Abstract
There is a high demand for the development of adjuvants that induce cytotoxic T lymphocytes, which are crucial for the elimination of intracellular pathogens and tumor cells. Toll-like receptor (TLR) agonists are prime candidates to fulfill this role because they induce innate immune activation and promote adaptive immune responses. The successful application of the TLR7 agonist R837 for treatment of basal cell carcinoma shows the potential for exploiting this pathway in tumor immunotherapy. Imidazoquinolines like R837 and stimulatory ssRNA oligonucleotides both trigger TLR7-mediated immune activation, but little is known about their comparative ability to promote immunity induction. We investigated differences in innate immune activation and adjuvant activity between the imidazoquinoline R848 and the ssRNA TLR7 agonist polyUs21. In contrast to R848, polyUs21 induced detectable levels of intracellular interferon-α (IFN-α) in plasmacytoid dendritic cells (PDCs). In immunization studies, only polyUs21 led to robust priming of type 1 T helper cells and cytotoxic T lymphocytes, and it was more efficient in inducing antitumor immunity than R848. Notably, exogenous IFN-α augmented the adjuvant activity of R848, whereas depletion of PDC abrogated the adjuvanticity of polyUs21. This study, therefore, identifies sufficient IFN-α production by PDC as an important determinant of vaccine efficacy.
Introduction
Cellular immune responses characterized by the induction of cytotoxic effector cells are crucial for therapeutic interventions in the context of tumor immunotherapy and for the induction of protective immunity against a variety of intracellular pathogens, such as the malaria parasite and HIV. A particular focus of novel vaccination strategies is the identification of adjuvants with the ability to skew adaptive immune responses toward a Th1 phenotype and thereby allow for the induction of cellular, in addition to humoral, immunity.1 Synthetic mimics of pathogen-associated molecular patterns in general, and especially those mimicking virus presence, appear particularly potent in promoting the induction of cellular immunity and might therefore constitute powerful adjuvants.2 Viral pathogen–associated molecular patterns can be detected by Toll-like receptors (TLRs) and cytoplasmic pattern recognition receptors.3,4 The virus-sensing TLRs sample the contents of specialized endosomal compartments, where they detect bacterial and viral genomes, as well as viral replication intermediates.3,4 Different classes of viral nucleic acids are detected by distinct TLRs with TLR3, TLR7/8, and TLR9 sensing dsRNA, ssRNA, and DNA, respectively.3,4
Although various synthetic TLR agonists have been tried as adjuvants,5 not many of them are approved for human use. In contrast, the TLR7/8 agonist R837 is approved for the topical treatment of genital warts, basal cell carcinoma, and bladder cancer.6-9 Imidazoquinolines such as R837 and R848 originally were developed as small immune response modifiers with antiviral activity, and it only became evident later that they stimulate innate immune activation via TLR7 and/or TLR8.10-13 In mouse studies, imidazoquinolines were shown to act as adjuvants able to promote an adaptive immune response to coadministered antigens.14,15 However, R837 also leads to TLR7-independent augmentation of inflammation by acting as an adenosine receptor antagonist.16 Furthermore, repeated systemic administration results in immune dysfunction caused by temporary depletion of peripheral leukocytes and altered lymphoid organ structure.17,18 Thus, systemic application of imidazoquinolines leads to adverse side effects,19 and the development of other TLR7 agonists suitable for nontopical use as adjuvants is desirable. The identification of suitable TLR7 agonists requires a systematic comparison of these candidates with imidazoquinolines for the ability to promote adaptive immunity.
Various RNA oligonucleotides, including siRNA constructs, have the capacity to trigger TLR7. There is no clear consensus on the motif that mediates recognition via TLR7, with uridine- and guanosine/uridine–rich sequences having been proposed in addition to guanosine/uridine–independent motives.20-23 A limiting factor for TLR7-mediated immune activation by ssRNA is the access of the latter to the endosomal compartments, in which recognition takes place. Because free ssRNA is quickly degraded by extracellular RNases, ssRNA TLR7 agonists have to be used in the form of complexes with cationic compounds to be effective both in vitro and in vivo.20-24 However, this does not preclude their use in vivo, and their potential as adjuvants for the induction of cytotoxic effector function has been suggested.24 Here, we explore the use of ssRNA TLR7 agonists as adjuvants for the induction of adaptive immunity. We demonstrate in a murine model system that a 21-mer of polyU previously reported to act as selective TLR7 stimulus is vastly superior to R848 at inducing CD4+ and CD8+ T-cell responses to coadministered antigen. Notably, we show that this potency is attributable to its superior ability to stimulate interferon-α (IFN-α) production by plasmacytoid dendritic cells (PDCs). This finding suggests that ssRNA TLR7 agonists could be developed as effective adjuvants for the induction of cellular immunity and that the ability to induce sufficient levels of IFN-α is a crucial determinant for the selection of potent adjuvants.
Methods
Mice
C57BL/6 and 129 Sv mice were obtained from Harlan UK. TLR7 knockout (KO; C57BL/6 background) and IFNRI KO (129 Sv background) mice were bred at the biological service unit at King's College London. All animal experiments were performed in accordance with United Kingdom governmental regulations (Animal Scientific Procedures Act 1986) and were approved by the United Kingdom Home Office.
Immunization studies
For dendritic cell (DC) activation, mice were injected intravenously with 30 μg of the indicated TLR agonists in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethansulfonic acid)–buffered saline if not specified otherwise. PolyU (Sigma-Aldrich) and polyUs21 (provided by Innate Pharma) were administered in form of complexes with 100 μL of DOTAP (Roche). For T- and B-cell activation, adjuvants were coadministered with 200 μg of endograde ovalbumin (OVA) from Profos AG or egg white preparation containing an equivalent of 600 μg of OVA.25 Control mice received OVA in combination with DOTAP but without TLR agonist. Exogenous IFN-α (Hycult Biotechnology) and OVA were coinjected intravenously with or without R848 (Invivogen). As control, mice were treated with 30 μg of polyI:C (GE Healthcare) with or without OVA. In specified experiments, mice were immunized with OVA plus 15 μg of anti-CD40 antibody (BD Biosciences) with or without R848.
DC activation analysis
Splenocyte suspensions were stained with fluorochrome-labeled antibodies specific for mouse CD11c, CD4, CD8α, B220 (BD Biosciences), and PDCA1 (eBiosciences). Intracellular cytokine staining of splenocytes was performed after a 3-hour incubation in the presence of 5μM brefeldin A (Invitrogen). Cells were fixed in 4% paraformaldehyde and stained for the DC markers listed previously. Subsequently, cells were permeabilized and stained for intracellular cytokines in phosphate-buffered saline containing 1% heat-inactivated fetal calf serum, 5mM EDTA (ethylenediaminetetraacetic acid), 0.1% saponin (Sigma-Aldrich), and 0.02% sodium azide with phycoerythrin-labeled IL-12p40– (clone C15.6; BD Biosciences), phycoerythrin-labeled interleukin-6– (IL-6; clone MP5-20F3; BD Biosciences), or fluorescein isothiocyanate–labeled IFN-α–specific antibody (clone RMMA-1; PBL). Data were acquired on a FACSCanto II (BD Biosciences).
Cytotoxic T lymphocyte priming
On day 6 after vaccination, mice were injected intravenously with a 1:1 mixture of splenocytes that had been pulsed with 500nM SIINFEKL peptide or left unpulsed and labeled with 0.5μM and 5μM carboxyfluorescein succinimidyl ester (CFSE), respectively. The next day, splenocytes were isolated and analyzed by flow cytometry. Antigen-specific killing was calculated using the following formula: (1 − % of CFSEpeptide/% of CFSEno peptide) × 100.
T helper cell and antibody responses
On day 7 after vaccination, splenocytes were isolated and cultured in vitro in the presence or absence of 300 μg/mL OVA for 72 hours. IFN-γ expression was determined in cells incubated for an additional 3 hours with 5μM brefeldin A by intracellular cytokine staining with IFN-γ–specific antibody (clone XMG1.2; BD Biosciences) as described previously. Supernatants from these cultures were assayed for IFN-γ, IL-5, IL-13, and IL-4 with the use of FlowCytomix kits from Bender MedSystems. Relative levels of OVA-specific antibodies in the serum of mice vaccinated 7 days earlier were determined by enzyme-linked immunosorbent assay (ELISA) with the use of anti-mouse immunoglobulin M (IgM; clone R6-60.2), IgG1 (clone A85-1), and IgG2c (clone R19-15) antibodies (all BD Biosciences).26
Tumor model
C57BL/6 mice were injected intravenously with 7.5 × 105 B14.3 cells (B16 melanoma cells expressing OVA/GFP fusion protein) 30 days after prophylactic vaccination, and the number of lung nodules was determined 18 days after tumor challenge. Up to 250 nodules were counted per lung.
PDC depletion
The hybridoma cell line for the monoclonal antibody 927 was kindly provided by Dr Marco Colonna, Washington University School of Medicine.27 The antibody was purified by protein G affinity chromatography. For depletion, mice were injected intraperitoneally with 500 μg of antibody 927 or control rat IgG2b antibody on 2 consecutive days. Mice were vaccinated 48 hours after start of the depletion. Depletion of PDC at the time of vaccination was confirmed by flow cytometry.
Statistics
Statistical analysis of in vivo cytotoxic T lymphocyte (CTL) assays and the B16 metastasis model was performed by 1-way analysis of variance followed by Bonferroni multiple comparison posttest. Data on cytokine induction were analyzed by the use of a 2-tailed Student t test.
Results
Stimulatory ssRNA and imidazoquinolines show differences in DC activation in vivo
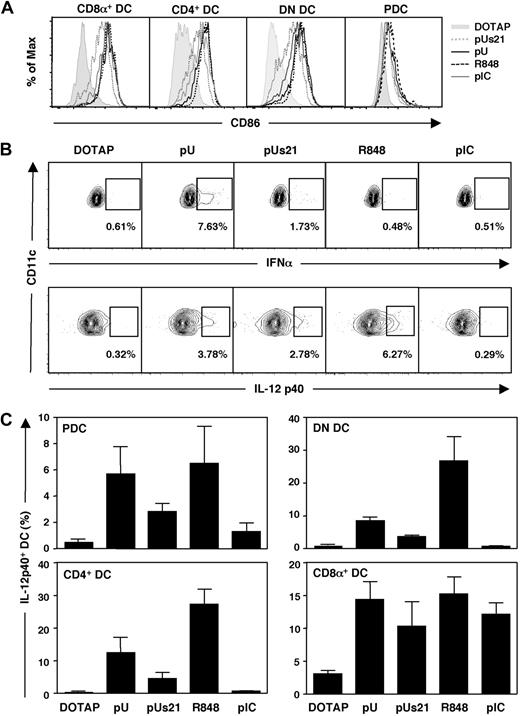
Adjuvants act in large measure by inducing the activation of DC, which is a prerequisite for induction of adaptive immunity. To compare the effects of distinct TLR7 agonists on DC subtypes in vivo, C57BL/6 mice were injected intravenously with polyU, polyUs21, R848, or the TLR7-independent control stimulus polyI:C. The imidazoquinoline R848 was chosen because it induces stronger innate immune activation of DC than R837.23 Because ssRNA is prone to degradation by extracellular RNases, polyU and polyUs21 were administered in the form of complexes with the cationic lipid DOTAP. We determined the optimal ratio of ssRNA oligonucleotides and DOTAP for complex formation by dose titration (data not shown). A dose of 30 μg of polyU or polyUs21 complexed with 100 μL of DOTAP led to maximal DC activation. For comparison, 30 μg of R848 and polyI:C were administered without DOTAP because at this dose robust innate immune activation was observed for both stimuli. In vivo activation of splenic DC was analyzed 4 hours after TLR agonist administration by gating on CD11cint PDCA1+ B220+ PDC versus CD11chigh conventional DC. Conventional DCs were further subdivided into CD4+, CD8α+, and double-negative (DN) DC subsets.
All 4 splenic DC subsets demonstrated up-regulation of the maturation marker CD86 after injection of TLR agonists (Figure 1A). The extent of CD86 up-regulation on the different DC subsets was comparable between polyU, R848, and slightly lower for polyUs21 on conventional DC (Figure 1A). Furthermore, TLR7-mediated CD86 up-regulation was comparable with the levels observed in response to the TLR3 agonist polyI:C (Figure 1A). Similar patterns were obtained for up-regulation of CD40, another marker of DC maturation (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, all DC subsets underwent maturation in response to systemic treatment with the tested TLR agonists.
ssRNA induces efficient DC activation in vivo. C57BL/6 mice were injected intravenously with the indicated TLR agonists. PolyU and polyUs21 were administered as complexes with DOTAP. Splenocytes were isolated and DC populations were analyzed by flow cytometry. (A) The up-regulation of the maturation marker CD86 on gated DC subsets was determined 4 hours after adjuvant administration. (B) The percentage of IFN-α– and IL-12p40–expressing PDCs was assessed by flow cytometry 4 hours after treatment. (C) The IL-12 p40 expression by PDC, CD4+, DN, and CD8α+ DC was analyzed as in panel B. The data are representative of 3 independent experiments (A-B) or are compiled to one figure with the SEM depicted (C).
ssRNA induces efficient DC activation in vivo. C57BL/6 mice were injected intravenously with the indicated TLR agonists. PolyU and polyUs21 were administered as complexes with DOTAP. Splenocytes were isolated and DC populations were analyzed by flow cytometry. (A) The up-regulation of the maturation marker CD86 on gated DC subsets was determined 4 hours after adjuvant administration. (B) The percentage of IFN-α– and IL-12p40–expressing PDCs was assessed by flow cytometry 4 hours after treatment. (C) The IL-12 p40 expression by PDC, CD4+, DN, and CD8α+ DC was analyzed as in panel B. The data are representative of 3 independent experiments (A-B) or are compiled to one figure with the SEM depicted (C).
TLR7 and TLR3 are differentially expressed on mouse splenic DC, with TLR7 being highly expressed by PDCs and absent in CD8α+ DC and TLR3 showing the inverse expression pattern.28 Nevertheless, all DC subsets up-regulated CD86 in response to the TLR7 agonists, indicating that activation in the case of TLR7-negative CD8α+ DC is indirect. Although up-regulation of costimulatory molecules can be induced in a bystander fashion, cytokine secretion by DC is regarded as a sign of direct signaling by TLRs.26 As expected, all TLR7 agonists induced direct activation of PDC, but the cytokine pattern that was induced varied (Figure 1B).
Similar to earlier in vitro observations,23 polyU and polyUs21 induced IFN-α in PDCs (6.94% ± 0.39% and 2.37% ± 0.49% IFN-α+ PDC, respectively), whereas the imidazoquinoline R848 was ineffective in triggering detectable levels of IFN-α (0.81% ± 0.49% IFNα+ PDCs compared with 1.13% ± 0.40% IFNα+ PDCs in mice treated with DOTAP only) in vivo (Figure 1B; supplemental Figure 1B). In contrast, IL-12 p40 induction by PDCs was similar or more pronounced in response to R848 (6.52% ± 2.81% vs 0.47% ± 0.19% IL-12 p40+ PDCs in control mice) than upon treatment with the ssRNA TLR7 agonists (5.68% ± 2.02% vs 2.83% ± 0.65% IL-12 p40+ PDCs for polyU and polyUs21, respectively). As expected PDCs did not produce IFN-α in response to the TLR3 agonist polyI:C (Figure 1B; supplemental Figure 1B), and IFN-α induction in response to polyU and polyUs21 administration was only detected in PDC and in none of the other DC subsets (data not shown).
The percentage of IL-12 p40-producing CD4+ and DN DCs was increased in response to the TLR7 agonists, with R848 stimulating more DCs to produce this cytokine than polyU or polyUs21 (Figure 1C). PolyUs21 was less potent in activating TLR7-expressing DC subsets than polyU in inducing IL-12 p40. The TLR3 agonist polyI:C failed to induce IL-12 p40 in CD4+ and DN DCs but led to an increase in IL-12 p40-expressing CD8α+ DCs (Figure 1C). Unexpectedly, there was a clear increase in the number of CD8α+ DCs, which produced IL-12 p40 in response to the TLR7 agonists polyU, polyUs21, and R848 (Figure 1C). It is currently unclear whether the induction of IL-12 p40 by CD8α+ DCs in response to TLR7 agonists is TLR7 dependent and is mediated via direct or indirect activation of this TLR7-negative DC subset. Direct activation of CD8α+ DCs by R848 has not been observed in vitro, suggesting that other cell types may be involved in the induction of IL-12 p40 by CD8α+ DCs in vivo.28
Thus, differences in TLR7-induced DC activation between ssRNA agonists versus imidazoquinolines that had been observed in vitro23 also are discerned after TLR7-mediated stimulation of splenic DC in vivo. For the subsequent detailed analysis of the adjuvanticity of TLR7 agonists, R848 was compared with polyUs21. Despite its lower activity as an innate stimulus for DC activation, polyUs21 was favored over polyU because it is of defined length and shows lower susceptibility to degradation by RNases. In addition, polyUs21 is entirely endotoxin free.
Differential adjuvanticity of distinct TLR7 agonists
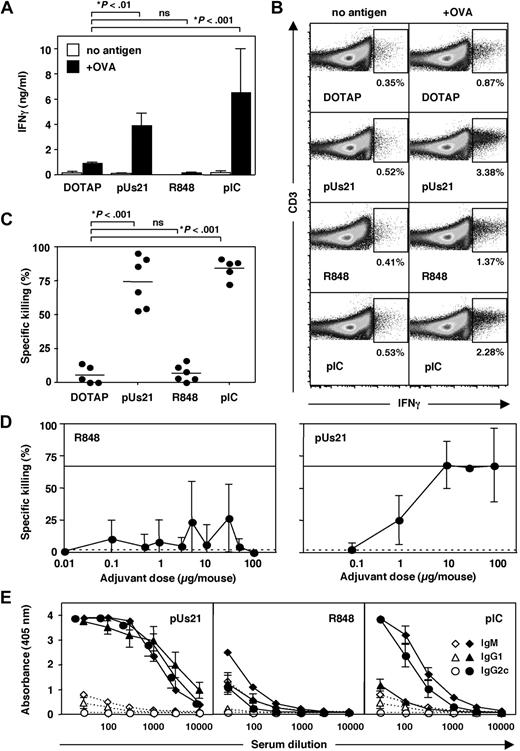
To determine how differences in innate immune activation by different TLR7 agonists influence the induction of adaptive immune responses, we coinjected TLR7 agonists with OVA to study their adjuvant properties. Splenocytes were isolated 7 days after immunization and were restimulated with antigen in vitro. Culture supernatants of restimulated splenocytes from polyUs21- and polyI:C-treated mice revealed high levels of IFN-γ, in contrast to splenocytes from R848-treated mice (Figure 2A). IFN-γ production was dependent on restimulation and was not observed in the absence of antigen (Figure 2A). We were unable to detect the Th2 cytokines IL-4, IL-5, and IL-13 in restimulated splenocyte cultures (data not shown).
polyUs21 induces potent T- and B-cell responses in vivo. C57BL/6 mice were immunized intravenously, and 7 days later T- and B-cell responses were analyzed. (A) Splenocytes were isolated and cultured in vitro for 72 hours in the presence or absence of OVA. IFN-γ levels in the culture supernatants were determined by bead-based ELISA assay. Data contain results from at least 2 independent experiments. (B) IFN-γ production by splenocytes cultured for 72 hours in the presence or absence of OVA was assessed by flow cytometry gating on CD4+ CD3+ T cells. One representative of 3 independent experiments is shown. (C) In vivo CTL killing assays were performed at day 7 after immunization. Pooled data of 2 independent experiments are shown. (D) The indicated doses of R848 or polyUs21 in form of complexes with DOTAP were administered intravenously in combination with egg white preparation (n = 3). In vivo CTL assay was performed 7 days after immunization. (E) OVA-specific antibody levels of IgM, IgG1, and IgG2c isotypes in serial dilutions of serum were determined by ELISA in mice treated with adjuvant (solid lines) or in OVA + DOTAP–treated control mice (dotted lines). Serum dilution curves represent the mean ± SEM of at least 3 independent experiments.
polyUs21 induces potent T- and B-cell responses in vivo. C57BL/6 mice were immunized intravenously, and 7 days later T- and B-cell responses were analyzed. (A) Splenocytes were isolated and cultured in vitro for 72 hours in the presence or absence of OVA. IFN-γ levels in the culture supernatants were determined by bead-based ELISA assay. Data contain results from at least 2 independent experiments. (B) IFN-γ production by splenocytes cultured for 72 hours in the presence or absence of OVA was assessed by flow cytometry gating on CD4+ CD3+ T cells. One representative of 3 independent experiments is shown. (C) In vivo CTL killing assays were performed at day 7 after immunization. Pooled data of 2 independent experiments are shown. (D) The indicated doses of R848 or polyUs21 in form of complexes with DOTAP were administered intravenously in combination with egg white preparation (n = 3). In vivo CTL assay was performed 7 days after immunization. (E) OVA-specific antibody levels of IgM, IgG1, and IgG2c isotypes in serial dilutions of serum were determined by ELISA in mice treated with adjuvant (solid lines) or in OVA + DOTAP–treated control mice (dotted lines). Serum dilution curves represent the mean ± SEM of at least 3 independent experiments.
To determine whether IFN-γ was produced by CD4+ T cells, activation of restimulated splenocytes was assessed by intracellular cytokine staining gating on CD4+CD3+ cells. After immunization with antigen plus polyUs21 or polyI:C, the frequency of IFN-γ–producing CD4+ T cells was elevated upon restimulation from 0.87% in the DOTAP control group to 3.38% and 2.28%, respectively (Figure 2B). In contrast, restimulated splenocytes from R848-treated mice showed only a slight increase in IFN-γ–producing CD4 T cells to 1.37% (Figure 2B). In the absence of restimulation, the frequency of IFN-γ–positive CD4 T cells was low and ranged from 0.35% to 0.53% for the different treatment groups (Figure 2B). In conclusion, treatment with polyUs21 complexed to DOTAP induces a Th1 response similar to the control adjuvant polyI:C, whereas R848 is relatively inefficient in priming this type of T helper cell response.
Effective priming of CTL is crucial for the induction of cellular immunity. We quantified the induction of CTL in response to the different adjuvants by performing an in vivo CTL assay. Immunization with OVA plus polyUs21-DOTAP complexes or polyI:C led to potent CTL responses, whereas OVA plus R848 failed to induce levels of CTL killing above the background levels observed for mice injected with DOTAP and OVA alone (Figure 2C). To rule out the possibility that the observed lack of in vivo CTL killing activity with R848 was the result of administering a suboptimal dose, a dose titration was performed. None of the tested doses of R848 (0.01-100 μg/mouse) led to reproducible induction of CTL activity, whereas titration of polyUs21 (0.1-100 μg/mouse) showed a clear dose response for CTL induction (Figure 2D). Coadministration of R848 with DOTAP as performed for polyUs21 also failed to improve the induction of CTL responses (data not shown). In contrast, coadministration of anti-CD40 antibody, which mimics T-cell help, increased levels of CTL induction by R848 (supplemental Figure 2).
We also analyzed the humoral response potentiated by these adjuvants. We determined the levels of OVA-specific antibodies by ELISA and analyzed IgM, IgG1, and IgG2c isotypes separately. Interestingly, immunization with OVA plus polyUs21-DOTAP complexes led to high antibody titers, whereas OVA plus R848 induced relatively low titers for all 3 isotypes (Figure 2E). A clear preference for class switching to IgG1 versus IgG2c antibodies could not be observed for either of the TLR7 agonists. In contrast to our findings, the ssRNA TLR7 agonist RNA40 has been shown to preferentially induce IgG1 antibodies upon subcutaneous administration.24 The discrepancy in the preference of B-cell class switching induced by these 2 ssRNA TLR7 agonists could be either caused by differences in their stimulatory activity or the route of administration. The control adjuvant polyI:C induced relatively low IgG1 in comparison with IgG2c antibody titers (Figure 2E), thereby displaying a preference for Th1-dependent class switching.
Our results suggest that the observed differences in DC-derived cytokine induction upon systemic administration of polyUs21 versus R848 result in differences in priming of both CD4+ and CD8+ T-cell responses. In addition, R848 is a poor inducer of B-cell class switching, in direct comparison with polyUs21, leading to a weaker humoral response. In summary, polyUs21 represents a more reliable and potent adjuvant upon systemic administration.
Induction of an antitumor immune response upon immunization with TLR7 agonists
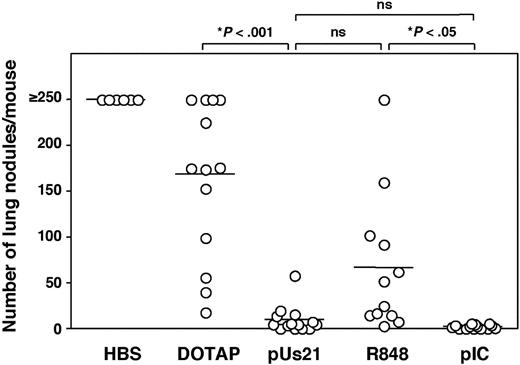
To evaluate the adjuvant activity of the ssRNA TLR7 agonist polyUs21 versus the imidazoquinoline R848 for induction of antitumor immunity, we assessed them in a prophylactic vaccination regimen in a mouse model of lung metastases. Mice were given OVA plus different TLR agonists and were challenged intravenously with OVA-expressing B16 melanoma cells 30 days after vaccination after the primary response had waned. The development of lung nodules was determined 18 days after tumor challenge. Control mice that had been treated with OVA and DOTAP in the absence of adjuvant were partially protected from tumor challenge, in contrast to mice injected with buffer alone (Figure 3). Mice vaccinated with OVA in combination with polyUs21-DOTAP complexes were completely protected from metastases as were mice that received OVA in combination with polyI:C (Figure 3). Mice vaccinated with a combination of OVA and R848 were only partially protected from tumor growth. Thus, although R848 can boost the induction of antitumor immunity, ssRNA agonists for TLR7 are more effective as adjuvants for generation of a protective antitumor immune response. The fact that any tumor protection is seen in R848-treated mice is surprising in view of the poor CTL induction. However, elimination of tumor cells in OVA plus R848-treated mice could be mediated by classes of cytotoxic effector cells other than CTL such as natural killer cells or cytotoxic DC.29,30 Future studies will have to clarify the mechanisms by which these different TLR7 agonists lead to the killing of tumor cells.
Prophylactic vaccination with polyUs21 prevents tumor growth. C57BL/6 mice were challenged intravenously with B14.3 melanoma cells 30 days after vaccination with OVA in combination with the indicated TLR agonists. Control mice were injected with HEPES-buffered saline or with a mixture of OVA and DOTAP. The tumor burden was determined 18 days after tumor challenge. Each dot represents an individual house. Pooled data from at least 3 independent experiments are shown.
Prophylactic vaccination with polyUs21 prevents tumor growth. C57BL/6 mice were challenged intravenously with B14.3 melanoma cells 30 days after vaccination with OVA in combination with the indicated TLR agonists. Control mice were injected with HEPES-buffered saline or with a mixture of OVA and DOTAP. The tumor burden was determined 18 days after tumor challenge. Each dot represents an individual house. Pooled data from at least 3 independent experiments are shown.
The adjuvant activity of polyUs21 is TLR7-dependent
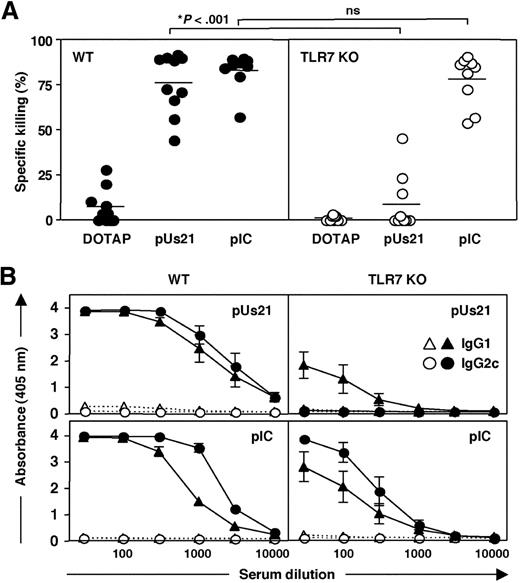
To determine whether the adjuvant activity of polyUs21 is exclusively mediated via TLR7, we evaluated the in vivo CTL killing responses in TLR7-deficient (TLR7 KO) mice. The CTL response in polyI:C-treated TLR7 KO mice was undiminished (Figure 4A). In contrast, the level of antigen-specific target cell killing in TLR7 KO mice immunized with polyUs21-DOTAP complexes was reduced to background levels, indicating that the adjuvant activity of polyUs21 is TLR7-dependent (Figure 4A). Similarly, the generation of antigen specific antibodies in response to polyUs21 as adjuvant was strongly inhibited in TLR7 KO mice, whereas antibody production in polyI:C-treated TLR7 KO mice was only marginally reduced (Figure 4B). Surprisingly, low IgG1 antibody titers greater than the levels seen for OVA plus DOTAP-treated control mice were still detected in TLR7 KO, indicating the presence of residual TLR7-independent Th2-skewing adjuvant activity.
Adaptive immune responses induced by immunization with polyUs21 are TLR7 dependent. (A) C57BL/6 (●) and TLR7 KO (○) mice were immunized with a combination of OVA and the indicated TLR agonists. PolyUs21 was administered in form of complexes with DOTAP, and control mice received a combination of OVA and DOTAP. In vivo CTL assays were performed 7 days after immunization. Data are a pooled from 4 independent experiments. (B) OVA-specific serum titers of IgG1 and IgG2c antibodies were determined by ELISA in wild-type and TLR7 KO mice immunized with (solid lines) or without (dotted lines) TLR agonists. Serum dilution curves represent the compiled data of 3 independent experiments with the SEM indicated.
Adaptive immune responses induced by immunization with polyUs21 are TLR7 dependent. (A) C57BL/6 (●) and TLR7 KO (○) mice were immunized with a combination of OVA and the indicated TLR agonists. PolyUs21 was administered in form of complexes with DOTAP, and control mice received a combination of OVA and DOTAP. In vivo CTL assays were performed 7 days after immunization. Data are a pooled from 4 independent experiments. (B) OVA-specific serum titers of IgG1 and IgG2c antibodies were determined by ELISA in wild-type and TLR7 KO mice immunized with (solid lines) or without (dotted lines) TLR agonists. Serum dilution curves represent the compiled data of 3 independent experiments with the SEM indicated.
The difference in adjuvant activity between polyUs21 and R848 is caused by differences in type I IFN induction
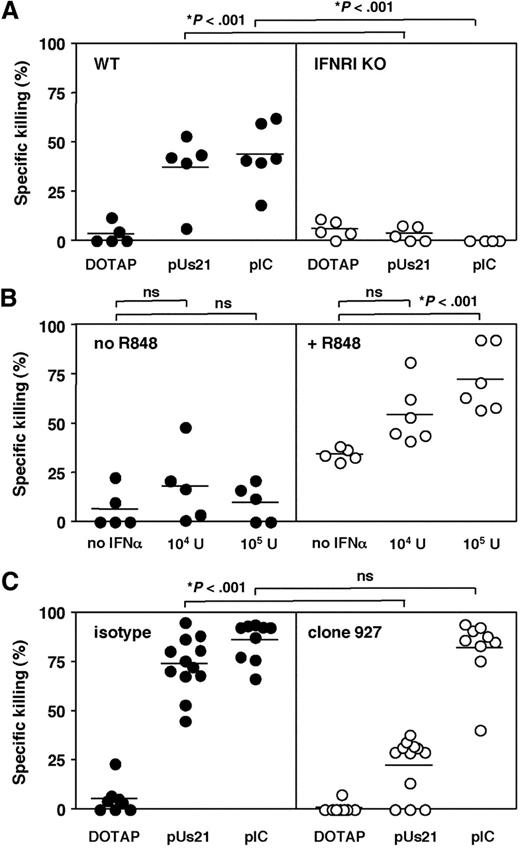
The results presented thus far provide evidence that ssRNA agonists are effective adjuvants whereas imidazoquinolines are less potent upon systemic administration. On the basis of the DC-derived cytokine induction patterns observed in vitro23 and in vivo (Figure 1), we hypothesized that differences in the ability to induce type I IFN (IFN-I) could be one of the factors that determine the adjuvanticity of TLR7 agonists. To test this hypothesis the induction of CTL responses was assessed in IFN-I receptor–deficient (IFNRI KO) mice. Interestingly, antigen-specific killing of target cells observed in 129 Sv wild-type mice was completely abolished in IFNRI KO mice irrespective of whether polyUs21 or polyI:C was used as adjuvant (Figure 5A). This finding indicates that signaling through the IFN-I receptor is indeed crucial for CTL priming in vivo in response to immunization with antigen plus TLR agonists. IFN-I acts on a variety of cell types and promotes cross-priming of CTL via direct and indirect mechanisms.31,32 Furthermore, the failure to mount antiviral CTL responses in IFNRI KO mice has been attributed to defects in the survival of antigen-specific CD8 T cells upon priming.33
CTL responses to polyUs21 are dependent on signaling through IFNRI. (A) 129 Sv mice (●) and IFNRI KO (○) were vaccinated with egg white preparation as a source of OVA in combination with the indicated TLR agonists. In vivo CTL killing assays were performed at day 7 after vaccination. Data are pooled from 2 independent experiments. (B) C57BL/6 mice were treated with egg white preparation in combination with IFN-α (104 or 105 U/mouse) in either the absence (●) or presence (○) of 100 μg of R848. On day 7 after vaccination, the in vivo CTL killing assay was performed. Data show the mean ± SEM from 3 independent experiments. (C) C57BL/6 mice were treated with PDC-depleting (○; clone 927) or isotype control antibody (●). Upon depletion of PDC, mice were vaccinated with OVA in combination with the indicated TLR agonists. The in vivo CTL killing assay was performed at day 7 after vaccination. The graph contains pooled data from 3 independent experiments with the SEM indicated.
CTL responses to polyUs21 are dependent on signaling through IFNRI. (A) 129 Sv mice (●) and IFNRI KO (○) were vaccinated with egg white preparation as a source of OVA in combination with the indicated TLR agonists. In vivo CTL killing assays were performed at day 7 after vaccination. Data are pooled from 2 independent experiments. (B) C57BL/6 mice were treated with egg white preparation in combination with IFN-α (104 or 105 U/mouse) in either the absence (●) or presence (○) of 100 μg of R848. On day 7 after vaccination, the in vivo CTL killing assay was performed. Data show the mean ± SEM from 3 independent experiments. (C) C57BL/6 mice were treated with PDC-depleting (○; clone 927) or isotype control antibody (●). Upon depletion of PDC, mice were vaccinated with OVA in combination with the indicated TLR agonists. The in vivo CTL killing assay was performed at day 7 after vaccination. The graph contains pooled data from 3 independent experiments with the SEM indicated.
Because R848 was found to be ineffective in inducing detectable levels of intracellular IFN-α in PDC we tested whether coinjection of IFN-α with antigen and R848 enables CTL cross-priming. Administration of antigen alone or in combination with either IFN-α or R848 induced minimal to low antigen-specific killing responses (Figure 5B). In contrast, coadministration of IFN-α and R848 together with antigen enhanced OVA-specific CTL activity (Figure 5B). The amplification of CTL induction by IFN-α was dose dependent, and administration of 105 U of IFN-α per mouse significantly increased CTL activity (Figure 5B). These results indicate that exogenous addition of IFN-α during priming can compensate for the inability of R848 to induce sufficient levels of this cytokine.
IFN-α induced in response to TLR7-mediated activation is exclusively PDC derived (Figure 1). We, therefore, examined the effect of PDC depletion on the induction of CTL responses in polyUs21-treated mice. Depletion of PDCs was carried out by the use of a PDC-depleting antibody (clone 927) and confirmed by flow cytometry on day 1 and 3 after immunization (data not shown). PDC-depleted mice showed a significant reduction in the level of antigen-specific killing compared with mice treated with isotype control antibody (Figure 5C). The ability of polyI:C to boost CTL priming was found to be unaltered by PDC depletion, in accordance with the ability of polyI:C to induce IFN-I in cell types other than PDC via TLR3-dependent and -independent mechanisms.3 In contrast, for TLR7-targeting vaccines, PDC activation is a key component of the immune response.
Discussion
Adjuvants are crucial for the induction of antigen-specific immune responses and determine the qualitative and quantitative phenotype of the adaptive immune response to a coadministered antigen. Therefore, the development of molecularly defined adjuvants such as TLR agonists for vaccine design will benefit from understanding the mechanisms of their adjuvanticity. In the present study, we qualitatively and mechanistically investigated the adjuvant activity of the ssRNA TLR7 agonist polyUs21 in comparison with the TLR7-activating imidazoquinoline R848 and the TLR3 agonist polyI:C. Our data demonstrate the potent adjuvanticity of polyUs21. When used in combination with antigen, polyUs21 promotes the induction of a Th1 response, CTL cross-priming, B-cell class switching, and is effective in the initiation of antitumor immunity. Our study indicates that the adjuvant activity of polyUs21 is dependent on PDC-derived IFN-I, suggesting that the development of TLR7 agonists as adjuvants needs to focus on the induction of a systemic IFN-I response in addition to the phenotypic and functional activation of professional antigen-presenting cells such as DC. Whether PDC are required solely for production of systemic IFN-I or also play a crucial role in antigen presentation and cross-priming of CTL in response to immunization with antigen and ssRNA TLR7 agonists is currently unclear and awaits further dissection of the underlying mechanisms of TLR7-mediated immunity induction.
This report is the first of a direct and systematic comparison of imidazoquinolines versus ssRNA TLR7 agonists with regard to quantitative and qualitative differences in innate immune activation and adjuvanticity upon systemic administration in vivo. In previous studies,11,12,34-37 imidazoquinolines such as R848 have been shown to induce IFN-I in vitro and in vivo, which seems to contradict our finding that R848 is inefficient in inducing IFN-α. However, there are differences in the frequency of DCs between different mouse strains that affect the serum levels of IFN-I that are observed upon TLR7 stimulation, and C57BL/6 mice as used in this study have a low frequency of these IFN-producing cells.38 Another contributing factor that may lead to discrepancies in IFN-I induction between different studies is the sensitivity of different IFN-α–detecting methods, which varies widely. Although the bioassay used in many of these previous studies is the most sensitive method for IFN-I detection,11,34,37 detection of IFN-α by ELISA is less robust, in particular for serum samples,39 and detection by intracellular staining requires high levels of IFN-α to be present in the cells. For the present study, we also tried to quantify IFN-α by ELISA, yet serum levels of IFN-α were very low to undetectable in all mice, including polyI:C- and polyUs21-treated mice, underlining the insufficient sensitivity of this method. Thus, R848 may induce low levels of this cytokine, which fall below the detection threshold of the intracellular cytokine staining in our experiments. In accordance with this interpretation of the data, we reported in a previous in vitro study that R848 induces 30 times less IFN-α than polyUs21.23
Although IFN-α was not detected in response to systemic administration of R848 in our study, we observed clear innate immune activation of various DC subsets, including PDCs on the level of IL-12 p40 induction and up-regulation of costimulatory molecules. The frequency of IL-12 p40-producing DCs was even greater for R848- than for polyUs21-treated mice. Nevertheless, innate immune activation induced by R848 provided insufficient adjuvant activity as demonstrated by its failure in inducing CTL responses and its weak induction of T helper cells and antibody production. R848 also induced partial protection from tumor challenge, underlining that it is not entirely devoid of adjuvant activity but that it compares unfavorably with the nucleic acid TLR7 agonist polyUs21 and the dsRNA mimic polyI:C. Although imidazoquinolines such as R848 have been shown to be poor adjuvants,40-42 this study shows for the first time that the nucleic acid TLR7 agonist polyUs21 has potent adjuvant activity upon systemic administration, which is comparable with the adjuvanticity of the viral dsRNA mimic polyI:C.
A recent study15 in which the authors compared different TLR agonists has identified polyI:C to be the most effective adjuvant for induction of Th1 responses. The study demonstrates that polyI:C induces greater levels of IFN-I than topically applied R848 and that the adjuvant activity of polyI:C is crucially dependent on IFN-I induction.15 Our data support the conclusion that the induction of IFN-I is crucial for adjuvant-induced induction of Th1 responses and CTL cross-priming. Although IFN-I in response to polyI:C is produced by cells of hematopoietic and nonhematopoietic origin,15 PDCs are the exclusive source of TLR7 agonist-induced IFN-I. Our study demonstrates that the ssRNA TLR7 agonist polyUs21, in contrast to R848, is a potent inducer of PDC-derived IFN-I upon intravenous administration and that its adjuvant activity is similar to that of polyI:C. Thus, ssRNA TLR7 agonists hold great potential to be developed as adjuvants in parallel to polyI:C. Because there are fundamental differences in the cell types that directly respond to polyUs21 versus polyI:C and in the signaling pathways that are triggered, the application of these adjuvants could be complementary allowing for synergy of the different innate activation pathways.43
For the development of TLR7 agonists such as polyUs21 as adjuvants, several technical aspects have to be considered and potentially improved. The stability of ssRNA oligonuceotides is much lower than that of DNA oligonucleotides and even though the introduction of phosphorothioate bonds decreases their susceptible to degradation, polycationic compounds such as DOTAP are still required to ensure efficient delivery and TLR7 activation. However, additional modifications of the ssRNA oligonucleotides that increase their stability allow for their use in the absence of nucleic acid-condensing reagents in vivo.44 Alternatively, the development and optimization of new compounds for binding and condensation of ssRNA TLR7 agonists could improve their adjuvanticity for in vivo application. When developing new reagents for complexing ssRNA TLR7 agonists, it also has to be taken into account that complex formation between immunostimulatory nucleic acids and cationic compounds has been shown to influence endosomal maturation and, thereby, affects TLR-mediated signaling and cytokine induction.45,46
The translation of findings on TLR7-mediated responses from the mouse model to the human system is complicated by the fact that murine TLR8 is nonfunctional or does not contribute to immune activation by ssRNA agonists and imidazoquinolines under the usual experimental conditions.13,47 In contrast to the mouse model in which ssRNA-mediated immune activation is restricted to TLR7, in humans TLR8 has an overlapping yet distinct sensitivity. Both R848 and ssRNA oligonucleotides, including polyUs21, are agonists for TLR7 and TLR8 in the human system.23 Taking this into account, it is very likely that ssRNA TLR agonists such as polyUs21 also act as potent adjuvants in the human system.
In summary, our data provide evidence that differences in the innate cytokine induction pattern between different classes of TLR7 agonists affect their ability to act as adjuvants. Our results point out the crucial role of PDC-derived IFN-I in skewing and boosting adaptive immunity in response to TLR7 agonists and demonstrate that polyUs21 is a potent TLR7-dependent adjuvant with the capacity to prime effective CTL responses. We conclude that polyUs21 and similar immunostimulatory ssRNA oligonucleotides represent potent candidates for the development of adjuvants for tumor immunotherapy and for vaccination against pathogens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Marco Colonna for 927 hybridoma cells and Roman Spörri for advice on the detection of OVA-specific antibodies. We also thank Amy Lewis, Martin Kreutz, Benoit Giquel, Claudia Kemper, and Caetano Reis e Sousa for helpful discussions and critical review of the manuscript.
This work was funded by a Cancer Research UK Career Development Award to S.S.D.; D.R. was funded by Innate Pharma.
Authorship
Contribution: D.R. and S.S.D. designed and performed experiments, analyzed data and generated the figures; C.P. and Y.M. provided supporting data and advised on study design and manuscript layout; S.U. and S.A. contributed genetically modified mice; and S.S.D. wrote and edited the manuscript.
Conflict-of-interest disclosure: C.P. and Y.M. are employees of Innate Pharma, which has a financial interest in TLR7 agonists. S.S.D. and C.P. are inventors on a patent application relating to TLR7 agonists, which has been licensed to Innate Pharma from Cancer Research Technology. The remaining authors declare no competing financial interests.
Correspondence: Sandra S. Diebold, Peter Gorer Department of Immunobiology, King's College London, Guy's, King's and St Thomas' Medical School, 2nd Floor Borough Wing, Guy's Hospital, London SE1 9RT, United Kingdom; e-mail: sandra.diebold@kcl.ac.uk.