Abstract
Negative regulatory mechanisms within the solid tumor microenvironment inhibit antitumor T-cell function, leading to evasion from immune attack. One inhibitory mechanism is up-regulation of programmed death-ligand 1 (PD-L1) expressed on tumor or stromal cells which binds to programmed death-1 (PD-1) on activated T cells. PD-1/PD-L1 engagement results in diminished antitumor T-cell responses and correlates with poor outcome in murine and human solid cancers. In contrast to available data in solid tumors, little is known regarding involvement of the PD-1/PD-L1 pathway in immune escape by hematopoietic cancers, such as acute myeloid leukemia (AML). To investigate this hypothesis, we used the murine leukemia, C1498. When transferred intravenously, C1498 cells grew progressively and apparently evaded immune destruction. Low levels of PD-L1 expression were found on C1498 cells grown in vitro. However, PD-L1 expression was up-regulated on C1498 cells when grown in vivo. PD-1−/− mice challenged with C1498 cells generated augmented antitumor T-cell responses, showed decreased AML burden in the blood and other organs, and survived significantly longer than did wild-type mice. Similar results were obtained with a PD-L1 blocking antibody. These data suggest the importance of the PD-1/PD-L1 pathway in immune evasion by a hematologic malignancy, providing a rationale for clinical trials targeting this pathway in leukemia patients.
Introduction
Cancer cells can express tumor antigens, rendering them susceptible to recognition and lysis by CD8+ T cells.1 However, spontaneous rejection of established cancers is a rare occurrence, in part due to negative regulatory mechanisms used by the tumor and its microenvironment,2-6 including engagement of programmed death-1 (PD-1) on activated T cells with its ligand programmed death-ligand 1 (PD-L1; B7-H1)7,8 expressed on macrophages, nonhematopoietic stromal cells, and tumor cells. In normal hosts, PD-1/PD-L1 interactions contribute to the maintenance of peripheral tolerance to self-antigens.9 PD-1 is expressed on activated T cells and functions to down-regulate T-cell activation.7,10 The demonstration that PD-1−/− mice developed strain-specific autoimmunity provided evidence of the negative regulatory function of this receptor and its ligands.11,12 PD-L17,8 and PD-L213,14 are the ligands for PD-1, and have quite different cellular expression patterns. Expression of PD-L2 is largely restricted to antigen presenting cells (APCs).13,14 Conversely, PD-L1 mRNA is broadly expressed in tissues,7,8 and protein expression has been detected on many tumor cell types,15 and can be further induced by exposure to interferon (IFN)-γ.16
Mounting evidence suggests that PD-L1 expression on solid tumor cells is capable of dampening antitumor T-cell responses.8,9,16-19 Blockade of PD-L1 inhibits tumor growth or delays progression in multiple murine models,15,18-20 and adoptive transfer of tumor-specific PD-1−/− T-cell receptor (TCR) transgenic (Tg) T cells can reject tumors even in settings where CTLA-4−/− Tg T cells cannot.16 Moreover, PD-L1 expression on tumor cells correlates with an inferior clinical outcome in various solid human malignancies.21-25 Although PD-1/PD-L1 interactions are important in suppressing immune responses against solid cancers, evidence supporting a functional role for this pathway in hematologic malignancies is lacking,26,27 and one could imagine that distinct immune evasion mechanisms may be active within the setting of a hematologic malignancy circulating through the blood and other tissues, in comparison to a solid tumor growing as a vascularized mass enmeshed in complex stromal elements. PD-L1 expression was not detected at baseline on human leukemia cell lines, but could be induced upon treatment with IFN-γ.15 Chen et al measured PD-L1 expression on bone marrow samples from patients with acute myeloid leukemia (AML) and found increasing levels upon disease progression, which was an independent negative prognostic factor for French-American-British type M5 AML.28
To investigate if the PD-1/PD-L1 pathway promotes immune escape in a murine AML model, C57BL/6 or PD-1−/− mice were challenged intravenously (IV) with a highly lethal, syngeneic AML cell line, C1498, transduced to express green fluorescent protein (C1498.GFP) to allow monitoring of tumor burden. We found low baseline expression of PD-L1 on C1498.GFP cells grown in culture, but PD-L1 was highly up-regulated when C1498.GFP cells were analyzed directly ex vivo. PD-1−/− mice harboring C1498.GFP had a significantly lower tumor burden, survived longer, and demonstrated augmented antitumor immune responses compared with wild-type mice. Treatment of C57BL/6 mice with a PD-L1 blocking antibody after tumor challenge yielded similar results. Tumor-antigen–specific T-cell responses were also higher in PD-1−/− mice injected with C1498 cells engineered to express a model peptide antigen, suggesting that the improved survival seen in PD-1−/− mice occurred as a result of T cell–mediated antitumor responses. These results confirm that the PD-1/PD-L1 pathway inhibits effective antitumor immune responses against murine AML, and support a rationale for clinical trials examining anti–PD-1 antibodies in patients with hematologic malignancies.
Methods
Mice and tumor cell lines
C57BL/6 (H-2b) mice, aged 6 to 12 weeks, were purchased from either The Jackson Laboratory or Taconic Laboratories. PD-1−/− mice were a gift from Tasuku Honjo (Kyoto University, Kyoto, Japan) and were bred onto a C57BL/6 background at our facility. Animals were maintained in a specific pathogen-free environment and used according to protocols approved by the Institutional Animal Care and Use Committee at the University of Chicago, according to National Institutes of Health guidelines for animal use. The C1498 murine AML cell line has been previously described,29 and was purchased from ATCC. C1498 cells were cultured in complete DMEM supplemented with 10% fetal calf serum (FCS). C1498.GFP cells were engineered by retroviral transduction using the pLEGFP plasmid. GFP expression by C1498.GFP was maintained with G418 (4 mg/mL) and periodically monitored by flow cytometry. C1498.SIY cells were engineered by retroviral transduction using the pLEGFP plasmid expressing cDNA for the SIYRYYGL (SIY) model peptide antigen in frame with eGFP. The SIYRYYGL peptide can be recognized by CD8+ T cells in the context of Kb. GFP-SIY expression was maintained with G418 (4 mg/mL) and monitored by flow cytometry.
Tumor challenge and peripheral blood monitoring of leukemia
After washing C1498.GFP or C1498.SIY cells 3 times with phosphate-buffered saline (PBS) to remove FCS, they were resuspended in PBS at a concentration of 107 cells/mL. A volume of 0.1 mL (106 tumor cells) was injected into the lateral tail vein of each mouse. Beginning on day 7 after injection of C1498.GFP, and periodically thereafter, blood was drawn from the retro-orbital venous plexus of mice and resuspended in ACK lysis buffer to remove red blood cells. Remaining cells were washed twice with PBS and analyzed by flow cytometry for GFP-expressing cells. The percentage of C1498.GFP cells in peripheral blood was calculated by gating on the entire white blood cell (WBC) population, followed by gating on GFP+ cells, and taking the fraction of GFP+ cells/total WBCs and multiplying by 100.
Analysis of PD-L1 expression
C1498.GFP cells were cultured for 48 hours in either complete Dulbecco modified Eagle medium (DMEM) or with complete DMEM supplemented with 20 ng/mL recombinant murine IFN-γ (R&D Systems) to stimulate PD-L1 expression. Cells were stained with a phycoerythrin (PE)–anti–PD-L1 antibody (MIH5; eBioscience) or a matched PE-conjugated isotype control monoclonal antibody (mAb; rat IgG 2aλ). Stained cells were washed twice in PBS containing 1% FCS and 0.1% sodium azide. Events were collected on a Becton Dickinson FACScanto flow cytometer, and were analyzed using FlowJo software (TreeStar). For ex vivo analysis of PD-L1 expression on C1498.GFP, tumor cells were injected into C57BL/6 mice as above in “Tumor challenge and peripheral blood monitoring of leukemia.” Sections of liver from killed, tumor-challenged mice were surgically resected, passed through a 70-μm mesh filter, stained with the anti–PD-L1 antibody as described earlier in this paragraph, and analyzed by flow cytometry for PD-L1 expression after gating of GFP+ cells.
Flow cytometry
Antibodies against the following molecules coupled to the indicated fluorochromes were purchased from BD PharMingen: APC-anti-CD8α (53-6.7), PerCP-anti–CD4 (RM4-5), PE-anti–CD3ϵ (2C11), and PE-anti–PD-1 (J43). In general, 106 cells were blocked with the anti-FcR mAb 2.4G2 and then stained with the indicated antibodies or appropriate isotype controls for 15 minutes at 4°C. Cells were subsequently washed twice in PBS containing 1% FCS, and resuspended for FACS analysis. Flow cytometry was performed on the FACScanto cytometer using BD FACSDiva software. Data analysis was performed using FlowJo software.
To analyze SIY-reactive CD8+ T cells, SIY-Kb tetramers (Beckman-Coulter) were used. Spleen cells from C57BL/6 or PD-1−/− mice challenged with C1498.SIY cells were resuspended at a concentration of 106/mL and stained with APC-anti–CD8α, PerCP-anti–CD4, and PerCP-anti–B220 antibodies, as well as with 10 μL of the SIY-Kb tetramers, according to the manufacturer's protocol. To calculate the frequency of CD8+SIY+ cells within the general CD8+ population, gating was performed on CD4−B220− and CD8+ cells, and the percentage of CD8+SIY+ cells/total CD8+ cells was calculated.
PD-L1 blockade in vivo
C57BL/6 mice were injected with 106 C1498.GFP tumor cells IV on day 0. Endotoxin-free PD-L1 antibody (10F.9G2) was purchased from BioXCell, and administered at a dose of 200 μg intraperitoneally (IP) on day 0 and also again on days 3, 6, 9, and 15. Isotype control rat IgG2b antibody was administered to tumor challenged C57BL/6 mice on the same dosing schedule.
IFN-γ enzyme-linked immunospot assay
The enzyme-linked immunospot assay (ELISPOT) was conducted with the BD PharMingen mouse IFN-γ ELISPOT kit according to the provided protocol. Briefly, ELISPOT plates were coated with anti–mouse IFN-γ antibody and stored overnight at 4°C. Plates were then washed and blocked with DMEM supplemented with 10% FCS for 2 hours at room temperature. Splenocytes from tumor-challenged mice were harvested at various indicated time points and plated at 106 cells/well. Stimulation was performed with irradiated (10 000 rad) C1498.GFP tumor cells at 5 × 104 cells/well, SIY peptide (80 nM), or PMA (50 ng/mL) and ionomycin (0.5 μM). Plates were stored at 37°C in an 8% CO2 incubator overnight, washed, and coated with detection antibody for 2 hours at room temperature. They were again washed and coated with avidin-peroxidase for 1 hour at room temperature. Plates were then washed and developed by addition of aminoethylcarbazole (AEC) substrate. Developed plates were dried overnight, read using an ImmunoSpot Series 3 Analyzer, and analyzed with ImmunoSpot software (Cellular Technology Ltd).
Immunohistochemistry
Frozen livers were cut into 6-μm sections and fixed in cold acetone and methanol for 20 minutes. After the sections were washed with PBC, they were incubated in 10% rabbit serum in 0.025% Triton X-100 in PBS for 30 minutes. Anti-CD4 (1 μg/mL, clone GK1.5) or CD8 (4 μg/mL, clone 53-6.7) were applied to the sections for 60 minutes at 25°C (room temperature) in a humidity chamber. The slides were blocked with 3% hydrogen peroxide, followed by incubation with a biotinylated secondary antibody (Vector Laboratories). Subsequently, slides were incubated for 30 minutes in ABC reagent (Vector Laboratories) according to the manufacturer's protocol, and antibody binding was detected with a diaminobenzidine (DAB) substrate chromogen kit according to the manufacturer's protocol. Slides were then briefly immersed in hematoxylin for counterstaining. Rag IgG was used as a negative staining control.
Statistical analysis
Percentages of C1498.GFP cells in the blood of differently treated cohorts of mice were compared statistically using unpaired Student t tests, anddifferences in survival were analyzed by the log-rank test on Kaplan-Meier curves.
Results
C1498 acute myeloid leukemia rapidly progresses in syngeneic C57BL/6 mice
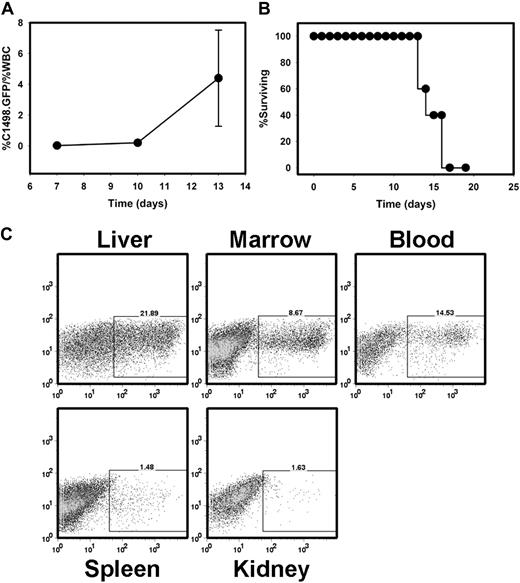
The C1498 cell line was originally derived from a C57BL/6 mouse and has been previously been described.29 It most closely resembles an AML. To develop a murine model of AML in which an estimate of in vivo tumor burden could be measured in a time-dependent manner, C1498 cells were transduced with a retroviral vector encoding the eGFP protein (C1498.GFP). Stable transductants expressing GFP were selected via cell sorting, and GFP expression was maintained with periodic exposure to the selectable antibiotic G418. The C1498.GFP cell line enabled us to follow the leukemia burden in the peripheral blood and other organs, such as the liver and spleen. When 106 C1498.GFP cells were injected intravenously into C57BL/6 mice, the percentage of GFP+ cells within the white blood cell population increased rapidly (Figure 1A). When naive, previously untreated C57BL/6 mice were injected with 106 C1498.GFP cells IV, they succumbed within approximately 15 days (Figure 1B), demonstrating marked involvement of the liver, bone marrow, and peripheral blood (Figure 1C) with leukemic cells.
C1498.GFP grows progressively when injected intravenously into immunocompetent, syngeneic mice. (A) C57BL/6 mice were injected with 106 C1498.GFP cells on day 0. On days 7, 10, and 13, peripheral blood was drawn from individual mice. Red blood cells were removed via exposure to ACK lysis buffer, and samples were analyzed by flow cytometry. The percentage of leukemia cells was calculated as percentage of GFP+ cells/percentage of total white blood cells. (B) Analysis of survival in a cohort of 5 C57BL/6 mice injected with C1498.GFP IV. Similar results were obtained in 2 independent experiments. (C) FACS analysis of organs from a C57BL/6 mouse 14 days after challenge with C1498.GFP.
C1498.GFP grows progressively when injected intravenously into immunocompetent, syngeneic mice. (A) C57BL/6 mice were injected with 106 C1498.GFP cells on day 0. On days 7, 10, and 13, peripheral blood was drawn from individual mice. Red blood cells were removed via exposure to ACK lysis buffer, and samples were analyzed by flow cytometry. The percentage of leukemia cells was calculated as percentage of GFP+ cells/percentage of total white blood cells. (B) Analysis of survival in a cohort of 5 C57BL/6 mice injected with C1498.GFP IV. Similar results were obtained in 2 independent experiments. (C) FACS analysis of organs from a C57BL/6 mouse 14 days after challenge with C1498.GFP.
PD-L1 is up-regulated on C1498.GFP cells in vivo
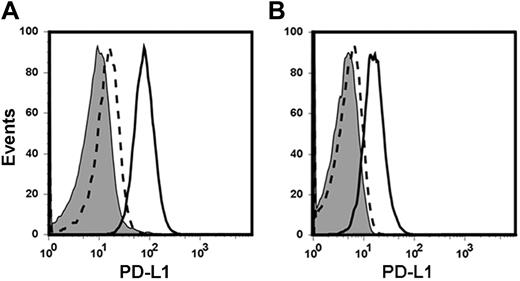
While there were multiple possibilities to explain the progressive growth and immune escape of C1498.GFP in vivo, including those related to the rapid intrinsic growth rate of the leukemia cells in vivo, it was attractive to consider the possibility that engagement of PD-1 on activated T cells with its ligand PD-L1 expressed by the C1498.GFP cells might be involved. As there have been conflicting reports regarding the expression patterns of the PD-L1 protein in human leukemia cell lines,15,28 it was of interest to determine whether PD-L1 was expressed on the cell surface of C1498.GFP cells grown in vitro, and/or after in vivo administration. C1498.GFP cells grown in culture expressed low baseline levels of PD-L1 (Figure 2A). However, after a 48-hour incubation with IFN-γ (20 ng/mL), PD-L1 expression was greatly increased, consistent with previous reports in solid tumor cells15,16 and demonstrating that these cells are capable of expressing this ligand. To investigate whether PD-L1 up-regulation occurred on C1498.GFP after in vivo injection, C57BL/6 mice were injected with 106 C1498.GFP cells. Twelve days later, portions of livers from tumor-challenged mice were resected and the prepared cell suspension was analyzed by flow cytometry. After gating on GFP+ cells, PD-L1 expression on C1498.GFP cells was determined. As shown in Figure 2B, C1498.GFP cells expressed high levels of PD-L1 when analyzed directly ex vivo, suggesting that the cytokine milieu within the leukemia “microenvironment” was capable of stimulating PD-L1 up-regulation. These results suggest that PD-L1 expression on C1498.GFP cells may be an inhibitory pathway used by leukemia cells to evade or diminish antitumor immune responses in vivo.
PD-L1 is up-regulated on C1498.GFP after injection into wild-type mice. (A) C1498.GFP cells grown in vitro were analyzed for expression of PD-L1 by flow cytometry either at baseline or after in vitro exposure to IFN-γ for 48 hours. The shaded histogram represents staining with an isotype control antibody. The dashed line represents PD-L1 expression at baseline, and the solid line represents PD-L1 expression after exposure to IFN-γ. (B) C57BL/6 mice were challenged with 106 C1498.GFP cells IV. Twelve days later, mice were killed and livers were surgically removed. After generation of single-cell suspensions, the specimens were analyzed by flow cytometry after staining with an anti–PD-L1 antibody and gating on GFP+ cells.
PD-L1 is up-regulated on C1498.GFP after injection into wild-type mice. (A) C1498.GFP cells grown in vitro were analyzed for expression of PD-L1 by flow cytometry either at baseline or after in vitro exposure to IFN-γ for 48 hours. The shaded histogram represents staining with an isotype control antibody. The dashed line represents PD-L1 expression at baseline, and the solid line represents PD-L1 expression after exposure to IFN-γ. (B) C57BL/6 mice were challenged with 106 C1498.GFP cells IV. Twelve days later, mice were killed and livers were surgically removed. After generation of single-cell suspensions, the specimens were analyzed by flow cytometry after staining with an anti–PD-L1 antibody and gating on GFP+ cells.
Absence of host PD-1 leads to improved leukemia control and augmented immune responses against C1498.GFP
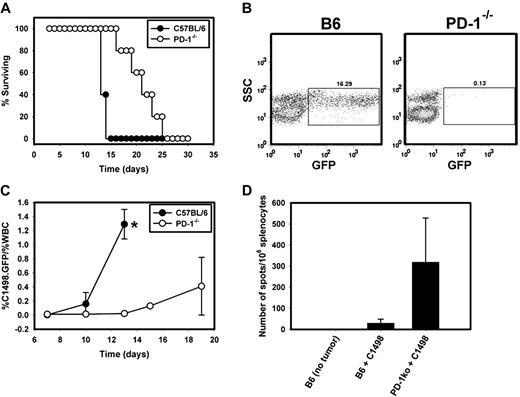
To explore the hypothesis that PD-L1/PD-1 interactions may restrain antitumor immunity in this leukemia model, PD-1−/− mice were used. C57BL/6 and syngeneic PD-1−/− mice were challenged with 106 C1498.GFP cells IV and were monitored for leukemic progression and survival. Interestingly, PD-1−/− mice receiving C1498.GFP survived significantly longer after tumor inoculation than did wild-type C57BL/6 mice (P = .002; Figure 3A). Median survival after C1498.GFP challenge was approximately 14 days in C57BL/6 mice, which was extended to 21.5 days in PD-1−/− mice. Tumor burden was assessed by flow cytometry in C57BL/6 and PD-1−/− mice through periodic analysis of the percentage of GFP+ cells in the blood and other tissues. An extreme example of markedly increased leukemic burden in the peripheral blood of a wild-type mouse compared with a PD-1−/− mouse 13 days after injection of C1498.GFP is shown in Figure 3B, and a time-based kinetic analysis of C1498.GFP burden in wild-type and PD-1−/− mice is depicted in Figure 3C. By day 13 after injection, the mean percentage of C1498.GFP cells in the peripheral blood was significantly lower in PD-1−/− versus C57BL/6 mice at 0.023% (± 0.021%) and 1.29% (± 0.21%), respectively (P < .001).
Improved survival and augmented immune responses in PD-1−/− mice challenged with C1498.GFP. (A) Survival rates of C57BL/6 (●) or PD-1−/− mice (○) challenged with 106 C1498.GFP cells IV (P = .002 for the comparison of survival between PD-1−/− and C57BL/6 mice). (B) Peripheral blood was sampled from a PD-1−/− mouse and a C57BL/6 mouse 13 days after IV injection of C1498.GFP and was analyzed by flow cytometry for GFP+ cells. Gating was initially performed on the total WBC population on the FSC versus SSC gate. GFP+ cells present in the WBC population were gated in a SSC versus GFP plot. The numbers above the GFP+ cell gate represent the percentage of GFP+ cells divided by the percentage of WBCs in the FSC versus SSC plot and multiplied by 100. (C) Cohorts of 5 PD-1−/− and C57BL/6 mice received 106 C1498.GFP cells IV. On days 10, 13, 15, and 19 after tumor challenge, peripheral blood was sampled from each mouse. Red blood cells were removed via exposure to ACK lysis buffer, and samples were analyzed by flow cytometry. The percentage of leukemia cells was calculated as the GFP+ cells/% total WBCs. Closed circles and open circles represent the mean percentage of GFP+ cells at each time point from C57BL/6 and PD-1−/− mice, respectively. (D) Spleens were harvested from C57BL/6 or PD-1−/− mice 12 days after IV injection with C1498.GFP cells. A total of 106 splenocytes from individual mice were restimulated overnight with either complete DMEM, 5 × 104 irradiated C1498.GFP cells (10 000 rad) or PMA + ionomycin in an IFN-γ ELISPOT assay. Bars represent the mean number of spot-forming cells (± SD) in each group (P < .001 for the comparison between PD-1−/− and C57BL/6 mice). Similar results were obtained in 2 independent experiments.
Improved survival and augmented immune responses in PD-1−/− mice challenged with C1498.GFP. (A) Survival rates of C57BL/6 (●) or PD-1−/− mice (○) challenged with 106 C1498.GFP cells IV (P = .002 for the comparison of survival between PD-1−/− and C57BL/6 mice). (B) Peripheral blood was sampled from a PD-1−/− mouse and a C57BL/6 mouse 13 days after IV injection of C1498.GFP and was analyzed by flow cytometry for GFP+ cells. Gating was initially performed on the total WBC population on the FSC versus SSC gate. GFP+ cells present in the WBC population were gated in a SSC versus GFP plot. The numbers above the GFP+ cell gate represent the percentage of GFP+ cells divided by the percentage of WBCs in the FSC versus SSC plot and multiplied by 100. (C) Cohorts of 5 PD-1−/− and C57BL/6 mice received 106 C1498.GFP cells IV. On days 10, 13, 15, and 19 after tumor challenge, peripheral blood was sampled from each mouse. Red blood cells were removed via exposure to ACK lysis buffer, and samples were analyzed by flow cytometry. The percentage of leukemia cells was calculated as the GFP+ cells/% total WBCs. Closed circles and open circles represent the mean percentage of GFP+ cells at each time point from C57BL/6 and PD-1−/− mice, respectively. (D) Spleens were harvested from C57BL/6 or PD-1−/− mice 12 days after IV injection with C1498.GFP cells. A total of 106 splenocytes from individual mice were restimulated overnight with either complete DMEM, 5 × 104 irradiated C1498.GFP cells (10 000 rad) or PMA + ionomycin in an IFN-γ ELISPOT assay. Bars represent the mean number of spot-forming cells (± SD) in each group (P < .001 for the comparison between PD-1−/− and C57BL/6 mice). Similar results were obtained in 2 independent experiments.
To investigate whether the improved survival seen in PD-1−/− mice was associated with improved antitumor immunity, an IFN-γ ELISPOT was performed from splenocytes isolated from wild-type and PD-1−/− mice 12 days after challenge with C1498.GFP cells. Irradiated tumor cells were used as an antigenic source for T-cell restimulation. Significantly greater numbers of IFN-γ spot-forming cells were detected from PD-1−/− compared with wild-type spleens (319 ± 209 spots vs 30 ± 19 spots, respectively; P < .001; Figure 3D). Collectively, these data suggest that in the C1498 AML model, PD-1 expression by host T cells restricts the priming and/or expansion and survival of tumor-specific T cells, thus contributing to leukemia progression.
Improved tumor antigen-specific T-cell priming in PD-1−/− mice
To investigate whether CD8+ antigen-specific T-cell responses were augmented in PD-1-deficient hosts, a C1498 cell line expressing a model tumor antigen (SIYRYYGL; SIY) was generated. T cell-mediated immune responses can be studied in mice harboring C1498.SIY using SIY-Kb tetramers to analyze the frequency of SIY-reactive CD8+ T cells generated after tumor challenge, and the function of such cells can be measured using IFN-γ ELISPOT after in vitro restimulation of spleen cells from tumor-challenged mice with SIY peptide.
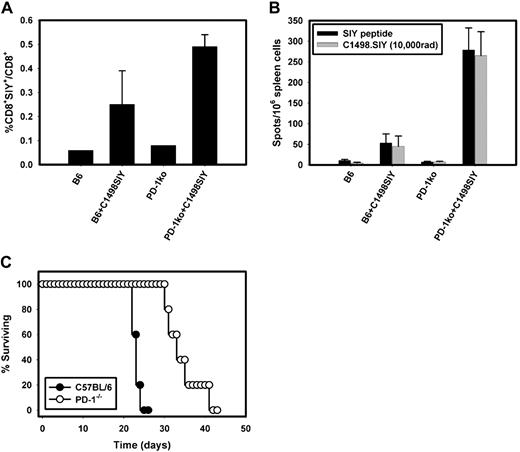
Cohorts of PD-1−/− or wild-type C57BL/6 mice were challenged with C1498.SIY cells IV, and immune responses generated in the spleens of tumor-challenged mice were analyzed 7 days later using SIY-Kb tetramers and IFN-γ ELISPOT. We found a significantly higher frequency of SIY+CD8+ T cells in PD-1−/− compared with C57BL/6 mice harboring C1498.SIY (Figure 4A). The frequency of SIY+CD8+ cells in PD-1−/− mice was 2-fold higher than that in C57BL/6 mice (0.49% ± 0.05% vs 0.25% ± 0.14%, respectively; P = .05). Similarly, significantly higher numbers of IFN-γ producing cells were present within the spleens of PD-1−/− compared with C57BL/6 mice harboring C1498.SIY (Figure 4B; 279 ± 53 spots vs 53 ± 22 spots; P < .001).
Augmented priming and effector function of tumor antigen-specific T cells in PD-1−/− mice challenged with C1498.SIY. (A) Groups of C57BL/6 or PD-1−/− mice were challenged with 106 C1498.SIY cells IV. Seven days later, spleens from tumor-bearing or control mice were harvested and the frequencies of SIY+CD8+ T cells present in each mouse were analyzed by flow cytometry after staining with SIY-Kb tetramers. Bars represent the mean frequency of SIY+CD8+/total CD8+ T cells (± SD) in each group (P = .05 for the comparison of the frequency of SIY+CD8+ cells present in PD-1−/− vs C57BL/6 mice). (B) IFN-γ ELISPOT performed on spleen cells harvested from tumor-bearing or control mice as in panel A. A total of 106 splenocytes from individual mice were stimulated with complete DMEM, SIY peptide (80 nM), irradiated C1498.SIY tumor cells, or PMA + ionomycin overnight; they were then analyzed for IFN-γ spot production. Bars represent the mean number of spot-forming cells (± SD) in each group (P < .001 for the comparison between IFN-γ spots produced by PD-1−/− vs C57BL/6 spleen cells after restimulation via SIY peptide and irradiated C1498.SIY cells). (C) Survival of PD-1−/− and C57BL/6 mice after IV injection of 106 C1498.SIY cells (P = .002 for the comparison of survival between PD-1−/− and C57BL/6 mice).
Augmented priming and effector function of tumor antigen-specific T cells in PD-1−/− mice challenged with C1498.SIY. (A) Groups of C57BL/6 or PD-1−/− mice were challenged with 106 C1498.SIY cells IV. Seven days later, spleens from tumor-bearing or control mice were harvested and the frequencies of SIY+CD8+ T cells present in each mouse were analyzed by flow cytometry after staining with SIY-Kb tetramers. Bars represent the mean frequency of SIY+CD8+/total CD8+ T cells (± SD) in each group (P = .05 for the comparison of the frequency of SIY+CD8+ cells present in PD-1−/− vs C57BL/6 mice). (B) IFN-γ ELISPOT performed on spleen cells harvested from tumor-bearing or control mice as in panel A. A total of 106 splenocytes from individual mice were stimulated with complete DMEM, SIY peptide (80 nM), irradiated C1498.SIY tumor cells, or PMA + ionomycin overnight; they were then analyzed for IFN-γ spot production. Bars represent the mean number of spot-forming cells (± SD) in each group (P < .001 for the comparison between IFN-γ spots produced by PD-1−/− vs C57BL/6 spleen cells after restimulation via SIY peptide and irradiated C1498.SIY cells). (C) Survival of PD-1−/− and C57BL/6 mice after IV injection of 106 C1498.SIY cells (P = .002 for the comparison of survival between PD-1−/− and C57BL/6 mice).
To measure effector function of tumor-specific CD8+SIY+ cells based upon their frequency within the spleens of PD-1−/− and C57BL/6 mice, the ratio of IFN-γ spots to the frequency of CD8+SIY+ cells was calculated. In PD-1−/− mice the ratio of function to frequency was 569 (279 IFN-γ spots/0.49% SIY+CD8+ cells; data not shown), whereas the ratio in C57BL/6 mice was only 212 (53 IFN-γ spots/0.25% SIY+CD8+ cells; data not shown). Collectively, these results suggest that not only was priming of tumor antigen-specific, CD8+ T cells greater in PD-1−/− mice, but also that their effector function on a per cell basis was augmented compared with control C57BL/6 mice.
It was important to determine whether the heightened antitumor immune responses generated against the SIY antigen in PD-1−/− mice would lead to superior mouse survival after tumor challenge. To this end, groups of PD-1−/− and wild-type mice were challenged with C1498.SIY cells IV and monitored over time for survival. As was seen for the non–SIY-expressing variant, PD-1−/− mice had a significant prolongation in median survival compared with C57BL/6 mice when challenged with C1498.SIY (33 days vs 23 days; P = .002; Figure 4C). Together, these results suggest that the augmented immune responses in PD-1−/− mice appear to be responsible for their improved survival after challenge with C1498.SIY leukemia cells.
In vivo blockade with anti–PD-L1 mAb leads to improved survival and augmented immune responses against C1498.GFP leukemia in wild-type mice
While immune-mediated tumor control occurred in genetically deficient PD-1−/− mice, it was of interest to confirm our findings in a more clinically relevant setting. Therefore, we also examined whether in vivo administration of a PD-L1 blocking antibody would improve outcome in tumor-challenged wild-type mice. C57BL/6 mice were inoculated with C1498.GFP cells IV followed by IP administration of a PD-L1 blocking antibody (10F.9G2) or an isotype control antibody at a dose of 200 μg on days 0, 3, 6, 9, 12, and 15. Treatment with the PD-L1 blocking antibody led to superior survival in mice compared with those receiving the isotype control antibody (P = .045, data not shown). Because the liver appears to be an important site for leukemic progression in the C1498 model, we assessed the tumor burden in the livers of C57BL/6 mice receiving either the anti–PD-L1 or isotype control antibody both histologically and using flow cytometry to detect GFP+ cells. For flow cytometric analysis, livers were resected from 5 mice in each group 12 days after tumor inoculation, and were disrupted by mechanical dissociation into a single-cell suspension. The percentage of C1498.GFP cells in the livers of mice receiving isotype control antibody was 10.5%, compared with 1.3% in the mice treated with the PD-L1 blocking antibody, an 8-fold difference (Figure 5A). Liver histology from the isotype control antibody treated mice revealed, at low power, a massive hepatic infiltration of leukemic cells appearing to originate from hepatic venules. Large numbers of leukemia cells could be visualized within the lumen of these vessels (Figure 5B). Conversely, livers from mice treated with the PD-L1 blocking antibody contained only scattered nests of leukemia cells, with minimal intravascular and visceral infiltration.
Anti–PD-L1 treatment decreases intrahepatic tumor burden and improves antitumor immune responses against C1498.GFP. (A) Cohorts of C57BL/6 mice were inoculated with 106 C1498.GFP cells IV, and received 200 μg of anti–PD-L1 or isotype control antibody IP on days 0, 3, 6, and 9. On day 12, livers were removed, and single-cell suspensions were generated, pooled between mice, and analyzed by flow cytometry for the percentage of GFP+ cells present. (B) Liver sections from C57BL/6 mice treated with isotype control (left) or PD-L1 blocking antibody (right) were stained with hematoxylin and eosin and imaged with a Zeiss Axioshop histology microscope using bright-field objective lenses. Top panels, 4× magnification; bottom panels, 20× magnification. Images were acquired with Zeiss Imaging Software, Version 4.7.1. (C) Spleens were harvested from tumor-challenged C57BL/6 mice treated with isotype control or PD-L1 blocking antibody 12 days after injection with C1498.GFP. A total of 106 splenocytes from individual mice were restimulated overnight with either complete DMEM, 5 × 104 irradiated C1498.GFP cells (10 000 rad), or PMA plus ionomycin in an IFN-γ ELISPOT assay. Bars represent the mean number of spot-forming cells (± SD) in each group. P = .001 for the comparison between mice treated with PD-L1 blocking antibody versus isotype control antibody. (D) Livers were harvested from C57BL/6 mice 12 days after C1498.GFP injection. Sections from various mice were stained with anti-CD4 and -CD8 antibodies, and the numbers of CD4+ and CD8+ cells from each liver were enumerated per high-power field (hpf; 40×). Similar results were obtained in 3 independent experiments.
Anti–PD-L1 treatment decreases intrahepatic tumor burden and improves antitumor immune responses against C1498.GFP. (A) Cohorts of C57BL/6 mice were inoculated with 106 C1498.GFP cells IV, and received 200 μg of anti–PD-L1 or isotype control antibody IP on days 0, 3, 6, and 9. On day 12, livers were removed, and single-cell suspensions were generated, pooled between mice, and analyzed by flow cytometry for the percentage of GFP+ cells present. (B) Liver sections from C57BL/6 mice treated with isotype control (left) or PD-L1 blocking antibody (right) were stained with hematoxylin and eosin and imaged with a Zeiss Axioshop histology microscope using bright-field objective lenses. Top panels, 4× magnification; bottom panels, 20× magnification. Images were acquired with Zeiss Imaging Software, Version 4.7.1. (C) Spleens were harvested from tumor-challenged C57BL/6 mice treated with isotype control or PD-L1 blocking antibody 12 days after injection with C1498.GFP. A total of 106 splenocytes from individual mice were restimulated overnight with either complete DMEM, 5 × 104 irradiated C1498.GFP cells (10 000 rad), or PMA plus ionomycin in an IFN-γ ELISPOT assay. Bars represent the mean number of spot-forming cells (± SD) in each group. P = .001 for the comparison between mice treated with PD-L1 blocking antibody versus isotype control antibody. (D) Livers were harvested from C57BL/6 mice 12 days after C1498.GFP injection. Sections from various mice were stained with anti-CD4 and -CD8 antibodies, and the numbers of CD4+ and CD8+ cells from each liver were enumerated per high-power field (hpf; 40×). Similar results were obtained in 3 independent experiments.
To examine the immune response in mice treated with anti–PD-L1 mAb, IFN-γ ELISPOT was performed on restimulated splenocytes 12 days after challenge with C1498.GFP. As shown in Figure 5C, significantly higher numbers of IFN-γ spot-forming cells were detected in spleens of mice treated with the PD-L1 blocking antibody compared with the isotype control antibody (50.1 ± 21.1 spots vs 25.9 ± 14.9 spots, respectively; P = .001). Immunohistochemical analysis was also performed on sectioned liver specimens from both groups of tumor-challenged mice to assess whether the number of infiltrating T cells was different. A greater number of CD8+ cells was found infiltrating livers from tumor-bearing C57BL/6 mice receiving anti–PD-L1 antibody compared with isotype control (54.2 ± 14.6 cells/high-power field [hpf] vs 5.2 ± 2.1 cells/hpf, respectively; P < .001; Figure 5D). CD4+ T cells were also increased in mice receiving anti–PD-L1 antibody versus isotype control, although the difference was not statistically significant (19.5 ± 10.7 cells/hpf vs 10.2 ± 6.1 cells/hpf, respectively; P = .09; Figure 5D). In addition, anti–PD-L1 did not significantly alter survival in RAG2−/− mice, arguing that the improved tumor control is lymphocyte-dependent (data not shown). Together, these results indicate a heightened immune response against leukemia cells when the PD-L1/PD-1 axis is interrupted.
Discussion
The discovery that tumor cells could display unique antigens rendering them recognizable by CD8+ T cells revolutionized the field of cancer immunotherapy,1 which has focused on augmenting adaptive immune responses against such antigens using cancer vaccines, adoptive T-cell transfer, and administration of immunomodulatory cytokines. Clinical trials using such therapies often can result in increased frequencies of antitumor T cells in the peripheral blood of cancer patients, but detection of such T cells has not generally correlated directly with objective tumor responses.30 More recently, multiple negative regulatory mechanisms have been described that appear to cooperate within the tumor microenvironment to potently inhibit even sufficiently primed antitumor T-cell responses.2,31 Putative inhibitory mechanisms include T-cell anergy due to poor costimulation by tumor cells,3 suppression of conventional T-cell function by regulatory T cells,4 up-regulation of CTLA-4 on T cells,5 tryptophan catabolism by IDO,6 and engagement of PD-1 on activated T cells with PD-L1.32 Interfering with any one or multiple of these pathways might improve antitumor immune responses in the setting of vaccination and adoptive T-cell transfer strategies in preclinical models of solid tumors.
Although evidence supporting the role of negative regulatory mechanisms within the solid tumor microenvironment has received much attention, the question whether these inhibitory pathways are important for immune escape in hematologic malignancies had been underexplored. Because of the unique growth patterns of solid and hematologic malignancies, it seems quite possible that the repertoire of immune evasion mechanisms used by each may be significantly different. Understanding subtle alterations in the mechanisms used by solid-organ and hematopoietic malignancies, as well as discovery of novel inhibitory pathways in preclinical models, is of great importance in moving successful cancer immunotherapeutic approaches forward to the treatment of leukemia patients in the clinic.
In murine solid tumor models, the PD-1/PD-L1 pathway has been well described to inhibit antitumor T-cell responses, leading to tumor progression.15,16,18-20 The major mechanism of PD-1/PD-L1 mediated T-cell inhibition lies in the ability of the PD-1 receptor to recruit and activate intracellular phosphatases, such as SHP-2, which negatively regulate TCR signaling,10 and thus, effector T-cell responses, such as cytokine secretion and target cell cytolysis.16 Other potential mechanisms that have been proposed include the induction of T-cell apoptosis after binding of PD-1 to PD-L1,15 and the provision of a molecular shield protecting tumor cells from cytolysis.20 In fact, PD-1 was originally isolated from T cells undergoing programmed cell death.33 In the context of the C1498 AML model, we found that these leukemia cells significantly up-regulated PD-L1 after disseminated growth in vivo. While the host signals that mediate this spontaneous up-regulation in vivo are not known, both type I IFNs and IFN-γ have been shown to have this capability in vitro.15,16,34
While PD-1−/− mice did not entirely reject C1498.GFP, the improvement in tumor control and survival was impressive, given that a single negative regulatory pathway was interrupted without antigen-specific vaccination or adoptive T-cell therapy. Presumably PD-L1/PD-1 blockade augmented the induction, expansion, or survival of endogenous antileukemia T cells. To clarify the mechanism through which PD-1 deficiency led to augmented antitumor immune responses, a C1498 cell line expressing a model tumor antigen was generated, which enabled an analysis of the frequency and function of tumor antigen-specific wild-type or PD-1−/− CD8+ T cells. Our results suggest that the absence of PD-1 on host cells leads to an increased frequency of IFN-γ-producing tumor-specific CD8+ T cells. Interestingly, at a relatively early time point after injection of C1498.SIY, a 2-fold higher frequency of SIY-reactive CD8+ T cells was present in PD-1−/− compared with C57BL/6 mice, supporting the notion that PD-1/PD-L1 interactions may negatively regulate T-cell priming against tumor antigens, in addition to their role in limiting the effector phase of the antitumor immune response.
Because multiple inhibitory mechanisms are likely active within the solid tumor microenvironment,2 it will be of interest to determine whether inhibition of additional negative regulatory mechanisms also can improve immune-mediated tumor control in the context of a leukemia model. Along these lines, indoleamine 2,3-dioxygenase (IDO) has been reported to be expressed in human leukemia patients,35 and the presence of CD4+CD25+FoxP3+ regulatory T cells also has been observed.36
In conclusion, our results suggest the PD-1/PD-L1 pathway plays an important role in limiting the host immune response against a hematologic malignancy in mice in vivo. Our results lend support for the evaluation of blocking antibodies against PD-L1 or PD-1 in patients with leukemia. Whether PD-L1 is expressed by various subsets of human AML, and if so, whether its expression leads to evasion of immune responses is currently underexplored. It would be important to clarify this before clinical trials testing the inhibition of the PD-1/PD-L1 pathway in AML patients are designed. If it is determined that particular AML subtypes regularly express PD-L1, then patient enrollment into trials examining PD-1 or PD-L1 blockade could be enriched for PD-L1-positive patients. Currently, 2 humanized anti–PD-1 antibodies (CT-011; Cure Tech; and MDX-1106; Medarex) have been evaluated in phase 1 clinical trials which have included patients with both solid and hematologic malignancies,37,38 and phase 2 studies are ongoing.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Tasuku Honjo (Kyoto University, Kyoto, Japan) for providing the PD-1–deficient mice, Michelle Gao for mouse breeding and screening, and Ramila Shah for assistance with retrovirus production.
This work was supported by National Institutes of Health grants R01 CA127475 (T.F.G.) and K23 CA133196 (J.K.).
National Institutes of Health
Authorship
Contribution: L.Z. planned and performed experiments; T.F.G. planned experiments and assisted in the drafting of the manuscript; and J.K. planned and performed experiments and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Justin Kline, Department of Medicine, Section of Hematology/Oncology, The University of Chicago Medical Center, 5841 S Maryland Ave, MC2115, Chicago, IL 60637; e-mail: jkline@medicine.bsd.uchicago.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal