Abstract
Protease nexin–1 (PN-1) is a serpin that inhibits plasminogen activators, plasmin, and thrombin. PN-1 is barely detectable in plasma but is expressed by platelets. Here, we studied platelet PN-1 in resting and activated conditions and its function in thrombosis. Studies on human platelets from healthy donors and from patients with a Gray platelet syndrome demonstrate that PN-1 is present both at the platelet surface and in α-granules. The role of PN-1 was investigated in vitro using human platelets incubated with a blocking antibody and using platelets from PN-1–deficient mice. Both approaches indicate that platelet PN-1 is active on thrombin and urokinase-type plasminogen activator. Blockade and deficiency of platelet PN-1 result in accelerated and increased tissue factor-induced thrombin generation as indicated by calibrated automated thrombography. Moreover, platelets from PN-1–deficient mice respond to subthreshold doses of thrombin, as assessed by P-selectin expression and platelet aggregation. Thrombus formation, induced ex vivo by collagen in blood flow conditions and in vivo by FeCl3-induced injury, is significantly increased in PN-1–deficient mice, demonstrating the antithrombotic properties of platelet PN-1. Platelet PN-1 is thus a key player in the thrombotic process, whose negative regulatory role has been, up to now, markedly underestimated.
Introduction
Platelets play an essential role in the normal hemostatic process by adhering and aggregating at sites of vascular injury. Platelets also participate in the generation and regulation of thrombin by providing a suitable membrane surface that localizes, amplifies, and modulates procoagulant enzymatic reactions.1 In response to vascular damage, a clot made of fibrin and platelets creates a plug that prevents bleeding.
Platelets contain granules, α-granules, dense granules, and lysosomes, the contents of which are released on platelet activation, triggered by many agonists, such as thrombin. Many of the compounds released by platelets play a role in thrombus formation or stabilization. Antithrombin and antifibrinolytic activities of platelets have been reported. Indeed, plasminogen activator inhibitor–1 (PAI-1), a serpin that inhibits plasmin formation and thrombin activity,2 is contained in α-granules and secreted during activation.3,4 Platelets may inhibit fibrinolysis by both PAI-1–dependent and PAI-1–independent mechanisms.5
Prior studies have suggested that platelets contain another serpin, protease nexin–1 (PN-1), secreted during activation.6,7 PN-1, also known as SERPINE2, is a powerful inhibitor of thrombin and also inhibits urokinase-type plasminogen activator (uPA), tissue plasminogen activator (tPA), and plasmin. Its action is potentiated by glycosaminoglycans (GAGs), such as heparan sulfates.8,9 In contrast to antithrombin and PAI-1, PN-1 is barely detectable in plasma10 but is produced by various cell types.11 Interestingly, PN-1 inhibits thrombin more rapidly than does either antithrombin12,13 or PAI-1.14
In the present study, we addressed the question of the behavior of platelet PN-1 in resting and activated conditions and of its function in thrombosis. Studies conducted on platelets from healthy human donors and from patients exhibiting a Gray platelet syndrome (GPS) allowed us to identify the storing organelles of PN-1 as α-granules. Our data provide direct evidence that platelets release active PN-1. Indeed, we demonstrate here, for the first time, by in vitro studies on platelet activation and thrombin generation, ex vivo studies on thrombus formation in blood flow conditions, and in vivo studies using a model of arteriolar and venular thrombosis in mice, that platelet PN-1 has relevant anticoagulant and antithrombotic properties. Platelet PN-1 is thus a key player in the thrombotic process whose negative regulatory role has been, up to now, markedly underestimated.
Methods
Patients
The 3 patients are from unrelated families and present hemorrhagic manifestations related to a well-characterized GPS. One case has already been reported in the literature,15 and 2 are new sporadic cases. All patients gave informed consent in accordance with the Declaration of Helsinki.
Animals
Mice homozygous for null mutations in the PN-1 or PAI-1 gene were generated as previously described.16,17 Experimental animals were 8 to 16 weeks of age. PN-1–deficient mice (PN-1−/−) were back-crossed for 12 generations into the C57BL/6 line. Heterozygous mating generated PN-1−/− and wild-type mice (WT). Mice were bred and maintained in our own laboratory (Paris, France). PAI-1–deficient mice (PAI-1−/−) come from HR Lijnen Laboratory (Leuven, Belgium). All animals were genotyped by polymerase chain reaction (PCR). All experiments were performed in accordance with European legislation on the protection of animals and were approved by the ethics committee of Inserm.
Preparation of washed platelets
Human platelets.
Human blood from healthy adult volunteers was collected into one-tenth volume ACD-A (38mM citric acid, 60mM sodium citrate, 136mM glucose). Washed platelets were isolated as previously described.18
Mouse platelets.
Blood was collected from anesthetized mice by cardiac puncture into syringes containing one-tenth volume ACD-C (130mM citric acid, 124mM sodium citrate, 110mM dextrose) as anticoagulant. Washing procedure is described in supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Western blot analysis
Washed human or mouse platelets were lysed using 100mM Tris, 150mM NaCl, 3mM ethylenediaminetetraacetic acid, and 2% sodium dodecyl sulfate–N-ethylmaleimide. Proteins were separated by electrophoresis in sodium dodecyl sulfate–polyacrylamide gels under reducing conditions and transferred to polyvinylidene difluoride membranes. After saturation with 5% lowfat milk in phosphate-buffered saline/0.1% Tween-20 for 1 hour, the membranes were incubated overnight at 4°C with the following antibodies: a polyclonal rabbit anti–PN-1 (gift from D. Hantai, Inserm U582, Paris, France19 ; 1.2 μg/mL), a monoclonal anti-GAPDH (Biovalley; 1.6 μg/mL), and a monoclonal anti-GPIb (SZ2; Immunotech; 2 μg/mL) in phosphate-buffered saline/0.1% Tween-20. The secondary antibody was a goat anti–mouse IgG or a goat anti–rabbit IgG conjugated with peroxidase (Beckman Coulter) revealed by chemiluminescence (Pierce Chemical). Recombinant PN-1, used as a positive control, was a generous gift from D. Hantai.20
Flow cytometry with human and mouse platelets
The monoclonal human anti–PN-1 antibody was purified and coupled to fluorescein isothiocyanate (FITC) as previously described.21,22 Flow cytometric analysis of human and mouse platelets was performed on a Beckman Coulter Epics XL-MCL. Human platelets (2 × 107cells/mL) were incubated with an irrelevant IgG or different primary antibodies coupled to FITC according to experimental conditions, for 30 minutes in the dark at room temperature. Mouse platelets (3 × 108/mL) were activated by increasing doses of thrombin for 15 minutes at 37°C or by a low dose of thrombin (0.1nM) for different times (0-3 minutes). Samples were stained with a FITC-coupled rat anti–mouse P-selectin IgG (Emfret Analytics) or with a FITC-coupled annexin V (Beckman Coulter) for 15 minutes at room temperature. For each sample, 50 000 events were analyzed.
Platelet aggregation
Platelet aggregation was measured on washed platelets. Platelets were resuspended in Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer and adjusted to a concentration of 3 × 108/mL. Platelets from WT or PN-1−/− mice were stimulated by increasing doses of thrombin, purified as previously described,23 at 37°C with stirring, and changes in light transmission were monitored with an aggregometer (Chronolog Inc).
Thrombin activity assay
Platelets (5 × 108/mL in reaction buffer) were activated by PAR1-AP (PAR1-activating peptide, SFLLRN, Neo MPS; 50μM) for human platelets or by PAR4-AP (PAR4-activating peptide, AYPGKF, Neo MPS; 250μM) for mouse platelets, for 30 minutes at 37°C. Control samples were obtained by incubating platelets for the same time with buffer. At the end of the incubation, samples were centrifuged and the supernatants (secreted fraction) were removed for analysis. The secreted fractions were incubated with 0.25nM thrombin for 30 minutes at 37°C with or without polybrene (hexadimethrine bromide; Sigma-Aldrich), or in the presence or absence of the blocking anti–PN-1 or anti–PAI-1 IgGs. The polyclonal anti-PN-1 IgG efficiently blocks the inhibitory activity of recombinant PN-1 on thrombin catalytic activity.21 The monoclonal anti–PAI-1 IgG is an inhibitory IgG to human and murine PAI-1 (MA-33B8-307; Molecular Innovations).24,25 At the end of the incubation, the thrombin-specific chromogenic substrate S2238 (Chromogenix) was added at a final concentration of 0.2mM, and changes in the absorbance were recorded at 405 nm. Residual thrombin activity is expressed as the ratio of the activity measured in the presence of the fraction secreted by activated platelets to the activity measured in the presence of the supernatant of resting platelets × 100.
uPA activity assay
Secreted fractions were obtained from human and mouse platelets as described in “Thrombin activity assay” and incubated with 1nM uPA (American Diagnostica) for 30 minutes at 37°C in the presence or absence of the blocking anti–PN-1 or anti–PAI-1 IgGs. At the end of the incubation, the urokinase-specific chromogenic substrate S2444 (Chromogenix) was added at a final concentration of 1mM and changes in the absorbance recorded at 405 nm. Residual uPA activity is expressed as the ratio of the activity measured in the presence of the fraction secreted by activated platelets to the activity measured in the presence of the supernatant of resting platelets × 100.
Thrombin generation in human and mouse PRP
Thrombin generation was continuously measured in platelet-rich plasma (PRP) using the thrombogram method as previously described.26 Human PRP (1.5 × 108 platelets/mL) was preincubated with the blocking anti–PN-1 or anti–PAI-1 IgGs (150 μg/mL) for 15 minutes at 37°C. Then PRP was transferred to microtitration plates containing 0.5pM tissue factor (TF; Diagnostica Stago). For measurements on mouse PRP, platelets were adjusted to 108/mL and thrombin generation was initiated by 1pM TF as previously described.27
Collagen-induced thrombus formation under flow conditions
Citrate-anticoagulated murine blood was labeled by Rhodamine 6G (Sigma-Aldrich) and perfused at a shear rate of 1000 seconds−1 over a collagen surface, as described elsewhere.28 A detailed protocol is given in supplemental data.
In vivo thrombosis
Ferric chloride injury was induced in 4- to 5-week-old mice as previously described.29 A detailed protocol is given in supplemental data.
Statistics
Results are shown as means plus or minus SD. Statistical analysis of differences between groups was performed using a Student t test. A P value less than or equal to .05 was considered significant.
Results
PN-1 is expressed by human platelets and is secreted during activation
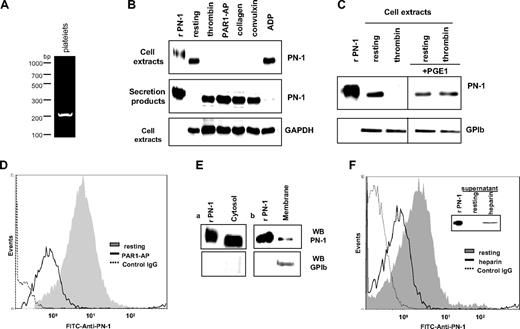
To investigate whether platelet PN-1 is an endogenous protein or results from the uptake of extracellular PN-1 by platelets, platelet mRNAs were analyzed by RT-PCR. The PN-1 amplification product detected (Figure 1A) and the fact that PN-1 is not present in the plasma strongly suggested an endogenous origin. PN-1 expression by platelets was analyzed by immunoblot on human washed platelets (Figure 1B). PN-1 was detected as a 50-kDa band in extracts of resting platelets, migrating slightly faster than recombinant PN-1 (rPN-1) because of differences in glycosylation, as previously reported.30 Platelet activation was induced by different agonists. After centrifugation, immunoblots were performed on platelet extracts obtained from pellets and on secretion products obtained from the supernatants. PN-1 was no longer detected in the platelet extracts after activation by 5nM thrombin, 50μM PAR1-AP, 5 μg/mL collagen, or 1 nM convulxin (Figure 1B). In contrast, a 50-kDa band corresponding to intact PN-1 was detected in the secretion products of activated platelets (Figure 1B), indicating that PN-1 was secreted during platelet activation by strong agonists. After activation by 10μM adenosine diphosphate (ADP), a weak agonist, or when platelets were incubated with prostaglandin E1, a cyclic adenosine monophosphate-increasing agent that inhibits platelet activation before activation by thrombin, PN-1 was still detected in platelet extracts and barely detectable in the secretion products (Figure 1B-C). These data indicate that platelet PN-1 is secreted by an activation-dependent mechanism.
Characterization of platelet PN-1. (A) RT-PCR analysis of PN-1 mRNA expression in platelets. (B) Washed platelets were activated by different agonists: 5nM thrombin, 50μM PAR1-AP, 5 μg/mL collagen, 1nM convulxin, and 10μM ADP, for 30 minutes at 37°C, and centrifuged to isolate platelet pellets from supernatants. Platelet pellets (cell extracts) and supernatants (secretion products) were analyzed by Western blot for PN-1 and GAPDH. (C) Washed platelets were pretreated or not with 1 μg/mL prostaglandin E1 before activation by thrombin. Cell extracts were analyzed by Western blot for PN-1 and GPIb. (D) Washed platelets activated by 50μM PAR1-AP were analyzed by flow cytometry with an FITC-coupled anti–PN-1 IgG. (E) Membrane and cytosol fractions of resting platelets analyzed by Western blot for PN-1 and GPIb. (F) Washed platelets treated with 150 μg/mL heparin were analyzed by flow cytometry. The supernatants of heparin-treated platelet were analyzed by Western blot for PN-1 (inset). Data are representative of 3 separate experiments from different donors.
Characterization of platelet PN-1. (A) RT-PCR analysis of PN-1 mRNA expression in platelets. (B) Washed platelets were activated by different agonists: 5nM thrombin, 50μM PAR1-AP, 5 μg/mL collagen, 1nM convulxin, and 10μM ADP, for 30 minutes at 37°C, and centrifuged to isolate platelet pellets from supernatants. Platelet pellets (cell extracts) and supernatants (secretion products) were analyzed by Western blot for PN-1 and GAPDH. (C) Washed platelets were pretreated or not with 1 μg/mL prostaglandin E1 before activation by thrombin. Cell extracts were analyzed by Western blot for PN-1 and GPIb. (D) Washed platelets activated by 50μM PAR1-AP were analyzed by flow cytometry with an FITC-coupled anti–PN-1 IgG. (E) Membrane and cytosol fractions of resting platelets analyzed by Western blot for PN-1 and GPIb. (F) Washed platelets treated with 150 μg/mL heparin were analyzed by flow cytometry. The supernatants of heparin-treated platelet were analyzed by Western blot for PN-1 (inset). Data are representative of 3 separate experiments from different donors.
PN-1 is bound to glycosaminoglycans at the platelet surface
To determine whether PN-1 was present on the platelet surface, flow cytometric analysis was performed using an FITC-coupled anti-PN-1 monoclonal antibody. A single population of labeled platelets was observed, indicating the presence of PN-1 at the surface of platelets (Figure 1D). Platelets analyzed were resting platelets as indicated by the absence of significant labeling with the anti–P-selectin IgG (< 1% positive platelets). After activation by PAR1-AP, fluorescence peak shifted to the left, indicating a decrease in the expression of PN-1 at the surface of activated platelets (Figure 1D).
Plasma membrane and cytosol fractions of resting platelets were analyzed by immunoblot. PN-1 was detected in the cytosol and the membrane fractions of resting platelets. However, the intensity of the band in the membrane fraction was considerably less intense than the one in the cytosol fraction, indicating that membrane-bound PN-1 represents a minor part of platelet PN-1 (Figure 1E).
PN-1 is known to bind to cell surface GAGs from which it is detached by heparin.31 Flow cytometric analysis of resting platelets showed that heparin induced a significant shift to the left of the platelet-associated fluorescence, indicating a decrease in PN-1 at the platelet surface (Figure 1F). In addition, an increased amount of PN-1 was detected by immunoblot analysis in the incubation medium (Figure 1F inset). These results indicate that the fraction of platelet PN-1 present at the platelet surface is mainly associated with membrane-bound GAGs.
PN-1 is stored in platelet α-granules
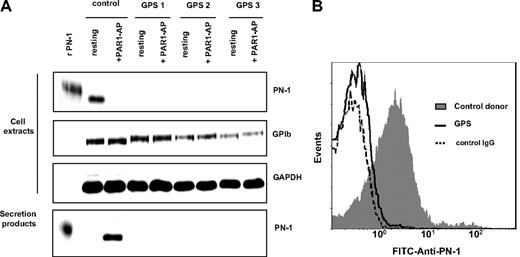
The results reported in “PN-1 is bound to glycosaminoglycans at the platelet surface” suggested that the major part of platelet PN-1 was stored in secretory granules. Platelets from 3 patients with a GPS, a rare platelet disorder characterized by thrombocytopenia with an absence of α-granules,32 were investigated. Washed platelets from healthy donors or GPS patients (5 × 108/mL) were activated by PAR1-AP (50μM) and analyzed by immunoblot. Contrasting with the observation obtained with platelets from healthy donors, PN-1 was detected neither in lysates of resting GPS platelets nor in the secretion products obtained after activation of these GPS platelets (Figure 2A). Moreover, flow cytometric analysis showed that, in contrast to the observation made with control platelets, PN-1 was not detected at the surface of GPS platelets (Figure 2B).
PN-1 is stored in platelet α-granules. (A) Washed platelets from control donors or patients with a GPS were activated by 50μM PAR1-AP for 30 minutes at 37°C and centrifuged to separate platelet pellets from supernatants. Platelet pellets (cell extracts) and supernatants (secretion products) were analyzed by Western blot for PN-1, GAPDH, and GPIb. (B) Washed platelets from control donor or GPS patients were analyzed by flow cytometry with an FITC-coupled anti–PN-1 IgG. Data are representative of 3 separate experiments from different donors.
PN-1 is stored in platelet α-granules. (A) Washed platelets from control donors or patients with a GPS were activated by 50μM PAR1-AP for 30 minutes at 37°C and centrifuged to separate platelet pellets from supernatants. Platelet pellets (cell extracts) and supernatants (secretion products) were analyzed by Western blot for PN-1, GAPDH, and GPIb. (B) Washed platelets from control donor or GPS patients were analyzed by flow cytometry with an FITC-coupled anti–PN-1 IgG. Data are representative of 3 separate experiments from different donors.
The data obtained with control and GPS platelets indicated that PN-1 contained in α-granules is released during activation.
Platelet PN-1 inhibits thrombin
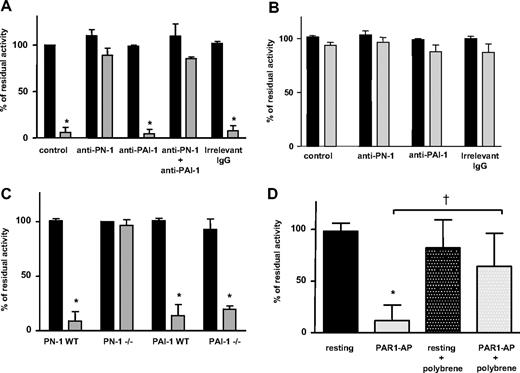
Washed platelets obtained from healthy human donors or GPS patients were activated by PAR1-AP. The efficacy of secreted PN-1 in inhibiting the amidolytic activity of purified thrombin was investigated. No inhibition of thrombin activity was observed in the presence of the supernatant of resting human platelets (Figure 3A). On the other hand, incubation of thrombin with the secretion products of activated platelets resulted in an almost complete thrombin inhibition; thrombin residual activity dropped to 5.9% plus or minus 5.4% of its initial activity (Figure 3A), indicating that activated platelets secreted a thrombin inhibitor. To determine the role of PN-1 in thrombin inhibition, experiments were performed in the presence of a PN-1–blocking antibody. This antibody abolished the inhibition of thrombin activity by the secreted products of activated platelets. Such an effect was not observed in the presence of irrelevant mouse immunoglobulins. Furthermore, the PAI-1–blocking antibody did not prevent the inhibition of thrombin activity by the platelet secretion products. Moreover, the simultaneous addition of the anti–PN-1 and anti–PAI-1 antibodies to the platelet secretion products did not result in a greater effect than the anti–PN-1 alone (Figure 3A). These results suggested that PN-1 was the major inhibitor of thrombin activity released by activated platelets and that platelet-derived PAI-1 was inefficient on thrombin activity. The effect of the secretion products obtained after activation of GPS platelets was thus analyzed on thrombin activity. In these conditions, no inhibition of thrombin activity was observed (Figure 3B). Taken together, these data strongly suggest, on one hand, that PN-1 is stored in α-granules and, on the other hand, that PN-1 is the main thrombin inhibitor released by activated platelets.
Inhibition of thrombin catalytic activity by released platelet PN-1. Thrombin catalytic activity was measured after incubation with the supernatants from resting platelets (■) or activated platelets ( ) as described in “Preparation of washed platelets.” Products of human platelet secretion from (A) control donors or (B) GPS patients were incubated with thrombin in the presence or absence of an anti-PN-1 IgG (150 μg/mL) and/or an anti-PAI-1 IgG (150 μg/mL). (C) Secretion products of platelets from mice deficient for PN-1 or PAI-1 and their WT littermates. (D) Secretion products of human platelets were pretreated with polybrene (50 μg/mL) before the incubation with thrombin. Data are presented as mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05, significantly different from products of resting platelet supernatant. †P < .05, significantly different from products of activated platelet supernatant.
) as described in “Preparation of washed platelets.” Products of human platelet secretion from (A) control donors or (B) GPS patients were incubated with thrombin in the presence or absence of an anti-PN-1 IgG (150 μg/mL) and/or an anti-PAI-1 IgG (150 μg/mL). (C) Secretion products of platelets from mice deficient for PN-1 or PAI-1 and their WT littermates. (D) Secretion products of human platelets were pretreated with polybrene (50 μg/mL) before the incubation with thrombin. Data are presented as mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05, significantly different from products of resting platelet supernatant. †P < .05, significantly different from products of activated platelet supernatant.
Inhibition of thrombin catalytic activity by released platelet PN-1. Thrombin catalytic activity was measured after incubation with the supernatants from resting platelets (■) or activated platelets ( ) as described in “Preparation of washed platelets.” Products of human platelet secretion from (A) control donors or (B) GPS patients were incubated with thrombin in the presence or absence of an anti-PN-1 IgG (150 μg/mL) and/or an anti-PAI-1 IgG (150 μg/mL). (C) Secretion products of platelets from mice deficient for PN-1 or PAI-1 and their WT littermates. (D) Secretion products of human platelets were pretreated with polybrene (50 μg/mL) before the incubation with thrombin. Data are presented as mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05, significantly different from products of resting platelet supernatant. †P < .05, significantly different from products of activated platelet supernatant.
) as described in “Preparation of washed platelets.” Products of human platelet secretion from (A) control donors or (B) GPS patients were incubated with thrombin in the presence or absence of an anti-PN-1 IgG (150 μg/mL) and/or an anti-PAI-1 IgG (150 μg/mL). (C) Secretion products of platelets from mice deficient for PN-1 or PAI-1 and their WT littermates. (D) Secretion products of human platelets were pretreated with polybrene (50 μg/mL) before the incubation with thrombin. Data are presented as mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05, significantly different from products of resting platelet supernatant. †P < .05, significantly different from products of activated platelet supernatant.
To confirm these findings, the same type of experiment was performed with platelets from PN-1−/− or PAI-1−/− and their littermate controls. As observed with human platelets, incubation of purified thrombin with the secretion products of activated platelets from WT mice resulted in a very significant inhibition of thrombin activity (Figure 3C). A similar result was obtained with activated platelets from PAI-1−/− mice. On the contrary, the products secreted by platelets from PN-1−/− mice did not inhibit thrombin activity (Figure 3C). These results are in good agreement with those obtained with the anti-PN-1 antibody in human platelets. Together, they indicate that PN-1 secreted by activated platelets has an efficient inhibitory effect toward thrombin whereas PAI-1 is devoid of such an effect.
Because thrombin inhibition by PN-1 is known to be potentiated by GAGs, we investigated whether PN-1, secreted by activated platelets, was associated with GAGs. Secretion products of human platelets activated by PAR1-AP were incubated with or without polybrene, a polycation that neutralizes GAG-dependent interactions. Residual thrombin activity observed after activation (11%) was partially restored in the presence of polybrene (64%) (Figure 3D). This result indicated that PN-1 released from platelet α-granules was associated with GAGs. On the other hand, thrombin inhibition with the secretion products of activated platelets was accelerated in the presence of heparin (not shown), indicating that a fraction of PN-1 released by platelets is free.
Platelet PN-1 inhibits urokinase
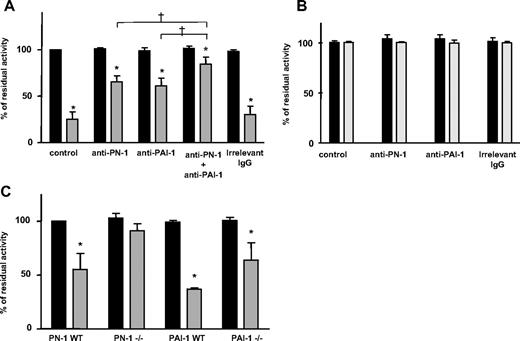
We then tested whether PN-1 secreted by activated platelets inhibits uPA activity. No inhibition of uPA was observed in the presence of supernatant from resting human platelets (Figure 4A). In contrast, the products secreted by activated platelets significantly inhibited uPA, with the residual activity dropping to 25.0% plus or minus 7.9% of the initial activity (Figure 4A), indicating that platelets secrete uPA inhibitor(s). To determine the respective role of PN-1 and PAI-1 in uPA inhibition, experiments were performed in the presence of anti–PN-1 or/and anti–PAI-1 antibodies. Both antibodies antagonized the inhibitory activity by 35.0% plus or minus 6.5% or 40.0% plus or minus 8.5%, respectively (Figure 4A). Their combination almost completely prevented inhibition of uPA by the platelet-secreted products, with uPA residual activity returning to 84.0% plus or minus 7.4% of the control value (Figure 4A). Products secreted by activated GPS platelets did not inhibit uPA activity (Figure 4B), in agreement with the localization of PN-1 and PAI-1 in platelet α-granules.
Inhibition of urokinase (uPA) catalytic activity by released platelet PN-1. uPA catalytic activity was measured after incubation with the supernatants from resting platelets (■) or activated platelets ( ) as described in “Thrombin activity assay.” Products of human platelet secretion from (A) control donors or (B) GPS patients were incubated with uPA in the presence or absence of an anti–PN-1 IgG and/or an anti–PAI-1 IgG. (C) Secretion products of platelets from mice deficient for PN-1 or PAI-1 or their WT littermates. Data are presented as mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05, significantly different from resting platelet supernatant. †P < .05 significantly different from activated platelet supernatant in the presence of an anti–PN-1 IgG or an anti–PAI-1 IgG.
) as described in “Thrombin activity assay.” Products of human platelet secretion from (A) control donors or (B) GPS patients were incubated with uPA in the presence or absence of an anti–PN-1 IgG and/or an anti–PAI-1 IgG. (C) Secretion products of platelets from mice deficient for PN-1 or PAI-1 or their WT littermates. Data are presented as mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05, significantly different from resting platelet supernatant. †P < .05 significantly different from activated platelet supernatant in the presence of an anti–PN-1 IgG or an anti–PAI-1 IgG.
Inhibition of urokinase (uPA) catalytic activity by released platelet PN-1. uPA catalytic activity was measured after incubation with the supernatants from resting platelets (■) or activated platelets ( ) as described in “Thrombin activity assay.” Products of human platelet secretion from (A) control donors or (B) GPS patients were incubated with uPA in the presence or absence of an anti–PN-1 IgG and/or an anti–PAI-1 IgG. (C) Secretion products of platelets from mice deficient for PN-1 or PAI-1 or their WT littermates. Data are presented as mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05, significantly different from resting platelet supernatant. †P < .05 significantly different from activated platelet supernatant in the presence of an anti–PN-1 IgG or an anti–PAI-1 IgG.
) as described in “Thrombin activity assay.” Products of human platelet secretion from (A) control donors or (B) GPS patients were incubated with uPA in the presence or absence of an anti–PN-1 IgG and/or an anti–PAI-1 IgG. (C) Secretion products of platelets from mice deficient for PN-1 or PAI-1 or their WT littermates. Data are presented as mean ± SD of 3 independent experiments, each performed in triplicate. *P < .05, significantly different from resting platelet supernatant. †P < .05 significantly different from activated platelet supernatant in the presence of an anti–PN-1 IgG or an anti–PAI-1 IgG.
To substantiate these results, experiments were performed using mouse platelets. The activity of uPA was inhibited in the presence of the products secreted by activated WT platelets (Figure 4C), in agreement with the data obtained with human platelets. In contrast, the secretion products from activated PN-1–deficient platelets did not inhibit uPA, the residual activity remaining at 91.0% plus or minus 6.5%. The products secreted by activated PAI-1–deficient platelets partially inhibited uPA (Figure 4C).
Taken together, our findings indicate that both PN-1 and PAI-1 released by activated platelets contribute to inhibit uPA.
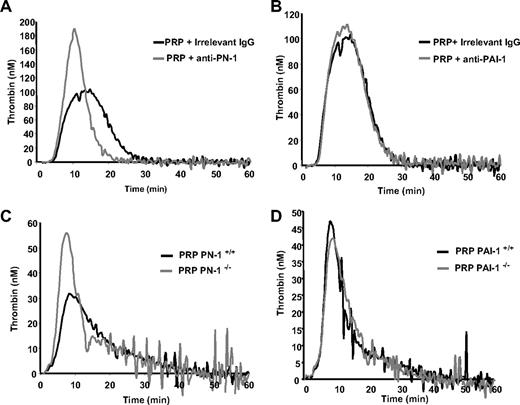
Platelet PN-1 delays time to peak and reduces the peak of thrombin generated by TF in PRP
The respective role of PN-1 and PAI-1 in the production of thrombin induced by TF in human and mouse PRP were investigated using the thrombogram method. The thrombin generation curve (thrombogram) is characterized by several parameters: (1) the lag phase related to the clotting time; (2) the peak height, which represents the maximal net thrombin production and reflects the maximum prothrombinase activity reached; (3) the time to peak, which reflects the velocity; and (4) the endogenous thrombin potential, namely, the area under the curve representing the total amount of active thrombin generated during the assay. The effects of the anti–PN-1 and anti–PAI-1 antibodies on thrombin generation in human PRP are shown in Figure 5A and B, and quantitative parameters are reported in Table 1. The anti–PN-1 antibody significantly shortened the thrombin burst, as indicated by the reduced time to peak, and significantly increased the peak height, but did not modify the endogenous thrombin potential (Figure 5A; Table 1). The anti–PAI-1 antibody and the irrelevant antibody had no significant effect on the thrombin generation parameters (Figure 5B; Table 1). Neither the anti–PN-1 nor the anti–PAI-1 antibodies affected TF-induced thrombin generation in platelet-poor plasma (PPP; not shown), confirming that PN-1 originated from platelets.
Thrombin generation in human and mouse PRP. TF-triggered thrombin generation was measured in PRP. Human PRP was preincubated with (A) a PN-1–blocking IgG (150 μg/mL) or (B) a PAI-1–blocking IgG (150 μg/mL). Mouse PRP was from (C) PN-1–deficient mice or (D) PAI-1–deficient mice on thrombin. Results are from one experiment performed in triplicate, representative of at least 3 independent experiments.
Thrombin generation in human and mouse PRP. TF-triggered thrombin generation was measured in PRP. Human PRP was preincubated with (A) a PN-1–blocking IgG (150 μg/mL) or (B) a PAI-1–blocking IgG (150 μg/mL). Mouse PRP was from (C) PN-1–deficient mice or (D) PAI-1–deficient mice on thrombin. Results are from one experiment performed in triplicate, representative of at least 3 independent experiments.
Parameters of thrombin generation in human PRP
| . | PRP alone . | PRP + anti–PN-1 . | PRP + anti–PAI-1 . | PRP + irrelevant IgG . |
|---|---|---|---|---|
| Lag time, min | 5.3 ± 0.3 | 5.4 ± 0.5 | 5.5 ± 0.2 | 5.2 ± 0.5 |
| Time to peak, min | 15.3 ± 1.8 | 11.3 ± 1.5* | 12.3 ± 1.6 | 13.9 ± 3.2 |
| Peak height, nM | 124.4 ± 32.6 | 187.9 ± 11.5* | 132.2 ± 33 | 124.5 ± 17.6 |
| ETP, nM thrombin × min | 1640.1 ± 244 | 1540.3 ± 233 | 1701.2 ± 415 | 1509.5 ± 81 |
| . | PRP alone . | PRP + anti–PN-1 . | PRP + anti–PAI-1 . | PRP + irrelevant IgG . |
|---|---|---|---|---|
| Lag time, min | 5.3 ± 0.3 | 5.4 ± 0.5 | 5.5 ± 0.2 | 5.2 ± 0.5 |
| Time to peak, min | 15.3 ± 1.8 | 11.3 ± 1.5* | 12.3 ± 1.6 | 13.9 ± 3.2 |
| Peak height, nM | 124.4 ± 32.6 | 187.9 ± 11.5* | 132.2 ± 33 | 124.5 ± 17.6 |
| ETP, nM thrombin × min | 1640.1 ± 244 | 1540.3 ± 233 | 1701.2 ± 415 | 1509.5 ± 81 |
Values are the mean ± SD of 5 experiments performed in triplicate.
P < .05.
Thrombinography was also performed on mouse PRP. TF-triggered thrombin generation was accelerated (shorter lag time and time to peak), and the peak height was significantly increased in the PRP from PN-1−/− mice (Figure 5C; Table 2). No significant differences were observed in the thrombogram parameters obtained with PRP from PAI-1−/− and WT mice (Figure 5D; Table 2). As observed with human PPP, no significant differences in the thrombogram parameters were observed between WT, PN-1– and PAI-1–deficient PPP (data not shown). Taken together, these results show, for the first time, that platelet PN-1, but not PAI-1, regulates TF-induced thrombin generation in PRP.
Parameters of thrombin generation in mouse PRP
| . | PN-1+/+ . | PN-1−/− . | PAI-1+/+ . | PAI-1−/− . |
|---|---|---|---|---|
| Lag time, min | 4.6 ± 0.5 | 3.2 ± 0.5* | 4.1 ± 0.4 | 3.9 ± 0.6 |
| Time to peak, min | 9.4 ± 0.4 | 6.9 ± 0.7* | 8.4 ± 0.3 | 8.7 ± 1.2 |
| Peak height, nM | 36.8 ± 4.3 | 51.5 ± 4.7* | 49.2 ± 2.2 | 40.4 ± 2.1* |
| ETP, nM thrombin × min | 499.8 ± 44 | 527.4 ± 17 | 541 ± 81 | 470 ± 10.6 |
| . | PN-1+/+ . | PN-1−/− . | PAI-1+/+ . | PAI-1−/− . |
|---|---|---|---|---|
| Lag time, min | 4.6 ± 0.5 | 3.2 ± 0.5* | 4.1 ± 0.4 | 3.9 ± 0.6 |
| Time to peak, min | 9.4 ± 0.4 | 6.9 ± 0.7* | 8.4 ± 0.3 | 8.7 ± 1.2 |
| Peak height, nM | 36.8 ± 4.3 | 51.5 ± 4.7* | 49.2 ± 2.2 | 40.4 ± 2.1* |
| ETP, nM thrombin × min | 499.8 ± 44 | 527.4 ± 17 | 541 ± 81 | 470 ± 10.6 |
Values are the mean ± SD of 3 experiments performed in triplicate.
P < .05.
Platelet PN-1 decreases mouse platelet activation and aggregation and reduces collagen-induced thrombus formation under blood flow conditions
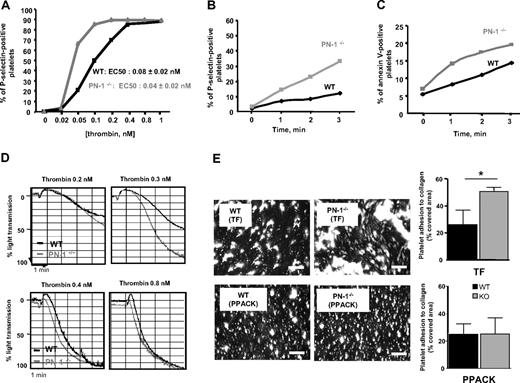
We investigated whether platelet PN-1 could regulate thrombin-induced platelet activation and aggregation. For this purpose, platelets from WT and PN-1−/− mice, activated by increasing concentrations of thrombin, were monitored for surface exposure of P-selectin using flow cytometry. The dose-response curve was displaced to the left for PN-1–deficient platelets compared with WT platelets (Figure 6A). Half-maximal P-selectin exposure was obtained with 0.08 plus or minus 0.02nM in WT versus 0.04 plus or minus 0.02nM thrombin in PN-1–deficient platelets (P < .05, n = 5 mice), indicating that PN-1–deficient platelets were twice as responsive to thrombin as WT platelets. However, activation of WT and PN-1–deficient platelets reached the same extent for concentrations of thrombin more than or equal to 0.4 nM (Figure 6A).
Platelet activation and aggregation, and thrombus formation on collagen under blood flow. (A) Washed platelets from WT (black line) or PN-1–deficient mice (gray line) were activated by increasing doses of thrombin, and P-selectin was measured by flow cytometry. (B-C) Washed platelets from WT mice (black line) or PN-1–deficient mice (gray line) were activated by 0.1nM thrombin. Time courses of (B) P-selectin and (C) phosphatidylserine exposure on platelets were measured by flow cytometry using FITC-labeled rat anti–mouse P-selectin IgG and FITC–annexin V. (D) Washed platelets from WT mice (black line) or PN-1–deficient mice (gray line) were activated by different doses of thrombin, and platelet aggregation was monitored. Data are from 1 experiment representative of at least 3 separate experiments. (E) Rhodamine 6G–labeled mouse platelets in whole blood were perfused in procoagulant conditions (2pM TF and 5mM Ca2+) or in anticoagulant conditions (80μM PPACK), at 1000/s over a collagen surface. After 2 minutes of perfusion, the formation of thrombi was observed at original magnification ×20 under an epifluorescent microscope (Nikon Eclipse TE2000-U; Champigny sur Marne, France), coupled to Metamorph 7.0r1 software (Universal Imaging Corporation). The mean percentage of the area covered by platelets ± SD of 3 independent experiments was calculated and expressed as the mean percentage of the total area covered by thrombi. Data are presented as mean ± SD. *Significant difference (P < .05) versus WT. Bars represent 100 μm.
Platelet activation and aggregation, and thrombus formation on collagen under blood flow. (A) Washed platelets from WT (black line) or PN-1–deficient mice (gray line) were activated by increasing doses of thrombin, and P-selectin was measured by flow cytometry. (B-C) Washed platelets from WT mice (black line) or PN-1–deficient mice (gray line) were activated by 0.1nM thrombin. Time courses of (B) P-selectin and (C) phosphatidylserine exposure on platelets were measured by flow cytometry using FITC-labeled rat anti–mouse P-selectin IgG and FITC–annexin V. (D) Washed platelets from WT mice (black line) or PN-1–deficient mice (gray line) were activated by different doses of thrombin, and platelet aggregation was monitored. Data are from 1 experiment representative of at least 3 separate experiments. (E) Rhodamine 6G–labeled mouse platelets in whole blood were perfused in procoagulant conditions (2pM TF and 5mM Ca2+) or in anticoagulant conditions (80μM PPACK), at 1000/s over a collagen surface. After 2 minutes of perfusion, the formation of thrombi was observed at original magnification ×20 under an epifluorescent microscope (Nikon Eclipse TE2000-U; Champigny sur Marne, France), coupled to Metamorph 7.0r1 software (Universal Imaging Corporation). The mean percentage of the area covered by platelets ± SD of 3 independent experiments was calculated and expressed as the mean percentage of the total area covered by thrombi. Data are presented as mean ± SD. *Significant difference (P < .05) versus WT. Bars represent 100 μm.
Platelet activation from PN-1−/− and WT mice was analyzed during the first minutes after thrombin addition by measuring P-selectin exposure and annexin V binding (Figure 6B-C). In the absence of PN-1, the rates of P-selectin and phosphatidylserine exposure were increased, indicating that PN-1 delays the initial phase of platelet activation.
To confirm the negative effect of PN-1 on the activation of platelets by thrombin, platelet aggregation assays were performed with WT and PN-1–deficient platelets. The rate and extent of aggregation were higher when PN-1–deficient platelets were stimulated with 0.2 or 0.3nM thrombin compared with WT platelets (Figure 6D). At higher thrombin concentrations (≥ 0.4nM), the aggregation profiles of WT and PN-1–deficient platelets became similar (Figure 6D). No difference in aggregation profiles between WT and PN-1–deficient platelets were observed in response to suboptimal concentrations of other agonists (collagen, convulxin, ADP, and PAR4-AP; data not shown). Thus, PN-1 deficiency specifically facilitates platelet aggregation induced by low doses of thrombin.
Because platelet-PN-1 was shown to modulate thrombin generation and platelet aggregation, we investigated the role of PN-1 in thrombus formation. Fluorescently labeled WT or PN-1−/− platelets were perfused at a shear rate of 1000/s over immobilized type I fibrillar collagen. After 2 minutes of perfusion, thrombi formed in procoagulant conditions (TF and Ca2+) were smaller with WT than PN-1−/− platelets, with the covered surface reaching 26% plus or minus 11% and 51% plus or minus 3%, respectively (Figure 6E). In the presence of the thrombin inhibitor PPACK, no differences were observed between both types of platelets (Figure 6E). These results demonstrate that the effect of PN-1 on thrombus formation under blood flow conditions is dependent on its effect on thrombin.
Platelet PN-1 modulates the thrombus formation in response to a vascular injury
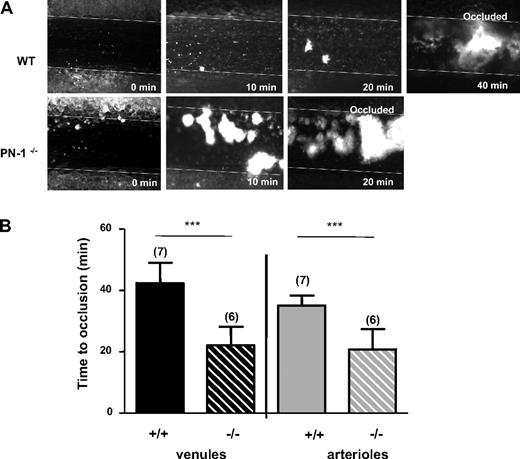
The role of PN-1 on in vivo thrombus formation was investigated in a murine model of thrombosis. Vessel occlusions were monitored in real-time under microscope. At 20 minutes, the mesenteric venules from PN-1−/− mice were completely occluded, whereas WT mice venules show small thrombi with persistent blood flow (Figure 7A). The complete occlusion of the mesenteric veins was twice as rapid in PN-1−/− mice, with the postinjury occlusion times being 22.1 plus or minus 6 minutes compared with 42.4 plus or minus 6.5 minutes in WT mice (Figure 7B). Mesenteric arterioles of PN-1−/− mice were also occluded more rapidly than arterioles of WT mice (Figure 7B). The time to occlusion after injury was 20.0 plus or minus 6.5 minutes for PN-1−/− mice versus 35.0 plus or minus 3.3 minutes for WT mice. Altogether, these data show, for the first time, that platelet PN-1 may have an antithrombotic role in both venules and arterioles.
In vivo thrombus formation. (A) Representative images of FeCl3-injured mesenteric venules in WT and PN-1–deficient mice. Thrombus formation was visualized in real time using an inverted epifluorescent microscope (Nikon Eclipse TE 2000-U) using a lens at 20×/0.5 (Nikon). Images were acquired using a CCD CoolSNAP HQ2 camera (Photometrics/Roper Scientific) and processed with Metamorph 7.0r1 software (Universal Imaging Corporation). (B) Time to occlusion of mesenteric venules (black histograms) and arterioles (gray histograms) was measured after application of FeCl3 in WT mice and PN-1−/− mice. Data are the mean ± SD for the number of mice indicated in parentheses. ***Significant difference (P < .001) versus WT.
In vivo thrombus formation. (A) Representative images of FeCl3-injured mesenteric venules in WT and PN-1–deficient mice. Thrombus formation was visualized in real time using an inverted epifluorescent microscope (Nikon Eclipse TE 2000-U) using a lens at 20×/0.5 (Nikon). Images were acquired using a CCD CoolSNAP HQ2 camera (Photometrics/Roper Scientific) and processed with Metamorph 7.0r1 software (Universal Imaging Corporation). (B) Time to occlusion of mesenteric venules (black histograms) and arterioles (gray histograms) was measured after application of FeCl3 in WT mice and PN-1−/− mice. Data are the mean ± SD for the number of mice indicated in parentheses. ***Significant difference (P < .001) versus WT.
Discussion
Experimental evidence has previously suggested that platelets store and secrete PN-1.6,7 Moreover, high amounts of PN-1 have been observed in platelet-rich areas of human atherosclerotic plaques.21,33 Here, we directly demonstrated that PN-1 is stored in the α-granules from where it is released during platelet activation. The absence of PN-1 in the plasma and the presence of its messenger in platelets strongly suggest the endogenous origin of PN-1 and its targeting to α-granules during megakaryocyte maturation.34 This packaging is further indicated by the absence of the serpin in Gray platelets lacking appropriate assembling of α-granules.32 In contrast to Gronke et al6,7 who detected PN-1 at the platelet surface only after activation, we provide direct evidence that PN-1 is present at the surface of resting platelets, but it represents a minor fraction of total PN-1 compared with the cytosol fraction. The apparent discrepancy between these results can be explained by the improvement of the methods, which allow detection of low levels of PN-1. We have previously reported that PN-1 is associated with membrane GAGs and proteoglycans on vascular cells from where it can be displaced by heparin.31 PN-1 bound to the platelet surface is also displaced by heparin. Because membrane-bound PN-1 is undetectable on Gray platelets, PN-1 is probably translocated to the membrane during platelet production. Surface exposure of PN-1 is unlikely the consequence of platelet activation because platelets were P-selectin negative and activating platelets in vitro did not result in an increased, but in contrast, in a decreased binding of the FITC-coupled anti–PN-1 antibody, indicating a shedding of membrane PN-1 on platelet activation.
Storage of PN-1 in α-granules allows its secretion in significant amounts. Our data show undoubtedly that platelet PN-1 is secreted in an active form during platelet activation because it very efficiently inhibits thrombin. As indicated by the experiments with polybrene, platelet PN-1 is secreted, at least in part, in association with heparin-like GAGs, which have been shown to enhance its interaction with thrombin.9
The inhibitory activity of PN-1, secreted during platelet activation, on both thrombin and uPA was evidenced on both human and mouse platelets using, respectively, a specific PN-1–blocking antibody and platelets from PN-1–deficient animals. These data suggest that platelet PN-1 is a potential player in the control of fibrinolysis. PAI-1 is also stored in α-granules,3 and de novo synthesis of PAI-1 has been reported to occur in human platelets.35 In humans, platelet PAI-1 released locally after activation is assumed to be a major contributor to the stabilization of the thrombus by inhibiting endogenous fibrinolysis,2,36 but PAI-1–independent mechanisms have also been proposed to contribute to platelet-dependent inhibition of fibrinolysis.5 In the present study, no role for secreted PAI-1 in inhibiting uPA was observed with mouse platelets. This is in agreement with a previous report demonstrating significant differences between human and mice fibrinolytic systems.37 Our data are in good agreement with the proposal of the existence of another fibrinolysis inhibitor in human and mouse platelets. The capacity of platelets to secrete active PN-1 provides a new explanation for how platelets regulate fibrinolysis. Further experiments are needed to clarify the respective implications of platelet PN-1 and PAI-1 in tPA-mediated fibrinolysis. However, because the second-order rate constant for the interaction of PN-1 with tPA is 3 orders of magnitude lower than that of PAI-1 with tPA,38 PN-1 appears much less efficient than PAI-1 to block fibrinolysis.
Studies with PN-1−/− and PAI-1−/− mice also point out the supremacy of platelet PN-1 compared with platelet PAI-1 in the regulation of thrombin activity. Indeed, the effect of platelet PN-1 is directed toward low concentrations of thrombin, but it is significant enough to regulate platelet response to thrombin. The deficiency in PN-1 significantly increased the rate and extent of platelet aggregation, as well as surface exposure of P-selectin in response to low doses of thrombin. Interestingly, the deficiency in PN-1 also leads to a faster exposure of negatively charged phospholipids on platelets. As previously suggested7 and as demonstrated now by our data, it can be assumed that PN-1 imposes a threshold concentration of thrombin below which platelet activation cannot proceed.
Platelet activation by trace amounts of thrombin amplifies thrombin generation by providing a catalytic surface suitable for the assembly of the enzymatic complexes tenase and prothrombinase, which lead to prothrombin activation.39 Thrombograms indicate that platelet PN-1 is an as yet unrecognized negative regulator of thrombin generation. Indeed, thrombin formation was accelerated and facilitated when PN-1 was blocked or absent. Nevertheless, the total amount of thrombin formed, which depends on the plasma concentration of clotting factors and inhibitors, was not changed. This is in agreement with previous observations showing that blocking platelet activation affects primarily the kinetics of the reaction rather than the total amount of thrombin produced.40
Vascular injury creates a thrombogenic surface on which platelets are activated and thrombin is generated. To address the question of the role of PN-1 in thrombosis, we have analyzed thrombus formation ex vivo and in vivo in PN-1−/− mice. Our data demonstrate, for the first time, that PN-1 modulates thrombus formation in response to vascular injury. PN-1−/− mice were indistinguishable from their WT littermates in weight and survival,16 and they did not exhibit spontaneous bleeding or thrombotic disorders. In contrast to the apparent normal phenotype of unchallenged PN-1−/− mice, the time required for the formation of platelet aggregates was significantly shorter in arterioles and venules when a vascular lesion was induced in these mice. Indeed, thrombus formation induced ex vivo by collagen in flow conditions and in vivo by FeCl3 injury indicates that the lack of PN-1 results in a loss of protection against thrombosis. Platelet adhesion and activation and thrombin generation contribute to thrombus formation on collagen. PN-1 does not alter the initial phase of platelet adhesion to collagen; but because it inhibits trace amounts of thrombin initially formed, it should alter the propagation phase of thrombus. The absence of difference in thrombi formed in the presence of PPACK between WT and PN-1−/− mice confirms that PN-1 is inhibitory when coagulation takes place.
In conclusion, our results indicate that PN-1 is a critical protective actor against thrombosis, whose role has been underestimated until now. It would be interesting to determine whether a modification in PN-1 expression or function could constitute a risk factor in vessel thrombosis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in abstract form during the Special Session on the Basic Science of Hemostasis & Thrombosis at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 9, 2008. This abstract received the Mary Rodes Gibson Memorial Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Liliane Louedec for animal experimentations and Dr Mary Osborne-Pellegrin for editing this paper.
This work was supported by Inserm, Université Paris VII, Fondation de France (grant 2006005672), Leducq Foundation, and the Bettencourt-Schueller foundation. Y.B. is supported by Région Ile de France.
Authorship
Contribution: Y.B. and L.V. performed experiments; F.A. and M.B. performed the in vivo thrombosis experiments; F.A., M.B., and V.O. performed the in vitro flow experiments; R.F. allowed the platelet studies of 3 GPS patients; M.-C.A. provided the PAI-deficient mice; S.T. and D.M. provided the PN-1–deficient mice; V.A. and B.R. participated in the discussion; and M.J.-P. and M.-C.B. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie-Christine Bouton, Unité Inserm U698, CHU Xavier Bichat, 46 rue Henri Huchard 75877 Paris Cedex 18, France; e-mail: marie-christine.bouton@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal