Abstract
After the introduction of highly active antiretroviral therapy (HAART), intensive treatment, including high-dose therapy (HDT) and peripheral blood stem cell transplantation (PBSCT), has become feasible in HIV-positive patients with Hodgkin (HL) and non-Hodgkin (NHL) lymphoma. Herein, we report the long-term results, on an intention-to-treat basis, of a prospective study on HDT and PBSCT in 50 HIV-positive HAART-responding patients with refractory/relapsed lymphoma. After debulking therapy, 2 patients had early toxic deaths, 10 had chemoresistant disease, 6 failed stem cell mobilization, 1 refused collection, and 4 progressed soon after PBSC harvest. Twenty-seven actually received transplant. Twenty-one patients are alive and disease-free after a median follow-up of 44 months (OS, 74.6%; PFS, 75.9%). Only lymphoma response significantly affected OS after transplantation. In multivariate analyses both lymphoma stage and low CD4 count negatively influenced the possibility to receive transplant. Median OS of all 50 eligible patients was 33 months (OS, 49.8%; PFS, 48.9%). Low CD4 count, marrow involvement, and poor performance status independently affected survival. PBSCT is a highly effective salvage treatment for chemosensitive AIDS-related lymphoma. It seems rational to explore its use earlier during the course of lymphoma to increase the proportion of patients who can actually receive transplant.
Introduction
Treatment of HIV-associated Hodgkin (HL) and non-Hodgkin (NHL) lymphoma have been limited during the first 2 decades of HIV epidemics because of potential toxicity and the possibility of worsening severe immunodeficiency leading to mortality.1-3 The introduction of highly active antiretroviral therapy (HAART) in 1996 has changed the natural history of HIV infection, reducing HIV-related rates of morbidity and mortality.4 Moreover, immune preservation with HAART has changed the therapeutic approach to AIDS-related lymphoma (ARL); intensive chemotherapy (CT) is now well tolerated,5,6 and efforts are underway to optimize lymphoma therapy to reap gains in lymphoma free and overall survival.7-9 Even if lymphomas have been reduced in incidence in HIV-positive subjects, ARLs are emerging as one of the major causes of death in patients with HIV who have access to HAART.10 ARL prognosis is nowadays tightly linked to lymphoma outcome more than related to HIV infection, as it was in the past.11 In the pre-HAART era few studies addressed the salvage treatment,12-14 and the reported results with conventional dose second-line CT were poor with low complete remission (CR) rate and short survival. High-dose therapy (HDT) with peripheral blood stem cell (PBSC) transplantation (PBSCT), which is the treatment of choice for relapsing or refractory HL or NHL in HIV-negative patients,15,16 has been recently explored by several centers in the HIV setting, showing the feasibility of the procedure in terms of stem cell mobilization, engraftment, and low regimen-related toxicity17,18 and showing antilymphoma efficacy.19-22 However, selection of patients in these studies is not known and might have been substantial. Within the Italian Cooperative Group on AIDS and Tumors (GICAT) we started in 2000 a prospective multicentric study with the use of HDT and PBSCs as salvage therapy in HIV-positive patients with refractory or relapsed HL or NHL. Our initial experience showed the feasibility of this treatment approach on a multi-institutional basis and in unselected HAART-responding patients,23 with encouraging clinical results. Here, we present the long-term results of the GICAT study in a larger series of patients with mature follow-up and analyze the feasibility of the program according to the intention to treat and the effect of HIV- and lymphoma-related factors on PBSCT outcome and on the prognosis of the eligible patients.
Methods
Patients
HIV-positive patients with biopsy-proven HL or NHL who failed to achieve CR after standard dose first-line CT, ie, primary refractory or with histologically confirmed partial remission (PR), or relapsed after initial CR (first or subsequent relapse) were eligible. Until April 2003 patients with HL in first relapse were eligible if previous CR lasted less than 1 year; afterward this requirement was deleted. Exclusion criteria included age older than 60 years; World Health Organization performance status (PS) higher than 2; left ventricular ejection fraction lower than 50%; creatinine level higher than 2 mg/dL, bilirubin level higher than 3 mg/dL, and/or total bilirubin level higher than 3 mg/dL and/or prothrombin test lower than 70%; diffuse capacity lower than 50% predicted; CD4 count fewer than 100 cells/μL before first-line CT in patients treated for at least 6 months with HAART; or no availability of potentially effective HAART. Patients with central nervous system or meningeal lymphoma were excluded as well as patients with active major opportunistic infections or previous CMV pneumonia. Previous AIDS-defining illness was not an exclusion criteria as well as positive hepatitis B or C serology. All patients had to be receiving HAART or starting HAART at study entry.
This study was a collaborative effort within the GICAT. The institutional review board of each participating center approved the protocol, and informed consent was obtained from each patient in accordance with the Declaration of Helsinki.
Findings from a preliminary analysis have been reported.23 The present analysis represents an updated report after the final accrual goal was achieved and with a median follow-up of censored patients of 45 months, which allows the evaluation of long-term efficacy with prognostic factors analysis, including an adequate intention-to-treat analysis.
Treatment
Patients with primary refractory or relapsed HL or NHL received debulking treatment with 2 to 4 courses of conventional-dose salvage CT, at center discretion. Those who showed CT sensitivity, defined as at least minor response (> 25% decrease in measurable disease or any amelioration of assessable disease or disappearance of disease-related symptoms) to debulking treatment, had PBSCs collected on recovery from salvage CT or after cyclophosphamide 4 g/m2 plus granulocyte colony-stimulating factor (G-CSF) 10 μg/kg/d. Patients in PR after first-line CT received cyclophosphamide 4 g/m2 + G-CSF 10 μg/kg/d to mobilize and collect PBSCs, without previous debulking treatment. A minimum of 5 × 106 CD34+ cells/kg of patient body weight was planned to be collected. Patients refractory to debulking treatment or with disease progression during mobilization and collection procedures were off protocol; they received treatment at center discretion and were analyzed for the study on an intention-to-treat basis. At least 1 month after stem cell harvest patients received HDT with carmustine 300 mg/m2 on day −7, cytarabine 200 mg/m2 on days −6 to −3, etoposide 200 mg/m2 on days −6 to −3, and melphalan 140 mg/m2 on day −2 (BEAM) as the conditioning regimen24 and PBSC reinfusion. G-CSF 5 μg/kg was started on day +1 until neutrophil recovery. All patients received HAART throughout the entire treatment program, regardless of CD4 count or HIV viral load, according to international guidelines, avoiding AZT to avoid myelosuppression (47 were maintained on their previous HAART regimen and 3 patients started HAART at study entry). From the beginning of the conditioning regimen until stable engraftment, patients received antibacterial, antifungal, and antiviral prophylaxis with quinolones, azoles, and acyclovir. Trimethoprim/sulfamethoxazole, used as prophylaxis for Pneumocystis carinii pneumonia during the entire treatment program, was suspended after stem cell infusion until hematologic recovery. Toxicity was graded according to the National Cancer Institute of Canada common toxicity grading criteria.
Statistical methods
Baseline characteristics were compared between patients receiving or not PBSC transplants with the use of the unpaired t test for continuous variables and the Fisher exact test for categoric variables. Overall survival (OS) and progression-free survival (PFS) from study entry was computed from the initiation of debulking CT, for refractory and relapsed patients, or mobilizing therapy, for patients in PR, until death or the last visit for OS and until disease progression, relapse, or the last time the patient was known to be alive and disease free for PFS.25 Survival after transplantation was computed from the date of PBSCT until death or the last visit for OS and until progression, relapse, or the last time the patient was known to be disease free and alive for PFS. OS and PFS were estimated with the use of the Kaplan-Meyer method, and differences between subgroups were assessed by the log-rank test. We evaluated a variety of prognostic factors for their effect on survival after transplantation, on survival of the entire series because the study entry and on the possibility to actually receive the transplant (intention-to-treat analysis), including demographic factors (age, sex), lymphoma-specific factors (stage, systemic symptoms, lymphoma subtype, status at entry [refractory and PR with histologically confirmed persistent disease versus relapsed], PS, LDH, bone marrow involvement), number of debulking cycles before PBSCT, achievement of CR before PBSCT or after PBSCT, radiotherapy (RTT) after PBSCT, and HIV-specific factors (risk factor for HIV, CD4 count at entry and at transplantation, detectable HIV viral load, prior opportunistic infection (OI) or other AIDS-defining illnesses). Multivariate Cox regression analysis was applied to evaluate prognostic factors for survival, and multivariate logistic regression analysis was applied to evaluate factors affecting the possibility to receive the transplant, including all variables that were statistically significant or borderline significant in univariate analysis or of particular clinical importance. In all cases, statistical significance was claimed for P values less than .05.
Results
Patient characteristics
From May 2000 to February 2007, 50 patients with ARL were enrolled. Median age at study entry was 39 years (range, 28-59 years). Thirty-one patients had NHL (13 refractory, 15 first relapse, 1 second relapse, 2 PR), and 19 patients had HL (7 refractory, 7 first relapse, 2 second relapse, 3 PR). The median duration of the last remission was 6 months (range, 1-53 months). Histologic subtypes of NHL were 22 diffuse large B cell (DLBCL), 4 plasmablastic, 3 anaplastic large-cell (2 null and 1 T cell), and 2 Burkitt/Burkitt-like. Histologic subtypes of HL were 9 mixed cellularity, 4 nodular scleroses, 3 lymphocyte depletion, and 3 classic HL not further classifiable. Within the patients with DLBCL, 8 were treated with rituximab-containing regimen in first-line therapy and 14 were not. Characteristics of the study population are shown in Table 1. At study entry 46 patients were on HAART, 2 started HAART at the time of enrollment and 2 at the time of PBSC mobilization. The median CD4 cell count at study entry was 218 cells/μL (range, 17-561 cells/μL); 11 patients had a CD4 count fewer than 100 cells/μL. Eleven patients had a detectable HIV viral load ranging between 204 and 750 000 copies/mL (median, 1390 copies/mL). Twenty-two patients (44%) had positive serology for hepatitis C virus (HCV) infection and 13 of 18 evaluable (in 4 cases data not known) patients had positive HCV viremia at study entry. Two patients had hepatitis B virus (HBV) infection with HBsAg positivity.
Characteristics of the study population at entry
| Patient characteristic . | n (%) . |
|---|---|
| Patients | 50 (100) |
| Sex | |
| Male | 43 (86) |
| Female | 7 (14) |
| WHO performance status | |
| 0 | 10 (20) |
| 1 | 27 (54) |
| 2 | 13 (26) |
| Risk group for HIV infection | |
| Intravenous drug use | 24 (48) |
| Homosexual male | 13 (26) |
| Heterosexual contact | 9 (18) |
| Unknown | 4 (8) |
| Previous AIDS-defining event (other than lymphoma) | 15 (30) |
| CD4+ count | |
| < 100 cells/mL | 11 (22) |
| < 200 cells/mL | 21 (42) |
| HIV viral load | |
| Undetectable | 39 (78) |
| Detectable | 11 (22) |
| HCV-positive | 22 (44) |
| Histology | |
| Non-Hodgkin | 31 (62) |
| Hodgkin | 19 (38) |
| Status of lymphoma | |
| First relapse | 22 (44) |
| Second relapse | 3 (6) |
| Refractory | 20 (40) |
| Partial remission | 5 (10) |
| Stage of lymphoma | |
| II | 7 (14) |
| III | 13 (26) |
| IV | 30 (60) |
| B symptoms | 20 (40) |
| Extranodal involvement | 31 (62) |
| Bone marrow involvement | 13 (26) |
| LDH greater than normal value | 17 (36) |
| Patient characteristic . | n (%) . |
|---|---|
| Patients | 50 (100) |
| Sex | |
| Male | 43 (86) |
| Female | 7 (14) |
| WHO performance status | |
| 0 | 10 (20) |
| 1 | 27 (54) |
| 2 | 13 (26) |
| Risk group for HIV infection | |
| Intravenous drug use | 24 (48) |
| Homosexual male | 13 (26) |
| Heterosexual contact | 9 (18) |
| Unknown | 4 (8) |
| Previous AIDS-defining event (other than lymphoma) | 15 (30) |
| CD4+ count | |
| < 100 cells/mL | 11 (22) |
| < 200 cells/mL | 21 (42) |
| HIV viral load | |
| Undetectable | 39 (78) |
| Detectable | 11 (22) |
| HCV-positive | 22 (44) |
| Histology | |
| Non-Hodgkin | 31 (62) |
| Hodgkin | 19 (38) |
| Status of lymphoma | |
| First relapse | 22 (44) |
| Second relapse | 3 (6) |
| Refractory | 20 (40) |
| Partial remission | 5 (10) |
| Stage of lymphoma | |
| II | 7 (14) |
| III | 13 (26) |
| IV | 30 (60) |
| B symptoms | 20 (40) |
| Extranodal involvement | 31 (62) |
| Bone marrow involvement | 13 (26) |
| LDH greater than normal value | 17 (36) |
WHO indicates World Health Organization; and HCV, hepatitis C virus.
Treatment, response, and survival
Forty-five patients with refractory or relapsed disease received debulking treatment; they received a median of 2 courses of CT (range, 1-4 courses). Patients with HL received ifosfamide, mitoxantrone, etoposide (MINE; 14 patients); mecloretamine, vincristine, procarbazine, prednisone (1 patient); and adriamycin, bleomycin, vinblastine, dacarbazine (1 patient). Patients with NHL received dexamethasone, high-dose cytarabine and oxaliplatin (16 patients) or other plastin-based CT (7 patients); cyclophosphamide, doxorubicin, etoposide (2 patients); MINE (2 patients); methotrexate, doxorubicin, cyclophosphamide, vincristine, bleomycin, prednisone (1 patient); ifosfamide, epirubicin, etoposide (1 patient). Twelve patients with DLBCL received rituximab as part of debulking treatment (6 had already received rituximab in the first-line treatment and 6 had not). Two patients with HL enrolled in PR received debulking therapy with MINE before mobilizing treatment according to physician decision.
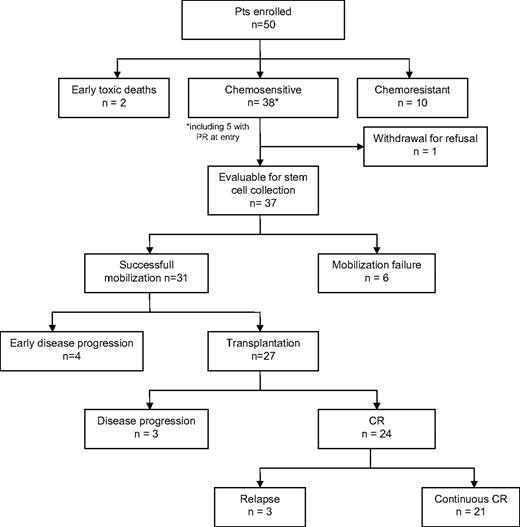
Figure 1 shows the main results of the study. Thirteen patients withdrew before stem cell mobilization because of early toxic deaths (2 patients), chemoresistant disease (10 patients), or refusal (1 patient). An adequate number of CD34+ cells (median, 5.9 × 106/kg of body weight; range 2.5-20 × 106/kg of body weight) were obtained in 31 (84%) of 37 evaluable patients, after a median of 2 apheresis treatments (range, 1-3 apheresis treatments). In 19 patients PBSCs were collected at recovery after debulking CT and in 12 after high-dose cyclophosphamide 4 g/m2 plus G-CSF. Four patients had early disease progression soon after PBSC collection (median, 13.5 days after last apheresis; range, 5-20 days), and finally 27 patients received PBSC transplant according to the protocol (time from PBSC collection to reinfusion, 42 days; range, 30-63 days), who represent the 54% of enrolled patients in the study. Nineteen patients had NHL (8 refractory, 1 PR, and 10 relapsed) and 8 had HL (3 refractory and 5 relapsed); the median age was 39 years (range, 31-59 years). The median CD4 count at the time of transplantation was 190 cells/μL (range, 88-545 cells/μL) compared with 230 cells/μL (range, 95-561 cells/μL) at baseline. All patients were on HAART (23 patients were on a protease inhibitor–based regimen, and the remainder were on a nonnucleoside reverse transcriptase inhibitor–based regimen). Four patients had detectable HIV viremia (median, 367.5 copies/mL; range, 60-14 262 copies/mL). Six patients have had previous major OIs, including intestinal atypical mycobacteriosis, mycobacterium pneumonia, P carinii pneumonia, cytomegalovirus encephalitis, esophagus candidosis, and 2 patients have had cutaneous Kaposi sarcoma. Eleven patients had positive HCV serology. All patients received BEAM regimen therapy and G-CSF 5 μg/kg until neutrophil recovery. Engraftment was prompt and satisfactory, with adequate long-term hematologic reconstitution, in all patients except one who had delayed platelet engraftment and suboptimal reconstitution (this patient had cytopenia before BEAM). Neutrophil engraftment, defined as an absolute neutrophil count greater than 0.50 × 109/L (500/mm3), occurred at a median of 10 days (range, 8-14 days), and platelet engraftment, defined as self-supporting platelet count greater than 20 × 109/L (20 × 103/mm3), occurred at a median of 12 days (range, 8-120 days).
Twenty-seven patients received a transplant according to the protocol (ie, with chemosensitive disease); 26 of them had previously received debulking treatment, achieving CR (8 patients), PR (17), and minor response (1), before starting the conditioning regimen. All patients are evaluable for response after PBSCT. Ten patients received RTT (1 patient, with lymphoma of the testis, on the contralateral testis, 5 on previous bulky disease, and 4 on residual disease). Overall, 24 of 27 patients achieved CR (1 patient with PR was converted to CR after RTT), 2 patients with PR, who progressed after RTT (months +5 and +6) and died, and 1 patient had early progression. Three patients relapsed after CR at months +5, +8, and +12 and died of lymphoma. Six patients died because of lymphoma, and no deaths were registered because of treatment-related toxicity or infectious complications. After a median follow-up of 44 months from transplantation (range, 4-70 months), 21 patients are alive in continuous CR with an OS and PFS of 74.6% plus or minus 9.0% and 75.9% plus or minus 8.6%, respectively (Figure 2).
Overall survival (OS) and progression-free survival (PFS) of 27 patients with AIDS-related lymphoma after PBSCT.
Overall survival (OS) and progression-free survival (PFS) of 27 patients with AIDS-related lymphoma after PBSCT.
Five patients received PBSC transplant after withdrawal from the protocol: 4 with chemoresistant NHL who died of disease progression after brief response with PBSCT and 1 with chemosensitive HL who had an initial relapse just before PBSCT and is still alive in CR after more than 2 years from transplantation.
Analyzing separately the results in 2 main lymphoma subtypes, DLBCL and HL, 13 (59%) of 22 patients with DLBCL received transplant, and 11 (42%) of 13 patients are alive in continuous CR, compared with 8 of 19 patients with HL who underwent transplantation, with 6 of 8 alive in continuous CR. OS and PFS after transplantation are 81.5% and 83%, respectively, in DLBCL and 75% (both OS and PFS) in HL.
PBSCT toxicity and effect on HIV disease
Antiretroviral therapy was intended to be given to all patients during the entire treatment program, but not all patients were able to tolerate it throughout. Two patients suspended HAART before starting the conditioning regimen because of cytopenia and hepatotoxicity, and they restarted it at day +20 and +75 after transplantation. Five patients suspended HAART after BEAM because of intolerance resulting from gastrointestinal toxicity (mucositis) in 4 and hepatotoxicity in 1, and they restarted the same HAART regimen after a median of 16 days (range, 5-28 days).
Nine (33%) of 27 patients experienced grade 3 to 4 treatment-related toxicity, including grade 3 gastrointestinal toxicity in 8 patients (oral mucositis in 6 and diarrhea in 3), grade 3 hepatic toxicity in 2 patients, and grade 4 oral mucositis in 1 patient. Another patient had a hypersensitivity reaction to dimethyl sulfoxide at PBSC reinfusion requiring parenteral medications. Nine patients had an episode of fever of unknown origin and were treated with broad-spectrum antibiotics. Six patients had documented bacterial infections during neutropenia, including 2 sepses (from Escherichia coli and Staphylococcus epidermidis), 2 reactivations of perianal abscess, 1 phlegmon of the neck, 1 Clostridium difficile colitis, 1 Pseudomonas aeruginosa pneumonia. One herpes zoster and one herpes simplex infection of the mouth were seen. All cases responded well to treatment.
The 7 patients who temporarily suspended antiretroviral therapy did not experience an excess in infectious complication. Postengraftment infections included CMV retinitis in one patient, 4 cases of asymptomatic CMV viremia (including the patient with previous CMV encephalitis), 6 herpes zoster infections, and 2 esophagus candidosis (in one case after lymphoma relapse and HAART suspension). All patients responded to therapy.
Of 24 long-term survivors, 9 required changes in HAART regimen during the observation period (because of viral failure in 4 and intolerance in 5).
CD4 counts decreased, as expected, reaching a nadir approximately 3 months after transplantation (median CD4 count at month +3, 119.5 cells/μL; range, 28-734 cells/μL); they recovered at month +6 (median, 159 cells/μL; range, 46-449 cells/μL) and overcame the baseline 1 year after transplantation (median, 254 cells/μL; range, 66-486 cells/μL). Patients who survived in CR at 2 years had a median CD4 count of 363 cells/μL (range, 101-490 cells/μL).
Of 4 patients with detectable viremia at the time of transplantation, 2 became undetectable early afterward, whereas of 23 with undetectable viral load, 8 were positive after transplantation: 1 patient died soon after of disease progression, 6 became negative within 6 months, and 1 patient remains positive at a low level 2 years after transplantation, with an acceptable CD4 count (340 cells/μL). Moreover, 2 patients had a late viral failure to HAART (in one case at the time of relapse).
No reactivation of HCV was seen as a consequence of transplantation or after transplantation. One patient was successfully treated with interferon plus ribavirin for chronic HCV hepatitis 2 years after transplantation. All patients with positive serology for HBV and 1 patient with positive HBsAg received lamivudine as part of their HAART, and no HBV reactivation was seen.
Prognostic factors and intention-to-treat analysis
The outcome of patients after transplantation was satisfactory with a low relapse rate (12.5%). Of note, the multivariate analyses of prognostic factors for survival after transplantation showed that only the achievement of CR after transplantation was significant for predicting both OS and PFS (Table 2) The median OS of patients who did not proceed to transplantation according to the protocol, who represent almost half of enrolled patients (46%), was 7 months from study entry, with a 3-year PFS of 13.0% (± 7.9%) compared with 76.3% (± 8.5%) for patients who received a transplant (P < .001, log-rank test).
Univariate and multivariate statistical analysis of the prognostic factors for overall survival and progression-free survival in 27 patients receiving transplant
| Prognostic factors . | Overall survival . | Progression-free survival . | ||
|---|---|---|---|---|
| Univariate . | Multivariate, HR (95% CI) . | Univariate . | Multivariate, HR (95% CI) . | |
| Systemic symptoms | 0.067 | 0.796, 1.35 (0.13-13.41) | 0.070 | 0.802, 1.33 (0.13-12.97) |
| Performance status 2 | 0.034 | 0.119, 3.94 (0.7-22.18) | 0.049 | 0.129, 3.66 (0.68-19.21) |
| Less than CR after PBSCT | 0.005 | 0.021, 7.66 (1.35-43.57) | 0.006 | 0.017, 8.29 (1.45-47.32) |
| Prognostic factors . | Overall survival . | Progression-free survival . | ||
|---|---|---|---|---|
| Univariate . | Multivariate, HR (95% CI) . | Univariate . | Multivariate, HR (95% CI) . | |
| Systemic symptoms | 0.067 | 0.796, 1.35 (0.13-13.41) | 0.070 | 0.802, 1.33 (0.13-12.97) |
| Performance status 2 | 0.034 | 0.119, 3.94 (0.7-22.18) | 0.049 | 0.129, 3.66 (0.68-19.21) |
| Less than CR after PBSCT | 0.005 | 0.021, 7.66 (1.35-43.57) | 0.006 | 0.017, 8.29 (1.45-47.32) |
Only those parameters that achieved statistical or borderline significance on at least 1 endpoint are listed.
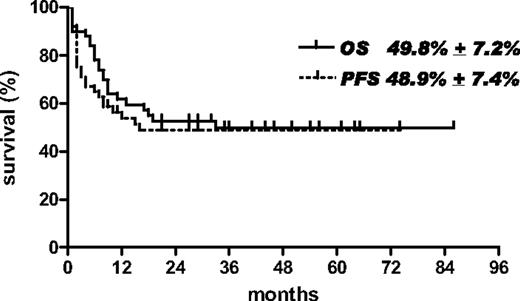
On an intention-to-treat basis, the median OS of the entire series of 50 patients was 33 months from study entry, with an OS and PFS of 49.8% (± 7.2%) and 48.9% (± 7.4%), respectively, after a median follow-up of 45 months (range, 9-86 months; Figure 3).
Overall survival (OS) and progression-free survival (PFS) of the entire series of 50 patients with AIDS-related lymphoma eligible for the study.
Overall survival (OS) and progression-free survival (PFS) of the entire series of 50 patients with AIDS-related lymphoma eligible for the study.
Table 3 shows the comparison of the characteristics of patients, between the groups who received the transplant and who did not. Significant differences were found in lymphoma stage and CD4 count, with a higher frequency of Ann Arbor stage IV and low CD4 count among patients who could not receive the transplant. The multivariate analyses of factors preventing patients from receiving the transplant confirmed that Ann Arbor stage IV (OR, 5.72; 95% CI, 1.24-26.38) and low CD4 count (OR, 25.44; 95% CI, 2.27-284.7) had a negative effect on the possibility to receive the transplant (Table 4). Interestingly, only 1 patient with CD4 fewer than 100 cells/μL at entry was able to receive a transplant; the causes of failure in the remainder were progressive disease in 7 patients, CD34+ cell collection failure in 2 patients, and early death during debulking treatment in 1 patient.
Comparison between characteristics of patients receiving a transplant or not
| . | PBSC transplant . | No PBSC transplant . | P . |
|---|---|---|---|
| No. of patients | 27 | 23 | |
| Median age, y (range) | 39 (31-59) | 39 (28-58) | NS |
| WHO performance status, n (%) | |||
| 0-1 | 22 (81) | 15 (65) | NS |
| 2 | 5 (19) | 8 (35) | |
| Risk group for HIV infection, n (%) | |||
| Intravenous drug use | 11 (41) | 13 (56) | NS |
| Homosexual male | 8 (30) | 5 (22) | |
| Heterosexual contact | 5 (18) | 4 (17) | |
| Unknown | 3 (11) | 1 (4) | |
| Previous AIDS-defining event, n (%) | 8 (30) | 6 (26) | NS |
| CD4 cell count, median (range), cells/mL | 230 (95-561) | 138 (17-451) | .003 |
| CD4 cell count < 100 cells/mL, n (%) | 1 (4) | 10 (43) | .001 |
| Detectable HIV viremia, n (%) | 5 (18) | 6 (26) | NS |
| HCV-positive, n (%) | 11 (41) | 11 (48) | NS |
| Histology, n (%) | |||
| Non-Hodgkin | 19 (70) | 12 (52) | NS |
| Hodgkin | 8 (30) | 11 (48) | |
| Status of lymphoma, n (%) | |||
| Refractory + partial remission | 12 (44) | 13 (57) | NS |
| Relapse | 15 (56) | 10 (43) | |
| Stage of lymphoma, n (%) | |||
| II-III | 16 (59) | 4 (17) | .03 |
| IV | 11 (41) | 19 (83) | |
| B symptoms, n (%) | 9 (33) | 11 (48) | NS |
| Bone marrow involvement, n (%) | 5 (18) | 8 (35) | NS |
| LDH greater than normal value, n (%) | 10 (40) | 7 (32) | NS |
| . | PBSC transplant . | No PBSC transplant . | P . |
|---|---|---|---|
| No. of patients | 27 | 23 | |
| Median age, y (range) | 39 (31-59) | 39 (28-58) | NS |
| WHO performance status, n (%) | |||
| 0-1 | 22 (81) | 15 (65) | NS |
| 2 | 5 (19) | 8 (35) | |
| Risk group for HIV infection, n (%) | |||
| Intravenous drug use | 11 (41) | 13 (56) | NS |
| Homosexual male | 8 (30) | 5 (22) | |
| Heterosexual contact | 5 (18) | 4 (17) | |
| Unknown | 3 (11) | 1 (4) | |
| Previous AIDS-defining event, n (%) | 8 (30) | 6 (26) | NS |
| CD4 cell count, median (range), cells/mL | 230 (95-561) | 138 (17-451) | .003 |
| CD4 cell count < 100 cells/mL, n (%) | 1 (4) | 10 (43) | .001 |
| Detectable HIV viremia, n (%) | 5 (18) | 6 (26) | NS |
| HCV-positive, n (%) | 11 (41) | 11 (48) | NS |
| Histology, n (%) | |||
| Non-Hodgkin | 19 (70) | 12 (52) | NS |
| Hodgkin | 8 (30) | 11 (48) | |
| Status of lymphoma, n (%) | |||
| Refractory + partial remission | 12 (44) | 13 (57) | NS |
| Relapse | 15 (56) | 10 (43) | |
| Stage of lymphoma, n (%) | |||
| II-III | 16 (59) | 4 (17) | .03 |
| IV | 11 (41) | 19 (83) | |
| B symptoms, n (%) | 9 (33) | 11 (48) | NS |
| Bone marrow involvement, n (%) | 5 (18) | 8 (35) | NS |
| LDH greater than normal value, n (%) | 10 (40) | 7 (32) | NS |
WHO indicates World Health Organization; HCV, hepatitis C virus; and NS, not significant.
Univariate and multivariate statistical analysis of the prognostic factors for actually receiving a transplant, according to the intention to treat and for overall survival and progression-free survival in 50 eligible patients
| Prognostic factors . | Possibility to receive a transplant . | Overall survival . | Progression-free survival . | |||
|---|---|---|---|---|---|---|
| Univariate . | Multivariate . | Univariate . | Multivariate . | Univariate . | Multivariate . | |
| Stage IV | 0.004 | 0.025 | 0.006 | 0.196 | 0.022 | 0.49 |
| OR (95% CI) | 5.72 (1.24-26.38) | 2.14 (0.67-6.84) | 1.48 (0.48-4.58) | |||
| Marrow involvement | NS | NS | 0.002 | <0.001 | 0.024 | 0.009 |
| OR (95% CI) | 5.28 (2.0-13.93) | 3.49 (1.32-8.96) | ||||
| Systemic symptoms | NS | NS | 0.011 | 0.964 | 0.082 | 0.755 |
| OR (95% CI) | 0.97 (0.35-2.68) | 0.85 (0.32-2.24) | ||||
| Performance status 2 | NS | NS | <0.001 | 0.001 | 0.018 | 0.067 |
| OR (95% CI) | 3.84 (1.66-8.86) | 2.23 (0.94-5.29) | ||||
| CD4+ count < 100/μL | 0.006 | 0.008 | 0.002 | <0.001 | 0.001 | <0.001 |
| OR (95% CI) | 25.44 (2.27-284.7) | 6.71 (2.5-18.01) | 6.14 (2.36-15.99) | |||
| Prognostic factors . | Possibility to receive a transplant . | Overall survival . | Progression-free survival . | |||
|---|---|---|---|---|---|---|
| Univariate . | Multivariate . | Univariate . | Multivariate . | Univariate . | Multivariate . | |
| Stage IV | 0.004 | 0.025 | 0.006 | 0.196 | 0.022 | 0.49 |
| OR (95% CI) | 5.72 (1.24-26.38) | 2.14 (0.67-6.84) | 1.48 (0.48-4.58) | |||
| Marrow involvement | NS | NS | 0.002 | <0.001 | 0.024 | 0.009 |
| OR (95% CI) | 5.28 (2.0-13.93) | 3.49 (1.32-8.96) | ||||
| Systemic symptoms | NS | NS | 0.011 | 0.964 | 0.082 | 0.755 |
| OR (95% CI) | 0.97 (0.35-2.68) | 0.85 (0.32-2.24) | ||||
| Performance status 2 | NS | NS | <0.001 | 0.001 | 0.018 | 0.067 |
| OR (95% CI) | 3.84 (1.66-8.86) | 2.23 (0.94-5.29) | ||||
| CD4+ count < 100/μL | 0.006 | 0.008 | 0.002 | <0.001 | 0.001 | <0.001 |
| OR (95% CI) | 25.44 (2.27-284.7) | 6.71 (2.5-18.01) | 6.14 (2.36-15.99) | |||
Only those parameters that achieved statistical or borderline significance on at least one endpoint are listed.
The multivariate analysis for prognostic factors for survival in the entire series of patients showed that marrow involvement, PS 2, and CD4 count fewer than 100 cells/μL had independent prognostic value for predicting OS and marrow involvement and CD4 count fewer than 100 cells/μL for predicting PFS (Table 4). OS was significantly worse among patients with CD4 count fewer than 100 cells/μL (18.1% vs 59.2%, with median OS of 7 months versus not reached), marrow involvement (19.2% vs 60.3%, with median OS of 8 months versus not reached), and PS 2 (15.2% vs 62.4%, with median OS of 5 months vs not reached). No other factors achieved statistical significance in multivariate analysis. The difference in OS seen in univariate analysis between Ann Arbor stage IV compared with stage I to III (35% vs 19.2%; P = .006) was lost in multivariate analysis, and the borderline statistical difference seen between patients enrolled with refractory versus with relapsed disease (OS 34.1% vs 67.2%; P = .054) could not be confirmed in multivariate analysis. Moreover, no significant difference was apparent between HL and NHL (OS 47.3% and 51.9%, respectively; P = .67).
For patients with DLBCL no difference was evident between those exposed to rituximab in the first-line treatment or not (OS 56.2% ± 19.8% and 56.2% ± 13.4%, respectively; P = .94).
Discussion
We report the results of a multi-institutional phase 2 trial of HDT and PBSCT as salvage treatment in 50 HIV-positive patients with HL or NHL. Previous studies and a recent analysis from the European Bone Marrow Transplantation Working Party on Lymphomas26 have shown the feasibility of stem cell collection, tolerability of HDT, and appropriate engraftment in this patient population.17,18,23 Moreover, they suggested an encouraging clinical efficacy of this procedure in patients with relapsed or refractory lymphoma or in high-risk CR, at least in small series of patients.19,20,22 These studies enrolled patients after response to salvage therapy, at the time of stem cell collection, and selection of patients might have been substantial. French investigators, in a recent retrospective analysis,21 reported less enthusiastic results in a heterogeneous group of patients, including subjects with chemorefractory disease. The present study, which represents the largest prospective study of HDT in ARL, confirms the feasibility and safety of this procedure on a multi-institutional basis in unselected HAART-responding patients. For many years, before the introduction of HAART, this aggressive treatment approach has been considered prohibitive in the HIV setting. In this study, with patients receiving effective antiretroviral therapy, the infectious risk was similar to the non-HIV setting with bacterial infections as the main complication during the preengraftment period and herpes zoster or CMV reactivation as the more frequent late complications. No significant detrimental effects on underlying HIV infection were noticed. The effect on CD4 cell count and HIV viral load does not seem superior than what is usually seen as a consequence of standard CT given with concomitant HAART27-29 ; within the first year after transplantation the CD4 count reached a higher level than the baseline, and the HIV viremia remained undetectable in most patients. Of note is that no patients receiving transplant died because of treatment-related toxicity, infections, or other HIV-related complications.
This study depicts HDT as a highly effective salvage treatment in chemosensitive HL and NHL, which is the widely accepted indication even in HIV-negative patients. Seventy-five percent of OS and 76% of PFS after 44 months of follow-up with HDT as salvage therapy in HL and NHL are promising results, also considering the high proportion in our study of primary refractory patients (40%) or with histologically confirmed PR (10%). The very low relapse rate (12.5%) and the plateau in PFS 12 months after transplantation, in the present study, are encouraging, as also reported by Krishnan et al19 ; the small numbers of patients in these series do not allow any definitive conclusion. Moreover, a recent case-control study within the European Bone Marrow Transplantation Lymphoma Working Party found no difference in OS and PFS after PBSCT in HIV-positive and -negative groups of patients, stratifying patients according to lymphoma subtype, stage, and disease status at transplantation.30 A nonstatistical significant increase in the first-year nonrelapse mortality was found in the HIV-positive group (8% vs 2%).
In the present study, the analysis of the entire series of eligible patients, enrolled at the time of first-line treatment failure, allows us to evaluate the real effect of HDT with PBSCT in the setting of ARL, according to the intention to treat. In fact not all patients with lymphoma eligible for the HDT salvage program can actually receive transplants, even in the HIV-negative population.15 In the HIV setting this aggressive approach is thought to have an even more difficult application. Nevertheless, in our study, a substantial proportion of patients (54%) could actually receive the transplant according to the protocol (8 [42%] of 19 patients with HL and 19 [61%] of 31 patients with NHL). This result, which can be considered satisfactory for the HIV-positive population, however, appears slightly inferior to the HIV-negative setting in which 50% to 70% of relapsed or refractory patients with chemosensitive disease are reported to receive a transplant.31-33 This could reflect the aggressive behavior of HIV-Ly, and particularly of HL, and the tendency to early relapse or rapid disease progression (2 patients with NHL and 2 with HL in our study progressed within 3 weeks after PBSC collection, before admission for transplantation).3 Nevertheless, the overall results in the present study remains highly satisfactory, with 50% OS in the entire series of patients, after a median follow-up of 45 months.
The multivariate analysis of factors preventing the possibility to proceed to transplantation in this study showed that low CD4 count and stage IV lymphoma were associated with a lower opportunity to receive a PBSC transplant according to the protocol. Moreover, the statistical analyses of prognostic factors for survival in the entire series showed that both lymphoma-related factors, such as stage IV and bone marrow involvement, and HIV-related factor (low CD4 count), together with low PS, had a negative prognostic effect on survival. On the contrary, after transplantation OS and PFS seem to be influenced only by lymphoma response. These data suggest that the immunologic status, together with lymphoma stage, is highly significant at enrollment to predict the possibility to receive a transplant and then the final outcome. However, receiving HDT and PBSC transplant with chemosensitive disease seems to offer a high possibility to achieve a long-lasting CR, irrespective of CD4 count at the time of transplantation, and the final outcome appears to be strongly related only to lymphoma chemosensitivity. This supports any effort to bring patients to transplantation, including optimization of HIV infection control and effective debulking treatment. We should also keep in mind that the possibility to receive effective HAART is an essential requirement to apply an aggressive treatment approach, including HDT, in the HIV setting. Patients not responding to HAART were excluded from this study and could represent a not negligible minority of patients.34 Nevertheless, even in the up-front therapy of NHL greater than 20% of patients in our experience cannot be adequately treated, because of poor clinical conditions, major OIs, or comorbidities.35 The prevalence of HCV infection, which usually excludes patients from HDT experimental program in the HIV-negative population, in HIV-positive patients is higher than in the general population and it was very high in our study (44% of enrolled patients). Fortunately, it does not seem to represent a substantial problem in HIV-positive patients receiving a transplant36 ; 11 patients in our series underwent transplantation with HCV-positive serology, and no one developed HCV-related complications.
The present study does not allow any conclusion about the use of a specific debulking treatment because it was at the center discretion in terms of the number of courses and type of combination CT. Again, the role of rituximab in salvage therapy could not be assessed, mainly because of the heterogeneous treatments received as front-line therapy and as pretransplantation therapy and because of the small number of evaluable patients. It may be that specific debulking strategies would help to increase the response rate before transplantation, contributing to bring a higher proportion of patients to HDT. As expected, and according with the experience in the HIV-negative setting, the results of PBSCT in patients with chemoresistant disease are highly unsatisfactory, in our as in other experience.20 No definitive conclusions could be drawn about the similar outcome of HL and NHL in this study, because of the relatively small number of patients; for the same reason several common prognostic factors, such as primary refractory compared with relapsed disease, might not have achieved statistical significance.
Taken together these results support the use of HDT and PBSCT as the treatment of choice for both relapsed and refractory chemosensitive ARL. Because of the high efficacy of this treatment strategy, any effort should be done to bring patients to transplantation, optimizing HIV infection control and debulking treatment. Adequate supportive therapy is required, and collaboration between oncologists or hematologists and specialists in HIV disease is mandatory. Because a significant proportion of patients, mainly those with advanced lymphoma or advanced HIV disease or both, could not benefit from this approach, it seems rational to explore the use of HDT and PBSCT earlier during the course of lymphoma disease, at least for patients with high-risk disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Annamaria Nosari (Division of Hematology, Niguarda Hospital, Milan, Italy), Clara Schiantarelli (Division of Infectious Disease, Niguarda Hospital, Milan, Italy), Mariagrazia Viganò (Division of Infectious Disease, San Raffaele Hospital, Milan, Italy), Immacolata Izzi (Clinic of Infectious Disease, Policlinico Gemelli, Rome, Italy), Dino Veneri (Division of Hematology, Policlinico GB Rossi, Verona, Italy), Simone Voltolini (Division of Oncology, Carlo Poma Hospital, Mantova, Italy), Marcello Mazzetti (Division of Infectious Disease, Careggi Hospital, Firenze, Italy), Silvia Franceschetti (Division of Hematology, Ospedale Maggiore della Carità, Novara, Italy).
This study was supported by grants from the Istituto Superiore di Sanità (ISS) and the Associazione Italiana per la Ricerca sul Cancro (AIRC).
Authorship
Contribution: A.R. and G.R. designed the study; A.R. was primarily responsible for the evaluation of the data; A.R. and S.C. performed statistical analysis; M. Michieli, B.A., M.R., and R.M. reported on the patients; A.R., M. Michieli, S.C., B.A., C.C., M.R., M.S., R.M., E.V., M. Mazzucato, L.A., P.F., G.C., U.T., and G.R. were involved in the conduct of the study at treatment centers and contributed to patient care; and A.R. wrote the paper, which was reviewed by all the authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the GICAT study appears as a data supplement (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Alessandro Re, Division of Hematologia, Spedali Civili di Brescia, Piazzale Spedali Civili n 1, 25100 Brescia, Italy; e-mail: sandrore@aruba.it.