Abstract
Although surface sialic acid is considered a key determinant for the survival of circulating blood cells and glycoproteins, its role in platelet circulation lifetime is not fully clarified. We show that thrombocytopenia in mice deficient in the St3gal4 sialyltransferase gene (St3Gal-IV−/− mice) is caused by the recognition of terminal galactose residues exposed on the platelet surface in the absence of sialylation. This results in accelerated platelet clearance by asialoglycoprotein receptor-expressing scavenger cells, a mechanism that was recently shown to induce thrombocytopenia during Streptococcus pneumoniae sepsis. We now identify platelet GPIbα as a major counterreceptor on ST3Gal-IV−/− platelets for asialoglycoprotein receptors. Moreover, we report data that establish the importance of sialylation of the von Willebrand factor in its function.
Introduction
Platelets, small anuclear blood cells essential for hemostasis, are produced from precursor bone marrow megakaryocytes and released into the circulation, where they have a well-defined life span of 7 to 10 days for human platelets and 3 to 5 days for mouse platelets. Senescent platelets are selectively removed by the scavenging system, but little is known about the molecular mechanisms controlling their clearance. Platelet life span in mice is defined by an intrinsic apoptotic mechanism controlled by an antagonistic balance between prosurvival Bcl-xL and proapoptotic Bak.1 Phosphatidylserine exposure is considered the ultimate death signal that targets apoptotic platelets for phagocytosis.2,3 Additional time-dependent surface modifications, such as loss of sialic acid or antibody binding, are also thought to influence platelet life span.4,5 Loss of sialic acid residues initiates removal of erythrocytes and plasma glycoproteins by exposing penultimate βgalactose (βGal) residues, effecting the recognition and phagocytosis by the lectin asialoglycoprotein receptor (ASGPR).6-9 Similar to erythrocytes, in vitro desialylated platelets are cleared rapidly from the circulation.4,10 As platelets lose sialic acid from membrane glycoproteins during aging and circulation, enhanced exposure of nonsialylated glycan chains may represent a physiologic phenomenon triggering clearance of senescent blood cells.11-13 Observations that platelets with increased exposure of βGal may serve as endogenous ligands for the ASGPR were recently substantiated in a study that demonstrated this clearance mechanism to induce thrombocytopenia during Streptococcus pneumoniae sepsis.14
Sialyltransferases are a family of 21 characterized glycosyltransferases transferring sialic acid from the donor substrate CMP-sialic acid to acceptor oligosaccharide substrates.15 The biosynthesis of oligosaccharide chains on glycoconjugates is generally completed by addition of sialic acid, fucose, or histo-blood group ABH glycans, which cap the structures and prevent further chain elongation. Because of their terminal position and negative charge, sialic acids potentially mediate a great variety of cell-cell and cell-matrix interactions. They are critical components of ligands recognized by sialic acid–specific lectins, such as Siglecs and selectins, but can also play the opposite role by shielding underlying immature carbohydrates that could otherwise serve as ligands for lectin receptors. Several specific sialyltransferase genes have been targeted in mice, and mice deficient in several of these enzymes exhibit defects in the hematopoietic cell lineage.16-18 In particular, the ST3Gal-IV enzyme, which transfers sialic acid in a α2,3 linkage to glycans with terminal Galβ1-4GlcNAc, Galβ1-3GlcNAc, and Galβ1-3GalNAc sequences,19,20 operates as a dominant modifier of hemostasis by concealing ASGPR ligands on platelets and von Willebrand factor (VWF).16 We addressed the impact of deficient sialylation on ASGPR-mediated platelet clearance after transfusion using a ST3Gal-IV knockout mouse model with autosomal recessive thrombocytopenia. Deficiency in the ST3Gal-IV sialyltransferase increases exposure of penultimate βGal on VWF and platelets and decreases plasma levels of these to 50% and 30% of normal values, respectively.16
In the present study, we provide evidence for rapid ASGPR-mediated clearance of transfused ST3Gal-IV−/− platelets by liver macrophages and hepatocytes. We identify platelet GPIbα, the major VWF receptor, as a key counterreceptor for the ASGPR. We demonstrate that deficient sialylation increases VWF-mediated platelet activation, and suggest that increased binding of deficiently sialylated VWF to platelets contributes to the accelerated clearance of ST3Gal-IV−/− platelets.
Methods
Mice
Wild-type (WT) C57BL/6J mice were purchased from The Jackson Laboratory. ST3Gal-IV+/− mice were provided by the Consortium for Functional Glycomics (www.functionalglycomics.org). Mice were maintained and treated as approved by the Harvard Medical Area Standing Committee on Animals according to National Institutes of Health standards as set forth in the Guide for the Care and Use of Laboratory Animals.21
Platelet preparation
Mouse blood was obtained and platelet-rich plasma (PRP) isolated as described previously.22 After separation from plasma, platelets were washed in a solution of 140 mM NaCl, 5 mM KCl, 12 mM trisodium citrate, 10 mM glucose, 12.5 mM sucrose, pH 6.0 (buffer A), and resuspended in 140 mM NaCl, 3 mM KCl, 0.5 mM MgCl2, 5 mM NaHCO3, 10 mM glucose, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4 (buffer B).
Flow cytometry
Platelets from age- and sex-matched ST3Gal-IV+/+, ST3Gal-IV+/−, and ST3Gal-IV−/− mice were washed and suspended in buffer B at 2 × 108/mL. Platelet surface galactose exposure was analyzed by incubation with fluorescein isothiocyanate (FITC)–conjugated Ricinus communis I (RCA-1; Vector Laboratories), Erythrina cristagalli (ECA; EY Laboratories), and Griffonia simplicifolia (GS-IB4; EY Laboratories). Platelets were fixed for 20 minutes at 37°C using BD Cytofix (BD Biosciences), washed, and incubated overnight at 4°C with the monoclonal antibodies 1B2 (specific for Galβ1-4GlcNAcβ-R),23 3C9 (specific for Galβ1-3GalNAcα-R; clone similar to HH8),24 or TH5 (specific for Galα1-3Galβ1-4GlcNAcβ-R).25 Unbound antibodies were removed by washing and bound antibodies detected by incubation with isotype-specific Alexa 488–labeled rabbit anti–mouse Ig (Invitrogen). Platelet surface glycoprotein receptor expression was determined by incubation with FITC-labeled anti-GPIX and phycoerythrin (PE)–labeled anti-GPIbα IgGs. Appropriate species control IgGs were used in all experiments (Emfret). To measure VWF binding, platelets were suspended in plasma, activated with increasing concentrations (0, 0.25, 0.5, 1, and 2 U/mL) of botrocetin (Centerchem), and incubated with FITC-labeled rabbit anti-VWF antibody (Emfret). The percentage of platelet reticulocytes was determined using Thiazole Orange staining of RNA according to the manufacturer's instructions (BD Biosciences). Reticulated and nonreticulated platelets were gated, and the respective forward scatter (FSC) determined and expressed as mean fluorescence intensities. Cell acquisition was performed on a FACSCalibur flow cytometer (BD Biosciences), and a total of 20 000 events analyzed using the CellQuest software (BD Biosciences). All experiments were repeated in triplicate.
Platelet circulation studies
Platelets were fluorescently labeled by incubation with 5 μM 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen) in buffer B containing 0.001% dimethyl sulfoxide (Sigma-Aldrich) for 20 minutes at 37°C. Unincorporated dye was removed by centrifugation and platelets suspended in buffer B. Platelet transfusions were performed by retro-orbital injection of 200 μL of 4 × 108 platelets/mL. In inhibition studies, 2 mg asialofetuin or fetuin (Sigma-Aldrich) was injected into the opposite eye plexus 1 minute before platelet transfusion. GPIbα was enzymatically removed from the surface of platelets suspended in buffer B containing 1 mM Ca2+ and 10 μg/mL O-sialoglycoprotein endopeptidase (Cerladane), and removal monitored by flow cytometry using PE-anti–mouse GPIbα Xia.G5. Treatment of platelets with O-sialoglycoprotein endopeptidase did not alter the surface expression of GPIX, GPIbβ, GPV, GPVI, or β3 (CD61), as determined by flow cytometry (not shown). After transfusion, blood was collected by retro-orbital eye bleeding at time points of 1, 3, 5, and 30 minutes and 2, 24, 48, and 72 hours, and the percentage of CMFDA+ platelets in PRP determined by flow cytometry. Data were normalized by designating the amount of CMFDA+ ST3Gal-IV+/+ platelets at the first time point (1 or 5 minutes) as 100% and expressing all other CMFDA+ values at different posttransfusion times as the percentage of this value. Data represent an average of 4 mice plus or minus SD per experiment and are representative of 3 separate experiments.
VWF binding to transfused platelets
WT platelets were fluorescently labeled by incubation with 1.8 μM CM-Orange (Invitrogen) and transfused into WT or ST3Gal-IV−/− mice. Blood samples were obtained immediately after transfusion, and VWF binding was measured in PRP by flow cytometry using FITC-conjugated rabbit anti-VWF antibody (Emfret). The percentage of platelets positive for green and orange fluorescence was determined.
Histology
Platelets, labeled with CMFDA, were transfused into WT mice as described in “Platelet circulation studies.” Organs were harvested after 30 minutes or 2 hours and cryosections prepared. To visualize macrophages, specimens were stained with rat anti–mouse F4/80 IgG (Serotec), washed, and incubated with AlexaFluor 594 goat anti–rat secondary antibody (Invitrogen). Hepatocytes were visualized with rabbit anti–human albumin antibody (Dako Denmark), followed by incubation with AlexaFluor 594 goat anti–rabbit secondary antibody (Invitrogen). Nuclei were visualized by 4,6-diamidino-2-phenylindole staining. Images were obtained using a Zeiss Axiovert S-100 equipped with a 100× differential interference contrast oil immersion objective (NA 1.4). Five nonoverlapping randomly selected fields of view from stained tissue sections were microscopically examined for semiquantitative analysis of differential hepatocyte and macrophage platelet uptake.
Macrophage depletion
Mice were injected intravenously with 0.02 mL clodronate liposomes (Cl2MDP-LIPs, Dr Van Rooijen, Vrije Universiteit Medical Center, Department of Molecular Cell Biology, Faculty of Medicine) per 10 g body weight 24 hours before platelet transfusions.26 Controls were injected with phosphate-buffered saline–containing liposomes. Staining for macrophages was performed using F4/80 antibody in tissue sections of liposome-treated mice. No F4/80 staining was detected in clodronate-liposome-treated animals (not shown). Hepatocytes appeared morphologically normal, and no evidence of hepatic necrosis was detected after clodronate-liposome treatment (not shown). Macrophage depletion was confirmed by transfusing FluoSpheres (Invitrogen), which are rapidly ingested by macrophages in vivo and in vitro.27 The percentage of injected FluoSpheres remained stable for at least 24 hours in mice treated with clodronate liposomes. FluoSphere clearance was immediate in mice injected with control liposomes.
In vitro HepG2-based platelet ingestion assay
Human HepG2 hepatocarcinoma cells were maintained in RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum, 0.3% l-glutamine, and penicillin/streptomycin solution. HepG2 cells (105/well) transferred to 24-well plates were allowed to adhere for 24 hours, starved 30 minutes in serum-free media, and assays initiated by adding 5 × 107/well CM-Orange–labeled WT or ST3Gal-IV−/− platelets. In some experiments, 0.1, 1, or 10 μg/mL asialofetuin or fetuin was added immediately before platelets and the effect on platelet ingestion determined. Hepatocytes and platelets were incubated together for 30 minutes at 37°C with gentle agitation. To stop the assay, HepG2 monolayers were washed 3 times by changing the buffer. HepG2 cells were dissociated from the plates and from adherent platelets by treatment with 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid in Hanks balanced salt solution (Invitrogen) at 37°C for 10 minutes. Trypsin/ethylenediaminetetraacetic acid incubation with CM-Orange–labeled platelets did not diminish intracellular staining or caused its release (not shown). Ingestion of CM-Orange–labeled platelet was quantified by flow cytometry. Unbound platelets were separated from hepatocytes by their forward and side scatter characteristics. HepG2 cells containing ingested platelets were identified by their orange fluorescence. HepG2 cells having adherent, but not ingested platelets were identified by labeling exposed platelets with FITC–anti-CD61 (Accurate Scientific). A total of 10 000 events were acquired for each sample.
Immunoblot analysis
Platelets were lysed with 4× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer containing 5% β-mercaptoethanol, proteins displayed by SDS-PAGE on 7.5% polyacrylamide gels (Lonza Rockland), and transferred onto Immobilon-P membranes (Millipore). Membranes were blocked and probed with rat anti–mouse-GPIbα (XiaG7, XiaB2, and XiaG5) or rabbit anti–human VWF antibody followed by peroxidase-tagged secondary antibodies, or with peroxidase-conjugated ECA. Detection was performed with an enhanced chemiluminescence system (Pierce Chemical).
Statistical analysis
All data are presented as mean plus or minus SD unless otherwise indicated. Numeric data were analyzed for statistical significance using 2-sample t test for means with SAS (Version 9.1) software. P values less than .05 were considered as statistically significant. Platelet half-lives were calculated from linear trend lines applied to mean platelet circulation curves. Initial clearance and delayed clearance half-times were determined based on platelet circulation from times of 0 to 5 minutes and 5 minutes to 72 hours, respectively.
Results
ST3Gal-IV−/− platelets have short circulation lifetimes
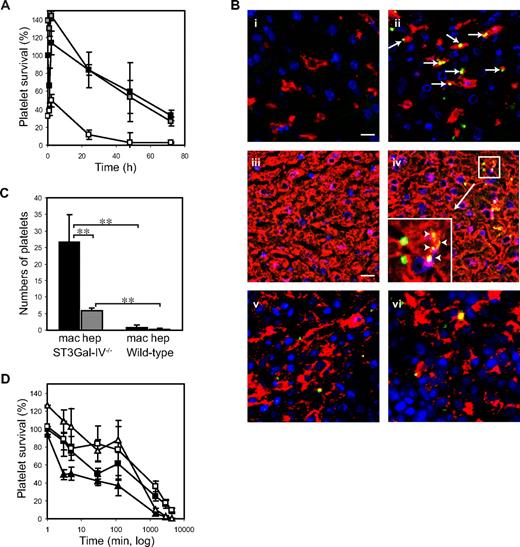
Transfusion studies were performed to investigate whether the thrombocytopenia in ST3Gal-IV−/− mice results from accelerated platelet clearance. Murine WT platelets are removed from the circulation of transfused animals at a linear rate with a t½ of 37.5 hours.28 We found no significant difference in the clearance rates of ST3Gal-IV+/+ (WT) and ST3Gal-IV+/− platelets (Figure 1A). In contrast, Figure 1A shows that ST3Gal-IV−/− platelets were removed rapidly from the circulation of WT recipient animals and that clearance followed biphasic kinetics with a fast initial clearance (t½ = 2.8 minutes) followed by a prolonged clearance phase (t½ = 22.5 hours). Clearance was so rapid that by the first withdrawal of blood (time = 1 minute), the amount of circulating ST3Gal-IV−/− platelets was reduced by more than two-thirds compared with littermate control platelets (27% ± 6% vs 82% ± 34%, P = .021). The life span of ST3Gal-IV−/− platelets was also significantly reduced compared with ST3Gal-IV+/+ platelets (P = .001 and .004 at 24 hours and 48 hours, respectively).
Clearance of transfused ST3Gal-IV−/− platelets. (A) Average survival curves for transfused ST3Gal-IV+/+ (WT, ■), ST3Gal-IV+/− ( ), or ST3Gal-IV −/− (□) platelets in WT mice. CMFDA-labeled platelets were injected into WT mice, blood samples collected at the indicated time points, and the percentage of labeled platelets remaining in the circulation determined by flow cytometry. ST3Gal-IV−/− platelet recovery and platelet survival were significantly reduced compared with WT and ST3Gal-IV+/− platelets (P = .021 and P = .001, respectively). Transfusion data represent an average of 4 mice ± SD and are representative of 3 separate experiments. (B) Immunofluorescent images of cryosections of liver and spleen obtained 30 minutes after transfusion of CMFDA-labeled (green) ST3Gal-IV+/+ platelets (i,iii,v) or ST3Gal-IV−/− platelets (ii,iv,vi). Liver (i-ii) and spleen (v-vi) macrophages were stained with F4/80 antibody (red); hepatocytes were stained with anti-albumin antibody (red; iii-iv). ST3Gal-IV−/− platelets colocalized (yellow) with both liver macrophages (arrows) and hepatocytes (arrowheads). (Inset) Original magnification (×3) of the region indicated in subpanel iv. A small number of CMFDA-labeled platelets were found in splenic macrophages (v-vi). Nuclei were 4,6-diamidino-2-phenylindole–labeled (blue). Bars represent 5 μm. Platelet staining was not observed in specimens from untransfused mice (not shown). (C) Quantification of fluorescently labeled ST3Gal-IV−/− and ST3Gal-IV+/+ platelets colocalizing with liver hepatocytes or macrophages (P < .001). Data are mean ± SD of 5 randomly selected fields from stained tissue sections. (D) Effect of asialofetuin on the clearance of ST3Gal-IV−/− platelets in WT mice. Coinjection of asialofetuin (open symbols), a competitive inhibitor of the ASGPR, restored the recovery and initial circulation of ST3Gal-IV−/− platelets (triangles) to normal levels (P = .173). Circulation of ST3Gal-IV+/+ platelets (squares) was also improved by asialofetuin injection (P = .072). Fetuin coinjections (closed symbols) had no effect on ST3Gal-IV−/− platelet recovery or circulation.
), or ST3Gal-IV −/− (□) platelets in WT mice. CMFDA-labeled platelets were injected into WT mice, blood samples collected at the indicated time points, and the percentage of labeled platelets remaining in the circulation determined by flow cytometry. ST3Gal-IV−/− platelet recovery and platelet survival were significantly reduced compared with WT and ST3Gal-IV+/− platelets (P = .021 and P = .001, respectively). Transfusion data represent an average of 4 mice ± SD and are representative of 3 separate experiments. (B) Immunofluorescent images of cryosections of liver and spleen obtained 30 minutes after transfusion of CMFDA-labeled (green) ST3Gal-IV+/+ platelets (i,iii,v) or ST3Gal-IV−/− platelets (ii,iv,vi). Liver (i-ii) and spleen (v-vi) macrophages were stained with F4/80 antibody (red); hepatocytes were stained with anti-albumin antibody (red; iii-iv). ST3Gal-IV−/− platelets colocalized (yellow) with both liver macrophages (arrows) and hepatocytes (arrowheads). (Inset) Original magnification (×3) of the region indicated in subpanel iv. A small number of CMFDA-labeled platelets were found in splenic macrophages (v-vi). Nuclei were 4,6-diamidino-2-phenylindole–labeled (blue). Bars represent 5 μm. Platelet staining was not observed in specimens from untransfused mice (not shown). (C) Quantification of fluorescently labeled ST3Gal-IV−/− and ST3Gal-IV+/+ platelets colocalizing with liver hepatocytes or macrophages (P < .001). Data are mean ± SD of 5 randomly selected fields from stained tissue sections. (D) Effect of asialofetuin on the clearance of ST3Gal-IV−/− platelets in WT mice. Coinjection of asialofetuin (open symbols), a competitive inhibitor of the ASGPR, restored the recovery and initial circulation of ST3Gal-IV−/− platelets (triangles) to normal levels (P = .173). Circulation of ST3Gal-IV+/+ platelets (squares) was also improved by asialofetuin injection (P = .072). Fetuin coinjections (closed symbols) had no effect on ST3Gal-IV−/− platelet recovery or circulation.
Clearance of transfused ST3Gal-IV−/− platelets. (A) Average survival curves for transfused ST3Gal-IV+/+ (WT, ■), ST3Gal-IV+/− ( ), or ST3Gal-IV −/− (□) platelets in WT mice. CMFDA-labeled platelets were injected into WT mice, blood samples collected at the indicated time points, and the percentage of labeled platelets remaining in the circulation determined by flow cytometry. ST3Gal-IV−/− platelet recovery and platelet survival were significantly reduced compared with WT and ST3Gal-IV+/− platelets (P = .021 and P = .001, respectively). Transfusion data represent an average of 4 mice ± SD and are representative of 3 separate experiments. (B) Immunofluorescent images of cryosections of liver and spleen obtained 30 minutes after transfusion of CMFDA-labeled (green) ST3Gal-IV+/+ platelets (i,iii,v) or ST3Gal-IV−/− platelets (ii,iv,vi). Liver (i-ii) and spleen (v-vi) macrophages were stained with F4/80 antibody (red); hepatocytes were stained with anti-albumin antibody (red; iii-iv). ST3Gal-IV−/− platelets colocalized (yellow) with both liver macrophages (arrows) and hepatocytes (arrowheads). (Inset) Original magnification (×3) of the region indicated in subpanel iv. A small number of CMFDA-labeled platelets were found in splenic macrophages (v-vi). Nuclei were 4,6-diamidino-2-phenylindole–labeled (blue). Bars represent 5 μm. Platelet staining was not observed in specimens from untransfused mice (not shown). (C) Quantification of fluorescently labeled ST3Gal-IV−/− and ST3Gal-IV+/+ platelets colocalizing with liver hepatocytes or macrophages (P < .001). Data are mean ± SD of 5 randomly selected fields from stained tissue sections. (D) Effect of asialofetuin on the clearance of ST3Gal-IV−/− platelets in WT mice. Coinjection of asialofetuin (open symbols), a competitive inhibitor of the ASGPR, restored the recovery and initial circulation of ST3Gal-IV−/− platelets (triangles) to normal levels (P = .173). Circulation of ST3Gal-IV+/+ platelets (squares) was also improved by asialofetuin injection (P = .072). Fetuin coinjections (closed symbols) had no effect on ST3Gal-IV−/− platelet recovery or circulation.
), or ST3Gal-IV −/− (□) platelets in WT mice. CMFDA-labeled platelets were injected into WT mice, blood samples collected at the indicated time points, and the percentage of labeled platelets remaining in the circulation determined by flow cytometry. ST3Gal-IV−/− platelet recovery and platelet survival were significantly reduced compared with WT and ST3Gal-IV+/− platelets (P = .021 and P = .001, respectively). Transfusion data represent an average of 4 mice ± SD and are representative of 3 separate experiments. (B) Immunofluorescent images of cryosections of liver and spleen obtained 30 minutes after transfusion of CMFDA-labeled (green) ST3Gal-IV+/+ platelets (i,iii,v) or ST3Gal-IV−/− platelets (ii,iv,vi). Liver (i-ii) and spleen (v-vi) macrophages were stained with F4/80 antibody (red); hepatocytes were stained with anti-albumin antibody (red; iii-iv). ST3Gal-IV−/− platelets colocalized (yellow) with both liver macrophages (arrows) and hepatocytes (arrowheads). (Inset) Original magnification (×3) of the region indicated in subpanel iv. A small number of CMFDA-labeled platelets were found in splenic macrophages (v-vi). Nuclei were 4,6-diamidino-2-phenylindole–labeled (blue). Bars represent 5 μm. Platelet staining was not observed in specimens from untransfused mice (not shown). (C) Quantification of fluorescently labeled ST3Gal-IV−/− and ST3Gal-IV+/+ platelets colocalizing with liver hepatocytes or macrophages (P < .001). Data are mean ± SD of 5 randomly selected fields from stained tissue sections. (D) Effect of asialofetuin on the clearance of ST3Gal-IV−/− platelets in WT mice. Coinjection of asialofetuin (open symbols), a competitive inhibitor of the ASGPR, restored the recovery and initial circulation of ST3Gal-IV−/− platelets (triangles) to normal levels (P = .173). Circulation of ST3Gal-IV+/+ platelets (squares) was also improved by asialofetuin injection (P = .072). Fetuin coinjections (closed symbols) had no effect on ST3Gal-IV−/− platelet recovery or circulation.
ST3Gal-IV−/− platelets are removed in the liver by ASGPRs on macrophages and hepatocytes
The liver is the major clearance site for transfused ST3Gal-IV−/− platelets. Fluorescent CMFDA-labeled ST3Gal-IV+/+ and ST3Gal-IV−/− platelets (green) were identified in tissue sections from recipient animals 30 minutes and 2 hours after transfusion (Figure 1B). Costaining of liver macrophages (Figure 1Bi-ii) or hepatocytes (Figure 1Biii-iv) with specific antibodies (red) revealed that platelets were detected inside both cell types. ST3Gal-IV+/+ and ST3Gal-IV−/− platelets were also occasionally found in splenic macrophages (Figure 1Bv-vi). As predicted from transfusion studies, more ST3Gal-IV−/− platelets were found in the livers of transfused animals compared with ST3Gal-IV+/+ platelets. We detected no difference in the uptake of platelets by splenic macrophages, which is in agreement with previous data reporting that splenectomy does not affect ST3Gal-IV−/− clearance.16 Figure 1C quantifies platelets colocalized with macrophages or hepatocytes. The uptake of ST3Gal-IV−/− platelets was significantly greater than that of ST3Gal-IV+/+ platelets in both liver macrophages (P < .001) and hepatocytes (P < .001). ST3Gal-IV−/− platelets were associated 4.4-fold more frequently with macrophages than with hepatocytes (P < .001).
Evidence that lectin receptors are involved in the removal of ST3Gal-IV−/− platelets from the circulation derives from the use of the competitive inhibitor of the ASGPR, asialofetuin. Coinjection of asialofetuin restored ST3Gal-IV−/− platelet recovery to levels similar to or even better than WT platelets (108% ± 20% vs 86% ± 21%, P = .173) and enhanced survival in the circulation for 2 hours (Figure 1D). The survival of ST3Gal-IV−/− platelets decreased rapidly thereafter in accordance with the short plasma half-life of asialofetuin (5.9 minutes).29 In comparison, the recovery (49% ± 20%) and initial circulation (2 hours: 37% ± 21%) of ST3Gal-IV−/− platelets coinjected into recipient animals with fetuin were significantly lower (P = .006 and P = .008). Transfusion of ST3Gal-IV+/+ platelets into recipients receiving asialofetuin resulted in a small improvement in the percentage of transfused platelets remaining in the circulation (2 hours: 77% ± 8% vs 61% ± 12%, P = .072), suggesting that a portion of the normal platelet population is recognized and removed by this mechanism.
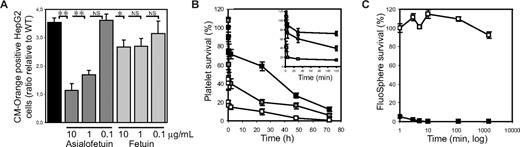
Platelet ingestion by hepatocytes was confirmed in independent experiments. Figure 2A shows that HepG2 cells, which express the ASGPR1/2, specifically ingested ST3Gal-IV−/− platelets and after 30 minutes contained 3.8-fold more CM-Orange–labeled ST3Gal-IV−/− platelets than WT platelets (P < .001). Critically, ST3Gal-IV−/− platelet ingestion by HepG2 cells in vitro was significantly inhibited by asialofetuin in a dose-dependent manner, whereas the control protein fetuin had no effect on ST3Gal-IV−/− platelet ingestion.
ST3Gal-IV−/− platelets are ingested by ASGPR-expressing liver macrophages and hepatocytes. (A) Human hepatocarcinoma cells (HepG2) cells ingest CM-Orange–labeled ST3Gal-IV−/− platelets in vitro. The uptake of ST3Gal-IV−/− platelets by HepG2 cells was 3.8-fold higher (P < .001) than that of WT platelets, as determined by flow cytometry. Platelets were counted as ingested when HepG2 cells contained CM-Orange–labeled platelets that did not label with FITC-labeled anti-β3 IgGs. Asialofetuin, but not fetuin, inhibited the ingestion of ST3Gal-IV−/− platelets by HepG2 cells (P < .001). WT platelet ingestion was set as 1 (not shown) and the ratio of platelet ingestion compared. Each point is the mean ± SD of 2 independent experiments in duplicates. Significance: *P < .05; **P < .01. (B) Macrophage depletion slows platelet clearance. The circulation of ST3Gal-IV−/− platelets (□) was significantly increased (P = .007) in macrophage-depleted mice ( ). ■ shows the survival of WT platelets in WT mice. (Inset) Platelet survival in the first 120 minutes after transfusion. (C) Macrophage depletion was confirmed by injecting FluoSpheres into the clodronate-liposome–treated mice (□). FluoSphere circulation in control (mock liposome-treated) mice was compared (■). The extended recovery and circulation of FluoSpheres demonstrated the effectiveness of the clodronate liposomes in depleting the resident phagocytic cell population. The recovery of FluoSpheres measured 1 minute after transfusion was set as 100%. Each time point is the mean ± SD of 4 mice.
). ■ shows the survival of WT platelets in WT mice. (Inset) Platelet survival in the first 120 minutes after transfusion. (C) Macrophage depletion was confirmed by injecting FluoSpheres into the clodronate-liposome–treated mice (□). FluoSphere circulation in control (mock liposome-treated) mice was compared (■). The extended recovery and circulation of FluoSpheres demonstrated the effectiveness of the clodronate liposomes in depleting the resident phagocytic cell population. The recovery of FluoSpheres measured 1 minute after transfusion was set as 100%. Each time point is the mean ± SD of 4 mice.
ST3Gal-IV−/− platelets are ingested by ASGPR-expressing liver macrophages and hepatocytes. (A) Human hepatocarcinoma cells (HepG2) cells ingest CM-Orange–labeled ST3Gal-IV−/− platelets in vitro. The uptake of ST3Gal-IV−/− platelets by HepG2 cells was 3.8-fold higher (P < .001) than that of WT platelets, as determined by flow cytometry. Platelets were counted as ingested when HepG2 cells contained CM-Orange–labeled platelets that did not label with FITC-labeled anti-β3 IgGs. Asialofetuin, but not fetuin, inhibited the ingestion of ST3Gal-IV−/− platelets by HepG2 cells (P < .001). WT platelet ingestion was set as 1 (not shown) and the ratio of platelet ingestion compared. Each point is the mean ± SD of 2 independent experiments in duplicates. Significance: *P < .05; **P < .01. (B) Macrophage depletion slows platelet clearance. The circulation of ST3Gal-IV−/− platelets (□) was significantly increased (P = .007) in macrophage-depleted mice ( ). ■ shows the survival of WT platelets in WT mice. (Inset) Platelet survival in the first 120 minutes after transfusion. (C) Macrophage depletion was confirmed by injecting FluoSpheres into the clodronate-liposome–treated mice (□). FluoSphere circulation in control (mock liposome-treated) mice was compared (■). The extended recovery and circulation of FluoSpheres demonstrated the effectiveness of the clodronate liposomes in depleting the resident phagocytic cell population. The recovery of FluoSpheres measured 1 minute after transfusion was set as 100%. Each time point is the mean ± SD of 4 mice.
). ■ shows the survival of WT platelets in WT mice. (Inset) Platelet survival in the first 120 minutes after transfusion. (C) Macrophage depletion was confirmed by injecting FluoSpheres into the clodronate-liposome–treated mice (□). FluoSphere circulation in control (mock liposome-treated) mice was compared (■). The extended recovery and circulation of FluoSpheres demonstrated the effectiveness of the clodronate liposomes in depleting the resident phagocytic cell population. The recovery of FluoSpheres measured 1 minute after transfusion was set as 100%. Each time point is the mean ± SD of 4 mice.
Next, experiments were performed in mice to dissect the relative contribution of Kupffer cells and hepatocytes to the clearance of ST3Gal-IV−/− platelets. Mice, purged of mature macrophages by injecting toxic clodronate encapsulated in liposomes, were transfused with ST3Gal-IV−/− platelets and survival lifetimes determined. Figure 2B shows that transfused ST3Gal-IV−/− platelets survived better, but not at normal levels, in the macrophage-depleted animals. Macrophage depletion increased the initial platelet recovery from 41% plus or minus 16% to 108% plus or minus 32% (P = .009) and significantly increased the percentage of transfused platelets remaining in the circulation after 48 hours (16% ± 6% vs 3% ± 2% in mock-treated recipients, P = .007). The extended circulation of FluoSpheres demonstrated the effectiveness of the clodronate liposome treatment in depleting the resident phagocytic cell population (Figure 2C). In summary, these findings demonstrate that both liver macrophages and hepatocytes contribute to the removal of the sialic acid-deficient platelets.
Platelet GPIbα is a major counterreceptor for ASGPRs
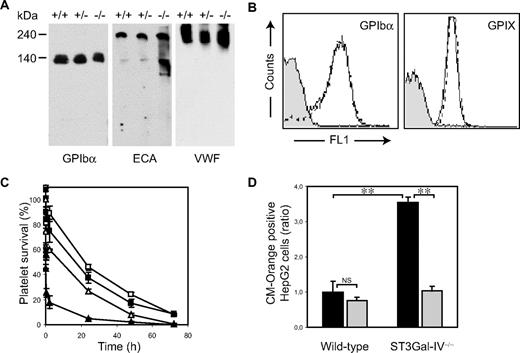
Exposed βGlcNAc residues on clustered GPIbα N-linked glycans bind to liver macrophage αMβ2 lectin domains.30 We investigated whether GPIbα plays a role in the removal of platelets with exposed βGal residues. As expected, immunoblotting of ST3Gal-IV+/+, ST3Gal-IV+/−, and ST3Gal-IV−/− platelet lysates using ECA lectin showed multiple glycoproteins with exposed βGal residues in all phenotypes (Figure 3A). Two major desialylated proteins were discernable in ST3Gal-IV−/− lysates. One had an apparent molecular weight of 140 kDa, a size consistent with GPIbα, which was confirmed by probing the lysates with a GPIbα specific antibody. The second major desialylated protein had an apparent molecular weight of 240 kDa and was identified as platelet-bound VWF (Figure 3A). Expression of the VWF receptor was similar between ST3Gal-IV+/+ and ST3Gal-IV−/− platelets, as evidenced by flow cytometry of platelets incubated with monoclonal antibodies to GPIbα and GPIX (Figure 3B). Electron microscopy also revealed the normal linear alignment of GPIbα on platelets from both genotypes, suggesting that receptor topology was unchanged in the ST3Gal-IV−/− platelets (not shown).
Role of GPIbα in the clearance of ST3Gal-IV−/− platelets. (A) A 140-kDa glycoprotein recognized by anti-GPIbα antibody contained increased amounts of βGal, as determined by ECA lectin blotting. The 240-kDa glycoprotein, also recognized on the ECA blot, was identified as VWF by anti-VWF antibody. (B) Surface expression of GPIbα and GPIX was not altered in ST3Gal-IV−/− platelets. Glycoprotein expression was determined with antibodies by flow cytometry. (C) Removal of the extracellular N-terminal portion of GPIbα with O-sialoglycoprotein endopeptidase enhanced the survival of transfused ST3Gal-IV−/− platelets (triangles; P < .001). Circulation of WT platelets is also shown (squares). Open symbols represent platelets treated with O-sialoglycoprotein endopeptidase; closed symbols, untreated platelets. (D) Ingestion of ST3Gal-IV−/− platelets by HepG2 cells in vitro was significantly reduced by removal of the extracellular N-terminal portion of GPIbα with O-sialoglycoprotein endopeptidase ( ). The data are mean ± SD of 2 independent experiments done in triplicate. Significance: **P < .01; NS, P ≥ .05.
). The data are mean ± SD of 2 independent experiments done in triplicate. Significance: **P < .01; NS, P ≥ .05.
Role of GPIbα in the clearance of ST3Gal-IV−/− platelets. (A) A 140-kDa glycoprotein recognized by anti-GPIbα antibody contained increased amounts of βGal, as determined by ECA lectin blotting. The 240-kDa glycoprotein, also recognized on the ECA blot, was identified as VWF by anti-VWF antibody. (B) Surface expression of GPIbα and GPIX was not altered in ST3Gal-IV−/− platelets. Glycoprotein expression was determined with antibodies by flow cytometry. (C) Removal of the extracellular N-terminal portion of GPIbα with O-sialoglycoprotein endopeptidase enhanced the survival of transfused ST3Gal-IV−/− platelets (triangles; P < .001). Circulation of WT platelets is also shown (squares). Open symbols represent platelets treated with O-sialoglycoprotein endopeptidase; closed symbols, untreated platelets. (D) Ingestion of ST3Gal-IV−/− platelets by HepG2 cells in vitro was significantly reduced by removal of the extracellular N-terminal portion of GPIbα with O-sialoglycoprotein endopeptidase ( ). The data are mean ± SD of 2 independent experiments done in triplicate. Significance: **P < .01; NS, P ≥ .05.
). The data are mean ± SD of 2 independent experiments done in triplicate. Significance: **P < .01; NS, P ≥ .05.
We next stripped the extracellular N-terminal domain of GPIbα carrying complex type N-linked glycans from WT and ST3Gal-IV−/− platelets with O-sialoglycoprotein endopeptidase and determined whether its removal enhanced platelet survival in WT recipient mice. Figure 3C shows that the recovery of ST3Gal-IV−/− platelets injected into WT recipient mice increased to WT levels after removal of the extracellular domain of GPIbα (103% ± 22% vs 100% ± 17%, P = .850). ST3Gal-IV−/− platelet survival times were also substantially increased after 24 hours (from 5% ± 1% to 27% ± 4% vs the WT values of 38% ± 9%, P < .001). These findings indicate that recognition of βGal on N-linked glycans of GPIbα by the ASGPR initiates platelet clearance.
ST3Gal-IV−/− platelet ingestion by HepG2 cells was also significantly reduced when platelets were treated with O-sialoglycoprotein endopeptidase (P < .001). O-sialoglycoprotein endopeptidase treatment did not change the uptake of WT platelets by the HepG2 cultures (P = .090; Figure 3D).
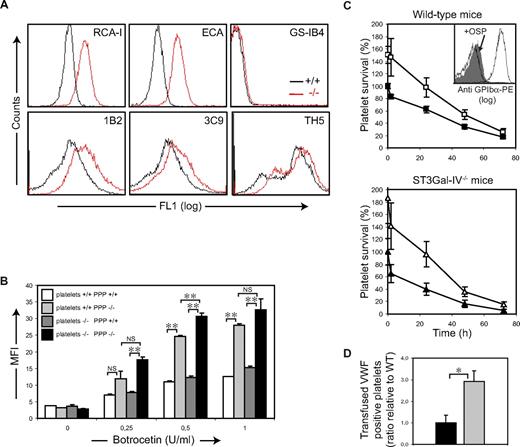
To investigate whether ST3Gal-IV depletion causes compensatory glycosylation, carbohydrate exposure was profiled by flow cytometry with sugar-specific lectins and monoclonal antibodies. As expected, binding of the galactose-recognizing lectins RCA-I and ECA (lectins specific for terminal βGal and Galβ1-4GlcNAc, respectively) to ST3Gal-IV−/− platelets was increased compared with ST3Gal-IV+/+ platelets (Figure 4A). Similar results were obtained when platelets were incubated with the monoclonal antibody 1B2 specific for Galβ1-4GlcNAc. Binding of the monoclonal antibody 3C9 (specific for Galβ1-3GalNAcα; T antigen) was increased on ST3Gal-IV−/− platelets compared with littermate controls, showing that ST3Gal-IV plays a role in N-linked and O-linked sialylation of platelets (Figure 4A). A potentially competing terminal modification could be mediated by a UDP-Gal:Galβ1-4GlcNAcβ1-R α1,3galactosyltransferase. Binding of the GS-IB4 lectin, which recognizes Galα1-3Gal, was only marginally increased in the ST3Gal-IV−/− platelets, demonstrating that α3Gal residues are not generated (Figure 4A), a finding confirmed using the monoclonal antibody TH5.
Deficient sialylation of VWF increases its binding to platelets and enhances platelet clearance. (A) ST3Gal-IV−/− platelets have increased βGal exposure. Staining of ST3Gal-IV−/− and ST3Gal-IV+/+ platelets with the βGal recognizing lectins RCA-I and ECA revealed increased βGal exposure on ST3Gal-IV−/− platelets (red lines) compared with WT (black lines) littermates. The level of Galα1-3Gal was only marginally increased on ST3Gal-IV−/− platelets as evidenced by binding of the GS-IB4 lectin. The lectin profile was confirmed using monoclonal antibodies 1B2 (specific for Galβ1-4GlcNAcβ-R), 3C9 (specific for Galβ1-3GalNAcα-OH), and TH5 (specific for Galα1-3Galβ1-4GlcNAcβ-R). (B) Botrocetin stimulation of platelets in ST3Gal-IV−/− plasma induced significantly higher levels of anti-VWF binding to both ST3Gal-IV−/− (■) and ST3Gal-IV+/+ ( ) platelets compared with control plasma (
) platelets compared with control plasma ( and □, respectively; P < .001). ST3Gal-IV−/− platelets (■ and
and □, respectively; P < .001). ST3Gal-IV−/− platelets (■ and  ) consistently bound more VWF than ST3Gal-IV+/+ platelets (
) consistently bound more VWF than ST3Gal-IV+/+ platelets ( and □) independently of the plasma type used (P = .008). VWF binding was not increased in resting ST3Gal-IV−/− platelets (botrocetin = 0 U/mL). Data are representative of 9 mice. (C) WT platelet life span was significantly reduced in ST3Gal-IV−/− mice (bottom panel ▲) compared with WT mice (top panel ■; P = .014). Removal of the extracellular domain of GPIbα by O-sialoglycoprotein endopeptidase (+OSP) doubled the percentage transfused WT platelets remaining in the circulation of ST3Gal-IV−/− mice at 24 and 48 hours (P = .003 and P = .014; bottom panel △) and significantly increased platelet life span in WT mice at these times (P = .022 and P = .021; top panel □). (C inset) Flow cytometry documenting the removal of the GPIbα N-terminus. Platelets before (white) and after (gray) treatment with O-sialoglycoprotein endopeptidase (+OSP), which removes the VWF binding domain of GPIbα carrying complex type N-linked glycans. Background labeling with IgG control is also shown. (D) Transfused platelets rapidly collect ST3Gal-VI−/− VWF on their surfaces. Three-fold more VWF-positive WT platelets were detected in ST3Gal-IV−/− mice compared with WT platelets transfused into WT mice (P = .021). The data are mean ± SD of 4 mice.
and □) independently of the plasma type used (P = .008). VWF binding was not increased in resting ST3Gal-IV−/− platelets (botrocetin = 0 U/mL). Data are representative of 9 mice. (C) WT platelet life span was significantly reduced in ST3Gal-IV−/− mice (bottom panel ▲) compared with WT mice (top panel ■; P = .014). Removal of the extracellular domain of GPIbα by O-sialoglycoprotein endopeptidase (+OSP) doubled the percentage transfused WT platelets remaining in the circulation of ST3Gal-IV−/− mice at 24 and 48 hours (P = .003 and P = .014; bottom panel △) and significantly increased platelet life span in WT mice at these times (P = .022 and P = .021; top panel □). (C inset) Flow cytometry documenting the removal of the GPIbα N-terminus. Platelets before (white) and after (gray) treatment with O-sialoglycoprotein endopeptidase (+OSP), which removes the VWF binding domain of GPIbα carrying complex type N-linked glycans. Background labeling with IgG control is also shown. (D) Transfused platelets rapidly collect ST3Gal-VI−/− VWF on their surfaces. Three-fold more VWF-positive WT platelets were detected in ST3Gal-IV−/− mice compared with WT platelets transfused into WT mice (P = .021). The data are mean ± SD of 4 mice.
Deficient sialylation of VWF increases its binding to platelets and enhances platelet clearance. (A) ST3Gal-IV−/− platelets have increased βGal exposure. Staining of ST3Gal-IV−/− and ST3Gal-IV+/+ platelets with the βGal recognizing lectins RCA-I and ECA revealed increased βGal exposure on ST3Gal-IV−/− platelets (red lines) compared with WT (black lines) littermates. The level of Galα1-3Gal was only marginally increased on ST3Gal-IV−/− platelets as evidenced by binding of the GS-IB4 lectin. The lectin profile was confirmed using monoclonal antibodies 1B2 (specific for Galβ1-4GlcNAcβ-R), 3C9 (specific for Galβ1-3GalNAcα-OH), and TH5 (specific for Galα1-3Galβ1-4GlcNAcβ-R). (B) Botrocetin stimulation of platelets in ST3Gal-IV−/− plasma induced significantly higher levels of anti-VWF binding to both ST3Gal-IV−/− (■) and ST3Gal-IV+/+ ( ) platelets compared with control plasma (
) platelets compared with control plasma ( and □, respectively; P < .001). ST3Gal-IV−/− platelets (■ and
and □, respectively; P < .001). ST3Gal-IV−/− platelets (■ and  ) consistently bound more VWF than ST3Gal-IV+/+ platelets (
) consistently bound more VWF than ST3Gal-IV+/+ platelets ( and □) independently of the plasma type used (P = .008). VWF binding was not increased in resting ST3Gal-IV−/− platelets (botrocetin = 0 U/mL). Data are representative of 9 mice. (C) WT platelet life span was significantly reduced in ST3Gal-IV−/− mice (bottom panel ▲) compared with WT mice (top panel ■; P = .014). Removal of the extracellular domain of GPIbα by O-sialoglycoprotein endopeptidase (+OSP) doubled the percentage transfused WT platelets remaining in the circulation of ST3Gal-IV−/− mice at 24 and 48 hours (P = .003 and P = .014; bottom panel △) and significantly increased platelet life span in WT mice at these times (P = .022 and P = .021; top panel □). (C inset) Flow cytometry documenting the removal of the GPIbα N-terminus. Platelets before (white) and after (gray) treatment with O-sialoglycoprotein endopeptidase (+OSP), which removes the VWF binding domain of GPIbα carrying complex type N-linked glycans. Background labeling with IgG control is also shown. (D) Transfused platelets rapidly collect ST3Gal-VI−/− VWF on their surfaces. Three-fold more VWF-positive WT platelets were detected in ST3Gal-IV−/− mice compared with WT platelets transfused into WT mice (P = .021). The data are mean ± SD of 4 mice.
and □) independently of the plasma type used (P = .008). VWF binding was not increased in resting ST3Gal-IV−/− platelets (botrocetin = 0 U/mL). Data are representative of 9 mice. (C) WT platelet life span was significantly reduced in ST3Gal-IV−/− mice (bottom panel ▲) compared with WT mice (top panel ■; P = .014). Removal of the extracellular domain of GPIbα by O-sialoglycoprotein endopeptidase (+OSP) doubled the percentage transfused WT platelets remaining in the circulation of ST3Gal-IV−/− mice at 24 and 48 hours (P = .003 and P = .014; bottom panel △) and significantly increased platelet life span in WT mice at these times (P = .022 and P = .021; top panel □). (C inset) Flow cytometry documenting the removal of the GPIbα N-terminus. Platelets before (white) and after (gray) treatment with O-sialoglycoprotein endopeptidase (+OSP), which removes the VWF binding domain of GPIbα carrying complex type N-linked glycans. Background labeling with IgG control is also shown. (D) Transfused platelets rapidly collect ST3Gal-VI−/− VWF on their surfaces. Three-fold more VWF-positive WT platelets were detected in ST3Gal-IV−/− mice compared with WT platelets transfused into WT mice (P = .021). The data are mean ± SD of 4 mice.
Deficient sialylation impacts VWF function
ST3Gal-IV deficiency increases galactose exposure not only in cells, but also on plasma glycoproteins. Because VWF and its major receptor GPIb-IX-V (vWfR) are highly sialylated and removal of GPIbα from the ST3Gal-IV−/− platelet surface greatly improved ST3Gal-IV−/− platelet survival in WT mice, we examined whether loss of sialylation impacts the function of VWF or alters its interaction with platelet vWfR. The influence of sialic acid deficiency on VWF-mediated platelet activation was tested in vitro after stimulating ST3Gal-IV−/− and ST3Gal-IV+/+ platelets with botrocetin. Mean fluorescence intensity of FITC-anti-VWF binding to ST3Gal-IV−/− PRP was significantly increased compared with WT PRP in response to all concentrations of botrocetin tested (Figure 4B). Increased binding was not the result of increased numbers of vWfRs on the ST3Gal-IV−/− platelets or the result of difference in platelet size, as VWF values were corrected for this parameter by comparing platelet size ratio.
To quantify further the differential response of VWF in ST3Gal-IV+/+ and ST3Gal-IV−/− PRP, and to determine the respective roles of platelet vWfR vs plasma VWF, crossed assays were performed. Platelets of each genotype were suspended and activated in plasma from ST3Gal-IV+/+ and ST3Gal-IV−/− blood. A 2-fold increase in anti-VWF binding was detected for both ST3Gal-IV+/+ and ST3Gal-IV−/− platelets activated in ST3Gal-IV−/− plasma compared with ST3Gal-IV+/+ plasma (P < .001). Part of the increased response to botrocetin was related to a functional difference in the vWfR on ST3Gal-IV−/− platelets, as these platelets consistently bound more VWF, independent of the plasma type used (P = .008; Figure 4B).
To investigate whether the increased binding of sialic acid-deficient VWF to platelets enhances platelet clearance in vivo, WT platelets were transfused into WT or ST3Gal-IV−/− mice and their circulation times measured. Although there was no significant difference in platelet recoveries transfused into WT (□) and ST3Gal-IV−/− mice (△; P = .130), the percentage of circulating platelets was significantly reduced in ST3Gal-IV−/− mice compared with WT mice at all time points measured (Figure 4C). Treatment with O-sialoglycoprotein endopeptidase doubled the percentage of circulating WT platelets in ST3Gal-IV−/− recipient mice (Figure 4C). O-sialoglycoprotein endopeptidase treatment also increased the percentage of circulating platelets in WT recipient mice as reported previously.31 When WT platelets were recovered from the blood of the ST3Gal-IV−/− recipient animals 1 minute after transfusion, the percentage of VWF-positive platelets was 3-fold higher compared with VWF-positive platelets recovered from WT mice (P = .021; Figure 4D). A significant increase in the VWF-positive platelets recovered from ST3Gal-IV−/− mice was also measured in blood samples collected 24 hours after transfusion (not shown). Taken together, these findings indicate that both carbohydrate structures on GPIbα and binding of deficiently sialylated VWF to circulating platelets contribute to the accelerated platelet clearance observed in the ST3Gal-IV−/− mice.
Platelet production is not increased in ST3Gal-IV−/− mice
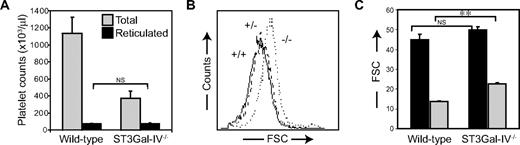
We investigated whether platelet production is increased in ST3Gal-IV−/− mice. Reticulated platelets in both genotypes were measured using Thiazole Orange RNA staining. Although the percentage of reticulated platelets was 3 times higher in the blood of ST3Gal-IV−/− mice compared with WT mice (not shown), the total number of reticulated platelets was similar in both genotypes, when adjusted for platelet counts (78.3 × 103/μL ± 5.6 in ST3Gal-IV−/− mice vs 79.2 × 103/μL ± 1.9 in WT mice, P = .490; Figure 5A). There was no measurable difference in plasma thrombopoietin levels in WT and ST3Gal-IV−/− mice (not shown). This suggests that megakaryopoiesis is not increased in ST3Gal-IV−/− mice despite accelerated platelet clearance. However, previous studies reported a trend toward increased megakaryocyte numbers with normal cellular morphology.16 The average ST3Gal-IV−/− platelet size was somewhat larger than that of WT platelets, as measured by forward scatter characteristics (Figure 5B). We further compared the size of reticulated and nonreticulated ST3Gal-IV−/− and WT platelets (Figure 5C). Interestingly, reticulated ST3Gal-IV−/− and WT platelets were of similar size (P = .150). With extended circulation, nonreticulated ST3Gal-IV−/− platelets remained larger than nonreticulated WT platelets (P < .001), suggesting that ST3Gal-IV−/− platelets do not undergo the aging process that diminishes WT cell size.
Effect of ST3Gal-IV sialyltransferase deficiency on platelet production. (A) Staining of reticulated platelets with Thiazole Orange revealed that the total number of circulating reticulocytes was similar between the 2 genotypes (P = .490). Total WT and ST3Gal-IV−/− platelet counts are also shown ( ). (B) The average ST3Gal-IV−/− platelet size (dotted line) was larger than that of ST3Gal-IV+/+ and ST3Gal-IV+/− platelets (solid and broken lines, respectively) as measured by FSC, indicating that the ST3Gal-IV−/− platelet population on average is younger. (C) Reticulated platelets (■) were larger than nonreticulated platelets (
). (B) The average ST3Gal-IV−/− platelet size (dotted line) was larger than that of ST3Gal-IV+/+ and ST3Gal-IV+/− platelets (solid and broken lines, respectively) as measured by FSC, indicating that the ST3Gal-IV−/− platelet population on average is younger. (C) Reticulated platelets (■) were larger than nonreticulated platelets ( ) in both genotypes. The nonreticulated platelets were larger in ST3Gal-IV−/− mice than in WT mice.
) in both genotypes. The nonreticulated platelets were larger in ST3Gal-IV−/− mice than in WT mice.
Effect of ST3Gal-IV sialyltransferase deficiency on platelet production. (A) Staining of reticulated platelets with Thiazole Orange revealed that the total number of circulating reticulocytes was similar between the 2 genotypes (P = .490). Total WT and ST3Gal-IV−/− platelet counts are also shown ( ). (B) The average ST3Gal-IV−/− platelet size (dotted line) was larger than that of ST3Gal-IV+/+ and ST3Gal-IV+/− platelets (solid and broken lines, respectively) as measured by FSC, indicating that the ST3Gal-IV−/− platelet population on average is younger. (C) Reticulated platelets (■) were larger than nonreticulated platelets (
). (B) The average ST3Gal-IV−/− platelet size (dotted line) was larger than that of ST3Gal-IV+/+ and ST3Gal-IV+/− platelets (solid and broken lines, respectively) as measured by FSC, indicating that the ST3Gal-IV−/− platelet population on average is younger. (C) Reticulated platelets (■) were larger than nonreticulated platelets ( ) in both genotypes. The nonreticulated platelets were larger in ST3Gal-IV−/− mice than in WT mice.
) in both genotypes. The nonreticulated platelets were larger in ST3Gal-IV−/− mice than in WT mice.
Discussion
Our results demonstrate the existence of a platelet clearance system, composed of macrophages and hepatocytes, which removes deficiently sialylated platelets using their ASGPRs. This conclusion is based on the following evidence: (1) lack of the ST3Gal-IV enzyme results in the exposure of βGal residues on platelet surfaces as determined using lectins and monoclonal antibodies; (2) βGal exposure increases platelet clearance rates; (3) immunohistology of livers from transfused animals reveals ST3Gal-IV−/− platelets in both hepatocytes and macrophages; (4) macrophage depletion in mice using clodronate-encapsulated liposomes prolongs ST3Gal-IV−/− platelet circulation; (5) coinjection of asialofetuin, a competitive inhibitor of the ASGPR, restores the survival of these deficiently sialylated platelets; and (6) cultured HepG2 cells selectively ingest deficiently sialylated platelets by ASGPRs, and their ingestion can be inhibited by addition of asialofetuin.
Masking of βGal terminating oligosaccharide chains by sialic acid prevents recognition by the ASGPR, a lectin receptor abundantly expressed on macrophages and hepatocytes. The ASGPR mediates the binding and removal of glycoconjugates having exposed βGal and GalNAc,32 although recognition of SAα2,6GalNAc and SAα2,6Gal has also been reported.33,34 The binding affinity of the ASGPR subtypes on hepatocytes or macrophages depends on ligand valency, spatial arrangement, and size.35,36 Small ligands (< 70 nm) are thought to be preferentially internalized via the hepatocyte ASGPR-mediated endocytic pathway.37 Larger ligands, such as cellular debris and platelets, are thought to be selectively ingested through the ASGPR on macrophages.37-39 Because depletion of macrophages in mice prolonged the circulation of ST3Gal-IV−/− platelets but did not restore it to normal, we determined whether hepatocytes could ingest platelets in vitro. Cultured human HepG2 cells were found to selectively bind and ingest ST3Gal-IV−/− platelets (Figure 2). Hepatocyte-mediated clearance of deficiently sialylated platelets thus negates the notion that only macrophages can ingest large cellular material and suggests that hepatic ASGPRs participate in clearing cells as well as plasma glycoproteins. Thus, it is probable that hepatocytes participate in the normal removal of senescent platelets, consistent with the findings of Grewal et al.14
Others have demonstrated that liver macrophages mediate the clearance of senescent cells, including platelets desialylated by a bacterial-derived trans-sialidase.40 We find that ST3Gal-IV−/− platelets are mainly associated with liver macrophages (Figure 1). Macrophage depletion would therefore be expected to enhance ST3Gal-IV−/− platelet survival in WT mice more than that measured here (Figure 2) and that reported by Grewal et al.14 An explanation might be that hepatocytic ingestion of platelets is less efficient than macrophage-based but becomes important when macrophages are absent. Moreover, removal of platelets by macrophages and hepatocytes at different rates could explain the biphasic clearance kinetics of sialic acid-deficient platelets. This mechanism could also explain the greatly improved recovery and slower initial rate of platelet removal in the macrophage-depleted animals (Figure 2). Similar biphasic clearance kinetics for VWF have been reported,41 which may reflect the use of multiple phagocytotic cells and lectin receptors. A second possibility is that ST3Gal-IV−/− platelets ingested by hepatocytes are more rapidly degraded, causing the platelet-associated fluorescence to be quenched by the time of analysis. A similar discrepancy in the clearance of VWF and FVIII by hepatocytes has been described.42 It is also possible that some macrophages escape depletion in the liposome-treated animals. Splenectomy of ST3Gal-IV−/− mice has no effect on ST3Gal-IV−/− platelet numbers,16 excluding splenic macrophages from playing a major role in the clearance of deficiently sialylated platelets. In summary, our data support the notion that ASGPRs on both macrophages and hepatocytes can recognize and clear deficiently sialylated platelets.
Despite our and others'16 measurements of accelerated ST3Gal-IV−/− platelet turnover, we found no difference in the total number of reticulated platelets in the circulation of either genotype, suggesting that platelet production is not markedly increased in the ST3Gal-IV−/− mice. In chronic immune thrombocytopenic purpura, a steady state is attained when a 10-fold increase of platelet production balances the accelerated splenic removal of platelets.43 A similar “elevated platelet production steady state” might therefore be expected in ST3Gal-IV−/− mice. However, the deficiently sialylated platelets in ST3Gal-IV−/− mice are probably rapidly detected and removed once they enter the circulation, in a process unrelated to platelet age. As opposed to immune thrombocytopenic purpura, rapid removal of young platelets might have the opposite effect, lowering the reticulated platelet percentage. In this situation, some increased platelet production would be required to maintain similar numbers of reticulated platelets. The increased mass of ST3Gal-IV−/− platelets could also influence equilibrium number of circulating platelets. The precise mechanism of compensation remains to be determined.
The counterreceptor for the ASGPR on ST3Gal-IV−/− platelets
Removal of GPIbα's N-terminal domain by O-sialoglycoprotein endopeptidase significantly improved the survival of deficiently sialylated platelets in vivo and in vitro, as it did to a small extent for transfused WT platelets (Figure 3). The GPIbα N-terminal domain contains the di-, tri-, and tetra-antennary N-linked glycans,44,45 whereas O-linked glycans reside within the C-terminal domain.46,47 It is probable that in vivo and in vitro removal of platelets occurs via exposed βGal residues on N-linked glycans rather than on O-linked glycans, consistent with the shown effects of ligand valency (tetra-, > tri-, > di-, > mono-antennary) and sugar spacing (20 Å > 10 Å > 4 Å) on glycan binding to ASGPRs.37
Restoration of ST3Gal-IV−/− platelet circulation by resialylation would provide direct evidence for the shielding effect of surface sialic acid on platelet clearance by ASGPRs. However, we were unable to resialylate ST3Gal-IV−/− platelets with several recombinant sialyltransferases, including ST3Gal-III, which acts on type I and II chains (not shown). Depletion of the ST3Gal-IV gene may expose only selective and/or partly cryptic acceptor sites that are substrates for specific sialyltransferase isoforms prohibiting resialylation by the enzymes used. Alternatively, βGal sites may undergo compensatory glycosylation by other glycosyltransferases competing for the same acceptor substrate. We excluded one compensatory pathway: α3galactosylation by UDP-Gal:Galβ1-4GlcNAcβ1-R α1,3 galactosyltransferase, which would prevent resialylation in ST3Gal-IV−/− platelets. The existence of other compensatory glycosylation cannot be excluded.
Role of sialic acid for GPIbα and VWF interaction
Despite a 50% reduction of VWF in ST3Gal-IV−/− plasma, binding of deficiently sialylated VWF to the vWfR on WT platelets is enhanced in vitro and in vivo (Figure 4). Both VWF and GPIbα are heavily glycosylated. Loss of sialic acid from VWF has been reported to both increase and decrease VWF-mediated platelet agglutination.48 Eight of the 10 putative O-glycosylation sites are located within the VWF A1 domain and its flanking regions49 and have been proposed to regulate platelet interactions50,51 by shielding of the GPIbα binding site.52,53 The majority of VWF O-glycans are disialyl-T with the sequence NeuAc2,3Galβ1,3[NeuAc (α2,6)]GalNAc.54 Loss of ST3Gal-IV that transfers sialic acid to core 1 O-glycans is expected to alter VWF O-linked glycan composition and could thereby deshield the GPIbα binding domain in the VWF A1 domain and promote platelet agglutination, supporting previous reports that failure to complete O-linked glycosylation in the VWF A1 domain promotes platelet agglutination.55-57 Removal of the N-terminal of GPIbα, the binding site for VWF, restored the circulation of ST3Gal-IV−/− platelets in mice. Hence, our results demonstrate that the loss of sialic acid alters the VWF–GPIb-IX-V interaction.
Platelet-type von Willebrand disease is a rare bleeding disorder that results from gain-of-function mutations in the GPIBA gene, leading to hyperaggregation of platelets and rapid clearance of platelet-VWF aggregates. Certain mutations cause a 9-amino acid deletion in the GPIbα macroglycopeptide domain, resulting in the loss of 4 potential sites of O-glycosylation.58 Because the O-linked glycans in the GPIbα macroglycopeptide domain are mainly hexasaccharides with the proposed structure NeuAcα2,3Galβ1,3/4GlcNAcβ1,6[NeuAcα2,3Galβ1,3]GalNAc and NeuAcα2,3Galβ1,3/4GlcNAcβ1,3Galβ1,3[NeuAcα2,6]GalNAc,59 deficiency in the ST3Gal-IV gene could result in a similar phenotype.
In conclusion, changes in platelet sialic acid content represent a potential pathogenic risk factor by facilitating platelet activation, particularly in patients with coronary heart disease.60 Our results suggest that the glycosylation state of GPIbα and VWF oligosaccharides plays central roles in defining platelet life span and function, providing a rationale for platelet and plasma factor glycoengineering to potentially alleviate thrombotic diseases and improve platelet products for transfusion.22,61
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Consortium for Functional Glycomics for providing the ST3Gal-IV+/− mice.
This work was supported by the National Institutes of Health (grant PO1 HL056949, J.H.H., K.M.H.; grant HL089224, K.M.H.), the Pew Scholars Award (K.M.H.), the Swedish Medical Research Council, Göteburg University Jubileums stipend (V.R.), the Blood Donors Research Fund (A.L.S.), the Danish Research Council (H.H.W., H.C.), the Benzon Foundation (H.C.), and University of Copenhagen Program of Excellence (H.C.); and supported in part by research funding from ZymeQuest Inc (A.L.S., V.R., H.C., H.H.W., K.M.H.).
National Institutes of Health
Authorship
Contribution: A.L.S., V.R., S.N.-H., and H.H.W. performed experiments; A.L.S., H.H.W., K.M.H., and J.H.H. analyzed results and made the figures; and A.L.S., H.C., J.H.H., H.H.W., and K.M.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: H.C. and K.M.H. are consultants for ZymeQuest. H.H.W. and H.C. have stock options in ZymeQuest. The remaining authors declare no competing financial interests.
Correspondence: Karin M. Hoffmeister, Translational Medicine Division, Brigham and Women's Hospital, Boston, MA 02115; e-mail: khoffmeister@rics.bwh.harvard.edu; or Hans H. Wandall, Copenhagen Center for Glycomics, Department of Cellular and Molecular Medicine, University of Copenhagen, DK-2200, Copenhagen, Denmark; e-mail: hhw@sund.ku.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal