Polycythemia vera (PV) is a myeloproliferative disorder characterized by a pronounced increase in the number of erythroid cells. However, despite this aberrant proliferation, the incidence of erythroleukemia is paradoxically rare in PV patients. In this study, we show that the progression of Friend virus–induced erythroleukemia is delayed in a mouse model of primary familial congenital polycythemia in which the wild-type Epo-receptor (EpoR) gene is replaced with a truncated human EPOR gene. Herein, we show that these mice exhibit enrichment of Sca1+/cKit− progenitors and several mature immune cells, such as dendritic cells and macrophages. In cotransplantation experiments, Sca1+/cKit− progenitors inhibit the tumorigenicity of Sca1−/cKit+ erythroleukemic cells. A cell line established from Sca1+/cKit− progenitors is also capable of inhibiting leukemic proliferation in culture and in mice. This phenomenon of leukemic inhibition, also detected in the serum of PV patients, is partially attributed to increased nitric oxide secretion. In addition, the administration of erythropoietin into leukemic mice induces a polycythemia-like state associated with the expansion of Sca1+/cKit− progenitors and derivative immune cells, thereby inhibiting leukemia progression. This study indicates that a combination therapy incorporating the enrichment of Sca1+/cKit− progenitors may serve as a novel approach for the treatment of leukemia.
Introduction
The progression of cancer is a multistage process arising from the additive or cooperative activities of multiple pathways, the activation of proto-oncogenes, and the inactivation of tumor suppressor genes. In addition to these events, the tumor microenvironment is also known to play an active role in cancer progression. Understanding both the genetic and microenvironmental factors is necessary to understand how disruption of the homeostatic balance between proliferation, differentiation, and apoptosis of hematopoietic stem cells (HSCs) and hematopoietic stem/progenitor cells (HSPCs) leads to the development of leukemia. Knowledge obtained from such studies is crucial to elucidate the underlying mechanisms of malignancy, as well as define potential therapeutic targets
Previous studies have shown the accumulation of HSCs within the microenvironment of various diseases, including myeloproliferative disorders (MPDs) and several types of leukemia.1,–3 Functional analyses of purified subpopulations of primitive hematopoietic cells have led to the development of procedures for isolating populations that are highly enriched in cells with in vivo stem cell activity.4,5 However, the mechanisms through which HSCs and HSPCs contribute to malignant transformation have yet to be fully delineated. Deciphering the interplay between HSCs and the tumor microenvironment should contribute to our understanding of the mechanisms underlying cancer progression.
MPDs are a group of hematologic malignancies caused by the abnormal production of myeloid cells in the bone marrow. Polycythemia vera (PV) is an acquired, clonal MPD arising in multipotential hematopoietic progenitor cells. This disorder is characterized by hyperplasia of 3 major myeloid lineages, with a pronounced increase in the erythroid lineage.6,–8 Previous studies have shown that polycythemic erythroid progenitor cells exert hypersensitivity to several cytokines, including erythropoietin (Epo), interleukin-3 (IL-3), granulocyte/macrophage-colony stimulating factor (GM-CSF), stem cell factor (SCF), and insulin growth factor-1.9,10 The recent identification of an acquired mutation within JAK-2 (JAK2V617F allele), leading to constitutive activation of Epo signaling, has greatly improved our understanding of the molecular pathogenesis of PV.11,–13 PV patients are predisposed to the development of life-threatening complications associated with augmented Epo signaling, such as thrombosis, hemorrhage, and myelofibrosis with myeloid metaplasia, or acute myeloid or lymphoid leukemias. However, transformation of PV to acute or chronic myeloid leukemia is very rare,14,15 suggesting the presence of a tumor inhibitory mechanism in these patients.16,17
Primary familial congenital polycythemia (PFCP), or familial erythrocytosis, is a hereditary MPD similar to PV.18,19 Mutations within the Epo receptor (EpoR) gene, leading to the expression of a truncated negative regulatory domain, are responsible for the dominantly inherited polycythemic phenotype.19 A PFCP mouse model expressing the truncated form of the human EpoR has been generated. This truncated EpoR lacks dephosphorylation sites for phosphatases, such as Shp-1 and -2, that modulate the phosphorylation of Jak-2. The tyrosine phosphorylation sites Y464 and Y479, which link the EpoR to the Ras and PI3K pathways, are also deleted. This, in turn, leads to increased Epo signaling and augmented erythropoiesis.20 These mice develop a polycythemic phenotype at approximately 3 to 6 weeks of age.20 The establishment of polycythemia in an animal model has provided an excellent experimental tool to study the balance between microenvironmental factors and the HSC or HSPC population in the development of leukemia.
The Friend murine leukemia virus (F-MuLV)–induced erythroleukemia mouse model has contributed to our understanding of the role of HSCs in the progression of leukemia. In this model, erythroid transformation commences with integration of the provirus at the Friend leukemia integration-1 (Fli-1) locus, driving Fli-1 overexpression.21 Constitutive expression of Fli-1 blocks erythroid differentiation and leads to a massive proliferation of erythroblasts in response to Epo.22,–24
Whereas erythroleukemic cells induced by F-MuLV proliferate in response to Epo, we have recently demonstrated that treatment of leukemic mice with Epo delays leukemogenesis.25 This result opens the possibility that changes in the tumor microenvironment can delay the proliferation of leukemic cells through an uncharacterized mechanism. Therefore, we hypothesized that interplay between the splenic microenvironment and leukemic cells is responsible for the lower incidence of leukemia in PV patients. In the present study, we show that F-MuLV–infected PFCP mice exhibit a delay in leukemogenesis. To determine the mechanism of inhibition, we demonstrated that the accumulation of Sca1+/cKit− progenitor cells in F-MuLV–infected PFCP mice has the capacity to suppress the tumorigenic ability of erythroleukemic cells. Moreover, leukemic inhibition may be partially mediated through the presence of immunoregulatory cells and increased production of nitric oxide (NO). Therefore, the unique hematopoietic composition present in PFCP mice may explain the rarity of leukemic transformation in PV patients.
Methods
Mice
H-H and Tr/Tr mice were crossed into Balb/c background (Charles River Canada) for 4 generations to confer susceptibility to F-MuLV–induced erythroleukemia, as described previously.21,25 Mice were genotyped using polymerase chain reaction (PCR) as described previously.20 CB17–severe combined immunodeficiency (SCID) mice were used for transplantation studies. All animal protocols were reviewed and approved by the Sunnybrook Health Sciences Center Animal Care Committee.
Tumor induction
Primary erythroleukemias in H/H or Tr/Tr mice were induced by intraperitoneal injection of F-MuLV at birth, as described previously.20 Mice with palpable spleens and low hematocrit values were sampled. Age-matched noninfected mice served as negative controls. Secondary tumors were developed by intravenous injection of 105 cKit+/Sca1− and cKit−/Sca1+ cells, isolated from the aforementioned primary erythroleukemias, into 1-month-old CB17-SCID mice. cKit−/Sca1+ cells were cotransplanted with 106 of CB3, HB60-5, H9, T5, or T6 cell lines and observed for leukemia development.
Hematocrit measurements
Hematocrit values of wild-type and erythroleukemic mice were measured by collection of tail blood in heparinized capillary tubes, centrifugation at 100g for 10 minutes, and comparison using a hematocrit gauge.
Flow cytometric analysis
Immunofluorescence staining was performed to determine expression of TER119, c-Kit (CD117), Sca1, CD71, Gr-1, Mac-1 (CD11b), B220 (CD45R), CD19, CD4, and CD8 molecules on the splenocytes of control (noninfected) and F-MuLV–infected H/H and Tr/Tr mice and their derivative cell lines, as described previously.26 In brief, 106 cells were incubated with anti-CD16/CD32 blocking antibody (eBioscience) for 10 minutes at 4°C. Cells were stained with primary antibodies for 30 minutes on ice. Primary antibodies were as follows: phycoerythrin-conjugated anti–mouse TER-119, c-Kit, CD71, Gr1, Mac1, CD4, or CD19, allophycocyanin-conjugated anti–mouse Sca1, B220, and CD8 (eBioscience). Cells were washed and resuspended in fluorescence-activated cell sorting (FACS) buffer. Propidium iodide (Sigma-Aldrich) was added (1 μg/mL) to exclude dead cells. A total of 104 events were collected using the FACSCalibur flow cytometer (BD Biosciences) and analyzed using CellQuest Pro software (BD Biosciences).
Cell lines
Erythroleukemia cell lines H7 and H9 were derived from the spleens of F-MuLV–infected H/H mice.27 Erythroleukemia cell lines T5 and T6 were derived from the spleens of F-MuLV–infected Tr/Tr mice. Briefly, splenocytes were washed twice in phosphate-buffered saline (PBS), cultured in α-minimum essential medium supplemented with 20% fetal bovine serum (FBS), 1 U/mL Epo (Boehringer Mannheim), and 10 ng/mL SCF (R&D Systems). Cells were maintained in culture in the presence of Epo and SCF until their proliferation rate indicated establishment in culture, as described elsewhere.27 F-MuLV–induced erythroleukemic cell lines CB3 and CB7 were maintained as described previously.27
Proliferation assay
T6 cells (105) were cultured in the presence of SCF, SCF plus GM-CSF, or SCF plus GM-CSF plus IL-4. Cytokines (R&D Systems) were added at a concentration of 100 ng/mL. Human Epo was added at a concentration of 2 U/mL. After 3-day incubations at 37°C and 5% CO2, supernatants from cytokine-treated T6 cells were collected and rinsed with PBS, and5 × 104 CB3 cells were added. Alternatively, the same number of CB3 cells was resuspended in 1 mL of the aforementioned collected supernatants. CB3 cells were grown for 3 days, and the number of nonadherent erythroleukemic cells was determined by direct cell counting, excluding trypan blue. Results from 3 independent experiments are presented as mean plus or minus SEM.
iNOS inhibitor experiments
CB3 (4 × 104) cells were grown in the presence of FBS plus 10% T6 supernatant with or without the addition of 10/20 μM inducible nitric oxide synthase (iNOS) inhibitor-dihydrochloride (1400W). Human erythroleukemia (HEL) cells (104) were grown in the presence of FBS plus 10% serum from either normal or PV patients (obtained from the clinic of J.T.P.) with or without the addition of 10 μM 1400W. After 3-day incubations, the number of viable cells was determined by direct cell counting, excluding trypan blue. This experiment was performed in triplicate.
SNP in vivo experiments
Balb/c neonates were infected with F-MuLV, as described previously.27 At 5 weeks after infection, mice were treated every day with either sodium nitroprusside (SNP; 10 μg/100 μL) or PBS for a period of 2 weeks. Mice were killed, and hematocrit and spleen weights were determined.
Differentiation of T6 cells using cytokines
T6 cells (105) were cultured in the presence of 100 ng/mL SCF, SCF plus GM-CSF, or SCF plus GM-CSF plus IL-4 for 3 days, then washed in PBS, and incubated for 2 hours at room temperature with normal goat serum to block nonspecific binding. Cells were incubated overnight, at 4°C, with rat anti–mouse CD68 (AbD Serotec) or rat anti–mouse 33D1 antibody (eBioscience), according to the manufacturer's recommendations, washed twice in PBS, and incubated with anti–rat horseradish peroxidase–conjugated IgG (BD Biosciences), counterstained with Carazzi hematoxylin, dehydrated in graded ethanol, and cover slips were applied. Primary antibodies were omitted for negative controls.
Tumor DNA extraction and molecular hybridization
High-molecular-weight DNA was extracted, digested with restriction enzymes, and electrophoresed and hybridized, with random-primed probe, 2 × 106 cpm/mL hybridization buffer as described previously.27
Reverse transcriptase–polymerase chain reaction
The method for reverse transcriptase–polymerase chain reaction (RT-PCR) and sequences for primers are included in the supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Immunoblotting
Cell lines and tumors were lysed with radioimmunoprecipitation assay buffer (0.5% Nonidet P-40, 50 mM Tris HCl, pH 8.0, 120 mM NaCl, 50 mM NaF, 10 μg/mL aprotinin, 100 μg/mL leupeptin, 10 mM phenylmethylsulfonyl fluoride); 50-μg protein lysates were resolved on sodium dodecyl sulfate–10% polyacrylamide gels and immunoblotted as described elsewhere.28 Fli-1 and β-actin antibodies were obtained from Santa Cruz Biotechnology.
Survival and statistical analysis
Survival rates of all mice were computed and plotted according to the nonparametric Kaplan-Meier analysis. Statistical analysis was performed using 2-tailed Student t test with significance considered at P > .05 and P < .005 by analysis of variance using Origin 3.5 software (Microcal Software).
Results
F-MuLV–injected polycythemic mice expressing the human truncated EPOR display delayed erythroleukemia progression
Mutations causing truncations in the cytoplasmic domain of the human EPOR are detected in a large percentage of PFCP patients.29 Recently, a mouse model for PFCP has been generated in which the murine EpoR is replaced with a wild-type or truncated (Tr) form of the human EPOR found in PFCP patients.20 Because the development of erythroleukemia is rare in PV patients, we hypothesized that mice expressing the human truncated form, designated Tr/Tr mice, may be resistant or less susceptible to F-MuLV–induced erythroleukemia compared with mice expressing the wild-type human EPOR, designated H/H mice. To this end, Tr/Tr and H/H neonates were infected with F-MuLV, and a significant delay in the rate of leukemia development was observed in Tr/Tr mice compared with control H/H mice (Figure 1A). Hematocrit levels, a characteristic marker of F-MuLV–induced erythroleukemia, were higher in Tr/Tr mice (Figure 1B). Because fli-1 is frequently activated in F-MuLV–induced erythroleukemias as a result of retroviral integration,30 we examined rearrangement of the fli-1 locus in Tr/Tr and H/H leukemias. Southern blot analysis revealed fli-1 retroviral integration in both mice (Figure 1C). Moreover, Fli-1 expression is elevated in both Tr/Tr and H/H leukemias (Figure 1D), compared with the uninfected controls (Figure 1E).
Mice expressing a truncated form of the EpoR exhibit a delay in the progression of F-MuLV–induced erythroleukemia. (A) Kinetics of erythroleukemia development in H/H and Tr/Tr mice. Age-matched H/H (n = 16) and Tr/Tr (n = 24) neonates were infected with F-MuLV. The survival rates of F-MuLV–infected H/H and Tr/Tr mice were calculated according to the Kaplan-Meier method using a Mann-Whitney U test with 2-sided P values and a significant difference reflecting P < .005. (B) Hematocrit levels of H/H and Tr/Tr mice during F-MuLV–induced erythroleukemia development. Hematocrit levels (%) were measured on a weekly basis in H/H-infected (n = 16) and Tr/Tr-infected (n = 24) mice. *Significant difference on week 11 (P < .005). Error bars represent ± SD. (C) Southern blot analysis of Fli-1 rearrangement in spleen cells isolated from F-MuLV–infected H/H and Tr/Tr mice. (D) Western blot analysis of Fli-1 protein expression in primary cells from H/H and Tr/Tr tumors compared with the F-MuLV–induced erythroleukemia cell line, CB3. (E) Fli-1 protein expression in normal spleens isolated from H/H and Tr/Tr mice, compared with CB3 cells.
Mice expressing a truncated form of the EpoR exhibit a delay in the progression of F-MuLV–induced erythroleukemia. (A) Kinetics of erythroleukemia development in H/H and Tr/Tr mice. Age-matched H/H (n = 16) and Tr/Tr (n = 24) neonates were infected with F-MuLV. The survival rates of F-MuLV–infected H/H and Tr/Tr mice were calculated according to the Kaplan-Meier method using a Mann-Whitney U test with 2-sided P values and a significant difference reflecting P < .005. (B) Hematocrit levels of H/H and Tr/Tr mice during F-MuLV–induced erythroleukemia development. Hematocrit levels (%) were measured on a weekly basis in H/H-infected (n = 16) and Tr/Tr-infected (n = 24) mice. *Significant difference on week 11 (P < .005). Error bars represent ± SD. (C) Southern blot analysis of Fli-1 rearrangement in spleen cells isolated from F-MuLV–infected H/H and Tr/Tr mice. (D) Western blot analysis of Fli-1 protein expression in primary cells from H/H and Tr/Tr tumors compared with the F-MuLV–induced erythroleukemia cell line, CB3. (E) Fli-1 protein expression in normal spleens isolated from H/H and Tr/Tr mice, compared with CB3 cells.
Variation in the hematopoietic composition of the tumor microenvironment delays erythroleukemia development in Tr/Tr mice
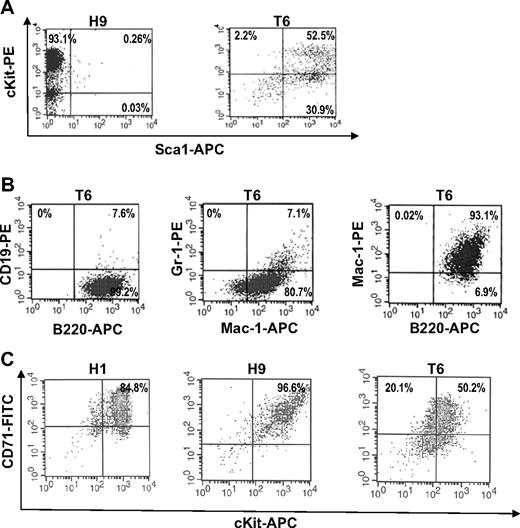
Splenocytes isolated from infected Tr/Tr and H/H mice were cultured in the presence of growth factors, such as SCF and Epo.22 Cell lines from F-MuLV–induced Tr/Tr leukemias, termed T5, T6, and T9, and H/H leukemias, termed H1, H7, and H9, were established. As expected, H/H tumor-derived cell lines treated with Epo induced growth and survival,27 whereas Tr/Tr tumor-derived cell lines, T5 and T6, are unresponsive because of the lack of truncated EPOR protein and transcript expression (data not shown) and display enhanced proliferation only when stimulated by SCF (Figure 2A). Comparative RT-PCR analysis showed that H7 and H9 erythroleukemic cell lines express markers of immature erythroid/megakaryocytic progenitor cells, and T5 and T6 cell lines express multilineage specific markers (Figure 2B). Flow cytometric analysis demonstrated that H9 cells are mostly Sca1−/cKit+, whereas T6 cells are mostly Sca1+/cKitlow (Figure 3A), B220+, Mac-1+, and Gr-1low (Figure 3B). In contrast, none of the H/H cell lines were positive for B220, Mac-1, or Gr-1 (data not shown). It is probable that H/H cell lines overexpressing Fli-1 (Figure 2C) originated from erythroleukemias capable of proliferating in response to Epo,28 whereas Tr/Tr cell lines originate from tumors overexpressing Fli-1 (Figure 1C-D), unresponsive to Epo and express negligible Fli-1 levels after prolonged culture (Figure 2C). These findings suggest that erythroleukemias isolated from Tr/Tr mice contain 2 cellular subsets: an erythroleukemia subpopulation overexpressing Fli-1 and an uncharacterized subpopulation not overexpressing Fli-1 that gave rise to the established cell lines.
Stem/progenitor cells derived from Tr/Tr tumors are sensitive to treatment with SCF. (A) Cell lines derived from H/H (H1, H9) and Tr/Tr (T5, T6) tumors were grown in 10% FBS and in the presence of Epo, SCF, or Epo + SCF. After 3 days of incubation, cells were collected, and the number of viable cells was determined on the indicated days using the trypan blue exclusion assay. (B) RT-PCR analysis of lineage-specific genes using H/H and Tr/Tr cell lines. (C) Fli-1 protein expression in cells derived from H/H and Tr/Tr tumors.
Stem/progenitor cells derived from Tr/Tr tumors are sensitive to treatment with SCF. (A) Cell lines derived from H/H (H1, H9) and Tr/Tr (T5, T6) tumors were grown in 10% FBS and in the presence of Epo, SCF, or Epo + SCF. After 3 days of incubation, cells were collected, and the number of viable cells was determined on the indicated days using the trypan blue exclusion assay. (B) RT-PCR analysis of lineage-specific genes using H/H and Tr/Tr cell lines. (C) Fli-1 protein expression in cells derived from H/H and Tr/Tr tumors.
T6 cells derived from Tr/Tr mice are Sca1+ stem/progenitors. (A) Flow cytometric analysis of H9 cells (derived from H/H tumors) and T6 cells (derived from Tr/Tr tumors) stained with anti-cKit and anti-Sca1 antibodies. (B) T6 cells are bipotent cells. Flow cytometric analysis of T6 cells stained with anti-CD19, anti-B220, anti-Gr1, and anti-Mac1 antibodies. (C) Flow cytometric analysis of H1, H9, and T6 cells stained with anti-CD71 and anti-cKit antibodies.
T6 cells derived from Tr/Tr mice are Sca1+ stem/progenitors. (A) Flow cytometric analysis of H9 cells (derived from H/H tumors) and T6 cells (derived from Tr/Tr tumors) stained with anti-cKit and anti-Sca1 antibodies. (B) T6 cells are bipotent cells. Flow cytometric analysis of T6 cells stained with anti-CD19, anti-B220, anti-Gr1, and anti-Mac1 antibodies. (C) Flow cytometric analysis of H1, H9, and T6 cells stained with anti-CD71 and anti-cKit antibodies.
To further explore why Tr/Tr mice display a delay in F-MuLV–induced erythroleukemia development, we examined the cellular composition of spleens isolated from these mice. Flow cytometric analysis revealed a dramatic difference in the cellular composition of spleens isolated from Tr/Tr and H/H mice (Table 1). Spleens from both Tr/Tr and H/H leukemic mice display a significant increase in the number of cKit+ cells, although H/H leukemic mice display a greater increase compared with uninfected controls (Table 1). In addition, H/H leukemic mice display a significant reduction in the number of immune cells, such as B cells (B220+/CD19+), T cells (CD4+/CD8+), granulocytic cells (Gr-1+), macrophages (Mac-1+), and dendritic cells (DCs; 33D1+; Table 1). Similarly, Tr/Tr leukemic mice display a reduction in the number of T cells (CD4+/CD8+), B cells (B220+/CD19+), and DCs (33 D1+), although to a lesser extent (Table 1). However, spleens isolated from leukemic mice are approximately 10-fold larger; therefore, the reduction in the number of immune cells may be explained by a dilution effect resulting from the expansion of leukemic cells within the spleen. Considering this dilution effect, Tr/Tr tumors display higher numbers of T cells (CD4+/CD8+), B cells (B220+/CD19+), macrophages (Mac-1+), granulocytic cells (Gr-1+), and DCs (33D1+) compared with H/H tumors as well as uninfected Tr/Tr mice (Table 1). Interestingly, although the increase in the percentage of cKit+ cells is greater in the leukemic spleens of H/H mice, the percentage of Sca1+ cells was significantly higher in the spleens of normal and leukemic Tr/Tr mice (Table 1; Figure 4A). These data provide evidence to suggest that the enrichment of Sca1+ progenitor cell population in Tr/Tr mice may be responsible for delayed leukemogenesis.
Expression of lineage-specific markers in the spleens of uninfected (control) and F-MuLV–infected H/H and Tr/Tr mice
| Mouse/treatment . | Marker . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| c-Kit . | Sca1 . | Ter-119 . | B220/CD19 . | CD4 . | CD8 . | Gr-1 . | Mac-1 . | 33D1 . | |
| H/H | |||||||||
| Control | 1.9 ± 1.4 | 8.5 ± 5 | 5.8 ± 3.9 | 38.8 ± 3.15 | 14 ± 3.9 | 10.4 ± 1.8 | 2.9 ± 0.4 | 3.2 ± 0.8 | 30.2 ± 5.0 |
| F-MuLV | 50.2 ± 24.8* | 5.0 ± 2.8 | 22.0 ± 7.5 | 1.95 ± 0.5 | 2.4 ± 0.7* | 1.25 ± 0.4* | 0.8 ± 0.3* | 0.9 ± 0.4* | 5.9 ± 1.1* |
| Tr/Tr | |||||||||
| Control | 1.7 ± 0.7 | 35.2 ± 12.9 | 17.7 ± 3.2 | 45.95 ± 3.2 | 14.4 ± 5.4 | 11.6 ± 0.95 | 4.2 ± 0.9 | 4.6 ± 0.5 | 28.1 ± 3.9 |
| F-MuLV | 14.1 ± 12.4* | 26.9 ± 12.4 | 30.7 ± 13.9 | 11.5 ± 2.0 | 10.0 ± 2.7 | 8.0 ± 3.4 | 7.9 ± 4.2 | 6.2 ± 1.5 | 15.8 ± 1.7 |
| Mouse/treatment . | Marker . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| c-Kit . | Sca1 . | Ter-119 . | B220/CD19 . | CD4 . | CD8 . | Gr-1 . | Mac-1 . | 33D1 . | |
| H/H | |||||||||
| Control | 1.9 ± 1.4 | 8.5 ± 5 | 5.8 ± 3.9 | 38.8 ± 3.15 | 14 ± 3.9 | 10.4 ± 1.8 | 2.9 ± 0.4 | 3.2 ± 0.8 | 30.2 ± 5.0 |
| F-MuLV | 50.2 ± 24.8* | 5.0 ± 2.8 | 22.0 ± 7.5 | 1.95 ± 0.5 | 2.4 ± 0.7* | 1.25 ± 0.4* | 0.8 ± 0.3* | 0.9 ± 0.4* | 5.9 ± 1.1* |
| Tr/Tr | |||||||||
| Control | 1.7 ± 0.7 | 35.2 ± 12.9 | 17.7 ± 3.2 | 45.95 ± 3.2 | 14.4 ± 5.4 | 11.6 ± 0.95 | 4.2 ± 0.9 | 4.6 ± 0.5 | 28.1 ± 3.9 |
| F-MuLV | 14.1 ± 12.4* | 26.9 ± 12.4 | 30.7 ± 13.9 | 11.5 ± 2.0 | 10.0 ± 2.7 | 8.0 ± 3.4 | 7.9 ± 4.2 | 6.2 ± 1.5 | 15.8 ± 1.7 |
Flow cytometric analysis of spleen cells from uninfected control (n = 8) and F-MuLV–infected (n = 9) Tr/Tr and H/H mice. Splenocytes were stained with cKit, Sca1, Ter119, B220, CD19, CD4, CD8, Gr-1, Mac-1, and 33D1. The numbers represent the percentage of stained cells. Values represent the mean ± SEM.
P < .05.
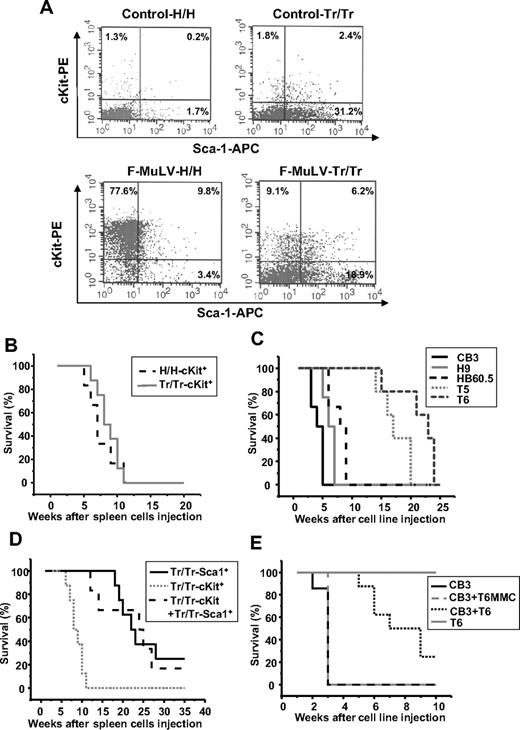
Sca1+ progenitor cells isolated from Tr/Tr tumors delay F-MuLV–induced erythroleukemogenesis. (A) Flow cytometric analysis of stem/progenitor markers in the spleens of uninfected (control) and erythroleukemic (F-MuLV) H/H and Tr/Tr mice. Spleen cells from control or tumorigenic spleens were stained with anti-cKit and anti-Sca1 antibodies. Survival analyses of CB17-SCID mice injected with (B) cKit+ (105) cells isolated from the spleens of F-MuLV–infected H/H (H/H-cKit+, n = 6) and Tr/Tr (Tr/Tr-cKit+, n = 6) mice by flow cytometry using anti-cKit and anti-Sca1 antibodies (“Methods”); (C) (106) erythroleukemic HB60-5 (n = 4), CB3 (n = 6), H/H-derived H9 (n = 6), or Tr/Tr-derived T5 (n = 5) and T6 (n = 6) cells. (D) Sca1+ cells isolated from Tr/Tr tumors (Tr/Tr-Sca1+, n = 8), cKit+ cells isolated from Tr/Tr tumors (Tr/Tr-cKit+, n = 6), and equal numbers of Tr/Tr-cKit+ and Tr/Tr-Sca1+ cells (n = 6). (E) Kinetics of tumor development in CB17-SCID mice injected with 106 CB3 cells (n = 8), T6 cells (n = 6), and equal numbers of CB3 + T6 cells (n = 5) or equal numbers of CB3 + T6 cells pretreated for 1 hour before injection with 10 μg/mL mitomycin C (CB3 + T6-MMC) (n = 3). Significant differences between survival curves of Tr/Tr-cKit+ and Tr/Tr-Sca1+ transplanted SCID mice were calculated according to the Kaplan-Meier method using a Mann-Whitney U test with 2-sided P values less than .05.
Sca1+ progenitor cells isolated from Tr/Tr tumors delay F-MuLV–induced erythroleukemogenesis. (A) Flow cytometric analysis of stem/progenitor markers in the spleens of uninfected (control) and erythroleukemic (F-MuLV) H/H and Tr/Tr mice. Spleen cells from control or tumorigenic spleens were stained with anti-cKit and anti-Sca1 antibodies. Survival analyses of CB17-SCID mice injected with (B) cKit+ (105) cells isolated from the spleens of F-MuLV–infected H/H (H/H-cKit+, n = 6) and Tr/Tr (Tr/Tr-cKit+, n = 6) mice by flow cytometry using anti-cKit and anti-Sca1 antibodies (“Methods”); (C) (106) erythroleukemic HB60-5 (n = 4), CB3 (n = 6), H/H-derived H9 (n = 6), or Tr/Tr-derived T5 (n = 5) and T6 (n = 6) cells. (D) Sca1+ cells isolated from Tr/Tr tumors (Tr/Tr-Sca1+, n = 8), cKit+ cells isolated from Tr/Tr tumors (Tr/Tr-cKit+, n = 6), and equal numbers of Tr/Tr-cKit+ and Tr/Tr-Sca1+ cells (n = 6). (E) Kinetics of tumor development in CB17-SCID mice injected with 106 CB3 cells (n = 8), T6 cells (n = 6), and equal numbers of CB3 + T6 cells (n = 5) or equal numbers of CB3 + T6 cells pretreated for 1 hour before injection with 10 μg/mL mitomycin C (CB3 + T6-MMC) (n = 3). Significant differences between survival curves of Tr/Tr-cKit+ and Tr/Tr-Sca1+ transplanted SCID mice were calculated according to the Kaplan-Meier method using a Mann-Whitney U test with 2-sided P values less than .05.
Accumulation of Sca1+ cells in the spleens of Tr/Tr mice delays leukemia development
To investigate the role of Tr/Tr Sca1+ cells in delayed leukemogenesis, c-Kit+ and Sca1+ cell populations from both H/H and Tr/Tr F-MuLV–injected mice were transplanted into immunodeficient CB17-SCID mice. PCR analysis confirmed expression of F-MuLV env sequences in the sorted cKit+ populations (data not shown). Injection of cKit+ cells from H/H and Tr/Tr leukemic mice gave rise to secondary tumors with similar kinetics (Figure 4B) and decreased hematocrit levels, characteristic of erythroleukemias induced by F-MuLV27 (data not shown). Similar results were observed when cKit+ erythroleukemic cell lines, CB3, H9, and HB60-5 were transplanted into CB17-SCID mice (Figure 4C). Conversely, transplantation of Sca1+ cells from F-MuLV–infected Tr/Tr mice produced leukemia with a significant (P < .005) delay, compared with mice transplanted with cKit+ cells (Figure 4D). These transplanted tumors display characteristics similar to tumors induced in Tr/Tr mice, exhibiting an increase in the percentage of cKit+, Gr-1+, and Mac-1+, and a decrease in B220/CD19+, CD4+, CD8+, and 33D1+ cells (data not shown). Cotransplantation of cKit+ and Sca1+ cells from Tr/Tr leukemic spleens into CB17-SCID mice also led to a significant delay in leukemia development (Figure 4D). These data indicate the potential of Sca1+ cells, carrying the gain-of-function human truncated EPOR, to inhibit the leukemogenic ability of cKit+ cells in vivo.
Sca1+ T6 cells delay leukemogenesis in SCID mice
The T6 cell line isolated from Tr/Tr leukemias proliferates in response to SCF and expresses cKit and Sca1 surface markers (Figure 3A). Whereas all H/H- and Tr/Tr-derived cell lines are clonal, based on their pattern of retroviral integration (data not shown), FACS analysis of T6 cells revealed heterogeneous Sca1 and cKit expression (ie, 30.9% are cKit negative or low; Figure 3A). These cells may have originated from a Sca1+ population within Tr/Tr leukemic mice. Similar to T6 cells, FACS analysis revealed a similar profile of B220+, Gr-1+, and Mac-1+ cells in the Sca1+/cKit− population isolated from normal or leukemic Tr/Tr mice (data not shown). The majority of H1 and H9 cells and a large percentage of T6 cells are also positive for CD71, an erythroid cell marker (Figure 3C). Thus, the Sca1+ leukemic inhibitory cells may represent multipotent progenitors that were induced as an indirect consequence of increased erythroid differentiation in Tr/Tr mice (see “Discussion”).
To examine whether, similar to Sca1+ cells isolated from Tr/Tr leukemias, T6 cells are capable of suppressing the leukemogenic ability of cKit+ cells, T6 cells were cotransplanted with the highly transformed F-MuLV–induced erythroleukemia cell line, CB3,30 into CB17-SCID mice. Cotransplantation suppressed the leukemogenic potential of CB3 cells (Figure 4E). Cotransplantation of T6 cells, pretreated with mitomycin C to induce growth arrest, with CB3 cells was unable to block tumor growth. The T6 cell line is probably derived from a progenitor Sca1+ population from Tr/Tr mice with antileukemic ability.
GM-CSF–inducible differentiation of bipotent stem/progenitor T6 cells to myeloid DCs suppresses leukemic growth
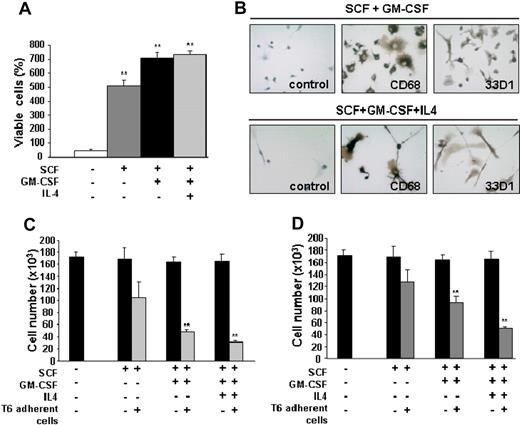
Because Tr/Tr tumors display accumulation of several mature hematopoietic cell types (Table 1), we investigated whether the differentiation of Sca1+ cells is responsible for leukemic inhibition. Sca1+ antileukemic T6 cells (Figure 4E) were treated with SCF, GM-CSF, and IL-4, which are necessary for the maturation of HSCs into granulocytes, macrophages, and DCs.31,32 Stimulation of T6 cells with SCF alone increased the proliferation rate (Figure 5A). This effect is synergized when GM-CSF and IL-4 are added (Figure 5A). Whereas T6 cells die in the presence of IL-4 alone (data not shown), stimulation with IL-4 in combination with SCF and GM-CSF results in differentiation to adherent 33D1+ cells (Figure 5B). These cells acquire a morphology that resembles DCs, with large multiple projections staining positive for both 33D1 and CD68 (Figure 5B).33,34 As expected, T6 cells stimulated with SCF and GM-CSF also stained positive for both 33D1 and CD68 but acquired morphology resembling macrophages and immature DCs (Figure 5B). T6 cells treated with SCF alone do not stain positive for these markers (data not shown). Because T6 cells stain positive for B220 and Mac-1 (Figure 3B) and have the ability to differentiate into macrophages and DCs, it is probable that these cells represent a population of bipotential precursors (see “Discussion”).
T6 cells undergo differentiation to macrophages and DCs in response to GM-CSF and inhibit proliferation of erythroleukemic cells in vitro. (A) T6 (5 × 104) cells were cultured for 3 days in the presence of SCF: SCF + GM-CSF and SCF + GM-CSF + IL-4. The number of viable cells was calculated using the trypan blue exclusion assay. Results from triplicate experiments (mean ± SEM) are presented as the percentage of viable cells (number of cells at day 3 to number cells at day 0). **Significant differences between the no cytokine control and cytokine-treated cells (P < .005). (B) Immunohistochemical staining of adherent T6 cells, after 3 days of incubation with SCF + GM-CSF or SCF + GM-CSF + IL-4 for CD68 (macrophage marker) and 33D1 (DC marker). Brown color represents positive staining. For negative controls, the primary antibody was omitted. Original magnification ×400. T6 cells were cultured with 100 ng/mL SCF, GM-CSF, or IL-4 for 3 days. The adherent cells from this treatment (C) or culture supernatant (D) was added to 5 × 104 CB3 cells for an additional 3 days. Viable cells were determined using the trypan blue exclusion assay. Results are reported as the mean ± SEM of 3 separate experiments. **P < .005. (B) Slides were viewed with a Leica DM LB2 microscope using a 40×/0.65NA air objective. Staining was done with Carazzi hematoxylin. Images were acquired using a Leica DFC300FX camera and Leica Application Suite software (Version 3.1.0).
T6 cells undergo differentiation to macrophages and DCs in response to GM-CSF and inhibit proliferation of erythroleukemic cells in vitro. (A) T6 (5 × 104) cells were cultured for 3 days in the presence of SCF: SCF + GM-CSF and SCF + GM-CSF + IL-4. The number of viable cells was calculated using the trypan blue exclusion assay. Results from triplicate experiments (mean ± SEM) are presented as the percentage of viable cells (number of cells at day 3 to number cells at day 0). **Significant differences between the no cytokine control and cytokine-treated cells (P < .005). (B) Immunohistochemical staining of adherent T6 cells, after 3 days of incubation with SCF + GM-CSF or SCF + GM-CSF + IL-4 for CD68 (macrophage marker) and 33D1 (DC marker). Brown color represents positive staining. For negative controls, the primary antibody was omitted. Original magnification ×400. T6 cells were cultured with 100 ng/mL SCF, GM-CSF, or IL-4 for 3 days. The adherent cells from this treatment (C) or culture supernatant (D) was added to 5 × 104 CB3 cells for an additional 3 days. Viable cells were determined using the trypan blue exclusion assay. Results are reported as the mean ± SEM of 3 separate experiments. **P < .005. (B) Slides were viewed with a Leica DM LB2 microscope using a 40×/0.65NA air objective. Staining was done with Carazzi hematoxylin. Images were acquired using a Leica DFC300FX camera and Leica Application Suite software (Version 3.1.0).
Antigen-presenting DCs are known to induce efficient immune responses and suppress the growth of leukemia.35,36 To directly investigate the antileukemic ability of T6 cells in vitro, this cell line was cultured in the presence of SCF, GM-CSF, and IL-4 or the combination of these cytokines. After 3 days of incubation, treated T6 adherent cells were cocultured with the erythroleukemia cell line, CB3. Results of 3 independent coculture experiments showed that cytokine stimulation of T6 cells was capable of suppressing the growth of CB3 cells. The growth rate of CB3 cells was significantly reduced after stimulation with both GM-CSF and IL-4 (Figure 5C). Similarly, supernatant from untreated T6 cells or GM-CSF/IL-4-stimulated T6 cells was able to inhibit proliferation of CB3 cells, although to a lesser extent than T6 cells (Figure 5D). These experiments indicate that T6 cells stimulated to differentiate into immunoregulatory cells can efficiently inhibit leukemic cell proliferation.
Leukemic inhibition in Tr/Tr mice is mediated in part through increased production of NO
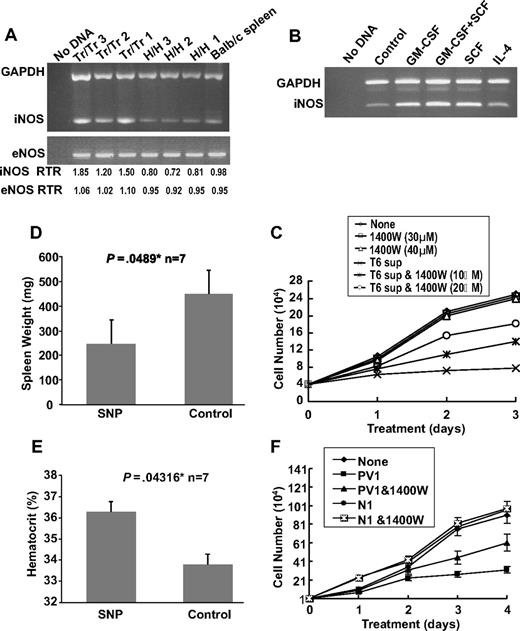
Previous studies have demonstrated that the NO pathway is activated in the polycythemia mouse model expressing exogenous levels of Epo.37 We examined the expression of endothelial nitric oxide synthase (eNOS) and iNOS in the spleens of Tr/Tr and H/H mice. RT-PCR analysis detected higher levels of iNOS in Tr/Tr splenocytes compared with H/H splenocytes (Figure 6A), suggesting that increased iNOS expression may be partially responsible for the leukemic inhibitory effect observed in Tr/Tr mice. Accordingly, RT-PCR analysis revealed higher iNOS expression in stimulated T6 cells treated with SCF or GM-CSF, but not in those treated with IL-4 alone (Figure 6B). Next we examined whether the inhibitory potential of T6 cells (Figure 5C) is partially mediated through iNOS activity. The addition of N-3-(aminomethyl) acetamine (1400W), a selective inhibitor of iNOS,38 to T6 supernatant partially blocked the ability of T6 cells to inhibit the proliferation of CB3 cells in a dose-dependent manner (Figure 6C). Moreover, F-MuLV–infected mice treated with the NO donor drug, SNP, display a partial inhibition of leukemogenesis and significantly decreased spleen weights and increased hematocrit levels (Figure 6D-E).39 Overall, this study suggests that the increased production of iNOS, and possibly other factor(s), by leukemic inhibitory cells plays a significant role in leukemic inhibition.
The inhibitory effect of T6 cells on the proliferation of erythroleukemic cells is partially mediated through the NO pathway. (A) RT-PCR analysis of iNOS and eNOS expression in spleens (n = 3) isolated from Tr/Tr and H/H mice. Normal Balb/c splenic RNA was used as control. (Bottom panel) Real-time PCR ratios (RTR) for iNOS and eNOS in the isolated spleens. (B) RT-PCR analysis of iNOS expression in T6 cells after 2 days of incubation with the indicated cytokines. An untreated T6 cell sample was designated as a control. (C) Effects of the iNOS inhibitor, 1400W, on the proliferation of erythroleukemic cells. CB3 cells were cultured in the presence of 10% FBS and T6 supernatant (10%), T6 supernatant + 1400W or 1400W alone. The number of viable cells was determined at the indicated time points using the trypan blue exclusion assay. (D) Balb/c neonates were infected with F-MuLV. At 5 weeks after infection, mice were treated daily with SNP (10 μg/100 μL) or PBS for a period of 2 weeks. At the end of treatment, mice were killed, and spleen weights (D) and hematocrit values (E) were determined. *Significant difference between the 2 groups. Error bars represent ± SD. (F) PV patient serum inhibits the growth of human erythroleukemia in culture. HEL cells (104) were cultured in the presence of 10% FBS and either normal or PV patient serum with or without 1400W (10 μM). Viable cells were counted on the indicated days using trypan blue exclusion assay. Graphs show the effect of serum from PV patient 1 (PV1) and normal person 1 (N1).
The inhibitory effect of T6 cells on the proliferation of erythroleukemic cells is partially mediated through the NO pathway. (A) RT-PCR analysis of iNOS and eNOS expression in spleens (n = 3) isolated from Tr/Tr and H/H mice. Normal Balb/c splenic RNA was used as control. (Bottom panel) Real-time PCR ratios (RTR) for iNOS and eNOS in the isolated spleens. (B) RT-PCR analysis of iNOS expression in T6 cells after 2 days of incubation with the indicated cytokines. An untreated T6 cell sample was designated as a control. (C) Effects of the iNOS inhibitor, 1400W, on the proliferation of erythroleukemic cells. CB3 cells were cultured in the presence of 10% FBS and T6 supernatant (10%), T6 supernatant + 1400W or 1400W alone. The number of viable cells was determined at the indicated time points using the trypan blue exclusion assay. (D) Balb/c neonates were infected with F-MuLV. At 5 weeks after infection, mice were treated daily with SNP (10 μg/100 μL) or PBS for a period of 2 weeks. At the end of treatment, mice were killed, and spleen weights (D) and hematocrit values (E) were determined. *Significant difference between the 2 groups. Error bars represent ± SD. (F) PV patient serum inhibits the growth of human erythroleukemia in culture. HEL cells (104) were cultured in the presence of 10% FBS and either normal or PV patient serum with or without 1400W (10 μM). Viable cells were counted on the indicated days using trypan blue exclusion assay. Graphs show the effect of serum from PV patient 1 (PV1) and normal person 1 (N1).
Similarly, sera from 3 PV patients (PV1-PV3) inhibited proliferation of the human erythroleukemia cell line, HEL (Figure 6F and supplemental Figure 1A-B). In addition, this growth arrest is partially blocked by incubation with 1400W. In contrast, sera from 5 normal persons (N1-N3) increased the proliferation of HEL cells (Figure 6F and supplemental Figure 1A-B). These results suggest that both human and murine PV share a similar mechanism of leukemic inhibition.
Recapitulation of the polycythemic phenotype through treatment with Epo delays leukemogenesis
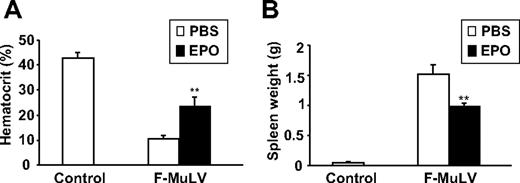
We have previously demonstrated that treatment of F-MuLV–induced erythroleukemic mice with Epo delays tumor progression.25 To examine whether the mechanism underlying Epo-induced leukemogenesis inhibition is similar to the inhibition observed in Tr/Tr mice, we analyzed the cellular profile of spleens isolated from normal and F-MuLV–induced leukemic mice before and after treatment with Epo. This experiment confirmed a previous observation in which administration of Epo in normal and leukemic mice increases hematocrit and reduces the size of splenic tumors (Figure 7). Flow cytometric analysis of splenocytes isolated from these mice displayed a decrease (from 49.9% to 15.2%) in the number of cKit+ leukemic cells (Table 2). Similar to F-MuLV–induced leukemias induced in Tr/Tr mice (Table 1), spleens of normal and leukemic mice treated with Epo were also enriched with a higher number of Sca1+, Ter119+, CD19+/B220+, CD4+, CD8+, Gr-1+, Mac-1+, and 33D1+ cells (Table 2). Thus, similar to Tr/Tr mice, increased erythroid differentiation induced through Epo administration promotes the enrichment of Sca1+ progenitors capable of leukemia inhibition.
Treatment of erythroleukemic mice with Epo results in accumulation of stem/progenitor Sca1+ cells and immune cells. At 6 weeks after F-MuLV infection, mice were separated into 2 experimental groups and injected intraperitoneally with PBS or 100 IU recombinant human Epo (3 times a week for a 2-week period). Simultaneously, a control group of uninfected age-matched mice were treated with PBS for the same time period. (A) Sixty days after infection, mice were bled from tail veins and hematocrit values were measured and represented as the mean ± SEM. **P < .005. (B) Spleens from all groups were harvested 60 days after infection, and their weight measurements are represented as the mean ± SEM. **P < .005.
Treatment of erythroleukemic mice with Epo results in accumulation of stem/progenitor Sca1+ cells and immune cells. At 6 weeks after F-MuLV infection, mice were separated into 2 experimental groups and injected intraperitoneally with PBS or 100 IU recombinant human Epo (3 times a week for a 2-week period). Simultaneously, a control group of uninfected age-matched mice were treated with PBS for the same time period. (A) Sixty days after infection, mice were bled from tail veins and hematocrit values were measured and represented as the mean ± SEM. **P < .005. (B) Spleens from all groups were harvested 60 days after infection, and their weight measurements are represented as the mean ± SEM. **P < .005.
Enrichment of Sca1+ cells in Epo-treated mice
| Marker . | Treatment/mouse . | ||
|---|---|---|---|
| PBS/control . | PBS/F-MuLV . | Epo/F-MuLV . | |
| cKit | 0.9 ± 0.3 | 49.9 ± 2.1 | 15.2 ± 4.0* |
| Sca1 | 8.0 ± 1.7 | 4.0 ± 0.8 | 18.2 ± 3.8† |
| Ter119 | 4.7 ± 1.1 | 13.95 ± 5.5 | 32.6 ± 4.7† |
| CD19/B220 | 30.0 ± 3.2 | 7.9 ± 3.1 | 9.4 ± 1.7 |
| CD4 | 19.8 ± 0.7 | 2.05 ± 0.15 | 10.1 ± 1.25* |
| CD8 | 11.2 ± 4.0 | 1.8 ± 0.6 | 3.7 ± 0.2† |
| Gr1 | 16.2 ± 9.0 | 5.0 ± 1.45 | 9.6 ± 0.5† |
| Mac1 | 4.4 ± 0.65 | 0.9 ± 0.3 | 5.4 ± 0.9* |
| 33D1 | 36.7 ± 6.5 | 5.8 ± 0.2 | 10.3 ± 1.4† |
| Marker . | Treatment/mouse . | ||
|---|---|---|---|
| PBS/control . | PBS/F-MuLV . | Epo/F-MuLV . | |
| cKit | 0.9 ± 0.3 | 49.9 ± 2.1 | 15.2 ± 4.0* |
| Sca1 | 8.0 ± 1.7 | 4.0 ± 0.8 | 18.2 ± 3.8† |
| Ter119 | 4.7 ± 1.1 | 13.95 ± 5.5 | 32.6 ± 4.7† |
| CD19/B220 | 30.0 ± 3.2 | 7.9 ± 3.1 | 9.4 ± 1.7 |
| CD4 | 19.8 ± 0.7 | 2.05 ± 0.15 | 10.1 ± 1.25* |
| CD8 | 11.2 ± 4.0 | 1.8 ± 0.6 | 3.7 ± 0.2† |
| Gr1 | 16.2 ± 9.0 | 5.0 ± 1.45 | 9.6 ± 0.5† |
| Mac1 | 4.4 ± 0.65 | 0.9 ± 0.3 | 5.4 ± 0.9* |
| 33D1 | 36.7 ± 6.5 | 5.8 ± 0.2 | 10.3 ± 1.4† |
Flow cytometric analysis of spleen cells from uninfected control mice treated with PBS (n = 5) and F-MuLV–infected mice (60 days after infection) treated with either PBS or Epo (n = 6). Splenocytes were stained with cKit, Sca1, Ter119, B220, CD19, CD4, CD8, Gr-1, Mac-1, and 33D1. Values represent the mean ± SE.
P < .005.
P < .05.
Discussion
A fundamental problem in cancer research is the inability to identify the cell capable of initiating and sustaining tumor growth, the CSC. Recent studies revealed the existence of functional heterogeneity within tumors and introduced the concept of the CSC.40 In this study, we propose the theory that cancer development, in many cases, depends on the balance between CSCs and cancer inhibitory cells surrounding the tumor.
The tumor microenvironment plays a major role in the progression of numerous malignancies. Previously, we have shown that the splenic microenvironment is necessary for the progression of F-MuLV–induced erythroleukemia because the progression of this disease is significantly delayed after splenectomy.26 In this study, we have shown that progression of erythroleukemia in polycythemic mice expressing a truncated human EPOR (Tr/Tr) is significantly delayed compared with control (H/H) mice. The majority of H/H and Tr/Tr primary tumors have acquired insertional activation at the fli-1 locus, a critical genetic event in F-MuLV–induced erythroleukemia.30 Detection of fli-1 retroviral integration indicates that the inhibitory effect observed in Tr/Tr mice is not associated with delayed initiation of transformation. Rather, initiation should be accelerated in Tr/Tr mice because they display increased numbers of BFU-E progenitors, the known target cell of F-MuLV.20 We observed that the splenic microenvironment of Tr/Tr mice is enriched with Sca1+ inhibitory progenitor cells. Cotransplantation experiments in CB17-SCID mice displayed the ability of these Sca1+/cKit− progenitors to delay the tumorigenic potential of transformed erythroleukemic cells, further implicating that the enrichment of Sca1+ progenitor cells in the spleens of Tr/Tr mice inhibits leukemia progression.
The human gain-of-function EpoR truncation present in Tr/Tr mice is associated with increased Epo signaling and erythroid differentiation through constitutive activation of the Jak2/STAT pathway because the EPOR truncation removes the ability of several phosphatases to modulate phosphorylation of Jak2.20 We have shown that these mice, along with increased erythropoiesis, also display an enrichment of Sca1+ progenitors and accumulation of immunoregulatory cells, including DCs, macrophages, and granulocytes, which are further increased on injection with F-MuLV. In addition, Tr/Tr tumor-derived cells do not respond to treatment with Epo because they lack EPOR expression. Although the origin of Sca1+ cells remains unclear, the lack of EpoR expression in these cells suggests that these multipotent progenitors are indirectly induced as a consequence of massive erythroid differentiation in Tr/Tr mice. In addition, the accumulation of immunoregulatory cells in Tr/Tr mice originated from Sca1+ progenitors capable of exerting potent antileukemic activity because injection of Tr/Tr Sca1+ cells into CB17-SCID mice induces their production. Moreover, treatment of T6 cells, a transformed derivative of Tr/Tr Sca1+ cells, with GM-CSF and/or IL-4 promotes differentiation to macrophages and DCs. In addition, the inhibitory potential of Sca1+ progenitors and immunoregulatory cells can also be observed after coculture experiments, seeing as both T6 cells and supernatant treated with growth factors are capable of inhibiting erythroleukemia growth. This inhibitory effect increases after differentiation to macrophages and DCs. Therefore, the presence of these immunoregulatory cells in the spleen could also play a major role in delaying leukemogenesis in Tr/Tr mice possibly through secretion of inhibitory factors and /or initiation of a potent immune response.
Because oxidative stress is induced in polycythemic mice overexpressing Epo37 and macrophages are known to produce iNOS,39 we suspected that this pathway is partially responsible for delayed leukemia progression. In support of this hypothesis, a recent study has demonstrated that, in response to γ-interferon and tumor necrosis factor-α, iNOS expression inhibits proliferation and induces apoptosis in human erythroleukemic cells.41 Accordingly, we have shown that in T6 cells the expression of iNOS increases in response to SCF or GM-CSF and treatment with the iNOS inhibitor, 1400W, partially removes their ability to inhibit proliferation. Moreover, treatment of erythroleukemic mice with the NO donor drug, SNP, results in a significant delay in cancer progression. Our data suggest that the production of NO from Sca1+ progenitors in F-MuLV–infected Tr/Tr mice is partially responsible for the delay in leukemogenesis. Therefore, identification and isolation of other factors secreted from Sca1+ progenitor cells and their derivatives may provide a basis for the development of novel therapies for the treatment of leukemia.
The leukemic inhibitory effect can also be detected when the sera of PV patients is added to the growth medium of a human erythroleukemia cell line, HEL. Sca1, a murine stem cell marker, cannot be used to identify a similar inhibitory stem cell population in patients with PV. However, although identification of specific inhibitory progenitor cells in PV patients is challenging at this stage, their characterization could further validate a role for these progenitor cells in the progression of leukemia. Interestingly, 2 recent reports have demonstrated that changing the microenvironment, either through the conditional knockout of retinoblastoma in hematopoietic cells3 or retinoic acid receptor–γ deficiency,2 causes myeloproliferative syndrome in mice. Increased myeloid proliferation in these knockout mice is intrinsically specific to the bone marrow microenvironment and not the stem cells. We have also recently reported that the progression of F-MuLV–induced erythroleukemia is delayed in Epo-transfused or vascular endothelial growth factor–overexpressing mice.26 Moreover, treatment of mice with Epo increases the number of Sca1+ cells and several other immune cells observed in the spleens of Tr/Tr mice leading to inhibition of leukemogenesis. Cancer development is also rare in patients expressing the unique R200W mutation in the von Hippel-Lindau tumor suppressor gene, which is associated with induction of Chuvash polycythemia and the presence of elevated serum levels of Epo and vascular endothelial growth factor.42,–44 Taken together, these studies demonstrate that alteration of the microenvironment through specific genetic manipulations induces the expansion of cancer inhibitory cells.
The origin of Sca1+ inhibitory cells in the hematopoietic hierarchy is still unclear. However, inhibitory T6 cells also stain positive for markers of early erythroid (CD71), early B-cell (B220), and myeloid (Mac-1) progenitors. Bipotential precursors of B cells and monocytes, with restriction to macrophage differentiation, have previously been identified in murine fetal liver and adults.45,46 Whether such cells have the ability to undergo differentiation into other cells, such as DCs, has yet to be revealed. Previous studies have shown that Sca1+/Mac1+ cells capable of producing NO may have an immunoregulatory role in vivo.47 Thus, the leukemic inhibitory cells identified in Tr/Tr mice could represent these bipotential progenitors, and their study could further increase our understanding of hematopoiesis.
Finally, we have shown that leukemic mice treated with Epo fail to develop anemia and display a delay in the development of erythroleukemia that is associated with a significant increase in Sca1+ cells as well as several mature immune cells. Because erythroleukemic cells overexpressing Fli-1 proliferate in response to Epo,48 this inhibitory effect may be attributed to cellular expansion after treatment with Epo. In support of this observation, a recent study has demonstrated that myelodysplastic syndrome patients treated with a combination of Epo and granulocyte-colony stimulating factor display improved survival rates.49 Indeed, leukemic mice treated with both Epo and GM-CSF further increases the number of inhibitory Sca1+ cells as well as several mature cells, compared with Epo alone treated mice (T.U. and Y.B.-D., unpublished results, May 2008). We therefore propose that administration of Epo and GM-CSF in combination with NO agonistic drugs could present a better therapy for the clinical treatment of myelodysplastic syndrome. Moreover, the inhibitory cells discovered in this study may eventually be used as a diagnostic marker to identify the therapy most suitable for the treatment of myelodysplastic syndrome.
In conclusion, we have demonstrated that enrichment of cancer inhibitory cells and immunoregulatory cells within the leukemic spleens of a polycythemic mouse model inhibits the progression of F-MuLV–induced erythroleukemia. The suppression of leukemogenesis is mediated in part through the presence of immunoregulatory cells and NO secretion that directly inhibits proliferation of erythroleukemic cells. Identification and isolation of a similar mechanism of inhibition in PV patients could have a major clinical application for the treatment of leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Mira Puri and David Spaner for the critical reading of this manuscript and Ms Melanie Suttar for her excellent secretarial work.
This work was supported by research funding from the Canadian Institute of Health Research (MOP84460; Y.B.-D.). M.H. was supported by a scholarship from the Ontario Graduate Scholarship.
Authorship
Contribution: T.U. designed and performed the research, analyzed the data, and wrote the manuscript; Y.-J.L. was involved in analyzing iNOS expression shown in Figure 6A-B; M.H. designed experiments, analyzed the data, and performed the experiments presented in Figure 7; Y.L. performed the experiment presented in Figures 6C and F and supplemental Figure 1; L.M.V.-F. designed experiments, provided constructive input on this project, and reviewed the manuscript; X.Z. performed the experiment shown in Figure 6D and E; J.T.P. provided us with Tr/Tr, H/H mice, and primers and was involved in reviewing the manuscript; and Y.B.-D. is the principal investigator, who was involved in designing the experiments and analyzing the overall data presented in this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yaacov Ben-David, Sunnybrook Health Sciences Centre, Division of Molecular and Cellular Biology, Rm S-216, 2075 Bayview Ave, Toronto, ON M4N 3M5, Canada; e-mail: bendavid@sri.utoronto.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal