Abstract
The ras/Raf/Mek/Erk pathway plays a central role in coordinating endothelial cell activities during angiogenesis. Transcription factors Ets1 and Ets2 are targets of ras/Erk signaling pathways that have been implicated in endothelial cell function in vitro, but their precise role in vascular formation and function in vivo remains ill-defined. In this work, mutation of both Ets1 and Ets2 resulted in embryonic lethality at midgestation, with striking defects in vascular branching having been observed. The action of these factors was endothelial cell autonomous as demonstrated using Cre/loxP technology. Analysis of Ets1/Ets2 target genes in isolated embryonic endothelial cells demonstrated down-regulation of Mmp9, Bcl-XL, and cIAP2 in double mutants versus controls, and chromatin immunoprecipitation revealed that both Ets1 and Ets2 were loaded at target promoters. Consistent with these observations, endothelial cell apoptosis was significantly increased both in vivo and in vitro when both Ets1 and Ets2 were mutated. These results establish essential and overlapping functions for Ets1 and Ets2 in coordinating endothelial cell functions with survival during embryonic angiogenesis.
Introduction
Angiogenesis, the biologic process by which endothelial cells (ECs) form new blood vessels from an existing vascular network, is a critical process in normal vertebrate embryonic development, as well as in processes like wound healing and inflammation in adults. Angiogenesis is also an essential element in many pathologic conditions, including cancer.1,2 Angiogenesis is regulated by a balance of both positive and negative signaling events mediated by growth factors and their receptors as well as by cell adhesion to the extracellular matrix.1–4 These complex signaling and cell adhesion interactions alter the growth, migration, survival, and differentiation of ECs through modulation of the intracellular signaling pathways that control these processes.1–5
Among these pathways, the ras/Raf/Mek/Erk pathway has been proposed to play a central role in coordinating these cellular activities during development and tumor angiogenesis. For example, gene knockouts of B-raf and Mek-1 point to their role in placental vascular formation during extraembryonic development, although their action in embryonic development is redundant.6,7 Expression of dominant-negative Raf in the tumor vasculature in a transplantation model increases EC apoptosis and decreases tumor growth,8 and sustained Erk activity is critical for EC migration and angiogenesis in the chick chorioallantoic membrane assay.9 In cell culture studies, Erk signaling has been implicated in EC survival.10–12 ECs are especially sensitive to apoptotic signals during angiogenesis, and the sustained activation of Erk signaling by the combination of growth factor receptors and integrin adhesion may be important in preventing cell death during this process.9,10
The downstream targets of Erks that mediate these effects on ECs remain largely ill-defined. The Raf/Mek/Erk pathway can prevent EC apoptosis and promote sprouting by antagonizing Rho-dependent signaling.13 The Ets-family transcription factor Net/Elk3 regulates genes like VEGF and HIF-1A in ECs in a ras-dependent fashion during development and in response to hypoxia.14,15 The transcription factors Ets1 and Ets2 may be additional effectors of the Erk pathway in ECs. Ets1 and Ets2 can be phosphorylated at conserved residues, threonine 38 and threonine 72, respectively, by Erks.16,17 The key phosphorylation event occurs within a region of Ets1 and Ets2 that is conserved through evolution with the Drosophila ortholog, Pointed P2 domain.18
An extensive literature implicates Ets1 in EC differentiation and function based chiefly on overexpression and dominant-negative approaches in cell culture systems.19,20 Ets1 has been proposed to regulate growth factors like VEGF, as well as EC receptors including Flk1 and Tie2 necessary for angiogenesis.21 In addition, Ets1 has been implicated in regulating extracellular proteases like Mmp9 involved in EC migration.22 Ets2 is activated by Erk signaling in ECs cultured in vitro, and small interfering RNA knockdown of Ets2 impairs gene expression and EC function.23 However, neither Ets1- nor Ets2-null mice have been reported to exhibit EC defects, and while Ets2 mutations are extraembryonic lethal, both genes are dispensable for the development of the embryo proper.24–27
The lack of severe embryonic or adult phenotypes in either Ets1 or Ets2 genetic models led us to test the hypothesis that these genes play overlapping, redundant roles during mouse development. Combining homozygous mutant alleles for these 2 genes resulted in embryonic lethality, consistent with this hypothesis. The double-mutant mice exhibited defective blood vessel branching, a defect that by genetic analysis was autonomous to ECs. Analysis of gene expression by quantitative real-time RT-PCR (qPCR) in highly enriched embryonic EC showed down-regulation of the extracellular protease Mmp9 and several antiapoptotic genes, including Bcl-XL and cIAP2 in cells from double-mutant embryos compared with controls. Studies on isolated aortic ECs in vitro support a role for Ets1 and Ets2 in directly regulating antiapoptotic genes and Mmp9. Analysis of cell viability revealed a significant increase in EC apoptosis in vivo and in vitro in the absence of both Ets1 and Ets2. These results indicate that Ets1 and Ets2 play redundant roles downstream of signaling pathways that coordinate EC branching and survival during embryonic development.
Methods
Mice
The Ets1−/− mice were described previously.26 The Ets2T72A mutation (Ets2tmA72Osh referred to here as Ets2A72) was generated by knockin gene targeting as described.28 An epiblast-specific Cre line, Mox2-Cre (termed MORE),29 was used as an alternative to tetraploid aggregation since Cre expression is restricted only to the embryonic tissues. Tie2-Cre mice30 were described previously. Tie2-GFP mice31 were obtained from The Jackson Laboratory. All animals were maintained on a mixed 129/Sv × C57BL/6 × FVB/N background. All mice were genotyped by PCR. Primers and conditions used are available upon request. Use and care of mice in this study was approved by The Ohio State University Institutional Animal Care and Use Committee.
Immunostaining and TUNEL
Whole-mount embryo immunohistochemical staining for platelet endothelial cell adhesion molecule (PECAM) was performed as described.32 For immunofluorescent staining, 5-μm embryo cryosections were prepared and stained with primary antibodies, followed by biotin-conjugated secondary antibody (Rockland) and Alexa Fluor dye 488 or 594 streptavidin (Molecular Probes). TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) staining was performed according to the manufacturer's instructions (Chemicon). Antibodies used were with rat anti–mouse PECAM (MEC13.3; BD Pharmingen), anti–smooth muscle actin antibody (Spring Bioscience), or rabbit anti–mouse Ets2 antibody. The staining was imaged under Zeiss microscope using a Carl Zeiss camera and was quantified using MetaMorph 6.0. The anti-Ets2 antibody was raised in rabbits against the peptide RGTLKRQPAFDT, representing amino acids 20-31 of mouse Ets2, and affinity purified using a column containing the target peptide (BioSource International). This antibody did not cross-react with Ets1 in immunoprecipitation assays or by Western blotting (data not shown).
Construction of targeting vectors
DNA corresponding to mouse Ets2 exons 4 to 5 was used to screen the mouse RPCI-22 129/sv BAC library. One positive clone, 210JS, was used to make the targeting vector. The 4.3-kb DNA fragment between the EcoRI site in intron 2 and the BamHI site in intron 5 was flanked by 2 loxP sites (see Figure 3A). A “floxed“ neomycin resistance cassette was inserted before the EcoRI site in intron 2. A 1-kb fragment immediately upstream of the EcoRI site in Ets2 intron 2 was generated by PCR and used as the short homologous recombination arm. The 8.5-kb fragment between the BamHI site in intron 5 and EcoRI site at the 3′ end of the gene was used as the long recombination arm. The linearized targeting construct was introduced into embryonic stem cells by electroporation, and after neomycin selection, homologous recombinants were identified by PCR and Southern blot analysis. The neomycin/Herpes thymidine kinase cassette was removed by transient expression of PGK-Cre and removal selected for by resistance to ganciclovir. Ets2fl/+ ES cells were injected into B6 blastocysts, and the resulting chimeras were used to establish the Ets2fl and Ets2KO strains.
Isolation of endothelial cells
Embryos were isolated at embryonic day 12.5 (E12.5) and DNA from dissected yolk sacs was used to genotype the embryos. A single-cell suspension of embryonic cells was prepared by established methods.31 Green fluorescent protein (GFP)–positive cells were enriched by cell sorting using the high-speed digital fluorescence activated cell sorter FACSVantage SE (BD Biosciences), upgraded with DiVA option (The Ohio State University Heart and Lung Research Institute Core Facility). Approximately 150,000 cells that were enriched more than 90% (as determined by an additional fluorescence-activated cell sorter (FACS) analysis) were obtained per embryo. Mouse aortic ECs were isolated as previously described.33 Briefly, thoracic aortas were isolated from anesthetized mice and cut longitudinally into 1- to 2-mm2 pieces. Four to 6 explants were placed in fibronectin (50 μg/mL)-coated dishes with a small volume of complete EC culture media (Dulbecco modified Eagle medium [DMEM]–F12 plus 20% heat-inactivated fetal bovine serum [FBS] plus penicillin-streptomycin plus 30 μg/mL endothelial cell growth supplement [Upstate Biotechnology] + 10 U/mL heparin [Sigma-Aldrich]) in 37°C incubator at 5% CO2. Migrating ECs were observed within 2 to 3 days and on day 7, the cells were trypsinized, labeled with 5 μg/mL di-I-acetylated low-density lipoprotein (LDL), and sorted. The purified EC population was characterized and maintained in complete EC culture media in 37°C incubator at 5% CO2.33
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed as described by Hu et al.35 Briefly, wild-type aortic EC precursors were cultured overnight at a density of 3 × 106 cells per 10-cm dish. Cells were cross-linked with formaldehyde at a final concentration of 1% at 37°C for 10 minutes before harvest. Soluble chromatin was prepared after sonication with a Branson 250 digital sonifier (Branson Ultrasonics) to an average DNA length of 200 to 1000 bp and precleared with tRNA-blocked protein G-agarose. Approximately 3 × 105 cell equivalents of the precleared chromatin was immunoprecipitated with 5 μg of antibodies as indicated in Figure 5. Ten percent of the precleared chromatin was set aside as input control. Immunoprecipitation was carried out overnight at 4°C. Immune complexes were pulled down using protein G-agarose, washed, and eluted twice with 250 μL of elution buffer (0.1 M NaHCO3, 1% sodium dodecyl sulfate [SDS]), and reverse cross-linked in 200 mM NaCl at 65°C overnight with 20 μg of RNase A (Sigma-Aldrich). DNA was purified after proteinase K treatment (Invitrogen) with the QIAGEN PCR purification kit using the manufacturer's instructions. Samples were analyzed by real-time PCR using the Roche Universal ProbeLibrary (Roche Diagnostics). The threshold for the promoter being studied was normalized to that of input values and represented as relative enrichment. All qPCR reactions were analyzed by agarose gel electrophoresis to confirm the presence of a single specific band.
Quantitative real-time PCR
Total RNA was extracted from embryonic ECs using the Cells-to-cDNA II kit (Ambion) and for aortic ECs by TRIzol (Invitrogen) according to the manufacturers' instructions. qPCR was performed exactly as previously described.34 For embryonic ECs, the threshold for the gene being studied was adjusted by that of the reference gene, PECAM. For in vitro studies, the threshold for the gene being studied was adjusted by that of the reference ribosomal L4 gene. All reactions were performed in duplicates. Primers used for qPCR are available upon request. The Student t test was used to determine the statistical significance of the differences between mutant and control genotypes.
Lentiviral transduction
ECs (5 × 105) were cultured overnight and infected with ecotropic lentivirus (pHAGE-IRES-GFP vectors with or without PGK-Cre, a gift from Dr N. Danial's laboratory at Harvard University). Infections were performed as described previously except that the endothelial cell medium decribed above was used.35
EC apoptosis assay
Serum starvation (0.1% serum, 24 hours) was used to induce apoptosis in ECs infected with the lentivirus vector with or without Cre. Apoptosis was assayed using the fluorescence-labeled LIVE/DEAD cell viability kit (Invitrogen) according to the manufacturer's protocol. The staining was imaged under a Zeiss microscope using a Carl Zeiss camera. Phycoerythrin-conjugated apoptotic cells were quantified in the lentiviral-infected cells (expressing GFP) using MetaMorph 6.0.
Results
Embryonic lethality in Ets1−/−;Ets2A72/A72 double-homozygous mutants
Ets1−/− mice are viable and appear normal.25,26 Ets2-deficient mice die due to placental insufficiency,27 but mice with a replacement of the threonine target of Erk phosphorylation (Ets2A72/A72) are viable.28 To determine whether Ets1 and Ets2 may have overlapping functions, mice of genotype Ets1+/−;Ets2A72/A72 were created and intercrossed, with homozygous double-mutant mice expected at a frequency of 25%. Instead of the expected Mendelian ratio, no Ets1−/−;Ets2A72/A72 double-mutant mice were found among 355 viable individuals obtained from the cross, while a compensating increase in the percentage of the other expected genotypes was observed (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results indicated that the combination of Ets1 and Ets2 mutant alleles were embryonic lethal.
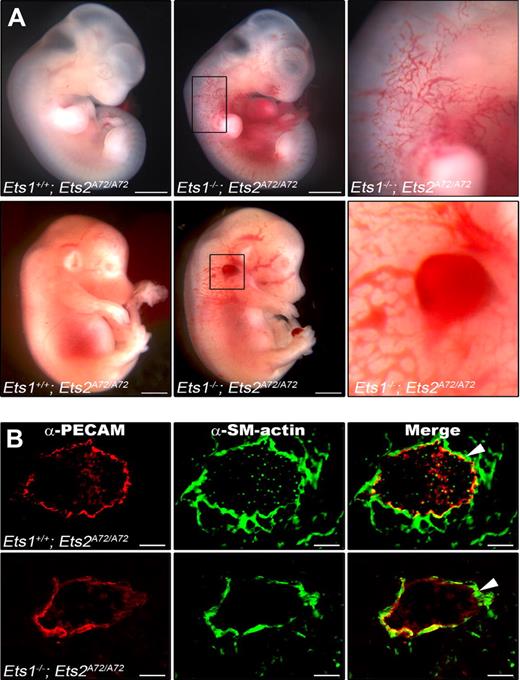
To determine when lethality occurred, embryos from this same cross were examined at different stages of development. This analysis demonstrated that viable double-mutant mice were found at a lower than expected Mendelian ratio beginning at E11.5, and by E15.5 the double-mutant genotype was no longer detected (supplemental Table 2). All other genotypes were detected at compensated ratios (data not shown). Beginning at E11.5 rare but viable (eg, heartbeat could be detected at dissection) Ets−/−;Ets2A72/A72 embryos could be visibly distinguished from littermates. These Ets1−/−;Ets2A72/A72 embryos had large, dilated blood vessels, and edema and hemorrhage were also frequently detected in the double-mutant embryos (Figure 1A). These visibly affected, nonresorbed embryos were infrequent especially at later stages of development accounting for less than 10% of double-mutant embryos recovered at all stages examined (data not shown). Because we wanted to uncover the causal mechanism of Ets1 and Ets2 function, subsequent studies described below were performed only with double-mutant embryos that had no visible phenotype, that is, the double-mutants used for subsequent experiments could be distinguished from controls only by genotyping.
Ets1−/−;Ets2A72/A72 mutant embryos have edema and hemorrhage, but blood vessel structure is unaffected. (A) E11.5 (top) and E14.5 (bottom) embryos; control embryo is at the left, mutant embryo is at the right. Boxed areas are enlarged to show region of edema at E11.5 or hemorrhage at E14.5. Bars, 0.5 mm. (B) Cryosections of control (top) and Ets1−/−;Ets2 A72/A72 (bottom) embryos were stained with anti–PECAM antibody (green) and anti–smooth muscle α-actin antibody (red). Representative data for E13.5 embryo are shown; a total of 4 embryos were analyzed. Bars, 50 μm
Ets1−/−;Ets2A72/A72 mutant embryos have edema and hemorrhage, but blood vessel structure is unaffected. (A) E11.5 (top) and E14.5 (bottom) embryos; control embryo is at the left, mutant embryo is at the right. Boxed areas are enlarged to show region of edema at E11.5 or hemorrhage at E14.5. Bars, 0.5 mm. (B) Cryosections of control (top) and Ets1−/−;Ets2 A72/A72 (bottom) embryos were stained with anti–PECAM antibody (green) and anti–smooth muscle α-actin antibody (red). Representative data for E13.5 embryo are shown; a total of 4 embryos were analyzed. Bars, 50 μm
A role for Ets2 in extraembryonic development is well established, and the Ets2A72 allele has been demonstrated to be haploinsufficient, with defects in the differentiation of the labyrinth layer of the placenta evident at E11.5.27,28 Thus, the histology of Ets1−/−;Ets2A72/A72 extraembryonic tissue was scrutinized to determine whether the observed phenotype resulted from placental defects (supplemental Figure 1A). The histologic analysis demonstrated that placenta, and in particular the labyrinth layer, from the double-mutant mice appeared indistinguishable from control littermates. Furthermore, the ratio of maternal and fetal blood vessels in the labyrinth layer of double-mutant embryos was indistinguishable from controls, representing additional evidence in support of the absence of a placental phenotype (supplemental Figure 1B).
Defective angiogenesis in Ets1−/−;Ets2A72/A72 double-homozygous mutants
Because of the visible phenotype present in some double-mutant mice, we focused our attention on the vasculature. Blood vessels are composed of both ECs and cells that provide vessel strength and support, the vascular smooth muscle cells and pericytes.36 To examine the blood vessel architecture in Ets1−/−;Ets2A72/A72 embryos, indirect immunofluorescence was performed on cryosections. EC marker PECAM (CD31) and smooth muscle marker α-actin antibodies were used to visualize ECs and vascular smooth muscle cells, respectively (Figure 1B). The analysis, carried out on 4 double-mutant embryos, indicated that there was no obvious defect in the organization of ECs and vascular smooth muscle cells within existing blood vessels in Ets1−/−;Ets2A72/A72 embryos compared with controls.
To determine whether the overall vascular architecture was altered in Ets1−/−;Ets2A72/A72 embryos, PECAM immunostaining was used to visualize the vascular system in whole mount. Representative results are presented in Figure 2 for Ets1−/−;Ets2A72/A72 embryos at E10.5, before the embryonic lethal phenotype appears and before any visible difference between genotypes can be detected. The analysis demonstrated a striking reduction of vascular complexity at E10.5 (Figure 2). Often the vessels in the double-mutant embryos were dilated, and there was no clear distinction between large and smaller vessels, as was apparent in the controls (Figure 2, head region). In total, 4 E10.5 Ets1−/−;Ets2A72/A72 double-mutant embryos without visible phenotype were examined by PECAM whole-mount staining, and all exhibited the defective blood vessel branching phenotype. In addition, the branching phenotype was observed in similar sized groups of E9.5 and E11.5 embryos (data not shown). Littermate controls were either wild type (shown in Figure 2) or heterozygous (data not shown) for the Ets1-null allele, and these control embryos showed no branching defects. In addition, embryos of genotype Ets1−/−;Ets2+/+, Ets1−/−;Ets2+/−, or Ets1+/−;Ets2+/−, produced in different crosses, had no obvious vascular defects (data not shown).
Ets1−/−;Ets2A72/A72 embryos have defects in vessel branching and reduced vascular complexity. E10.5 mouse embryos were analyzed by immunohistochemistry with anti–PECAM antibody in whole mount. Controls are in top row (WT), and Ets1−/−;Ets2A72/A72 embryos are in lower row (DM). Arrowheads highlight examples of vessel branching in control embryos that are absent in the double-mutant embryos. Bars, 0.25 mm.
Ets1−/−;Ets2A72/A72 embryos have defects in vessel branching and reduced vascular complexity. E10.5 mouse embryos were analyzed by immunohistochemistry with anti–PECAM antibody in whole mount. Controls are in top row (WT), and Ets1−/−;Ets2A72/A72 embryos are in lower row (DM). Arrowheads highlight examples of vessel branching in control embryos that are absent in the double-mutant embryos. Bars, 0.25 mm.
Conditional deletion of Ets2 in endothelial cells in combination with the Ets1-null allele results in angiogenesis defects
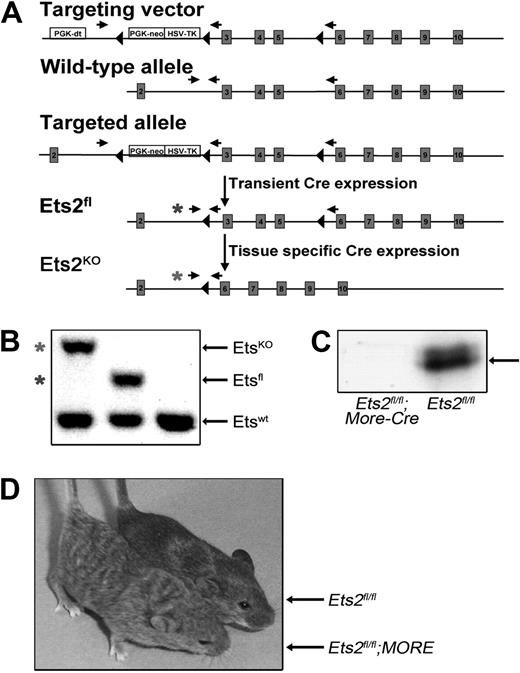
To circumvent extraembryonic lethality and thus better study the role of Ets2 in ECs, a conditional Ets2 allele containing loxP sites was created (Ets2fl, Figure 3A). In this allele, exons 3 to 5 of Ets2 were flanked by loxP sites. Ets2 gene exon 1 encodes the 5′ untranslated region (UTR), and exon 2 encodes the first 24 amino acids of the protein including the initiation ATG codon. Removal of exons 3 to 5 by Cre would result in a knockout allele (termed the Ets2-KO allele, Ets2KO), because this allele would produce spliced RNA products in which the exon 2 short reading frame would be out of frame with codons in exons 6 to 10, resulting in multiple stop codons present immediately downstream of the exon 2 coding sequence (Figure 3A). This strategy is different from that used in the previously published Ets2db1 knockout allele and Ets2flox allele, in which exons 8 to 10, encoding the DNA binding domain, were deleted.27,37 The Ets2db1 knockout allele leads to production of a C-terminal truncated product that lacks nuclear localization signals and DNA binding activity.27
Generation of Ets2 conditional knockout mice. (A) Illustration of targeting strategy. The position of the PCR primers used to distinguish the various alleles are indicated, and asterisks highlight primers used to distinguish Ets2fl and EtsKO alleles. (B) PCR genotyping results for Ets2ko/+, Ets2fl/+, and Ets2+/+ mice, as indicated. Asterisks indicate the PCR products that distinguish the Ets2fl and Ets2ko alleles, and they are coded to the appropriate primer sets represented in panel A. (C) Western blot prepared using embryonic fibroblast extracts derived from MORE;Ets2fl/fl or Ets2fl/fl, as indicated. (D) Waved hair phenotype of adult: Ets2fl/fl;MORE mice (left) versus Ets2fl/fl control littermate (right).
Generation of Ets2 conditional knockout mice. (A) Illustration of targeting strategy. The position of the PCR primers used to distinguish the various alleles are indicated, and asterisks highlight primers used to distinguish Ets2fl and EtsKO alleles. (B) PCR genotyping results for Ets2ko/+, Ets2fl/+, and Ets2+/+ mice, as indicated. Asterisks indicate the PCR products that distinguish the Ets2fl and Ets2ko alleles, and they are coded to the appropriate primer sets represented in panel A. (C) Western blot prepared using embryonic fibroblast extracts derived from MORE;Ets2fl/fl or Ets2fl/fl, as indicated. (D) Waved hair phenotype of adult: Ets2fl/fl;MORE mice (left) versus Ets2fl/fl control littermate (right).
After successful homologous recombination of the Ets2fl allele in embryonic stem cells, mice carrying either the Ets2fl allele or the Ets2KO allele could be generated and successfully identified by PCR genotyping (Figure 3B). Ets2fl/fl mice were born at the expected Mendelian frequencies and were normal and viable. However, mice homozygous for the Ets2KO allele created by deletion of exons 3 to 5 in the germline died during embryogenesis. The Ets2KO allele appeared indistinguishable from the published phenotype of the Ets2db1 allele by 3 criteria. First, embryonic lethality occurred by day E8.5 when Ets2KO is homozygous in the germline (not shown), the same as in the published model.27 Second, the combination of Ets2KO with EtsA72 results in embryonic lethality with no embryos surviving after E11.5 (not shown), again the same as for the previously published model.28 Third, genetic rescue of the extraembryonic lethal phenotype using epiblast-specific Mox2-cre (MORE) mice to restrict the Ets2KO/KO genotype to the embryo29 resulted in mice that did not express Ets2 (Figure 3C) but that were viable and had a waved hair phenotype (Figure 3D). This phenotype is very similar to the phenotype of Ets2db1/db1 mice obtained by tetraploid rescue.27
To further characterize the Ets2fl allele, MORE;Ets1+/−;Ets2fl/+ mice were created and mated to Ets1−/−;Ets2fl/fl mice. Mice of genotype MORE;Ets1−/−;Ets2fl/fl would be predicted at a frequency of 12.5% from this mating; however, no pups of this genotype were recovered among 78 pups genotyped, indicating embryonic lethality (data not shown). Analysis of embryos obtained from timed matings indicated that MORE;Ets1−/−;Ets2fl/fl embryos died about the same age as the Ets1−/−;Ets2A72/A72 embryos (supplemental Table 2). A visible but infrequent phenotype similar to the Ets1−/−;Ets2A72/A72 embryos was also detected in viable MORE;Ets1−/−;Ets2fl/fl embryos (Figure 4A first panel). These genetic results are consistent with the lack of obvious placental malformations and suggest that placental defects are not likely the cause of the phenotype of Ets1−/−;Ets2A72/A72 embryos.
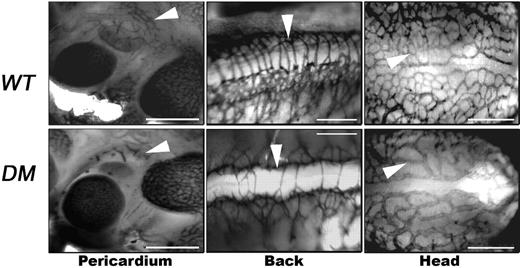
Tie-2-Cre;Ets1−/−;Ets2fl/fl embryos have obvious defects in angiogenesis. (A) MORE;Ets1−/−;Ets2fl/fl (left) and Tie2-Cre;Ets1−/−;Ets2fl/fl (right) E11.5 embryos with edema and hemorrhage. Bars, 0.5 mm. (B) PCR products from DNA extracted from yolk sac of E11.5 Tie2-Cre;Ets1−/−;Ets2fl/fl (lane 1), Ets2fl/+ (lane 2), or Ets2fl/KO (lane 3) mice. (C) PECAM (red) and Ets2 (green) antibody staining of cryosections from E11.5 embryos of genotype Ets1−/−;Ets2fl/fl (top panels) or Tie2-Cre;Ets1−/−;Ets2fl/fl (bottom panels). The third panel is the merged image of PECAM and Ets2 staining. Bars, 50 μm (D) PECAM antibody staining in whole mount of Tie2-Cre;Ets1−/−;Ets2fl/fl embryos and Ets1−/−;Ets2fl/fl controls, as indicated. Representative data are shown, including pericardium (E11.5, first left panel set), back (E10.5, second middle panel set), and head (E10.5, third right panel set). Arrowheads highlight examples of vessel branching in control embryos that are absent and/or abnormal in the double-mutant embryos. Bars, 0.25 mm.
Tie-2-Cre;Ets1−/−;Ets2fl/fl embryos have obvious defects in angiogenesis. (A) MORE;Ets1−/−;Ets2fl/fl (left) and Tie2-Cre;Ets1−/−;Ets2fl/fl (right) E11.5 embryos with edema and hemorrhage. Bars, 0.5 mm. (B) PCR products from DNA extracted from yolk sac of E11.5 Tie2-Cre;Ets1−/−;Ets2fl/fl (lane 1), Ets2fl/+ (lane 2), or Ets2fl/KO (lane 3) mice. (C) PECAM (red) and Ets2 (green) antibody staining of cryosections from E11.5 embryos of genotype Ets1−/−;Ets2fl/fl (top panels) or Tie2-Cre;Ets1−/−;Ets2fl/fl (bottom panels). The third panel is the merged image of PECAM and Ets2 staining. Bars, 50 μm (D) PECAM antibody staining in whole mount of Tie2-Cre;Ets1−/−;Ets2fl/fl embryos and Ets1−/−;Ets2fl/fl controls, as indicated. Representative data are shown, including pericardium (E11.5, first left panel set), back (E10.5, second middle panel set), and head (E10.5, third right panel set). Arrowheads highlight examples of vessel branching in control embryos that are absent and/or abnormal in the double-mutant embryos. Bars, 0.25 mm.
The EC autonomous role of Ets2 was genetically tested by using a Tie2-Cre transgene in combination with Ets2fl/fl alleles to inactivate Ets2 in ECs.30 Tie2-Cre;Ets1+/−;Ets2fl/+ mice were generated and mated to Ets1−/−;Ets2fl/fl mice. Mice of genotype Tie2-Cre;Ets1−/−;Ets2fl/fl would be predicted at a frequency of 12.5% from this mating; however, no live mice of this genotype were recovered out of 231 individuals genotyped, again indicating embryonic lethality (data not shown). Embryos obtained from timed matings demonstrated that Tie2-Cre;Ets1−/−;Ets2fl/fl did not survive past E14.5 (supplemental Table 2) and that some embryos of this genotype had the visible blood vessel phenotype described (Figure 4A second panel).
To demonstrate Cre expression in Tie2-Cre;Ets1−/−;Ets2fl/fl ECs, the genotype for Ets2 in the yolk sac, which is rich in embryonic-derived ECs, was determined. This analysis demonstrated that the deletion allele produced by Cre action, Ets2KO, was present (Figure 4B lane 1). Further evidence that Ets2 was mutated in ECs in the embryo was provided by indirect immunofluorescence using an Ets2-specific antibody produced by our laboratory (Figure 4C). The results demonstrated that Ets2 expression was detected in the nucleus of PECAM-positive cells in control embryos that lacked the Tie2-Cre transgene, but that expression was not detected in Tie2-Cre;Ets1−/−;Ets2fl/fl embryos (Figure 4C, compare top panels with bottom panels).
The vascular network was examined by PECAM staining in whole mount in Tie2-Cre;Ets1−/−;Ets2fl/fl and control littermates (Figure 4D). This analysis demonstrated a similar branching phenotype as that observed in Ets1−/−;Ets2 A72/A72 embryos at locations throughout the embryo on E10.5 and E11.5 (Figure 4D). The density of blood vessels, determined as the area of light (inter–blood vessel space) versus dark regions (PECAM-stained), was used as a means to quantify differences between the mutant Tie2-Cre;Ets1−/−;Ets2fl/fl, Ets1−/−;Ets2A72/A72 embryos, and controls. For this analysis, 4 mutant embryos of each genotype were used, and images from 3 similar locations (head and back, as in Figure 4; and trunk, as in supplemental Figure 2A) were selected. This analysis revealed a significant difference in the inter–blood vessel space between the controls and both mutant genotypes. However, the comparison of the 2 mutants revealed no significant difference (supplemental Figure 2B).
Identification of target genes down-regulated in embryonic ECs isolated from Ets1−/−;Ets2A72/A72 double-mutants
To address the mechanism of Ets1 and Ets2 action in ECs, expression of potential Ets target genes in ECs was studied. For this analysis, a well-characterized Tie2-GFP transgene31 was introduced into Ets1−/−;Ets2A72/A72 double-homozygous mutants, allowing isolation of ECs from the mutant mice by high-speed FACS (supplemental Figure 2C). For this purpose, Tie2-GFP;Ets1+/−;Ets2A72/+ mice were generated and mated to Ets1+/−;Ets2A72/A72 mice. Matings were timed and embryos isolated at E12.5. For these experiments, again only live embryos that did not exhibit the visible blood vessel and edema phenotype were used.
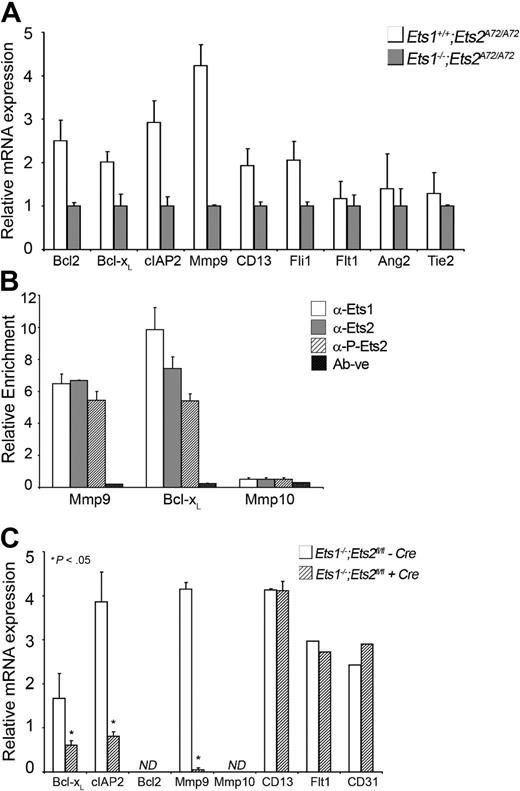
Using high-speed digital FACS, 100 000 to 150 000 GFP-positive cells that were more than 90% enriched were isolated from individual embryos (supplemental Figure 2C). RNA was prepared from the enriched ECs and subjected to qPCR analysis (Figure 5A). Genes selected for analysis contained Ets-binding sites within defined regulatory regions, and they have been reported to be potential target genes for Ets1 or Ets2.19,38 In particular, genes were selected to address whether the Ets factors were regulating ligands and receptors upstream of signaling pathways necessary for angiogenesis19–21 or whether Ets targets represented genes downstream that contribute to EC functions. The analysis demonstrated that expression of genes encoding the apoptosis regulator Bcl2, Bcl-XL, cIAP2, extracellular protease Mmp9, membrane-bound metallopeptidase Cd13, and the Ets family factor Fli1 were all down-regulated in Ets1−/−;Ets2A72/A72 embryos compared with Ets1+/+;Ets2A72/A72 (Figure 5A) or Ets1+/−;Ets2A72/A72 embryos (data not shown). In contrast, expression of genes encoding tyrosine kinase receptors critical for vascularization and angiogenesis such as Tie2, and the vascular endothelial growth factor receptors Flt1, as well as Ang2, encoding a negative ligand for Tie2, were expressed at the same level in double-mutant embryos and controls (Figure 5A). In addition, extracellular proteases like Mmp3 and urokinase plasminogen activator (uPA), and proapoptotic genes like Bak and Bax were not significantly affected in the double-mutant ECs (supplemental Figure 2C).
Ets1 and Ets2 directly regulate antiapoptotic genes in vivo and in vitro. (A) Target gene expression in GFP-tagged embryonic endothelial cells isolated by high-speed cell sorting from double-mutant and control mice as indicated. mRNA levels of target genes were standardized to PECAM/CD31 mRNA levels. Cells from 3 individual embryos for each genotyped were analyzed in duplicate experiments. Error bars indicate standard deviation. An asterisk indicates a significant difference between control and double mutant (P < .05) as determined by Student t test. (B) ChIP experiments in cultured aortic ECs, quantified by qPCR. Anti-Ets1, anti-Ets2, and anti–Ets-pThr72 phospho-specific antibodies were used. (C) Analysis of target gene expression by qPCR in cultured aortic ECs with and without Ets1/Ets2 as indicated. ND indicates not detected.
Ets1 and Ets2 directly regulate antiapoptotic genes in vivo and in vitro. (A) Target gene expression in GFP-tagged embryonic endothelial cells isolated by high-speed cell sorting from double-mutant and control mice as indicated. mRNA levels of target genes were standardized to PECAM/CD31 mRNA levels. Cells from 3 individual embryos for each genotyped were analyzed in duplicate experiments. Error bars indicate standard deviation. An asterisk indicates a significant difference between control and double mutant (P < .05) as determined by Student t test. (B) ChIP experiments in cultured aortic ECs, quantified by qPCR. Anti-Ets1, anti-Ets2, and anti–Ets-pThr72 phospho-specific antibodies were used. (C) Analysis of target gene expression by qPCR in cultured aortic ECs with and without Ets1/Ets2 as indicated. ND indicates not detected.
Bcl-XL and Mmp9 are direct targets of Ets1 and Ets2 in ECs
To further directly test the relationship between Ets1 and Ets2 and putative target genes identified in the mutant embryos, we prepared primary EC cultures for in vitro studies. ECs from the aorta of adult Ets1−/−;Ets2fl/fl and Ets1+/+;Ets2+/+ mice were isolated, purified, and cultured. These aortic ECs formed cobblestone-like monolayers at confluence, stained positive for PECAM/CD-31, imported fluorescently labeled low-density lipoproteins (Di-I-Ac-LDL), and formed tubelike structures when grown in a Matrigel, all properties consistent with their identity as ECs (see supplemental methods and supplemental Figure 3A-C).
The wild-type aortic ECs that contain both Ets1 and Ets2 were used to perform ChIP experiments. The ChIP experiments demonstrated that both Ets1 and Ets2 were enriched at regulatory sequences in both Bcl-XL and Mmp9 genes (Figure 5B and supplemental Figure 4A-B). In addition, the activated, phosphorylated form of Ets2 pThr72 was also detected at the promoters of both the genes, as determined by using antibody that specifically recognizes this form of Ets2 (Figure 5B). In contrast, Ets1 and Ets2 failed to associate with Mmp10, a gene that also contained conserved Ets-binding sites in its promoter.
Aortic ECs with Ets1−/−;Ets2fl/fl genotype were infected with lentivirus that expressed Cre to generate ECs that lack both Ets1 and Ets2 (supplemental Figure 4C-D). Analysis of expression of selected target genes in the double-knockout cells by qPCR demonstrated that expression of Bcl-XL, cIAP2, and Mmp9 were significantly reduced when both Ets1 and Ets2 were absent in these cells compared with control, while Bcl2 expression was not detected in the cultured aortic ECs (Figure 5C). Other potential target genes like CD13 were not affected by the absence of Ets1 and Ets2, perhaps indicating differences between embryonic and aortic ECs.
Ets1 and Ets2 support survival of ECs in vivo and in vitro
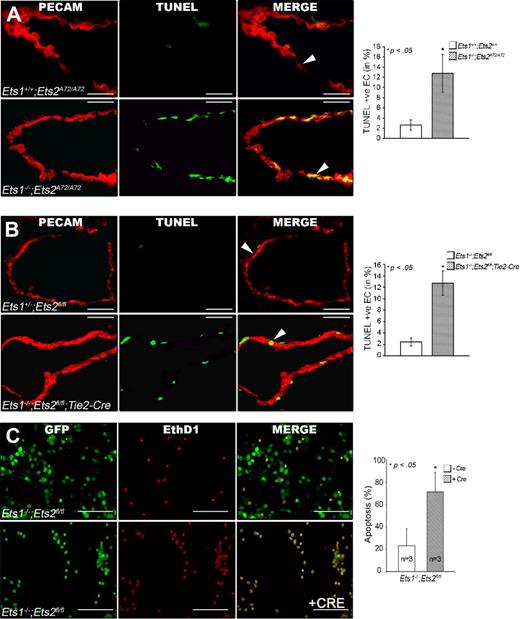
The down-regulation of genes like Bcl-XL and cIAP2 in ECs with mutated Ets1 and Ets2 suggested that these transcription factors might affect EC survival during angiogenesis. To test this hypothesis, apoptosis in ECs in E11.5 and E13.5 embryos was measured by TUNEL labeling in combination with PECAM staining (Figure 6A-B). The experiments revealed an obvious increase in apoptosis in Ets1−/−;Ets2A72/A72 embryos (Figure 6A) as well as in Tie2-Cre; Ets1−/−;Ets2fl/fl embryos (Figure 6B) compared with controls. Importantly, there was no significant increase in apoptosis in cells that were PECAM/CD31-negative (Figure 6A-B and data not shown). Quantification of the number of apoptotic ECs indicated a 4-fold increase in apoptosis in both Ets1−/−;Ets2A72/A72 embryos (Figure 6A) and in Tie2-Cre;Ets1−/−;Ets2fl/fl embryos (Figure 6B) at E11.5 compared with controls. Similar results were obtained for E13.5 embryos (data not shown). Consistent with the results obtained in vivo, aortic ECs that lack functional Ets1 and Ets2 were more sensitive to apoptosis induced by growth factor starvation than were control cells not infected with the lentivirus Cre (Figure 6C). Furthermore, cells with wild-type Ets1 and Ets2 infected with lentivirus Cre also showed no increase in apoptosis due to Cre expression (supplemental Figure 4E-F)
Increased EC apoptosis in Ets1/Ets2 double-mutant mice and in cultured cells deprived of growth factor. (A-B) Anti-PECAM (red) and TUNEL (green) double staining of thin frozen sections from E11.5 embryo of genotype Ets1−/−;EtsA72/A72 (A) or Tie2-Cre Ets1−/−;Ets2fl/fl (B). The third figure in each panel is the merged image, as indicated. The graphic panel shows the ratio of apoptotic endothelial cells (staining for both PECAM and TUNEL) to total PECAM-positive cells, expressed as percentage of apoptotic cells. At least 300 cells were counted in sections obtained from 2 different E11.5 embryos of genotype Ets1−/−;EtsA72/A72 (C) or Tie2-Cre Ets1−/−;Ets2fl/fl (D), and appropriate controls as indicated in the figure. Bars, 50 μm. (C) LIVE/DEAD staining of cultured aortic ECs of indicated genotype without Cre (top panels) or with Cre (bottom panels), that is, Ets1/Ets2-null condition. Graph at right is quantification of the results. Cells staining red indicate apoptotic/dead cells. Bars, 50 μm. For all panels, error bars indicate SD. * P < .05 (significant difference) between control and double mutant as determined by Student t test.
Increased EC apoptosis in Ets1/Ets2 double-mutant mice and in cultured cells deprived of growth factor. (A-B) Anti-PECAM (red) and TUNEL (green) double staining of thin frozen sections from E11.5 embryo of genotype Ets1−/−;EtsA72/A72 (A) or Tie2-Cre Ets1−/−;Ets2fl/fl (B). The third figure in each panel is the merged image, as indicated. The graphic panel shows the ratio of apoptotic endothelial cells (staining for both PECAM and TUNEL) to total PECAM-positive cells, expressed as percentage of apoptotic cells. At least 300 cells were counted in sections obtained from 2 different E11.5 embryos of genotype Ets1−/−;EtsA72/A72 (C) or Tie2-Cre Ets1−/−;Ets2fl/fl (D), and appropriate controls as indicated in the figure. Bars, 50 μm. (C) LIVE/DEAD staining of cultured aortic ECs of indicated genotype without Cre (top panels) or with Cre (bottom panels), that is, Ets1/Ets2-null condition. Graph at right is quantification of the results. Cells staining red indicate apoptotic/dead cells. Bars, 50 μm. For all panels, error bars indicate SD. * P < .05 (significant difference) between control and double mutant as determined by Student t test.
Discussion
Formation of the branched endothelial network during development involves a complex series of events that include sprouting, cell proliferation, directed cell migration, tubule formation, vessel fusion, and vessel pruning.39 In addition to modulating these events, EC growth factor receptors like VEGFR2 and Tie2 are also recognized as mediators of EC survival.3,40 For example, both cell-cell and integrin-matrix adhesion must be disrupted during the initial sprouting stage of angiogenensis, events that if uncoupled from receptor signaling can trigger cell apoptosis.3 In addition, programs for cell motility and replication must be tightly coordinated with alterations in cell adhesion and survival. Our studies implicate Ets1 and Ets2 as signaling effectors that act in an EC-autonomous fashion to coordinate cell survival with other molecular events required during angiogenesis.
Several previous studies have strongly implicated Ets1, as well as several other ETS family factors, including Fli1, Erg, and Nerf-2, as regulators of EC function.19–21 Previous studies have also indicated that Ets-1 can activate ligands and receptors, like Ang2, Tie2, and Flt1, that regulate angiogenesis.20,21,41 However, these in vitro studies were based on overexpression and dominant-negative approaches, and thus were not capable of adequately addressing issues of target specificity and redundancy inherent in studying the complex 27-member Ets gene family. Both genetic and biochemical evidence presented herein demonstrate that Ets1 and Ets2 act in a redundant fashion to regulate target genes in ECs. This is to our knowledge the first demonstration of redundant function within the ETS family in mammals. Furthermore, the results show that Ets1 and Ets2 act primarily downstream of receptor signaling, regulating target genes that promote cell survival. However, it is difficult to completely rule out compensation by other member of the large ETS family when either Ets1 or Ets2 are deleted in ECs. Further analysis will be required to better define all interactions among members of this large gene family.
Ets1 and Ets2 have been implicated in both promoting and preventing cell apoptosis in different cell types.25,26,34,42–45 A remarkable feature of our results is that apoptosis was selectively increased only in ECs in the double-mutant embryos at the stages of development studied. The results argue that the ability of Ets1 and Ets2 to promote cell survival is restricted to ECs and is not a general mechanism for preventing cell death during development.
While the striking reduction in vascular branching observed in mice doubly mutant for Ets1 and Ets2 is the phenotype on which we focused, our studies do not conclusively demonstrate that the vascular defect is directly related to embryonic lethality. Tie2-Cre is expressed in a panendothelial-specific fashion, but it is also expressed in hematopoietic stem cells and in mesenchymal cells of the atrioventricular canal that may derive from an endocardial-mesenchymal transformation.30 Expression of Cre in these other cell types complicates the interpretation of the cause of lethality in the double-mutant embryos, and further genetic and molecular studies will be required to fully address this issue. However, despite the hurdle created by the embryonic lethality of the double-mutants, the combination of in vivo and in vitro approaches used herein allows the redundant role of Ets1 and Ets2 in EC function to be clearly discerned.
Ets1 and Ets2 are protooncogenes, and it has been demonstrated that mammary tumor progression is restricted when Ets2 is mutated in the tumor stroma.28 Studies reported herein are consistent with the hypothesis that Ets1 and Ets2 may act from the tumor microenvironment, specifically in ECs, to affect tumor angiogenesis and metastasis. Because increasing EC apoptosis may provide an attractive therapeutic approach to blocking tumor angiogenesis,5,46 defining the cell-autonomous action of Ets1 and Ets2 during tumorigenesis may lend new insights into mechanisms involved in tumor angiogenesis, as well as provide new molecular targets of potential clinical interest.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge The Ohio State University Comprehensive Cancer Center's Transgenic/Knockout Shared Resource, Real-time PCR Shared Resource, and Flow Cytometry Shared Resource for their roles in supporting this work. We also acknowledge the Cincinnati Children's Hospital Medical Center Viral Vector Core. We thank Jianping Guo and Martina Gutik for excellent technical assistance. We thank the University Laboratory Animal staff for providing excellent animal care.
This work was supported by National Cancer Institute grants R01-CA53271 and P01-CA097189 (to M.C.O.).
National Institutes of Health
Authorship
Contribution: G.W., R.S., C.Z.C., S.M.S., and R.S. performed the experiments. G.W., R.S., C.Z.C., S.M.S., and M.C.O. analyzed the results. G.W. and R.S. created the figures. N.M., M.W., A.K.M., R.G.O., and G.L. provided critical reagents, including mice. M.W., G.L., and M.C.O. designed the research. G.W., R.S., C.Z.C, R.G.O., N.M., G.L., and M.C.O. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Present address for G.W.: The Broad Institute of Harvard University and Massachusetts Institute of Technology, Cambridge, MA 02142.
Correspondence: Michael C. Ostrowski, The Ohio State University, 810 Biomedical Research Tower, 460 W 12th Ave, Columbus, OH 43210; e-mail: michael.ostrowski@osumc.edu.
References
Author notes
*G.W. and R.S. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal