Abstract
T lymphopoiesis requires settling of the thymus by bone marrow–derived precursors throughout adult life. Progenitor entry into the thymus is selective, but the molecular basis of this selectivity is incompletely understood. The chemokine receptor CCR9 has been demonstrated to be important in this process. However, progenitors lacking CCR9 can still enter the thymus, suggesting a role for additional molecules. Here we report that the chemokine receptor CCR7 is also required for efficient thymic settling. CCR7 is selectively expressed on bone marrow progenitors previously shown to have the capacity to settle the thymus, and CCR7−/− progenitors are defective in settling the thymus. We further demonstrate that CCR7 sustains thymic settling in the absence of CCR9. Mice deficient for both CCR7 and CCR9 have severe reductions in the number of early thymic progenitors, and in competitive assays CCR7−/−CCR9−/− double knockout progenitors are almost completely restricted from thymic settling. However, these mice possess near-normal thymic cellularity. Compensatory expansion of intrathymic populations can account for at least a part of this recovery. Together our results illustrate the critical role of chemokine receptor signaling in thymic settling and help to clarify the cellular identity of the physiologic thymic settling progenitors.
Introduction
All blood lineages are derived from hematopoietic stem cells (HSCs) in the bone marrow (BM). Unlike other blood lineages, T cells continue the majority of their development outside the BM, in the thymus. As the thymus does not contain self-renewing progenitors, it must import BM-derived precursors during adult life.1-5 This process can be regarded as 3 steps: generation of T-lineage progenitors in the BM, mobilization of progenitors out of the BM into the blood, and settling of blood-borne progenitors into the thymus. Thymic settling progenitors (TSPs) have not yet been definitively identified due to their presumed rarity.6-9 After thymic settling, TSPs generate Lineage-marker (Lin)–negative, Kit+CD25– early thymic progenitors (ETPs), which constitute the earliest defined T-cell precursor population within the thymus.4,10 ETPs in turn undergo proliferative expansion to give rise to CD4–CD8–Kit+CD25+ double-negative 2 (DN2) and CD4–CD8–KitloCD25+ DN3 cells. DN3 cells undergo additional proliferation before differentiating into CD4+CD8+ double-positive (DP) cells, which constitute the majority of thymocytes. DP thymocytes subsequently undergo T-cell receptor–dependent selection to generate CD4 or CD8 single-positive (SP) cells, which emigrate from the thymus to populate the periphery.11
The BM contains multiple progenitors with T-lineage potential that may contribute to T lymphopoiesis.12-15 The most primitive hematopoietic progenitors in the BM have a Lin−Sca1+Kit+ (LSK) phenotype and can be differentiated into subsets on the basis of expression of the cytokine receptor Flt3. These subsets include multipotent and self-renewing HSCs (LSKFlt3−), multipotent progenitors (MPPs), which do not possess self-renewal capacity (LSKFlt3lo),16 and lymphoid-primed multipotent progenitors (LMPPs; LSKFlt3hi).17 LMPPs overlap extensively with early lymphoid progenitors that express mRNA for several lymphoid-specific genes including Rag, Tdt, and Il-7rα.18,19 More differentiated BM progenitors include common lymphoid progenitors (CLPs; Lin−KitloSca1loFlt3hiIL-7RαhiB220−)13 and more downstream CLP-2s that are phenotypically similar to CLPs but express B220,20-22 both of which can generate T cells. In addition, circulating T lineage–committed progenitors have been identified.23 Hence, many progenitors in BM and blood have the potential to contribute to T-cell generation.
In addition to T-lineage potential, BM progenitors must have the ability to settle the thymus to contribute to physiologic T-cell development.24 We have recently shown that thymic settling is selective, with HSCs and MPPs unable to settle the adult thymus.13 Downstream LMPPs and CLPs likely acquire the ability to settle the thymus through the expression of necessary homing molecules.13,21,25 It appears probable that progenitor entry into the thymus is analogous to mature lymphocyte entry into lymph nodes, during which cells use selectins, chemokine receptors, and integrins to arrest at the endothelial wall and extravasate into target organs.21,26 Recent work has begun to identify molecules that regulate progenitor entry into the thymus. Multiple groups have reported that the chemokine CCL25 and its receptor CCR9 are involved in this process.13,25,27-30 CCR9 is selectively expressed on subsets of LMPPs and CLPs,13 and CCR9+ LMPPs have been shown to directly home to sublethally irradiated thymi.25 In addition, the selectin/ligand pair P-selectin/PSGL-1 has also been implicated in thymic settling.31,32 Yet BM progenitors lacking these molecules show relatively modest defects in T lymphopoiesis even when competitive assays were used.13,31 These data indicate that other molecules are likely to contribute to thymic settling, and may account for its selectivity. Indeed, the chemokine receptor CCR7 has been demonstrated to recruit progenitors to the fetal thymus.33 Mice doubly deficient for both CCR7 and CCR9 have severely reduced fetal thymic cellularity as late at embryonic day 16.5, but by postnatal day 1 cellularity is normal.28 These data were interpreted to mean that CCR7 and CCR9 are involved in embryonic thymic settling of progenitors, but that their involvement ceases in the late embryonic period.
In this study, we investigated whether CCR7 mediates thymic homing of BM-derived progenitors in adult mice. We found that CCR7 is selectively expressed on progenitor populations downstream of HSCs and MPPs, corresponding with the acquisition of thymic settling capacity, and recruits progenitors into the adult thymus. We generated CCR7/CCR9 double knockout (DKO) mice and found that they had severely reduced numbers of ETPs. In addition, CCR7/CCR9 DKO progenitors were almost completely defective at thymic settling in competitive assays. Despite these defects, the CCR7/CCR9 DKO thymus possessed near-normal total thymic cellularity, suggesting that the thymus compensates for reduced progenitor settling. Supporting this hypothesis, progenitors intrathymically injected into CCR7/CCR9 DKO mice were able to undergo compensatory population expansion. Together, these data identify the critical role of CCR7 and CCR9 in thymic settling in adult mice. Furthermore, our results demonstrate the remarkable ability of the thymus to maintain near-normal size despite highly impaired settling by progenitors.
Methods
Mice
C57Bl/6 and B6.Ly5SJL were purchased from the National Cancer Institute animal facility or The Jackson Laboratory. CCR7−/− mice were purchased from The Jackson Laboratory. CCR9−/− mice were a gift of Dr Paul Love (National Institutes of Health [NIH]). CCR7−/−CCR9−/− mice were generated by crossing these 2 strains. Mice used were 4 to 10 weeks old. Live animal experiments were performed according to approved protocols of the Office of Regulatory Affairs at the University of Pennsylvania in accordance with NIH guidelines.
Cell preparations, flow cytometry, and cell sorting
BM and thymocytes were prepared as previously described.13 Cell preparations were stained with optimized antibody (Ab) dilutions. Abs in the Lin cocktail included anti-B220 (RA3-6B2), anti-CD19 (1D3), anti-CD11b (M1/70), anti-Gr-1 (8C5), anti-CD11c (HL3), anti-NK1.1 (PK136), anti–Ter-119 (Ter-119), anti-CD3 (2C11), anti-CD8α (53.6-7), anti-CD8β (53-5.8), anti-TCRβ (H57), and anti-γδTCR (GL-3). Additional Abs used included anti-CD45.2 (104), anti-CD45.1 (A20), anti-Kit (2B8), anti-Sca1 (D7), anti-CD4 (GK1.5), anti-Flt3 (A2F10), anti-IL7Rα (A7R34), anti-CD25 (PC61.5), and anti-CCR9 (CW-1.2). For CCR7 staining, a CCL19-Fc fusion protein (eBioscience) was used, followed by allophycocyanin-conjugated anti-Fc (Jackson ImmunoResearch). Abs were purchased from eBioscience or BD Pharmingen except for anti-CD45.2 Pacific Blue (Biolegend).
For cell sorting, BM was first enriched for progenitors, then stained and sorted on a FACSAria (BD Biosciences). Aliquots of sorted cells were reanalyzed to ensure purity, which was usually greater than 90%. An LSRII (BD Biosciences) was used for fluorescence-activated cell sorting (FACS) analysis. Dead cells were excluded through 4,6 diamidino-2-phenylindole uptake. Doublets were excluded through forward scatter–height by forward scatter–width and side scatter–height by side scatter–width parameters. Data were analyzed using FlowJo software (TreeStar).
Chemotaxis transwell assays
BM was harvested and cultured at 37° for 90 minutes to allow for surface recycling of internalized chemokine receptors and to remove adherent cells. Cells were placed in the top well of chemotaxis plates (Corning), with chemokine in the bottom well. After 2 hours at 37°, cells in the bottom wells were collected, stained, and analyzed by FACS.
Intravenous and intrathymic transfers
For intravenous transfers, 2 to 3 × 107 T cell–depleted BM cells were injected retro-orbitally. To prevent rejection, mice were given 0.1 mg of anti-CD4 (GK1.5) the day before BM transfer and every 4 days thereafter. Previous work by our laboratory has shown that this treatment does not affect BM or thymic engraftment by donor cells.13 For intrathymic adoptive transfer studies, 106 unfractionated BM or 2 to 3 × 103 sorted LMPPs (defined as BM Lin−Sca1+Kit+Flt3hi cells) were injected intrathymically as previously described.2 To generate mixed BM chimeras, host wild-type (WT) mice (expressing CD45.1) were lethally irradiated (9.5 Gy) and subsequently injected with T cell–depleted host-type and WT or knockout BM (expressing CD45.2) at a 1:3 ratio. T cells were depleted from BM by incubation with anti-CD4 and anti-CD8α Abs followed by removal of Ab-bound cells with magnetic beads (QIAGEN).
Real-time PCR
RNA was prepared from sorted populations using the RNeasy kit (QIAGEN). cDNA was generated using the Superscript II kit (Invitrogen). Real-time polymerase chain reaction (PCR) was performed using TaqMan Universal PCR Master Mix and primer/probe mixtures for Ccr7 and 18s (Applied Biosystems) and analyzed on an ABI Prism 7900 (Applied Biosystems). Relative expression levels were normalized using 18s transcript levels and calculated using the 2–ΔΔCT method.
OP9-DL1 cultures
Sorted LMPPs or ETPs (defined as Lin−CD25−Kit+ thymocytes) from WT or CCR7/CCR9 DKO were placed onto an OP9-DL1 stromal layer (gift of Juan C. Zúñiga-Pflücker, University of Toronto) as previously described.34
Cell cycle analysis
Cells were incubated at 106/mL in RPMI 1640 media with 10% fetal calf serum and 20 μg/mL Hoechst 33342 dye (Invitrogen) for 30 minutes at 37°. After this incubation, cells were washed twice in FACS buffer (phosphate-buffered saline with 0.1% bovine serum albumin and 0.1% sodium azide) followed by surface antigen staining and FACS analysis.
Apoptosis analysis
Thymocytes were collected and stained for surface antigens followed by a wash. Annexin V–phycoerythrin (BD Pharmingen) staining was then conducted as per the manufacturer's instructions.
Statistics
P values were calculated using Microsoft Excel by Student t test.
Results
CCR7 is up-regulated on progenitors downstream of HSCs
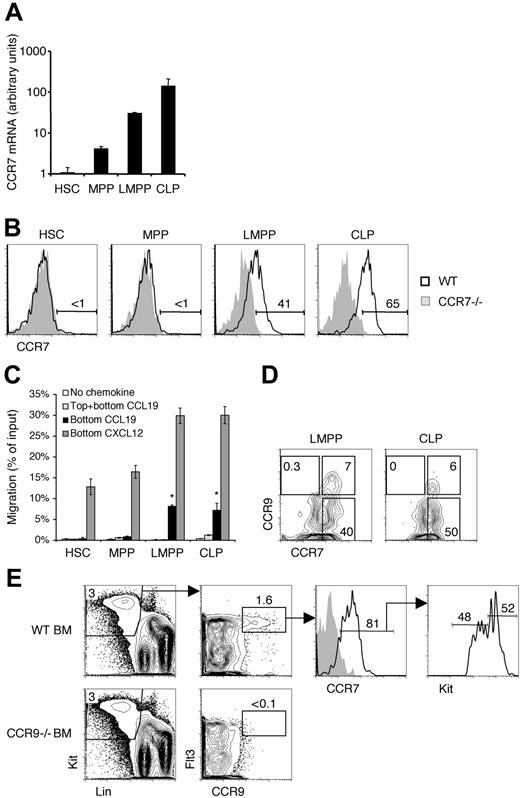
Our studies intended to determine whether CCR7 supports the thymic settling of BM-derived progenitors. Prior work has established a role for CCR7 in thymic settling during the embryonic period before thymic vascularization.28 CCR7 has 2 known ligands, CCL19 and CCL21. Both of these ligands are expressed in the adult thymus by medullary epithelial cells, and CCL19 is additionally associated with the endothelium.33,35-37 We first examined BM progenitor populations to identify whether this receptor was expressed. We found that HSCs had the lowest level of Ccr7 mRNA expression, and Ccr7 levels increased as progenitors matured through the MPP, LMPP, and CLP stages successively (Figure 1A). Cell surface staining revealed CCR7 protein expression on LMPPs and CLPs, but not on more primitive HSCs and MPPs (Figure 1B). Hence, those progenitors previously suggested to be able to settle the thymus from blood express CCR7 on the cell surface.13
Functional CCR7 is selectively expressed by bone marrow progenitors. (A) The indicated populations were sorted from WT mice and cDNA was prepared. Real-time PCR was then used to quantify the relative levels of CCR7 transcripts. Shown is the mean ± SEM for 3 independent experiments. (B) Bone marrow (BM) from wild-type (WT) and CCR7−/− mice were analyzed by flow cytometry for CCR7 expression on the indicated populations. Numbers represent the percentage of WT cells in the indicated gate. (C) For chemotaxis assays, WT BM cells were added to transwells with the indicated chemokines in the bottom and/or top wells. After a 2-hour incubation, the migrated cells were collected and quantified by flow cytometry. Shown is the mean ± SEM for data pooled from 3 experiments. *P < .05 for the indicated condition compared with the no-chemokine control condition. (D) BM from WT mice was analyzed for the coexpression of CCR7 and CCR9. Shown are cells previously gated as LMPPs (left panel) and CLPs (right panel). The gates showing CCR7−CCR9+, CCR7+CCR9+, and CCR7+CCR9− subpopulations were placed according to CCR7−/− and CCR9−/− controls (data not shown). (E) WT and CCR9−/− BM were analyzed by flow cytometry for CCR7 and CCR9 coexpression. The gray histogram in the third column represents the CCR7−/− control.
Functional CCR7 is selectively expressed by bone marrow progenitors. (A) The indicated populations were sorted from WT mice and cDNA was prepared. Real-time PCR was then used to quantify the relative levels of CCR7 transcripts. Shown is the mean ± SEM for 3 independent experiments. (B) Bone marrow (BM) from wild-type (WT) and CCR7−/− mice were analyzed by flow cytometry for CCR7 expression on the indicated populations. Numbers represent the percentage of WT cells in the indicated gate. (C) For chemotaxis assays, WT BM cells were added to transwells with the indicated chemokines in the bottom and/or top wells. After a 2-hour incubation, the migrated cells were collected and quantified by flow cytometry. Shown is the mean ± SEM for data pooled from 3 experiments. *P < .05 for the indicated condition compared with the no-chemokine control condition. (D) BM from WT mice was analyzed for the coexpression of CCR7 and CCR9. Shown are cells previously gated as LMPPs (left panel) and CLPs (right panel). The gates showing CCR7−CCR9+, CCR7+CCR9+, and CCR7+CCR9− subpopulations were placed according to CCR7−/− and CCR9−/− controls (data not shown). (E) WT and CCR9−/− BM were analyzed by flow cytometry for CCR7 and CCR9 coexpression. The gray histogram in the third column represents the CCR7−/− control.
We evaluated the functional status of CCR7 on BM progenitors through chemotaxis transwell assays. Whereas all populations were capable of migrating to a CXCL12 gradient (using the CXCR4 receptor), only LMPPs and CLPs chemotaxed to a CCL19 gradient (Figure 1C). To distinguish chemokinesis (random migratory behavior in response to chemokine) from chemotaxis (directional migration toward a chemokine gradient), CCL19 was placed in both the top and bottom wells. Neither LMPPs nor CLPs migrated into the bottom well in this case, confirming that these cells indeed were undergoing chemotaxis in response to CCL19. Together these data show that CCR7 is selectively expressed and functional only on LMPPs and CLPs, but not on more primitive HSCs or MPPs. Therefore CCR7 expression correlates with thymic settling capacity among early BM progenitors.
Previous work has reported a role for CCR9 in thymic settling of progenitors.13,25,27-30 Like CCR7, CCR9 is expressed on subsets of LMPPs and CLPs, but not on upstream HSCs or MPPs.13 The CCR9 on these cells is functional as demonstrated through chemotaxis assays (data not shown).21,25 We therefore asked whether CCR7 and CCR9 were coexpressed by BM progenitors on the cell surface. We found that whereas many CLPs and LMPPs express CCR7, only rare subsets of these CCR7+ cells also coexpress CCR9 (Figure 1D). We also identified CCR7+CCR9+ progenitors through an alternative gating strategy designed to be more inclusive than the LMPP and CLP gating scheme. BM cells within the Lin−Flt3hi pool possess the ability to efficiently and rapidly reconstitute the thymus after intravenous transfer.13,15 We identified a rare population (0.05% of total BM) of CCR9+ cells within this pool, the majority of which also expressed CCR7 (Figure 1E). CCR9+CCR7+ progenitors were heterogeneous for Kit expression, including both Kithi LMPPs and a Kitlo population that contained CLPs. Together these data indicate that CCR7+ progenitors include LMPPs and CLPs, and a small fraction of these coexpress CCR9.
A role for CCR7 in thymic settling
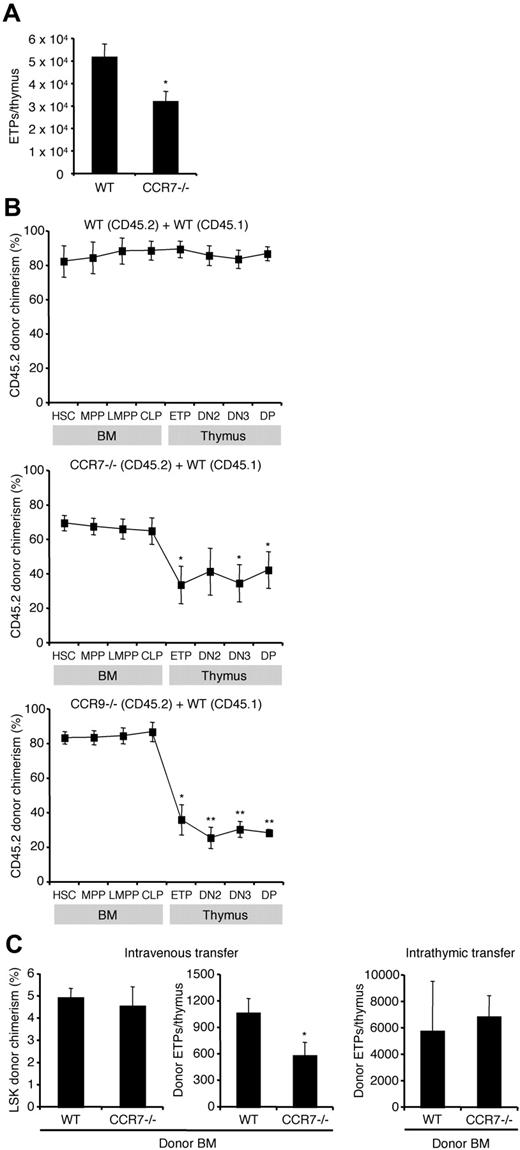
After establishing that CCR7 is expressed on BM progenitors with known T-lineage potential, we asked whether this molecule was important for early steps in T-cell development. ETPs are the earliest defined T-lineage progenitors within the thymus.4,10 Previous work has shown that mice lacking molecules involved in thymic settling have fewer ETPs.13,31 We examined CCR7−/− mice and found that BM progenitor frequencies and thymic cellularity were normal (data not shown), consistent with previous reports.28 However, they possessed slightly fewer ETPs than WT mice (Figure 2A), which could suggest a thymic settling defect. Yet ETP number may not directly correlate with thymic settling efficiency. To better examine the role of CCR7, we sought to evaluate thymic settling using assays in which the CCR7−/− cells are in competition with WT cells.
CCR7 mediates thymic settling by T-lineage progenitors. (A) Thymi from WT and CCR7−/− mice were analyzed by flow cytometry to calculate absolute numbers of ETPs. Absolute numbers were obtained by multiplying the frequency of ETPs among live singlets by total thymic cellularity. Shown is the mean ± SEM for 21 WT (8 males, 13 females) and 18 CCR7−/− (7 males, 11 females) mice all between the ages of 4 and 9 weeks. These data were pooled from 4 separate experiments, but for each experiment the mice were age and sex matched. *P < .05 compared with the WT control. (B) Mixed BM chimeras were generated using CD45.2 WT BM and CD45.1 WT BM mixtures as controls (top panel), CD45.2 CCR7−/− BM and CD45.1 WT BM mixtures (middle panel), or CD45.2 CCR9−/− BM and CD45.1 WT BM mixtures (bottom panel). Chimeras were analyzed by flow cytometry after 10 weeks using antibodies to CD45.1 and CD45.2 to determine donor chimerism. Shown is the mean CD45.2 donor chimerism ± SEM for each indicated population. *P < .05 and **P < .01 for the CD45.2 donor chimerism of the indicated population compared with HSC CD45.2 donor chimerism. (C) For adoptive transfer experiments, BM from either WT or CCR7−/− mice (both CD45.2) was administered intravenously (left 2 panels) or intrathymically (right panel) to unirradiated WT recipient mice (CD45.1). After 2 weeks (intrathymic transfer) or 3 weeks (intravenous transfer), donor-derived cells were quantified by flow cytometry. Results show the mean ± SEM for 5 recipients in each group. *P < .05 compared with the WT control.
CCR7 mediates thymic settling by T-lineage progenitors. (A) Thymi from WT and CCR7−/− mice were analyzed by flow cytometry to calculate absolute numbers of ETPs. Absolute numbers were obtained by multiplying the frequency of ETPs among live singlets by total thymic cellularity. Shown is the mean ± SEM for 21 WT (8 males, 13 females) and 18 CCR7−/− (7 males, 11 females) mice all between the ages of 4 and 9 weeks. These data were pooled from 4 separate experiments, but for each experiment the mice were age and sex matched. *P < .05 compared with the WT control. (B) Mixed BM chimeras were generated using CD45.2 WT BM and CD45.1 WT BM mixtures as controls (top panel), CD45.2 CCR7−/− BM and CD45.1 WT BM mixtures (middle panel), or CD45.2 CCR9−/− BM and CD45.1 WT BM mixtures (bottom panel). Chimeras were analyzed by flow cytometry after 10 weeks using antibodies to CD45.1 and CD45.2 to determine donor chimerism. Shown is the mean CD45.2 donor chimerism ± SEM for each indicated population. *P < .05 and **P < .01 for the CD45.2 donor chimerism of the indicated population compared with HSC CD45.2 donor chimerism. (C) For adoptive transfer experiments, BM from either WT or CCR7−/− mice (both CD45.2) was administered intravenously (left 2 panels) or intrathymically (right panel) to unirradiated WT recipient mice (CD45.1). After 2 weeks (intrathymic transfer) or 3 weeks (intravenous transfer), donor-derived cells were quantified by flow cytometry. Results show the mean ± SEM for 5 recipients in each group. *P < .05 compared with the WT control.
We first generated competitive BM chimeras by intravenously transferring both CCR7−/− BM (CD45.2) and WT BM (CD45.1) at a 3:1 ratio into lethally irradiated WT hosts. As a control, we injected mixtures of CD45.2 WT BM and CD45.1 WT BM. After waiting 10 weeks to allow hematopoietic reconstitution, BM and thymi were analyzed by FACS to determine the donor chimerism within defined progenitor populations (Figure 2B). CCR7−/− HSCs were able to engraft in the BM and differentiate into MPPs, LMPPs, and CLPs normally. Yet there was a significant reduction in CCR7−/− donor chimerism between BM populations and thymic ETPs, indicating that the absence of CCR7 conferred a disadvantage in the generation of ETPs (Figure 2B middle panel). This is consistent with a role in thymic settling for CCR7. The unchanged CCR7−/− donor chimerism among intrathymic populations indicates that the absence of CCR7 was not disadvantageous toward development between the ETP and DP stages. In addition, control chimeras made with CD45.2 CCR9−/− and CD45.1 WT BM confirmed that the absence of CCR9 confers a disadvantage on thymic settling (Figure 2B bottom panel), as expected from past work13,27,30 ; this disadvantage was greater than that seen with the loss of CCR7. Together these data establish a role for CCR7 in adult T-cell development at or upstream of the ETP stage, but not between the ETP and DP stages.
For the mixed BM chimeras we had used lethally irradiated recipient mice to ablate host hematopoietic cells. Yet irradiation generates nonphysiologic conditions, including an altered cytokine milieu in the BM and thymus and extramedullary hematopoiesis.13,38,39 In addition, the sites and kinetics of T-cell development are altered after irradiation.40 Therefore, we sought to complement the mixed BM chimera studies with short-term adoptive transfer assays using unirradiated hosts. We addressed the possibility of rejection of the injected cells, which can occur even as a result of allelic differences between CD45.1 and CD45.2,41 by depleting CD4+ T cells throughout the length of the experiment with anti-CD4 antibody.41 We used WT hosts, as expression of homing and adhesion molecules such as P-selectin and CCL25 on the thymic endothelium varies across different mutant strains of mice.32 WT or CCR7−/− BM cells (both CD45.2) were injected intravenously into unirradiated WT recipients (CD45.1). Three weeks later, donor-derived cells were quantified in the BM and thymus (Figure 2C). The donor-derived fraction of BM LSKs was equivalent in both recipient cohorts, indicating that CCR7−/− cells were neither rejected nor possessed a defect in BM engraftment (Figure 2C left panel). Yet CCR7−/− donor cells generated significantly fewer thymic ETPs than WT donor cells (Figure 2C middle panel). The generation of ETPs requires thymic settling of progenitors followed by Notch1-dependent intrathymic development.42,43 To distinguish which of these steps is defective for CCR7−/− cells, we intrathymically injected unfractionated CCR7−/− or WT BM into unirradiated WT hosts, thus bypassing the thymic settling step. CCR7−/− and WT progenitors were equally capable at generating ETPs (Figure 2C right panel), as well as downstream DN2 and DN3 cells (data not shown) after intrathymic transfer. This indicates that CCR7 is not required for early intrathymic development. To evaluate potential progenitor mobilization defects in CCR7−/− mice, we examined the rare LSK cells that can be found in blood.7 Circulating LSK cells were present at normal frequency in adult CCR7−/− mice (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). We also examined 1-week-old mice that possess higher frequencies of circulating progenitors, thus allowing for more accurate measurements. We again found that CCR7−/− mice possessed normal frequencies of circulating LSK cells (supplemental Figure 1B). These data suggest that the impaired ability of CCR7−/− progenitors to contribute to T lymphopoiesis is not due to defective mobilization of progenitors from the BM into the blood. Together these data demonstrate a requirement for CCR7 in efficient thymic settling.
CCR7/CCR9 DKO mice have a severe ETP defect
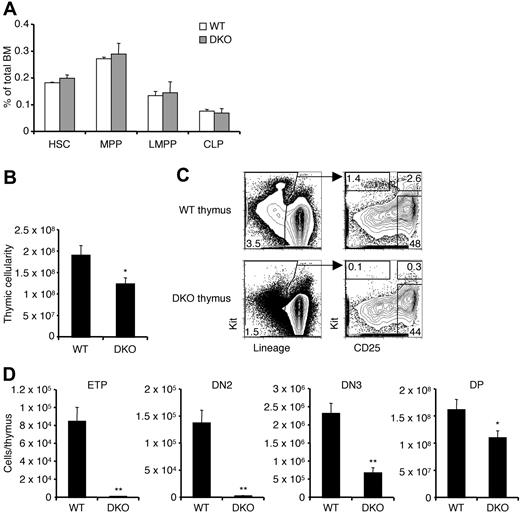
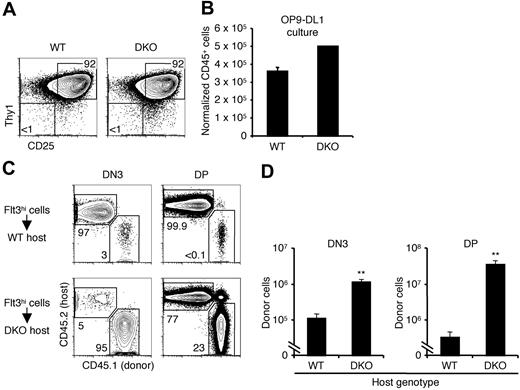
Previous reports have indicated a role for CCR9 in thymic settling and our studies now indicate that CCR7 too is involved in this process. To examine thymic settling in the absence of both receptors, we bred CCR7/CCR9 DKO mice. These mice have been previously generated and they produce mature T cells.28 A thymic settling defect was reported to exist in these mice only in the embryonic period before the thymus is vascularized.28 Analysis of early BM progenitors in adult CCR7/CCR9 DKO mice revealed no defect in the frequencies of these populations compared with WT controls (Figure 3A). The total thymic cellularity of CCR7/CCR9 DKO mice was modestly reduced by approximately 35% compared with WT controls (Figure 3B). However, the numbers of early intrathymic progenitors in CCR7/CCR9 DKO thymi were severely decreased (Figure 3C-D). Whereas the reduction in ETPs was approximately 100-fold, the magnitude of the defect was partially restored at later stages, becoming only 3-fold for DN3 cells, and less than 2-fold for DP cells. These results suggest that the CCR7/CCR9 DKO thymus is able to partially recover from reduced progenitor settling by the DN3 and DP stages. Such recovery has previously been observed in CCR9−/− mice13 and PSGL-1−/− mice,31 both of which have reduced ETPs but normal DN3 cell numbers. Compared with CCR9−/−13 or CCR7−/− (Figure 2A) single knockout mice, the CCR7/CCR9 DKO mice had more severe reductions in ETP and DN2 cell numbers, indicating that either receptor can sustain thymic settling in the absence of the other.
CCR7/CCR9 DKO mice have a severe ETP defect. (A) BM from WT and CCR7/CCR9 DKO mice were analyzed by flow cytometry to determine frequencies of the indicated progenitors. Shown is the mean ± SEM for 9 WT and 10 CCR7/CCR9 DKO mice. (B) Thymic cellularities from WT and CCR7/CCR9 DKO mice were calculated. Shown is the mean ± SEM. *P < .05 compared with the WT control. (C) WT and CCR7/CCR9 DKO thymi were analyzed by flow cytometry for thymic progenitors. Representative FACS profiles are shown. (D) Absolute numbers of thymic progenitor populations were calculated. Shown is the mean ± SEM. *P < .05 and **P < .01 compared with the corresponding WT controls.
CCR7/CCR9 DKO mice have a severe ETP defect. (A) BM from WT and CCR7/CCR9 DKO mice were analyzed by flow cytometry to determine frequencies of the indicated progenitors. Shown is the mean ± SEM for 9 WT and 10 CCR7/CCR9 DKO mice. (B) Thymic cellularities from WT and CCR7/CCR9 DKO mice were calculated. Shown is the mean ± SEM. *P < .05 compared with the WT control. (C) WT and CCR7/CCR9 DKO thymi were analyzed by flow cytometry for thymic progenitors. Representative FACS profiles are shown. (D) Absolute numbers of thymic progenitor populations were calculated. Shown is the mean ± SEM. *P < .05 and **P < .01 compared with the corresponding WT controls.
CCR7/CCR9 DKO progenitors are near absolutely restricted from thymic settling
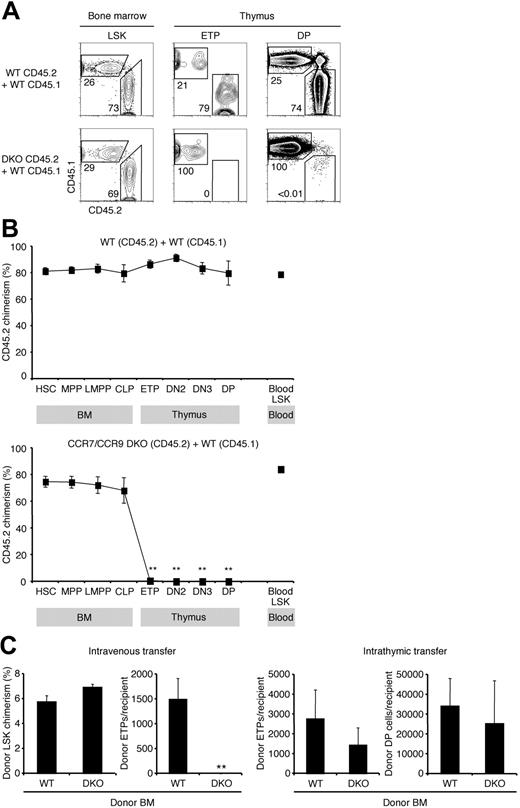
The previous experiments strongly suggested that CCR7 and CCR9 are involved in thymic settling, and that the lack of one can be partially compensated by the presence of the other. We therefore predicted that in the absence of both CCR7 and CCR9 thymic settling would be essentially abolished. To test this, we generated competitive mixed BM chimeras using a mixture of CCR7/CCR9 DKO and WT BM. After 10 weeks, BM and thymic subpopulations of chimeric mice were analyzed for their donor chimerism (Figure 4A-B). CCR7/CCR9 DKO progenitors were capable of BM engraftment, and early hematopoietic development in the BM was unaffected. Analysis of blood LSK progenitors demonstrated that CCR7 and CCR9 were not required for the mobilization of these cells out of the BM. This observation further suggests that CCR7 is unlikely to function as a BM progenitor mobilization signal. Despite high CCR7/CCR9 DKO donor chimerism in BM and blood progenitor populations, chimerism was nearly zero among the early intrathymic T-cell progenitors (ETPs: 0.6% ± 0.4%, DN2 cells: 0.2% ± 0.1%, DN3 cells: 0.1% ± 0.1%, and DP cells: 0.1% ± 0.03%; Figure 4A-B). Compared with CCR7−/−/WT and CCR9−/−/WT mixed BM chimeras (Figure 2B and Schwarz et al13 ), CCR7/CCR9 DKO/WT chimeras display a more severe defect in T-cell development beginning at or before the development of ETPs. Taken together, these results indicate that in a competitive situation, either CCR7 or CCR9 is required for the generation of thymocytes, and the expression of both CCR7 and CCR9 results in the most efficient T lymphopoiesis.
CCR7/CCR9 DKO progenitors have an absolute defect in thymic settling in the competitive scenario. (A) Mixed BM chimeras were generated using CD45.2 WT BM and CD45.1 WT BM mixtures (top row) or CD45.2 CCR7/CCR9 DKO BM and CD45.1 WT BM mixtures (bottom row). Chimeric mice were analyzed by flow cytometry 10 weeks after BM transfer for donor chimerism of the indicated populations using antibodies to CD45.1 and CD45.2. Representative FACS plots of BM LSKs (left column), thymic ETPs (middle column), and thymic DPs (right column) are shown. (B) Shown is the mean CD45.2 donor chimerism ± SEM for each indicated population from control WT chimeras (top panel) and CCR7/CCR9 DKO chimeras (bottom panel) described in panel A. **P < .01 for the CD45.2 donor chimerism of the indicated population compared with HSC CD45.2 donor chimerism. (C) For adoptive transfer experiments, BM from either WT or CCR7/CCR9 DKO (both CD45.2) was administered intravenously (left 2 panels) or intrathymically (right 2 panels) into unirradiated WT recipient mice (CD45.1). After 2 weeks (intrathymic transfer) or 3 weeks (intravenous transfer), donor-derived thymocytes were quantified by flow cytometry. Results show the mean ± SEM for 5 recipients in each group. **P < .01 compared with the WT control.
CCR7/CCR9 DKO progenitors have an absolute defect in thymic settling in the competitive scenario. (A) Mixed BM chimeras were generated using CD45.2 WT BM and CD45.1 WT BM mixtures (top row) or CD45.2 CCR7/CCR9 DKO BM and CD45.1 WT BM mixtures (bottom row). Chimeric mice were analyzed by flow cytometry 10 weeks after BM transfer for donor chimerism of the indicated populations using antibodies to CD45.1 and CD45.2. Representative FACS plots of BM LSKs (left column), thymic ETPs (middle column), and thymic DPs (right column) are shown. (B) Shown is the mean CD45.2 donor chimerism ± SEM for each indicated population from control WT chimeras (top panel) and CCR7/CCR9 DKO chimeras (bottom panel) described in panel A. **P < .01 for the CD45.2 donor chimerism of the indicated population compared with HSC CD45.2 donor chimerism. (C) For adoptive transfer experiments, BM from either WT or CCR7/CCR9 DKO (both CD45.2) was administered intravenously (left 2 panels) or intrathymically (right 2 panels) into unirradiated WT recipient mice (CD45.1). After 2 weeks (intrathymic transfer) or 3 weeks (intravenous transfer), donor-derived thymocytes were quantified by flow cytometry. Results show the mean ± SEM for 5 recipients in each group. **P < .01 compared with the WT control.
We further investigated the thymic settling capacity of CCR7/CCR9 DKO progenitors through intravenous transfers of unfractionated BM into unirradiated WT hosts. CCR7/CCR9 DKO progenitors had no defect in engrafting the BM, as demonstrated by the equivalent donor chimerism within the BM LSK population as WT progenitors (Figure 4C first panel). However, no CCR7/CCR9 DKO-derived ETPs (0 ± 0; Figure 4C second panel) were detected in the recipient cohort 3 weeks after intravenous transfer. When intrathymically injected, CCR7/CCR9 DKO progenitors gave rise to comparable numbers of ETPs and DP cells as did WT control progenitors (Figure 4C right 2 panels), thereby indicating that the absence of ETPs after intravenous transfer was due to impaired thymic settling, rather than defective intrathymic development. As an additional control, we cultured LMPPs in vitro on OP9-DL1 stromal cells, which express the Notch1 ligand delta-like 1 (DL1) to support T-cell development.44 We found that CCR7/CCR9 DKO LMPPs generated comparable numbers of cells to WT controls, of which more than 90% were double positive for the T-lineage markers CD25 and Thy1 (data not shown). Hence, CCR7/CCR9 DKO progenitors are as capable as WT progenitors at generating T-lineage progeny in vitro. Together these experiments firmly establish complementary roles for both CCR7 and CCR9 in thymic settling and affirm that either CCR7 or CCR9 is required for thymic settling in the competitive situation.
Compensatory proliferation in CCR7/CCR9 DKO mice
CCR7/CCR9 DKO mice possess thymi of near-normal total cellularity despite having a 100-fold defect in ETP numbers. The ability of thymocytes to compensate for reduced numbers of upstream progenitors has previously been demonstrated.41,45 To investigate whether compensatory population expansion occurs in CCR7/CCR9 DKO thymi, we first examined whether the very few Lin−Kit+CD25− cells detected in CCR7/CCR9 DKO thymi were functional T-lineage progenitors. We sorted Lin−Kit+CD25− cells from 3 CCR7/CCR9 DKO thymi (177 cells recovered) and compared them with sorted WT ETPs by culturing them on OP9-DL1 stromal cells. After 2 weeks, more than 90% of the progeny coexpressed the T-lineage markers CD25 and Thy1, thereby indicating that the sorted CCR7/CCR9 DKO cells had been bona fide ETPs with T-lineage potential (Figure 5A). CCR7/CCR9 DKO ETPs generated comparable numbers of CD25+Thy1+ T-lineage cells with WT controls on a per-cell basis (Figure 5B). These findings further confirm that CCR7/CCR9 DKO intrathymic progenitors do not possess an intrinsic defect in T-lineage differentiation or expansion in vitro.
Compensatory proliferation accounts for near-normal CCR7/CCR9 DKO thymic cellularity. (A) Sorted ETPs from WT or CCR7/CCR9 DKO mice were cultured on OP9-DL1 stromal layers. WT cells were cultured in triplicate wells, whereas CCR7/CCR9 DKO cells were cultured in a single well. After 2 weeks, cultured cells were analyzed by flow cytometry for expression of CD25 and Thy1. Representative FACS plots of cells previously gated to be CD45+ are shown. (B) Graphs show the relative numbers of cells obtained from cultures described in panel A. Shown is the mean ± SEM for WT and CCR7/CCR9 DKO cultures. (C) Lin−Flt3hi cells (3 × 103) sorted from WT BM (CD45.1) were intrathymically injected into WT or CCR7/CCR9 DKO recipients (both CD45.2). After 15 days, recipient thymi were analyzed for donor-derived DN3 cells (left column) or DP cells (right column). Shown are representative donor/host FACS plots. (D) Graphs show the total numbers of donor DN3 cells (left panel) and donor DP cells (right panel) found in recipient thymi for the experiment described in panel C. Shown is the mean ± SEM for 5 WT recipients and 3 CCR7/CCR9 DKO recipients. **P < .01 compared with WT controls.
Compensatory proliferation accounts for near-normal CCR7/CCR9 DKO thymic cellularity. (A) Sorted ETPs from WT or CCR7/CCR9 DKO mice were cultured on OP9-DL1 stromal layers. WT cells were cultured in triplicate wells, whereas CCR7/CCR9 DKO cells were cultured in a single well. After 2 weeks, cultured cells were analyzed by flow cytometry for expression of CD25 and Thy1. Representative FACS plots of cells previously gated to be CD45+ are shown. (B) Graphs show the relative numbers of cells obtained from cultures described in panel A. Shown is the mean ± SEM for WT and CCR7/CCR9 DKO cultures. (C) Lin−Flt3hi cells (3 × 103) sorted from WT BM (CD45.1) were intrathymically injected into WT or CCR7/CCR9 DKO recipients (both CD45.2). After 15 days, recipient thymi were analyzed for donor-derived DN3 cells (left column) or DP cells (right column). Shown are representative donor/host FACS plots. (D) Graphs show the total numbers of donor DN3 cells (left panel) and donor DP cells (right panel) found in recipient thymi for the experiment described in panel C. Shown is the mean ± SEM for 5 WT recipients and 3 CCR7/CCR9 DKO recipients. **P < .01 compared with WT controls.
We had earlier observed that the magnitudes of the numeric defects of CCR7/CCR9 DKO intrathymic progenitor populations were lessened among the more differentiated populations (Figure 3D). Having established the presence of rare ETPs in CCR7/CCR9 DKO thymi, we investigated whether the CCR7/CCR9 DKO thymic environment supports the compensatory proliferation of intrathymic T-lineage progenitors, which could account for near-normal total thymic cellularity. Lin−Flt3hi cells were sorted from CD45.1 WT BM and intrathymically injected into WT or CCR7/CCR9 DKO mice (both CD45.2). After 15 days, recipient thymi were analyzed for donor-derived DN3 cells and DP cells (Figure 5C-D). There were approximately 10-fold more donor DN3 cells in CCR7/CCR9 DKO hosts than WT hosts, and this difference increased to 100-fold at the DP stage. These data suggest that compensatory expansion in the CCR7/CCR9 DKO hosts was occurring before or during the DN3 stage, and also between the DN3 and DP stages. Hence, compensatory population expansion by progenitors can explain how CCR7/CCR9 DKO thymi attain near-normal cellularity despite highly inefficient thymic settling (Figure 3B-D). These findings also support the notion that the expansion of some intrathymic progenitors is not programmed, but rather is at least partially environmentally dependent.
These results indicated that the CCR7/CCR9 DKO thymus provides an environment in which progenitors can overcome early defects by increased population expansion during development. We investigated the mechanisms by which this compensatory expansion may occur. Cell cycle analysis revealed comparable fractions of cycling cells between WT and CCR7/CCR9 DKO progenitors at the DN3, immature single-positive, and DP stages of development (supplemental Figure 2A). Annexin V staining indicated that the fraction of progenitors undergoing apoptosis was not decreased in CCR7/CCR9 DKO thymi (supplemental Figure 2B). These results suggest that the compensatory population expansion seen in CCR7/CCR9 DKO thymi may be due to an increase in the total number of intrathymic cell divisions, though at a normal rate.
Discussion
We investigated the chemokine receptor signals required for thymic settling by BM-derived hematopoietic progenitors. Earlier work established a role for CCR9 in this process, yet the ability of CCR9−/− progenitors to settle the thymus suggested that other molecules, and chemokine receptors in particular, are likely active in this process. We now show that CCR7 is selectively expressed on BM progenitors downstream of HSCs and MPPs and is important for efficient thymic settling. Progenitors lacking both CCR7 and CCR9 are almost completely unable to contribute to T lymphopoiesis in a competitive environment. We determined that the compensatory expansion of rare intrathymic progenitors in these mice can account for the recovery of overall thymic cellularity despite such impaired settling. Together these data illustrate the critical importance of chemokine receptors in thymic settling and also point to the exceptional ability of the thymus to recover from early defects.
The ability to settle the thymus is selectively attained by progenitors downstream of HSCs and MPPs.13 One of the molecules shown to have a role in thymic settling—PSGL-1—is functionally expressed on a fraction of HSCs (data not shown and Rossi et al31 ) and therefore cannot be the basis for this selectivity. CCR7 and CCR9 are absent on HSCs and MPPs but are up-regulated on subsets of LMPPs and CLPs, which have been shown to rapidly and efficiently produce thymocytes after intravenous transfer. Hence, there exists a correlation between thymic settling ability and expression of these 2 chemokine receptors.
The earliest steps in T-cell development include the generation of BM progenitors competent to settle the thymus, their mobilization from BM into blood, their entry into the thymus by transmigrating across the thymic endothelium, and their differentiation into ETPs. When CCR7−/− and CCR7/CCR9 DKO BM were transferred intravenously, thereby bypassing the mobilization step, defects in ETP generation were still observed. Yet intrathymic injections that bypass the transmigration step resolved these defects, indicating that CCR7 and CCR9 function at the level of thymic entry or shortly thereafter. The specific mechanisms by which CCR7 and CCR9 function there have not been explored in detail, but they may act similarly to chemokine receptors on mature lymphocytes traveling through high endothelial venules of lymph nodes—namely, that their signaling induces the activation of integrins and consequently the firm arrest of the cell at the endothelium. The integrin dimers α4β1 (very late antigen 4) and αLβ2 (leukocyte function-associated antigen 1) have been implicated in thymic settling,21 but additional work is needed to identify whether these are the key integrins that are activated downstream of CCR7 and CCR9.
Whereas thymic settling is almost completely abrogated when CCR7/CCR9 DKO cells are in competition with WT cells, some settling must be occurring in the noncompetitive scenario to generate the few ETPs found in CCR7/CCR9 DKO mice. It is not clear how CCR7/CCR9 DKO cells gain access to the thymus in this case. P-selectin expression is dynamically regulated at the thymic endothelium.31,32 Up-regulation of these molecules may allow thymic settling to occur in the CCR7/CCR9 DKO thymus, perhaps in concert with other chemokine receptors also expressed by hematopoietic progenitors such as CXCR4.46,47 Another possibility is that inefficient thymic settling progenitors that normally do not contribute substantially to T-cell development are capable of thymic settling in the absence of competing progenitors expressing CCR7 and CCR9.
Whereas CCR7 and CCR9 are here demonstrated to be critical for thymic settling in the adult vascularized thymus, earlier studies reported the use of these receptors during the embryonic period during which the thymus is not yet vascularized.28,33 Thus it now appears that despite the substantial changes in thymic anatomy that occur during development, the same signals function during fetal and adult life. The usage of CCR7 and CCR9 for thymic settling after fetal life was previously overlooked because CCR7/CCR9 DKO mice have near-normal thymic cellularity. Our data suggest that this is due to compensatory population expansion of intrathymic progenitors. This expansion may be the result of an increase in the total number of intrathymic cell divisions occurring at a normal rate, as no differences in cell cycle were detected. Whether this increased number of intrathymic divisions affects the later function of mature CCR7/CCR9 DKO T cells remains to be determined. As suggested by past observations,41,45 we find that such compensatory expansion can take place before the DN3 stage and continues between the DN3 and DP stages. However, it also remains possible that progenitors settle the CCR7/CCR9 DKO thymus at stages downstream of the ETP. Whatever the mechanism, the ability of the thymus to recover from early defects indicates that decreased thymic settling alone cannot explain the decrease in thymic cellularity seen with aging.48,49
This study has focused on the key chemokine signals involved in thymic settling. Identifying these signals now allows us to better localize where thymic settling capacity may reside within the broad pool of BM progenitors. Previous work has identified that only minor subsets of both LMPPs and CLPs express CCR9.13,25 We now show that CCR7 is expressed on large fractions of these progenitors. Hence, there exist rare CCR7+CCR9+ progenitors, which are approximately equally split between Kithi and Kitlo cells, and a much larger pool of singly CCR7+ cells. The relative contribution of these populations toward T lymphopoiesis remains unclear. In addition, other progenitors that were not the focus of this study have been shown to express CCR7 and/or CCR9 and may contribute to T-cell development.21,23 It is possible that more than one progenitor contributes to T-cell production,6 but it seems that they must express either CCR7 or CCR9 to do so. Although expression of various molecules has been used to define potential TSP populations in the past, including Kit, IL-7Rα, L-selectin, and RAG,14,18,20,50 defining BM progenitors of T cells by their expression of homing molecules is also a useful strategy.29 As future work better identifies the panel of molecular signals that together are sufficient to confer thymic settling capacity onto progenitors, we will be able to molecularly define the physiologically relevant thymus settling progenitors.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Gudrun Debes and Brad Johnson for advice regarding these experiments and Tao Zou and Taku Kambayashi for their critiques of the paper.
This work was supported by NIH grants AI080091 (J.J.B.), AI059621, HL086900, and RC1HL099758 (A.B.), and a Scholar Award from the Leukemia & Lymphoma Society (A.B.).
National Institutes of Health
Authorship
Contribution: D.A.Z. performed most of the experimental work with contributions from A.S., T.D.L., and J.J.B.; D.A.Z., B.A.S., and A.B. together planned the project; D.A.Z. and A.B. analyzed data; and D.A.Z., B.A.S., and A.B. prepared the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Avinash Bhandoola, 266 John Morgan Bldg, 3620 Hamilton Walk, Philadelphia, PA 19104; e-mail: bhandooa@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal