figure 2A). Of note, anti–third-party CTLs grown using the conventional method (ie, 6 days of stimulation and subsequent culture with IL-2 and reactivation with their cognate Ag) displayed mostly an effector phenotype (96%, Figure 2A), in accordance with their peripheral homing pattern (Figure 1).
In vitro induction of anti–third-party Tcms, and their homing to recipient LNs. (A) BALB/c or CB6 splenocytes were stimulated with irradiated FVB splenocytes for 60 hours or 6 days in the absence of cytokines. Subsequently, CD8+ T cells were positively selected and further cultured with rhIL-2 or rhIL-15 in an Ag-free environment. Alternatively, anti–third-party CTLs were grown by culturing the cells with rhIL-2 and reactivating them with FVB splenocytes, after the 6-day cytokine deprivation period. On day 15 of the culture (end of culture), the cells were evaluated for percentage of Tcms (CD44+CD62L+) using FACS analysis. Data represent average ± SD of at least 3 independent experiments for each group. ***P < .001 compared with the cells cultured with only IL-15 after 60 hours of cytokine deprivation. (B-E) Lethally irradiated (8 Gy) BALB/c mice received 4 × 106 C57BL/6-NUDE BM cells and 1 × 104 syngeneic T cells. Mice then received a transplant of 1 × 107 DIR-labeled, CB6-derived, purified anti–third-party Tcms (B,D) or CTLs (C,E). After 2 (B-C) or 7 (D-E) days, selected recipients were killed and images were taken ex vivo using IVIS. A representative mouse, of 6 mice in 2 independent experiments, is displayed for each group.
In vitro induction of anti–third-party Tcms, and their homing to recipient LNs. (A) BALB/c or CB6 splenocytes were stimulated with irradiated FVB splenocytes for 60 hours or 6 days in the absence of cytokines. Subsequently, CD8+ T cells were positively selected and further cultured with rhIL-2 or rhIL-15 in an Ag-free environment. Alternatively, anti–third-party CTLs were grown by culturing the cells with rhIL-2 and reactivating them with FVB splenocytes, after the 6-day cytokine deprivation period. On day 15 of the culture (end of culture), the cells were evaluated for percentage of Tcms (CD44+CD62L+) using FACS analysis. Data represent average ± SD of at least 3 independent experiments for each group. ***P < .001 compared with the cells cultured with only IL-15 after 60 hours of cytokine deprivation. (B-E) Lethally irradiated (8 Gy) BALB/c mice received 4 × 106 C57BL/6-NUDE BM cells and 1 × 104 syngeneic T cells. Mice then received a transplant of 1 × 107 DIR-labeled, CB6-derived, purified anti–third-party Tcms (B,D) or CTLs (C,E). After 2 (B-C) or 7 (D-E) days, selected recipients were killed and images were taken ex vivo using IVIS. A representative mouse, of 6 mice in 2 independent experiments, is displayed for each group.
Anti–third-party Tcms exhibit marked LN homing and proliferate extensively in vivo
Next, we evaluated whether anti–third-party cells, expressing a Tcm phenotype (termed Tcms, and generated after a 60-hour stimulation and subsequent culture in the presence of IL-15), indeed exhibit improved homing to the LNs. To that end, CB6 (H2bd)–derived CTLs or Tcms were labeled with DIR and adoptively transferred into BALB/c recipients in the context of the graft rejection model described in the first section. To restrict our results exclusively to effects mediated by purified Tcms in this experiment and in all following experiments described, the highly enriched Tcm population was positively selected at the end of the culture, using magnetic beads, for CD62L+ cells (> 95% CD44+CD62L+ Tcm purity, compared with > 95% CD44+CD62L− cells among the CTLs). Mice were killed 2 or 7 days after the adoptive transfer, and the distribution of the labeled cells in different organs was determined using whole-body ex vivo imaging (Figure 2B-E), as well as by FACS analysis, that allowed for monitoring of the presence of infused cells by their expression of both host and donor major histocompatibility complex (Figure 3).
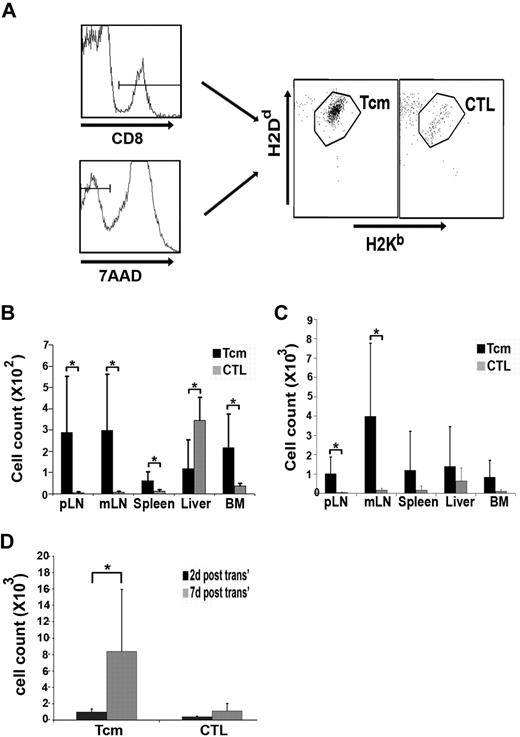
Tcms, in contrast to CTLs, populate the recipient LNs and proliferate extensively shortly after the BMT. Lethally irradiated (8 Gy) BALB/c (H-2d) mice received 4 × 106 C57BL/6-NUDE (H-2b) BM cells and 1 × 104 syngeneic HTCs. Mice then received a transplant of 107 DIR-labeled, CB6 (H-2bd)–derived, purified anti–third-party Tcms or CTLs. After 2 or 7 days, selected recipients were killed; the peripheral LNs (pLNs), mesenteric LNs (mLNs), spleen, liver, and BM were extracted; cells were purified from the different organs; and the purified cells were analyzed by FACS. To obtain absolute values of cells, samples were suspended in constant volume and flow cytometric counts for each sample were obtained during a constant, predetermined period of time and were compared with flow cytometric counts obtained from control samples that were set up with fixed volume and fixed numbers of input cells. (A) Representative FACS analysis demonstrating the presence of CD8+ and alive (7AAD−) CB6-derived cells (Tcms or CTLs) in the peripheral LNs of the recipients 2 days after transplantation. (B-C) Quantification of the FACS analysis as described in panel A, demonstrating the distribution of CB6-derived cells in various organs at 2 (B) and 7 (C) days after transplantation. (D) The sum of the total number of Tcms or CTLs, harvested from all organs tested, at 2 or 7 days after transplantation. In panels B-D, results shown represent average ± SD of pooled data from 6 animals from each group in 2 independent experiments. *P < .05.
Tcms, in contrast to CTLs, populate the recipient LNs and proliferate extensively shortly after the BMT. Lethally irradiated (8 Gy) BALB/c (H-2d) mice received 4 × 106 C57BL/6-NUDE (H-2b) BM cells and 1 × 104 syngeneic HTCs. Mice then received a transplant of 107 DIR-labeled, CB6 (H-2bd)–derived, purified anti–third-party Tcms or CTLs. After 2 or 7 days, selected recipients were killed; the peripheral LNs (pLNs), mesenteric LNs (mLNs), spleen, liver, and BM were extracted; cells were purified from the different organs; and the purified cells were analyzed by FACS. To obtain absolute values of cells, samples were suspended in constant volume and flow cytometric counts for each sample were obtained during a constant, predetermined period of time and were compared with flow cytometric counts obtained from control samples that were set up with fixed volume and fixed numbers of input cells. (A) Representative FACS analysis demonstrating the presence of CD8+ and alive (7AAD−) CB6-derived cells (Tcms or CTLs) in the peripheral LNs of the recipients 2 days after transplantation. (B-C) Quantification of the FACS analysis as described in panel A, demonstrating the distribution of CB6-derived cells in various organs at 2 (B) and 7 (C) days after transplantation. (D) The sum of the total number of Tcms or CTLs, harvested from all organs tested, at 2 or 7 days after transplantation. In panels B-D, results shown represent average ± SD of pooled data from 6 animals from each group in 2 independent experiments. *P < .05.
As shown in Figure 2B and C and Figure 3B, 40-fold more Tcms were found in the LNs 2 days after transplantation, compared with the CTLs, which were located primarily in the liver. Thus, 60% of the Tcms, recovered from all organs tested, were located at the LNs in contrast to only 3.5% of the CTLs (85% of the CTLs were located at the liver). The superior homing of the Tcms to the LNs was also evident at day 7 after transplantation (Figures 2D-E, 3C). Moreover, the total number of Tcms harvested from all organs tested had increased by 9-fold from days 2 to 7 after BMT, in sharp contrast to the CTLs, which displayed insignificant proliferation (Figure 3D). Therefore, we concluded that the Tcms not only home to the LNs in BM transplant recipients but also proliferate extensively in the early posttransplantation period.
Tcms display low veto activity in vitro, but upon reactivation acquire an effector phenotype, accompanied by potent and specific veto activity
Although anti–third-party Tcms home efficiently to the LNs, tolerance induction by these cells also requires that these cells be endowed with a strong veto activity. In general, CD8+ T cells bearing a Tcm phenotype were previously shown to exhibit weak CTL effector properties,23,24,26,36 but their veto activity has never been described.
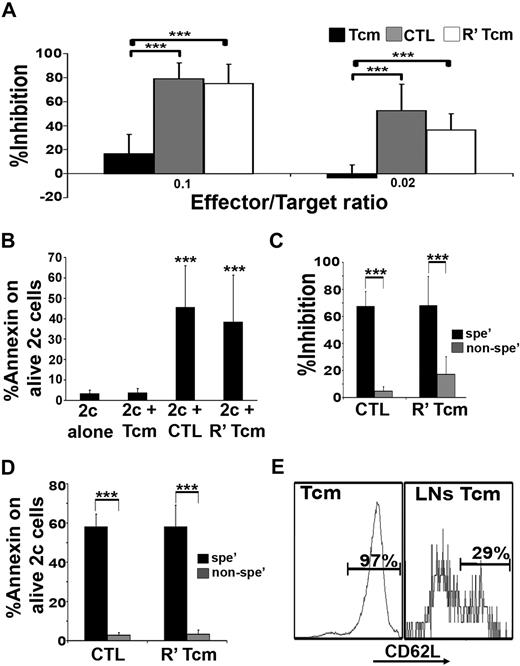
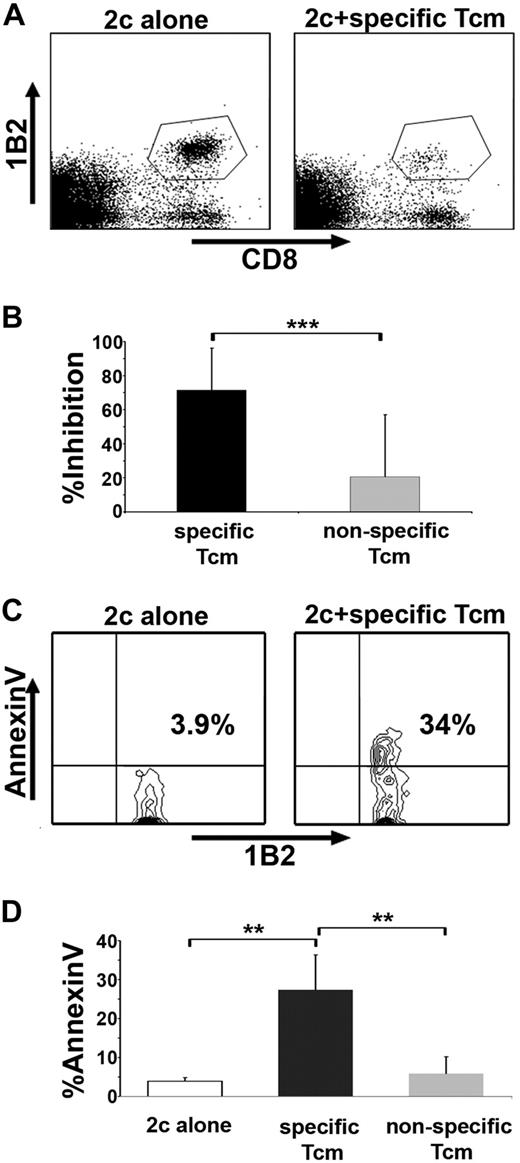
To evaluate the veto activity of Tcms, we used an assay based on TCR transgenic CD8+ effector cells (the 2c model). 2c CD8+ cells express a transgenic TCR against H-2d, and are therefore prone to veto deletion only by veto CTLs of H-2d background and not by nonspecific CTLs carrying different H-2 type, not recognized by the 2c cells.19 The 2c cells can be specifically identified using the clonotypic 1B2 antibody. CB6 mice were used as a source for veto cells to exclude hypothetical residual alloreactivity against the 2c cells. In accordance with previous studies,30,37 the CTLs, bearing an effector phenotype, exhibited efficient inhibition of the 2c cell expansion (Figure 4A), caused by apoptosis as indicated by annexin V staining (Figure 4B). In contrast, the Tcms exhibited poor inhibitory effect (Figure 4A) and failed to induce apoptosis upon the 2c cells, as suggested by lower levels of annexin V staining (Figure 4B).
Tcms display low veto activity in vitro, but upon reactivation acquire an effector phenotype, which is associated with potent and specific veto activity. (A) 2c splenocytes were stimulated with irradiated BALB/c (H-2d) splenocytes in the presence or absence of CB6 (H-2bd)–derived purified Tcms, CTLs, or purified Tcms reactivated in vitro with their cognate third-party FVB stimulators for 60 hours (R′ Tcms). The veto cells were added at the indicated veto-effector ratios. Veto activity was analyzed by FACS analysis 3 days after the initiation of the mixed lymphocyte reaction (MLR), to monitor the inhibition of CD8+1B2+ 2c cell expansion. Results are presented as mean ± SD of percentage inhibition from 5 independent experiments. (B) FACS analysis of annexin V levels on living (7AAD−) CD8+1B2+ 2c cells, at the end of the MLR, plated with or without veto cells at a veto-effector ratio of 0.02. Results are presented as mean ± SD of percentage annexin V in 7 independent experiments. (C) MLR was established as in panel A. The inhibition of 2c cell expansion was evaluated when veto cells derived from specific CB6 (H-2bd, spe′) or nonspecific C3B6F1 (H-2bk, nonspe′) mice were added at a 0.02 veto-effector ratio. Results are presented as mean ± SD of percentage inhibition from 4 independent experiments. The 2c cells were also analyzed for annexin V levels (D). (E) Lethally irradiated (8 Gy) BALB/c mice received 4 × 106 C57BL/6-NUDE BM cells and 1.25 × 104 syngeneic T cells. Mice then received a transplant of 2 to 10 × 106 purified CB6 CD8+ Tcms, which were analyzed for CD62L phenotype before the adoptive transfer (left panel, Tcms). Mice were killed 4 days after adoptive transfer, and LNs were harvested and mashed; CD62L expression on CB6 CD8+ T cells, isolated from the LNs, was analyzed by FACS (right panel, LN Tcms). Representative result of Tcms isolated from LNs of 1 of 20 mice tested in 4 independent experiments is displayed. (A-E) ***P < .001.
Tcms display low veto activity in vitro, but upon reactivation acquire an effector phenotype, which is associated with potent and specific veto activity. (A) 2c splenocytes were stimulated with irradiated BALB/c (H-2d) splenocytes in the presence or absence of CB6 (H-2bd)–derived purified Tcms, CTLs, or purified Tcms reactivated in vitro with their cognate third-party FVB stimulators for 60 hours (R′ Tcms). The veto cells were added at the indicated veto-effector ratios. Veto activity was analyzed by FACS analysis 3 days after the initiation of the mixed lymphocyte reaction (MLR), to monitor the inhibition of CD8+1B2+ 2c cell expansion. Results are presented as mean ± SD of percentage inhibition from 5 independent experiments. (B) FACS analysis of annexin V levels on living (7AAD−) CD8+1B2+ 2c cells, at the end of the MLR, plated with or without veto cells at a veto-effector ratio of 0.02. Results are presented as mean ± SD of percentage annexin V in 7 independent experiments. (C) MLR was established as in panel A. The inhibition of 2c cell expansion was evaluated when veto cells derived from specific CB6 (H-2bd, spe′) or nonspecific C3B6F1 (H-2bk, nonspe′) mice were added at a 0.02 veto-effector ratio. Results are presented as mean ± SD of percentage inhibition from 4 independent experiments. The 2c cells were also analyzed for annexin V levels (D). (E) Lethally irradiated (8 Gy) BALB/c mice received 4 × 106 C57BL/6-NUDE BM cells and 1.25 × 104 syngeneic T cells. Mice then received a transplant of 2 to 10 × 106 purified CB6 CD8+ Tcms, which were analyzed for CD62L phenotype before the adoptive transfer (left panel, Tcms). Mice were killed 4 days after adoptive transfer, and LNs were harvested and mashed; CD62L expression on CB6 CD8+ T cells, isolated from the LNs, was analyzed by FACS (right panel, LN Tcms). Representative result of Tcms isolated from LNs of 1 of 20 mice tested in 4 independent experiments is displayed. (A-E) ***P < .001.
However, short reactivation of the Tcms led to down-regulation of CD62L expression (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and to markedly enhanced suppressive activity (Figure 4A), likely mediated through a deletion-based mechanism as indicated by annexin V staining (Figure 4B). Moreover, reactivated Tcms, similarly to CTLs, displayed specific veto activity as shown by comparing reactivated “specific” Tcms or CTLs derived from CB6 (H2bd) to “nonspecific” (C57 × C3H)F1 (H2bk) CD8+ T cells that do not express the H-2d molecules and are not recognized by the 2c CD8+ T cells. Thus, only specific reactivated Tcms or CTLs effectively inhibited the expansion of 2c cells (Figure 4C) and were able to induce annexin V expression in the 2c effector cells (Figure 4D).
Collectively, these results suggest that Tcms do not possess intrinsic veto activity, but upon reactivation, acquire an effector phenotype and veto activity. This is consistent with the rapid induction of effector phenotype and function previously described for CD8+ T cells bearing a Tcm phenotype upon reactivation.24,28
Tcms isolated from the LNs of BM transplant recipients exhibit an effector phenotype
Collectively, the data described in the previous section suggest that enhanced LN homing and efficient veto activity in vitro are mutually exclusive. Nevertheless, because reactivation of Tcms dramatically improved their veto activity, it was of interest to determine whether adoptively transferred Tcms may be reactivated in the LNs of BM allograft recipients, in which adoptively transferred HTCs can mount antidonor responses. Indeed, 4 days after adoptive transfer of the purified Tcms, a significant number of the infused cells recovered from the LNs exhibited markedly reduced expression of CD62L (Figure 4E), indicating that reactivation might have occurred, possibly as a result of stimuli induced by the lethal conditioning and/or alloreactivity of HTCs.
Tcms specifically delete antidonor T cells in vivo
Considering that Tcms home effectively to the LNs of recipient mice and exhibit a veto-associated phenotype, which might enable tolerization of colocalized HTCs, we assessed the ability of adoptively transferred Tcms to suppress Ag-specific T cells in vivo. To evaluate exclusively the tolerizing activity of the Tcms, without interference from the well-documented veto activity of BM cells and BM-derived cells,13,15,18 we established a syngeneic BMT model in which lethally irradiated C57BL/6 (H2b) host mice were radioprotected with syngeneic C57BL/6-NUDE BM cells. In addition, recipient mice received 2c (H2b) CD8+ T cells and irradiated BALB/c (H2d) splenocytes, which induce the expansion of 2c cells in vivo. One day after the transfer of the 2c cells and their stimulators, the recipients received either specific CB6 derived (H2bd) purified Tcms, or nonspecific C57BL/6-derived purified Tcms, which do not express the H-2d molecule and therefore are not recognized by the 2c CD8+ T cells. Eight days after transplantation, recipients' spleens were harvested and evaluated for the presence of 2c cells. Strikingly, the specific Tcms effectively inhibited (71% inhibition) the expansion of the 2c cells (Figure 5A-B), compared with nonspecific Tcms, which exhibited only 20% inhibition. Moreover, annexin V staining indicated that the mechanism of inhibition displayed by the specific Tcms was mediated through induction of apoptosis upon recognizing 2c cells (Figure 5C-D).
Tcms specifically delete antidonor T cells in vivo. (A-B) Lethally irradiated (10 Gy) C57BL/6 mice received 1 × 105 purified CD8+ 2c cells and 5 × 105 irradiated BALB/c splenocytes. The following day, the mice received a transplant of 1 × 106 C57BL/6-NUDE BM cells or received, in addition, 5 × 106 specific, derived from CB6, or nonspecific, derived from C57BL/6, purified Tcms. Recipients were killed 8 days after transplantation, their spleens were harvested, and the deletion of 2c T cells was monitored by FACS. (A) Representative result demonstrating the level of surviving (7AAD−) 2c cells in the absence (left panel, 2c alone) or presence (right panel, 2c + specific Tcms) of specific Tcms. (B) Quantification of results measuring the inhibition of the 2c cells by specific and nonspecific Tcms. Data represent average ± SD of percentage inhibition from at least 10 animals in each group, pooled from 2 independent experiments. (C-D) Syngeneic BMT model was established as in panels A and B, but 5 × 105 purified CD8+ 2c cells and 2.5 × 106 irradiated BALB/c splenocytes were administrated. Recipients were killed 8 days after transplantation, their spleens were harvested, and FACS analysis of annexin V levels on living (7AAD−) CD8+1B2+ 2c cells was conducted. (C) Representative result demonstrating annexin V levels on 2c cells in the absence (left panel, 2c alone) or presence (right panel, 2c + specific Tcms) of specific Tcms. (D) Quantification of results measuring annexin V levels on the 2c cells after interactions with specific or nonspecific Tcms. Data present average ± SD of percentage annexin V levels in at least 4 animals from each group, in 1 representative experiment of 3 performed. **P < .01; ***P < .001.
Tcms specifically delete antidonor T cells in vivo. (A-B) Lethally irradiated (10 Gy) C57BL/6 mice received 1 × 105 purified CD8+ 2c cells and 5 × 105 irradiated BALB/c splenocytes. The following day, the mice received a transplant of 1 × 106 C57BL/6-NUDE BM cells or received, in addition, 5 × 106 specific, derived from CB6, or nonspecific, derived from C57BL/6, purified Tcms. Recipients were killed 8 days after transplantation, their spleens were harvested, and the deletion of 2c T cells was monitored by FACS. (A) Representative result demonstrating the level of surviving (7AAD−) 2c cells in the absence (left panel, 2c alone) or presence (right panel, 2c + specific Tcms) of specific Tcms. (B) Quantification of results measuring the inhibition of the 2c cells by specific and nonspecific Tcms. Data represent average ± SD of percentage inhibition from at least 10 animals in each group, pooled from 2 independent experiments. (C-D) Syngeneic BMT model was established as in panels A and B, but 5 × 105 purified CD8+ 2c cells and 2.5 × 106 irradiated BALB/c splenocytes were administrated. Recipients were killed 8 days after transplantation, their spleens were harvested, and FACS analysis of annexin V levels on living (7AAD−) CD8+1B2+ 2c cells was conducted. (C) Representative result demonstrating annexin V levels on 2c cells in the absence (left panel, 2c alone) or presence (right panel, 2c + specific Tcms) of specific Tcms. (D) Quantification of results measuring annexin V levels on the 2c cells after interactions with specific or nonspecific Tcms. Data present average ± SD of percentage annexin V levels in at least 4 animals from each group, in 1 representative experiment of 3 performed. **P < .01; ***P < .001.
Adoptive transfer of Tcms effectively overcomes T cell–mediated BM allograft rejection
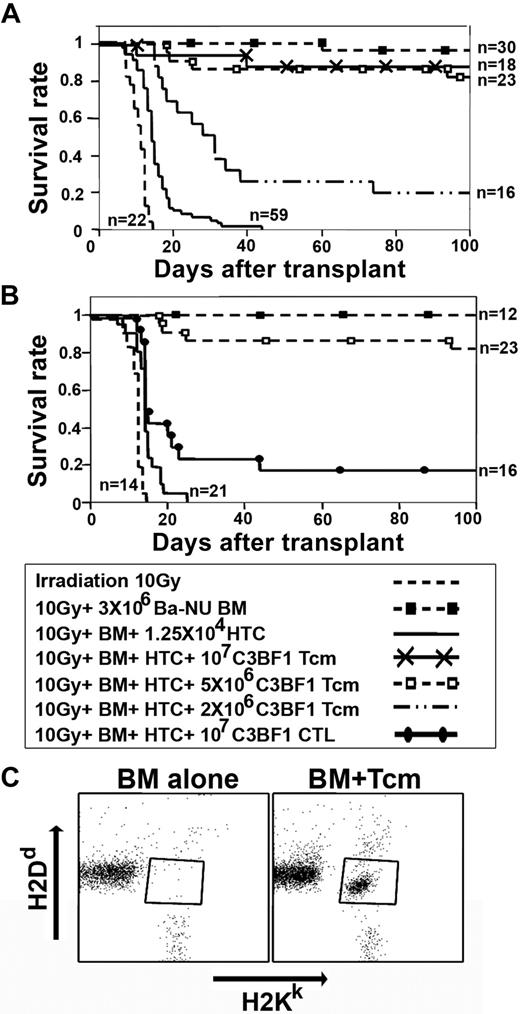
To assess the long-term ability of adoptively transferred donor-type anti–third-party Tcms to overcome BM allograft rejection, we used the graft rejection model described in the first section, which is specifically designed to measure T cell–mediated TDBM allograft rejection, without interference from stem cell competition, which might occur in mice exposed to RIC. Thus, C3H mice were lethally irradiated and infused with HTCs before transplantation of a TDBM allograft from BALB/c-NUDE donors, in the presence or absence of purified Tcms. (C3H × BALB/c)F1 mice were used as a source of Tcms to exclude potential effects of enhanced engraftment due to residual alloreactivity, and/or increased mortality due to GVHD. As can be seen in Figure 6A, all mice in the irradiation control group, not radioprotected with the BM graft, died shortly after irradiation. In contrast, 29 of 30 mice receiving TDBM survived, although the addition of HTCs led to graft rejection and lethality of all 59 mice that received this treatment. Strikingly, this HTC-mediated rejection was overcome in 16 of 18 mice and in 19 of 23 mice upon adoptive transfer of 1 × 107 or 5 × 106 Tcms, respectively. Lower numbers of Tcms were less effective (Figure 6A). All surviving mice displayed a durable and sustained donor chimerism (> 90%), which persisted for more than 1 year (supplemental Figure 2). Considering the marked polymorphism in human transplantation, we sought to test whether these findings can be extended to another strain combination. As shown in supplemental Figure 3, similar effect of Tcm-induced tolerance toward donor TDBM transplant was also demonstrated upon transplant of C57BL/6 TDBM into BALB/c recipients. These results are in contrast to our previous demonstration that adoptively transferred CTLs failed to overcome rejection unless administered in conjunction with rapamycin.19 Indeed, as can be seen in Figure 6B, only 3 of 16 of the recipient mice receiving 1 × 107 CTLs without rapamycin have survived (P < .001). Furthermore, the Tcms displayed striking persistence in vivo and could be detected in the recipients' peripheral blood for a prolonged period of time. Thus, 1 year after transplantation, H2kd-positive CD8+ T cells (ie, progeny of the infused Tcms) comprised 19% (± 6%) of the total CD8+ T-cell compartment in the peripheral blood (Figure 6C).
Tcms are endowed with marked tolerance induction capabilities, and persist in vivo at least 1 year after BMT. (A) Lethally irradiated (10 Gy) C3H (H-2K) mice received 1.25 × 104 syngeneic HTCs. Mice then received a transplant of 3 × 106 BALB/c-NUDE BM cells (H-2d,BA-NU BM) in the presence or absence of different doses of (C3H × BALB/c)F1 (H-2Kd, C3BF1) purified CD8+ Tcms. Data were pooled from 6 independent experiments. (B) Graft rejection model was established as in panel A. Mice received a transplant of 3 × 106 BALB/c-NUDE BM cells (H-2d, BA-NU BM) in the presence or absence of 5 × 106 (C3H × BALB/c)F1 (H-2Kd, C3BF1) purified Tcms or 1 × 107 (C3H × BALB/c)F1 CTLs. Data were pooled from 5 independent experiments. (C) Peripheral blood levels of Tcms were analyzed 1 year after BMT by FACS measuring H2KkH2Dd double-positive cells in the CD8+ gate. The figure shows representative mice of 7 mice that received a transplant of BM only (BM alone) or 7 mice that received a transplant of BM + HTCs + 5 × 106 (C3H × BALB/c)F1 CD8+ Tcms (BM + Tcms).
Tcms are endowed with marked tolerance induction capabilities, and persist in vivo at least 1 year after BMT. (A) Lethally irradiated (10 Gy) C3H (H-2K) mice received 1.25 × 104 syngeneic HTCs. Mice then received a transplant of 3 × 106 BALB/c-NUDE BM cells (H-2d,BA-NU BM) in the presence or absence of different doses of (C3H × BALB/c)F1 (H-2Kd, C3BF1) purified CD8+ Tcms. Data were pooled from 6 independent experiments. (B) Graft rejection model was established as in panel A. Mice received a transplant of 3 × 106 BALB/c-NUDE BM cells (H-2d, BA-NU BM) in the presence or absence of 5 × 106 (C3H × BALB/c)F1 (H-2Kd, C3BF1) purified Tcms or 1 × 107 (C3H × BALB/c)F1 CTLs. Data were pooled from 5 independent experiments. (C) Peripheral blood levels of Tcms were analyzed 1 year after BMT by FACS measuring H2KkH2Dd double-positive cells in the CD8+ gate. The figure shows representative mice of 7 mice that received a transplant of BM only (BM alone) or 7 mice that received a transplant of BM + HTCs + 5 × 106 (C3H × BALB/c)F1 CD8+ Tcms (BM + Tcms).
Fully allogeneic anti–third-party Tcms are depleted of GVH reactivity and support engraftment of TDBM allografts
After demonstrating the ability of the Tcms to overcome T cell–mediated BM allograft rejection, using (host × donor) F1 donors, devoid of alloreactivity, further assessment of the full potential of these cells was carried out using fully major histocompatibility complex–incompatible allogeneic donors. In particular, considering that anti–third-party Tcms are generated through initial allogeneic stimulation for only 60 hours under cytokine deprivation (so as to avoid down-regulation of CD62L), instead of the conventional 6-day deprivation method19 for generating anti–third-party CTLs, it was important to evaluate the GVH reactivity of fully allogeneic Tcms.
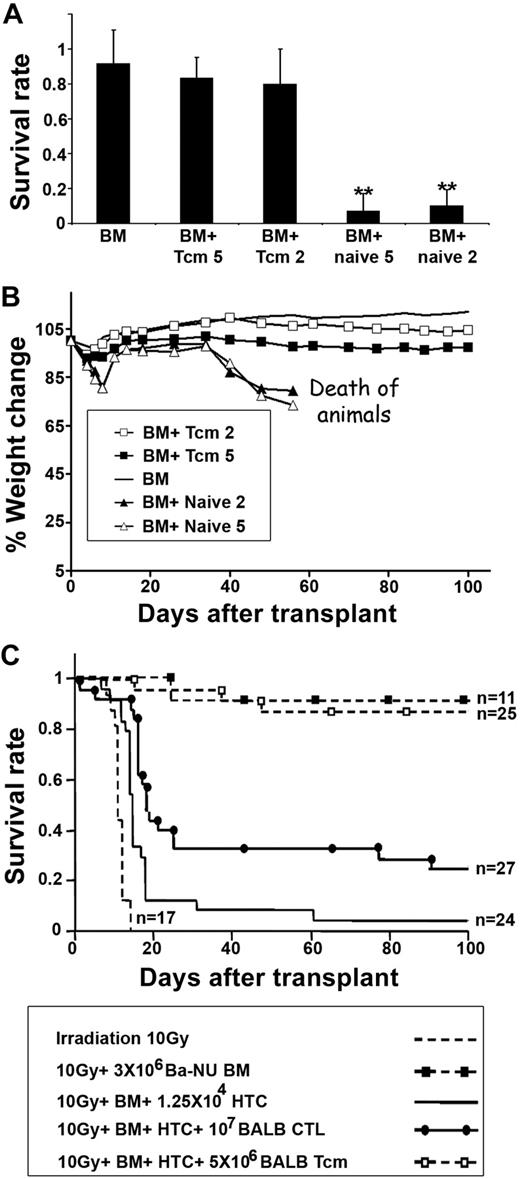
Thus, BALB/c-derived purified anti–third-party Tcms were compared with BALB/c-derived naive CD8+ cells for their GVH reactivity upon adoptive transfer into supralethally irradiated C3H recipients, which also received a transplant of BALB/c-NUDE BM. The mice were monitored for survival and signs of GVHD: ruffled fur, hunched back, and reduced body weight. As can be seen in Figure 7A, infusion of 5 × 106 or 2 × 106 Tcms did not induce lethal GVHD and 14 of 17 of the mice in each of the groups survived, similar to recipients of NUDE BM transplant without additional T cells (18/20 of the mice survived). In sharp contrast, only 1 of 15 and 2 of 22 of the mice receiving 5 × 106 or 2 × 106 naive CD8+ T cells, respectively, survived. Furthermore, the weight and overall appearance of mice receiving Tcms were not significantly different from that exhibited by control mice that received a transplant of NUDE BM alone (Figure 7B). This too is in contrast to the results in mice receiving naive T cells that displayed significant weight loss (P < .05) and had an overall appearance compatible with GVHD.
Fully allogeneic anti–third-party Tcms are depleted of GVH reactivity and support engraftment of TDBM allografts. (A-B) Supralethally irradiated (11 Gy) C3H mice were radioprotected with 5 × 106 BALB/c-NUDE BM cells in the presence or absence of 5 × 106 or 2 × 106 BALB/c-derived CD8+ purified Tcms (BM + Tcm 5 or BM + Tcm 2, respectively) or naive cells (BM + naive 5 or BM + naive 2, respectively). The GVH reactivity of the Tcms or the naive cells was reflected by survival percentage (A) or by average weight change (B) during 100 days after transplantation. Data represent averages of 3 independent experiments, with at least 5 mice for each group, in each experiment. **P < .01 compared with the group of mice that received only BM. (C) Lethally irradiated (10 Gy) C3H mice received 1.25 × 104 syngeneic HTCs. Mice then received a transplant of 3 × 106 BALB/c-NUDE BM cells (BA-NU BM) in the presence or absence of 5 × 106 BALB/c CD8+ purified Tcms or 1 × 107 BALB/c CTLs. Data were pooled from 3 independent experiments.
Fully allogeneic anti–third-party Tcms are depleted of GVH reactivity and support engraftment of TDBM allografts. (A-B) Supralethally irradiated (11 Gy) C3H mice were radioprotected with 5 × 106 BALB/c-NUDE BM cells in the presence or absence of 5 × 106 or 2 × 106 BALB/c-derived CD8+ purified Tcms (BM + Tcm 5 or BM + Tcm 2, respectively) or naive cells (BM + naive 5 or BM + naive 2, respectively). The GVH reactivity of the Tcms or the naive cells was reflected by survival percentage (A) or by average weight change (B) during 100 days after transplantation. Data represent averages of 3 independent experiments, with at least 5 mice for each group, in each experiment. **P < .01 compared with the group of mice that received only BM. (C) Lethally irradiated (10 Gy) C3H mice received 1.25 × 104 syngeneic HTCs. Mice then received a transplant of 3 × 106 BALB/c-NUDE BM cells (BA-NU BM) in the presence or absence of 5 × 106 BALB/c CD8+ purified Tcms or 1 × 107 BALB/c CTLs. Data were pooled from 3 independent experiments.
Finally, the ability of these fully allogeneic anti–third-party Tcms, depleted of GVH reactivity, to overcome T cell–mediated rejection was evaluated in the context of the previously described graft rejection model. As can be seen in Figure 7C, 21 of 25 of the mice that received 5 × 106 BALB/c-derived Tcms survived after transplantation and displayed stable donor chimerism (> 90%). In contrast, infusion of 1 × 107 BALB/c-derived CTLs could rescue only 7 of 27 of the recipient mice (P < .001). Lower CTL numbers (5 × 106 or 2 × 106) were completely ineffective in preventing this HTC-mediated rejection (data not shown).
Discussion
Our results demonstrate that CD8+ T cells activated against third-party stimulators for a short period of time, and thereafter expanded in the presence of IL-15, exhibit striking tolerizing activity in vivo. Our data suggest that these cells, expressing CD44 and CD62L typical of a Tcm phenotype, in accordance with previous studies23,28,38 also exhibit enhanced LN homing, as well as marked proliferation and prolonged in vivo persistence in BM transplant recipients. Critically, although we showed that the acquisition of veto activity in vitro and the expression of a Tcm phenotype are mutually exclusive, it was also found that Tcms recovered from LNs after infusion into mice receiving a transplant of TDBM significantly down-regulate their CD62L and regain strong suppressive activity. This rapid acquisition of effector phenotype by the Tcms is likely induced by the milieu created in the TDBM recipient animals, as suggested by studies showing that naive CD8+ cells also acquire effector-memory phenotype upon adoptive transfer into lymphopenic hosts.31,39 Therefore, it is possible that Tcms can localize to the LNs shortly after being transferred to the recipients, and undergo reactivation upon their arrival so as to acquire suppressive activity critical for the deletion of donor-specific HTCs.
In line with this notion, adoptive transfer of anti–third-party Tcms, in the absence of rapamycin treatment, significantly abolished HTC-mediated rejection of NUDE BM, thereby enabling a high survival rate and long-term donor-type chimerism. This is in sharp contrast to anti–third-party CTLs, which fail to enable engraftment unless administered in higher numbers and in conjunction with rapamycin treatment.19 A similar advantage was documented for CD62LhighCD4+CD25+ regulatory cells in preventing a pathogenic immune response.40,41 Similarly to the Tcms, these cells exhibited enhanced LN homing, compared with CD62Llow regulatory cells. Previous studies have also shown that CD8+ T cells expressing a Tcm phenotype can more effectively confer protection against pathogens and tumors, compared with CD8+ Tem/effector cells.23,28,29 The enhanced protective potential of Tcms was correlated with their greater proliferative capacity and persistence in vivo upon Ag re-encounter, compared with effector cells.23,27,28,38 However, our demonstration that anti–third-party Tcms, derived from F1 mice, which do not reencounter their cognate Ag in vivo, also proliferate extensively during the initial days after BMT suggests that this expansion might not be driven by reactivation against cognate antigen, but rather by cytokines regulating homeostatic expansion in lymphopenic mice. A previous study by Wherry et al also demonstrated that Tcms adoptively transferred into irradiated syngeneic mice display substantially more cell divisions than Tem cells.28 Considering that Tcms rapidly generate effector-like cells with veto activity after their transfer into irradiated recipient mice, this enhanced proliferative potential may allow the generation of a large number of effector T cells that can mediate a veto effect both at the LNs and at peripheral sites.
It could be argued that enhancement of BM allografting by the Tcms might be mediated by a nonspecific mechanism such as competition for niches/spaces by homeostatically proliferating T cells.42 Moreover, a recent study by Wan et al has shown that bystander syngeneic CD8+ T cells bearing a Tcm phenotype may suppress T-cell proliferation and delay allograft rejection,43 not only by niche competition but also by secretion of transforming growth factor-β. However, although we did detect some nonspecific in vivo deletion of host antidonor TCR transgenic T cells by Tcms not expressing the donor H-2, the superior in vivo deletion exhibited by donor Tcms in the present study clearly illustrates the advantage of using donor-type Tcms for tolerance induction. Thus, in contrast to currently used immunosuppressants, the specific effect displayed by these cells may allow the elimination of antidonor T-cell clones, while substantially sparing clones directed against pathogens or tumor cells critical for immune reconstitution in BM transplant recipients.
Most importantly, and of clinical relevance, when evaluated specifically for GVHD, the mice that received a therapeutic dose of anti–third-party Tcms displayed long-term survival, accompanied by a healthy overall appearance, in sharp contrast to the mice that received naive CD8+ T cells. Moreover, the similar survival rates, displayed by mice undergoing BM allograft rejection that received either fully allogeneic or F1-derived Tcms, indicate that allogeneic Tcms do not induce a harmful alloreactive effect in recipient mice. Furthermore, this similar survival suggests that the mechanism of tolerance induction displayed by the Tcms is indeed based on veto activity, as opposed to potential ablation of host resistance by donor alloreactive clones. Nevertheless, considering that human patients may be more prone to GVHD, compared with inbred mice (which are maintained under sterile conditions from birth), clinical translation of this approach must be pursued with caution; additional allodepletion steps, such as photodepletion44 or selection of activated cells45 at the end of the allostimulation period against the third-party stimulators, might be required to further reduce the risk of GVHD in haploidentical transplantation.
Although the major focus of our study is related to tolerance induction, a secondary objective that could be attained by administration of CD8+ T cells depleted of GVHD reactivity is related to their additional potential to enhance immune reconstitution in recipients of BM transplant. For example, anti–third-party CTLs were shown to possess, in addition to their tolerance induction potential, potent antileukemic reactivity.46 Likewise, several reports show that CD8+ Tcms exhibit enhanced response against pathogens and tumors, compared with Tem/effector cells.23,28,29 Moreover, Kaneko et al have suggested that human suicide gene–modified Tcm-like cells, induced ex vivo using IL-7 and IL-15, may elicit a stronger antileukemia response than Tem cells.47 Our demonstration in the present study that anti–third-party Tcms can persist in vivo for more than a year after their administration into recipients of TDBM transplant is in line with these suggestions. Further attempts to define whether adoptive transfer of anti–third-party Tcms can be useful not only for tolerance induction but also for the important goal of immune reconstitution during the early posttransplantation period are warranted. Furthermore, our results could be of particular relevance to the growing number of studies attempting to develop donor-derived Ag-specific Tcms for antileukemia or antiviral cell therapy. Thus, donor-derived Tcms specifically generated against Epstein-Barr virus or cytomegalovirus might also prove useful for tolerance induction and thereby could potentially afford a way to address 2 most desirable goals in BMT with one agent.
In conclusion, our study demonstrates several important attributes of anti–third-party CD8+ T cells generated by short stimulation under cytokine deprivation and subsequently expanded by treatment with IL-15. The marked LN homing, the potent veto activity in vivo leading to a specific deletion of host antidonor T cells, as well as reduced GVH reactivity and marked proliferative capacity strongly suggest that this Tcm population likely represents an important and novel tool for tolerance induction in general and for facilitating BM allografts in particular. Considering that recipients of haploidentical megadose stem cell transplants are currently conditioned using a strong ablative protocol including antithymocyte globulin (ATG), which has an adverse impact on posttransplantation immune reconstitution, one immediate application could be replacing ATG with anti–third-party Tcms. Furthermore, the use of such tolerizing cells could also lead to the development of a much safer protocol for the establishment of donor-type chimerism in patients conditioned with RIC. The latter, if indeed established with very low risk of lethality, would be ethically justified for tolerance induction in organ recipients48-50 or for allogeneic stem cell transplantation in nonmalignant disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Y.R. holds the Henry H. Drake Professorial Chair in Immunology.
This work was supported in part by National Institutes of Health grant CA-100265, project 5, and grants from Mrs E. Drake and the Gabriella Rich Center for Transplantation Biology Research and the M. D. Moross Institute for Cancer Research.
National Institutes of Health
Authorship
Contribution: E.O. and Y.E. designed research, performed research, analyzed data, and wrote the paper; R.A. and E.B.-L. designed research, performed research, and analyzed data; and Y.R. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yair Reisner, Department of Immunology, Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: yair.reisner@weizmann.ac.il.
References
Author notes
E.O. and Y.E. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal